Recent Analytical Approaches for the Study of Bioavailability and Metabolism of Bioactive Phenolic Compounds
Abstract
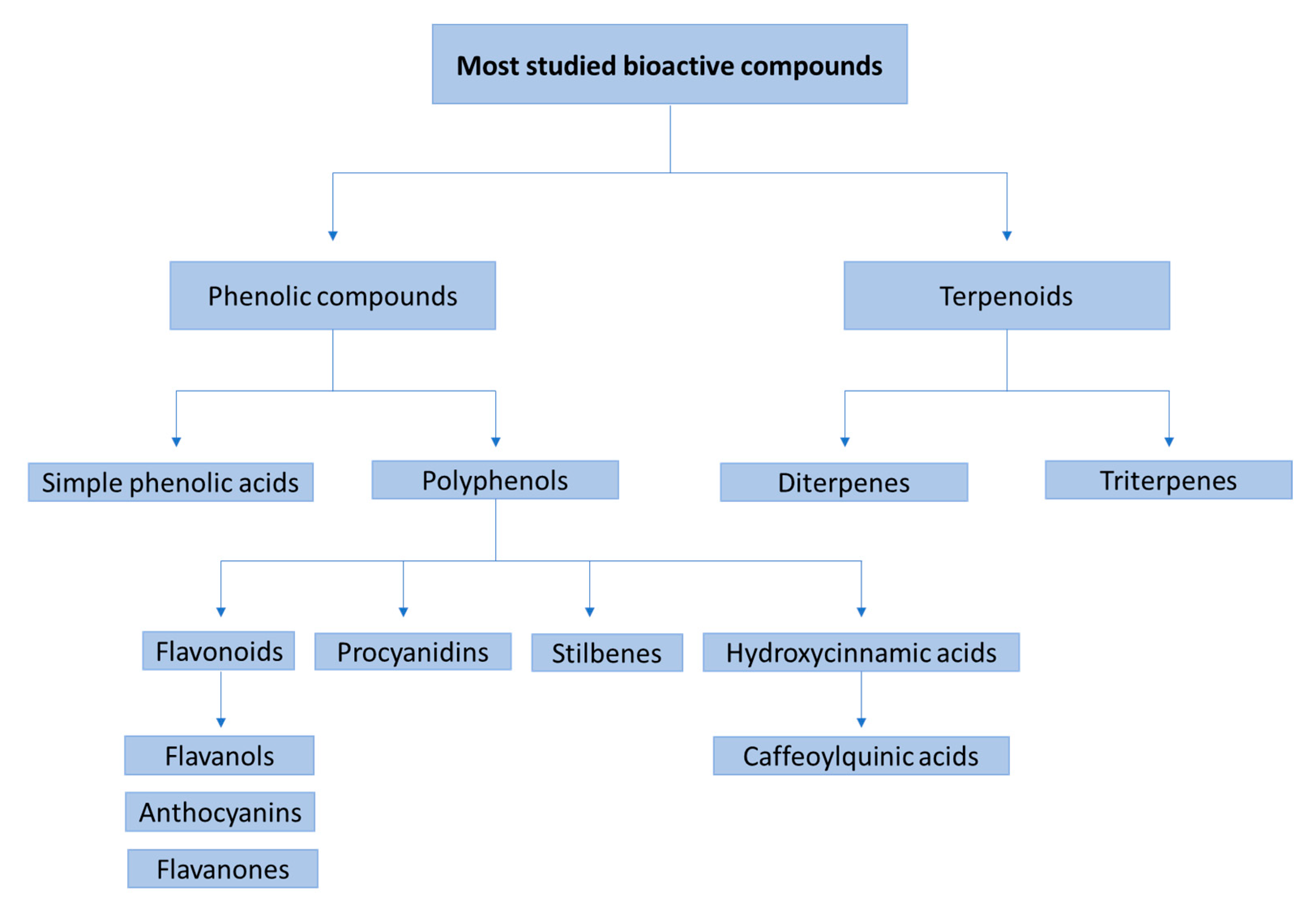
:1. Introduction
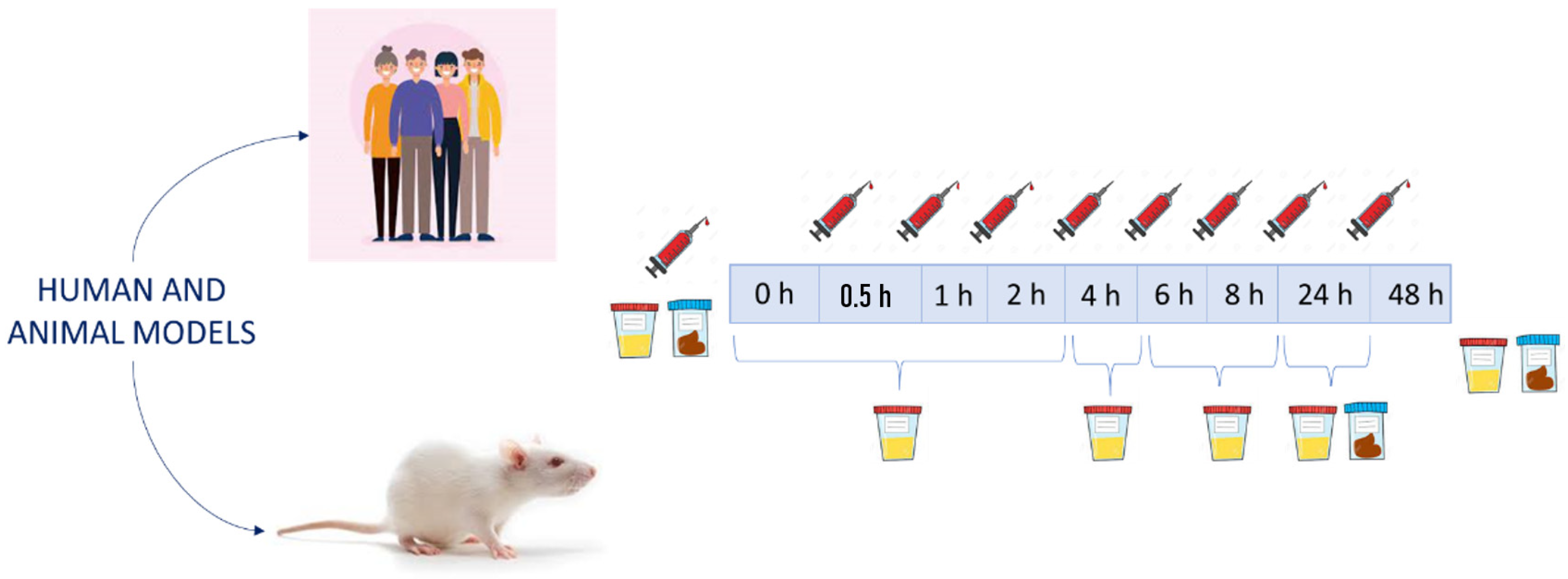
2. Experimental Designs
3. Biological Samples
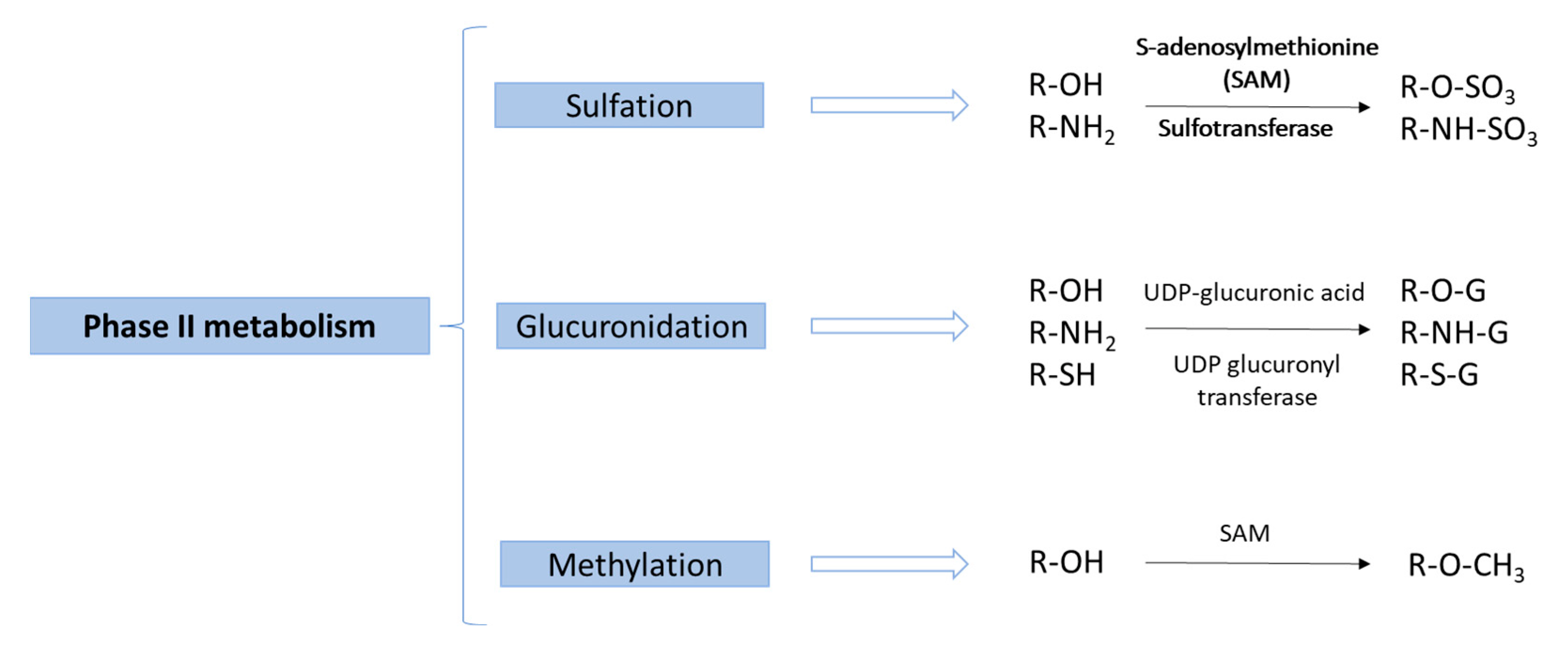
4. Instrumental Techniques
5. Data Processing
6. Recent Advances and Future Trends
| Matrix | Bioactive Compounds | Model (Nº Animals/Volunteers) | Biological Samples | Collection Times | Technique (Column) | Relevant Results (Metabolites, Reactions, etc.) | Reference |
|---|---|---|---|---|---|---|---|
| Rosemary extract | Flavonoids, diterpenes and triterpenes | Mice model (in situ perfusion assay) (n = 7) | Gastrointestinal liquid | 5, 10, 15, 20, 25, 30 min | HPLC–ESI–QTOF-MS (RP-C18) | Several diterpenes and four new metabolites detected in plasma. Sulfation and glucuronidation reactions. | [37] |
| Plasma | End of the assay | ||||||
| Ginsenoside Rb1 | Ginsenoside Rb1, Impact of 3 different fibers | Male Sprague Dawley rats (n = 32) | Plasma | 0, 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 24, and 48 h | UHPLC–ESI–QQQ-MS (RP-C18) | Secondary ginsenosides, especially ginsenoside CK, are the major active metabolites. Prebiotics promote the proliferation of certain bacterial strains that improve the biotransformation and bioavailability of ginsenosides. | [76] |
| Feces | -14 d, 0 h and 48 h | ||||||
| Quercetin glucoside mixture supplement | Quercetin glucoside (Quercetin-3-O-glucoside) and its glucose adducts | Male Wistar/ST rats (n = 35) | Plasma | Once in weeks 2, 4, 6, 8 | HPLC–ESI–QQQ-MS (RP-C18) | Three phases of quercetin metabolism, including cumulative, transient, and stable phases revealed. Water-soluble dietary fibers, especially soybean fiber, enhanced quercetin bioavailability. | [69] |
| Urine | Two times in weeks 2, 4, 6, 8 | ||||||
| Feces | Three times in weeks 2, 4, 6, 8 | ||||||
| Tomato juice | Lycopene, naringenin and chlorogenic acid | Sprague Dawley rats (n = 16) | Plasma | End of experiment | HPLC–ESI–IT-MS (RP-C18) | Total cholesterol was lower after the intervention. Low bioavailability of chlorogenic acid and naringenin. | [30] |
| Urine | Daily for 5 weeks | ||||||
| Feces | Daily for 5 weeks | ||||||
| Liver | End of experiment | ||||||
| Arbequina table olives | Hydroxytyrosol, tyrosol, verbascoside, luteolin, salidroside and p-coumaric acid | Male Sprague-Dawley rats (n = 7) | Plasma | 0, 30 min | HPLC–ESI- QIT-MS (RP-C18) | The possible metabolism suffered in the enterocytes cannot be underestimated. Importance of different mechanisms of absorption depending on the hydrophilic or lipophilic nature of the analyte. | [90] |
| Red raspberry | Raspberry Ketone (4-(4-hydroxyphenyl)-2-butanone)) | Mice (Non-specified) | Plasma | End of experiment | UHPLC–ESI– QQQ-MS (RP-C18) | 25 analytes identified as RK-derived metabolites. | [31] |
| Brain | End of experiment | ||||||
| Extra virgin olive oil (EVOO) | Oleocanthal (OLC) | Sprague-Dawley rats (n = 4) | Intestinal fluid | Every 5 min for 60 min | UHPLC–ESI-QQQ-MS (RP-C18) | Metabolism of phase I and II. Higher levels of OLC are expected to reach human plasma vs. rat plasma. | [36] |
| Plasma and intestinal lumen | End of experiment | ||||||
| Red grape polyphenols | Flavanols, phenolic acids, cinnamic acids, valerolactone and valeric acid | Wistar rats (n = 12) | Serum | 0, 2, 4, 7, 24, 48 h | HPLC–ESI–QTOF-MS (RP-C18) | Organic cultivation system influences the bioavailability and metabolism of polyphenols. Phase II metabolites. | [80] |
| Red grape polyphenols | Cinnamic acid, benzoic acid, flavonoid, phenylpropionic and phenylacetic acid | Male Fischer-344 rats (n = 54) | Serum | End of experiment | HPLC–ESI–QTOF-MS (RP-C18) | Flavonoid phase II metabolites. 6 h of light per day improves bioavailability of phenolic compounds. | [81] |
| Calafate berry extract | Anthocyanins and hydroxycinnamic acids | Gerbils (n = 18) | Plasma | 0, 1, 2, 4, 8, 12 h | GC–EI- QQQ-MS (HP-5MS) | ß-oxidation products were detected. Hydroxycinnamic, benzoic, and phenylacetic acids derivatives. No parental anthocyanins were detected. | [74] |
| Red wine extract. | Flavan-3-ols, proanthocyanidins | Male Sprague-Dawley rats (n = 3) | Plasma | 24 h | UHPLC–ESI–Q-Orbitrap-MS (RP-C18) | Phase II metabolism. Importance of the colonic microbiota in the transformation of proanthocyanidins. | [60] |
| Urine | 24 h | ||||||
| Feces | 24 h | ||||||
| Corylin extract supplement | Corylin metabolites | Male SPF grade KM mice (n = 18) | Plasma | 0.5, 6 h | UHPLC–ESI–QTOF-MS (RP-C18) | Phase I metabolism of corylin. Oxidation, hydration, glucuronidation and sulfation reactions. | [34] |
| Urine | End of experiment | ||||||
| Feces | End of experiment | ||||||
| Bile | End of experiment | ||||||
| Grape pomace | Phenolic acids and anthocyanins | Male rats (n = 30) | Urine | 0, 6 and 14 months | UHPLC–ESI–QTOF-MS (RP-C18) | Methylated, sulfated and glucuronidated metabolites. Growth inhibition of Clostridium. | [98] |
| Malaxinic acid and its aglycone | Malaxinic acid (MA) and its aglycone (MAA) | Male Sprague-Dawley rats (n = 50) | Plasma | 0, 15, 30, 60, 120, 240, 480 min | HPLC–ESI–Q-IT-MS (RP-C18) | Absence of intact forms of MA and MAA. Glucuronide metabolites were detected. | [73] |
| Rice bran enzymatic extract | Ferulic acid | Male Wistar rats (n = 50) | Plasma | 0, 15, 30, 60 min 3, 6, 12, 18, 24 h | UHPLC–ESI–QQQ-MS (RP-C18) | Sulfated metabolites and unconjugated simple aromatic acids. Phase II metabolites. | [61] |
| Urine | 0, 1, 2, 3, 4, 5, 6, 9, 24, 48 h | ||||||
| Feces | 0, 24, 36, 48 h | ||||||
| Specific phenolic compounds | Hydroxytyrosol, hydroxytyrosol acetate, DOPAC | Sprague-Dawley rats (n = 120) | Plasma | 0, 0.5, 1, 2, 4, 8, 24 h | UHPLC–ESI–QQQ-MS (RP-C18) | Influence of the sex-linked metabolism on the excretion pattern. The amounts of bioactive compounds did not result in a proportional increase in their plasma concentrations. | [83] |
| Matrix | Bioactive Compounds | Model (Nº/Volunteers) | Biological Samples | Collection Times | Technique (Column) | Relevant Results (Metabolites, Reactions, etc.) | Reference |
|---|---|---|---|---|---|---|---|
| Rosemary tea | Phenolic acids, flavonoids, | Healthy human volunteers (n = 12) | Plasma | 0, 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 9, 10 h | HPLC–ESI–QTOF-MS(RP-C18) | Phase II metabolites bioavailables. Metabolism by colonic microbiota. | [86] |
| Urine | (−2,0), (0–2), (2–5), (5–8), (8–12), (12–24) h | ||||||
| Two cocoa products | Flavanols | Healthy human volunteers (n = 13) | Plasma | 0, 0.5, 1, 2, 3, 4, 6, 8 h | HPLC–ESI–QTOF-MS(RP-C18) | Phase II derivatives of epicatechin, phenyl-valerolactone and phenylvaleric acid. Importance of colonic reactions. | [43] |
| Urine | (−2,0), (0–4), (4-8), (8–12), (1–-24) h | ||||||
| Cocoa products | Phenolics, flavanols | Healthy human volunteers (n = 13) | Urine | 0, 6, 9, 12, 24, 30, 36, 48 h | UHPLC–ESI–QTOF-MS (RP-C18) | Use of multivariate analyses (PCA and PLS-DA) to identify bioavailable compounds Phenyl-valerolactone metabolites. Phase II conjugated metabolites. | [96] |
| Bilberry pomace extract | Anthocyanins | Healthy women and women with Crohn’s disease (n = 10) | Plasma | 0, 1, 2, 4, 8 h | HPLC–ESI–QQQ-MS/MS (RP-C18) | Glucuronides and sulfated metabolites were detected in plasma and urine samples. Higher bioavailability in presence of an intact gut, revealing its potential site of action. | [45] |
| Urine | (−24–0), (0–2), (2-–4), (4–8), (8-–24) h | ||||||
| Ileostomy fluid | (−12–0), (0–1), (1–2), (2–4), (4–6), (6–8) h | ||||||
| Cranberry juice cocktail | Flavonoids, phenolic acids and proanthocyanidins | Healthy men and postmenopausal women (n = 10) | Plasma | 0, 0.25, 0.5, 1, 2, 3, 4, 5, 6, 10 h | HPLC–ESI–QQQ-MS (RP-C18, RP-C12) | Presence of PAC-A2 dimers in urine. Rapid phase II transformation and excretion of anthocyanins. | [70] |
| Urine | 0, 2, 4, 6, 8, 10, 24 h | ||||||
| Instant green/ roasted coffee | Hydroxy-cinnamates | Healthy human volunteers (n = 12) | Plasma | 0, 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 9, 10, 12 h | HPLC–ESI–QTOF-MS (RP-C18) | Sulfate, methyl and glucuronides metabolites were detected. Dihydrohydroxycinnamate esters have been identified for the first time in both plasma and urine. | [57] |
| Urine | (−2–0), (0–2), (2–5), (5–8), (8–12), (12–24) h | ||||||
| Yerba mate infusion | Caffeoylquinic acids, ferulic acids and hydroxyl-cinnamic acids | Healthy human volunteers (n = 12) | Plasma | 0, 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 9, 10, 12 h | HPLC–ESI–QTOF-MS (RP-C18) | Sulfated conjugates of caffeic and ferulic/isoferulic acids. Phase II flavanol and phenolic acids metabolites. | [46] |
| Urine | (−2–0), (0–2), (2–5), (5–8), (8–12), (12–24) h | ||||||
| Mixed berry fruit pureé | Caffeoylquinic acids and anthocyanins | Healthy human volunteers (n = 13) | Plasma | 0, 0.5, 1, 2, 4, 6 h | HPLC-ESI-QQQ-MS/MS (RP-C18) | Presence of methylated, sulfated and some dual conjugated compounds. Importance of catabolism in the colon. | [56] |
| Beverage enriched with grape pomace extract | Procyanidins, phenolic acids and flavanols | Healthy human volunteers (n = 12) | Urine | 0, 24 h | HPLC–ESI–Q-Orbitrap-MS (RP-C18) | Methylation, sulfation, glucuronidation, hydroxylation, dehydrogenation and glycine conjugation reactions. Seventy metabolites identified. | [50] |
| Red wine enriched with a grape pomace extract | Phenolic acids, flavanols, stilbenes, anthocyanins and phenyl alcohols. | Healthy human volunteers (n = 12) | Plasma | 0, 0.5, 1, 2, 4, 6 h | UHPLC–ESI–QQQ-MS (RP-C18) | Intense phase II metabolism. Sulfated form predominated over the glucuronidated one. Novel endogenous production pathway of hydroxytyrosol metabolites. | [47] |
| Urine | (0–6), (6–12), (12–24) h | ||||||
| Orange juice | Flavanones, flavones and phenolic acids | Healthy human volunteers (n = 9) | Plasma | 0, 1, 2, 3, 4, 5, 6, 8 h | UHPLC–ESI–QQQ-MS (RP-C18) | Phase II sulfate, glucuronide, and methyl metabolites. Dehydroxylation and demethoxylation mediated by the gut microflora. | [79] |
| Urine | (0–2), (2–5), (5–10), (10–15), (15–24) h | ||||||
| Cocoa rich in polyphenols | Epicatechin, valerolactones and flavonols | Healthy human volunteers (n = 15) | Urine | 0, 3, 6, 9, 12, 24, 30, 36, 48 h | UHPLC–ESI–QTOF-MS (RP-C18) | Phase II conjugation into sulfated and glucuronide derivatives. Bacterial metabolism of cocoa major flavanols. | [49] |
| Cranberry extract | Phenolic acids, anthocyanins | Healthy human volunteers (n = 13) | Urine | Day 1: 0 h Day 7: 1, 2, 4, 6, 8, 10, 12, 24 h | HPLC–ESI–Q-Orbitrap-MS (RP-C18). | Identification of 42 analytes highlighting the detection of six valerolactones/valeric acid derivatives | [48] |
| Common beans (Phaseolus vulgaris L.) | Flavanols, phenolic acids, catechols and pyrogallols. | Healthy human volunteers (n = 7) | Plasma | 0, 1, 2, 4, 6, 8 h | UHPLC–ESI–QTOF-MS (RP-C18) | Glucuronidation and sulfation reactions. Colonic bacterial metabolism of the phenolic compounds was detected. Hippuric acids was the most abundant class of metabolites in urine | [58] |
| Urine | 0, (0–2), (2–4), (4–6), (6–8), (8–24) h | ||||||
| Orange juice | Phenolic acids | Healthy human volunteers (n = 3) | Urine | 0–24 h | GC–MS and HPLC–ESI-Q-Orbitrap-MS (RP-C18) | Free phenolics and glucuronide and sulfate conjugates were detected. GC–MS was not suitable for the analysis of phenolic sulfate and glucuronide metabolites. | [59] |
| Maqui berry extract | Anthocyanins (>35%) and delphinidins (>25%) | Healthy human volunteers (n = 12) | Plasma | 0, 0.5, 1, 1.5, 2, 3, 4, 6, 8 h | UHPLC–DAD–ESI–QQQ-MS/MS (RP-C18) | Extensive and fast first-pass metabolism. Phenolic acids as breakdown products of anthocyanins were observed. | [28] |
| Brown seaweed extract | Phlorotannin metabolites | Overweight and obese volunteers (n = 80) | Plasma | Weeks 0, 8, 16, 24 | UHPLC–ESI–Q-Orbitrap-MS (RP-C18) | Phase II sulfated and glucuronidated metabolites. | [99] |
| Urine | 24 h | ||||||
| Red grape pomace | Anthocyanins, flavan-3-ol monomers, procyanidins | Healthy human volunteers (n = 10) | Plasma | 0, 8, 16, 24 h | UHPLC–ESI–QQQ-MS (RP-C18) | Glucuronide and sulfate forms. High inter-individual variability (importance of gut microbiota). | [87] |
| Urine | (0–3), (3–6), (6–10), (10–24), (24–36), (36–48) h | ||||||
| Green tea | Phenyl-γ-valerolactones | Healthy human volunteers (n = 16) | Urine | Day 0, day 8 | UHPLC–ESI–QQQ-MS (RP-C18) | Large inter-individual variability due to differences in microbiota patterns. Colonic catabolism of (–)-epigallocatechin and (–)-epigallocatechin-3-gallate. | [89] |
| Wild blueberry drinks | Anthocyanins, proanthocyanidins, flavonols and chlorogenic acids. | Healthy human volunteers (n = 9) | Plasma | 0, 1, 2, 4, 6 h | UHPLC–ESI–QTOF-MS (RP-C18) | 23 phenolic acid metabolites were quantified in plasma. Interindividual variability was high (age, dose-dependent effects, gender, gut microbiota and genetic polymorphisms). | [44] |
| Cranberry juice | Proanthocyanidins, anthocyanins, flavonols and phenolic acids | Healthy human volunteers (n = 10) | Plasma | 0, 1, 2, 4, 6, 8, 24 h | UHPLC–ESI–QTOF-MS (RP-C18) | Conjugated and non-conjugated phenolic acid derivatives were detected. Sulfated and glucuronidated metabolites. Phase I and phase II metabolism. | [29] |
| Urine | (0–8), (8–24) h | ||||||
| Seed/fruit extract (fraxinus angustifolia vahl) | Secoiridoid glucosides | Healthy human volunteers (n = 9) | Plasma | 0, 1, 2, 4, 8, 24 h | UHPLC–ESI–QTOF-MS (RP-C18) | Metabolic conversion by esterases, glycosidases, and phase II sulfo- and glucuronosyl transferases to form smaller conjugated derivatives. Metabolism by phase I and (or) microbial enzymes. | [84] |
| Urine | 0, (0–8), (8–24) h | ||||||
| Hard gelatine capsule containing phenolic compounds | Flavan-3-ols (epicatechin, procyanidin B1, and polymeric procyanidins) | Healthy human volunteers (n = 7) | Plasma | 0, 1, 2, 4, 8, 24, 48 h | GC–EI-QQQ-MS (DB-5MS) HPLC–DAD–ESI-Q-MS (RP-C18) | Glucuronidated, sulfated and methylated (-)-epicatechin and 5-(3′,4′-dihydroxyphenyl)-valerolactone were the dominant metabolites in blood and urine. High importance of the gut microbiota in flavan-3-ol metabolism. | [63] |
| Urine | (0–4), (4–8), (8–24) h | ||||||
| Feces | (0–24) h |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hosseinzadeh, S.; Jafarikukhdan, A.; Hosseini, A.; Armand, R. The Application of Medicinal Plants in Traditional and Modern Medicine: A Review of Thymus vulgaris. Int. J. Clin. Med. 2015, 06, 635–642. [Google Scholar] [CrossRef] [Green Version]
- Singh, R. Medicinal Plants: A Review. J. Plant Sci. 2015, 3, 50–55. [Google Scholar] [CrossRef]
- Perez-Gregorio, R.; Simal-Gandara, J. A Critical Review of Bioactive Food Components, and of their Functional Mechanisms, Biological Effects and Health Outcomes. Curr. Pharm. Des. 2017, 23, 2731–2741. [Google Scholar] [CrossRef] [PubMed]
- Cádiz-Gurrea, M.d.l.L.; Villegas-Aguilar, M.d.C.; Leyva-Jiménez, F.J.; Pimentel-Moral, S.; Fernández-Ochoa, Á.; Alañón, M.E.; Segura-Carretero, A. Revalorization of bioactive compounds from tropical fruit by-products and industrial applications by means of sustainable approaches. Food Res. Int. 2020, 138, 109786. [Google Scholar] [CrossRef]
- Mitra, S.; Naskar, N.; Chaudhuri, P. A review on potential bioactive phytochemicals for novel therapeutic applications with special emphasis on mangrove species. Phytomedicine Plus 2021, 1, 100107. [Google Scholar] [CrossRef]
- Gonzales, G.B. In vitro bioavailability and cellular bioactivity studies of flavonoids and flavonoid-rich plant extracts: Questions, considerations and future perspectives. Proc. Nutr. Soc. 2017, 76, 175–181. [Google Scholar] [CrossRef] [Green Version]
- del Carmen Villegas-Aguilar, M.; Fernández-Ochoa, Á.; de la Luz Cádiz-Gurrea, M.; Pimentel-Moral, S.; Lozano-Sánchez, J.; Arráez-Román, D.; Segura-Carretero, A. Pleiotropic biological effects of dietary phenolic compounds and their metabolites on energy metabolism, inflammation and aging. Molecules 2020, 25, 596. [Google Scholar] [CrossRef] [Green Version]
- Long, F.; Yang, H.; Xu, Y.; Hao, H.; Li, P. A strategy for the identification of combinatorial bioactive compounds contributing to the holistic effect of herbal medicines. Sci. Rep. 2015, 5, 12361. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.H.; Wang, D.M.; Fan, S.F.; Li, D.W.; Luo, Z.W. Synergistic effects and related bioactive mechanism of Potentilla fruticosa L. leaves combined with Ginkgo biloba extracts studied with microbial test system (MTS). BMC Complement. Altern. Med. 2016, 16. [Google Scholar] [CrossRef] [Green Version]
- Lozoya-Agullo, I.; González-Álvarez, I.; González-Álvarez, M.; Merino-Sanjuán, M.; Bermejo, M. In Situ Perfusion Model in Rat Colon for Drug Absorption Studies: Comparison with Small Intestine and Caco-2 Cell Model. J. Pharm. Sci. 2015, 104, 3136–3145. [Google Scholar] [CrossRef]
- Qusa, M.H.; Siddique, A.B.; Nazzal, S.; El Sayed, K.A. Novel olive oil phenolic (−)-oleocanthal (+)-xylitol-based solid dispersion formulations with potent oral anti-breast cancer activities. Int. J. Pharm. 2019, 569, 118596. [Google Scholar] [CrossRef]
- Rodríguez-López, P.; Lozano-Sanchez, J.; Borrás-Linares, I.; Emanuelli, T.; Menéndez, J.A.; Segura-Carretero, A. Structure–biological activity relationships of extra-virgin olive oil phenolic compounds: Health properties and bioavailability. Antioxidants 2020, 9, 685. [Google Scholar] [CrossRef]
- Ulaszewska, M.M.; Weinert, C.H.; Trimigno, A.; Portmann, R.; Andres Lacueva, C.; Badertscher, R.; Brennan, L.; Brunius, C.; Bub, A.; Capozzi, F.; et al. Nutrimetabolomics: An Integrative Action for Metabolomic Analyses in Human Nutritional Studies. Mol. Nutr. Food Res. 2019, 63, 1800384. [Google Scholar] [CrossRef]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.-L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Velderrain-Rodríguez, G.R.; Palafox-Carlos, H.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Chen, C.-Y.O.; Robles-Sánchez, M.; Astiazaran-García, H.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Phenolic compounds: Their journey after intake. Food Funct. 2014, 5, 189–197. [Google Scholar] [CrossRef]
- Fernández-Ochoa, Á.; Leyva-Jiménez, F.J.; Cádiz-Gurrea, M.d.l.L.; Pimentel-Moral, S.; Segura-Carretero, A. The role of high-resolution analytical techniques in the development of functional foods. Int. J. Mol. Sci. 2021, 22, 3220. [Google Scholar] [CrossRef]
- Misra, B.B. New software tools, databases, and resources in metabolomics: Updates from 2020. Metabolomics 2021, 17, 49. [Google Scholar] [CrossRef]
- Lea, T. Caco-2 Cell Line. Impact Food Bioact. Heal. Vitr. Ex Vivo Model. 2015, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Sánchez, A.; Borrás-Linares, I.; Barrajón-Catalán, E.; Arráez-Román, D.; González-Álvarez, I.; Ibáñez, E.; Segura-Carretero, A.; Bermejo, M.; Micol, V. Evaluation of the intestinal permeability of rosemary (Rosmarinus officinalis L.) extract polyphenols and terpenoids in Caco-2 cell monolayers. PLoS ONE 2017, 12, e0172063. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.X.; Liu, G.Y.; Yang, Y.F.; Wu, X.W.; Xu, W.; Yang, X.W. Intestinal Absorption of Triterpenoids and Flavonoids from Glycyrrhizae radix et rhizoma in the Human Caco-2 Monolayer Cell Model. Molecules 2017, 22, 1627. [Google Scholar] [CrossRef] [Green Version]
- Kamiloglu, S.; Capanoglu, E.; Grootaert, C.; van Camp, J. Anthocyanin Absorption and Metabolism by Human Intestinal Caco-2 Cells—A Review. Int. J. Mol. Sci. 2015, 16, 21555–21574. [Google Scholar] [CrossRef] [Green Version]
- Ou, K.; Percival, S.S.; Zou, T.; Khoo, C.; Gu, L. Transport of cranberry A-type procyanidin dimers, trimers, and tetramers across monolayers of human intestinal epithelial Caco-2 cells. J. Agric. Food Chem. 2012, 60, 1390–1396. [Google Scholar] [CrossRef]
- Lee, H.J.; Cha, K.H.; Kim, C.Y.; Nho, C.W.; Pan, C.H. Bioavailability of Hydroxycinnamic Acids from Crepidiastrum denticulatum Using Simulated Digestion and Caco-2 Intestinal Cells. J. Agric. Food Chem. 2014, 62, 5290–5295. [Google Scholar] [CrossRef]
- Ding, X.; Hu, X.; Chen, Y.; Xie, J.; Ying, M.; Wang, Y.; Yu, Q. Differentiated Caco-2 cell models in food-intestine interaction study: Current applications and future trends. Trends Food Sci. Technol. 2021, 107, 455–465. [Google Scholar] [CrossRef]
- Alminger, M.; Aura, A.M.; Bohn, T.; Dufour, C.; El, S.N.; Gomes, A.; Karakaya, S.; Martínez-Cuesta, M.C.; Mcdougall, G.J.; Requena, T.; et al. In Vitro Models for Studying Secondary Plant Metabolite Digestion and Bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2014, 13, 413–436. [Google Scholar] [CrossRef] [Green Version]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- World medical association declaration of helsinki: Ethical principles for medical research involving human subjects. J. Korean Med. Assoc. 2014. [CrossRef] [Green Version]
- Schön, C.; Wacker, R.; Micka, A.; Steudle, J.; Lang, S.; Bonnländer, B. Bioavailability study of maqui berry extract in healthy subjects. Nutrients 2018, 10, 1720. [Google Scholar] [CrossRef] [Green Version]
- Feliciano, R.P.; Boeres, A.; Massacessi, L.; Istas, G.; Ventura, M.R.; Nunes Dos Santos, C.; Heiss, C.; Rodriguez-Mateos, A. Identification and quantification of novel cranberry-derived plasma and urinary (poly)phenols. Arch. Biochem. Biophys. 2016, 599, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, I.; Pérez-Sánchez, H.; Martín-Pozuelo, G.; García-Alonso, J.; Periago, M.J. The inhibitory effects of bioactive compounds of Tomato juice binding to hepatic HMGCR: In vivostudy and molecular modelling. PLoS ONE 2014, 9, 1–11. [Google Scholar] [CrossRef]
- Yuan, B.; Zhao, D.; Kshatriya, D.; Bello, N.T.; Simon, J.E.; Wu, Q. UHPLC-QqQ-MS/MS method development and validation with statistical analysis: Determination of raspberry ketone metabolites in mice plasma and brain. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1149, 122146. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yuan, B.; Kshatriya, D.; Polyak, A.; Simon, J.E.; Bello, N.T.; Wu, Q. Influence of Diet-Induced Obesity on the Bioavailability and Metabolism of Raspberry Ketone (4-(4-Hydroxyphenyl)-2-Butanone) in Mice. Mol. Nutr. Food Res. 2020, 64, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ochoa, Á.; Cázares-Camacho, R.; Borrás-Linares, I.; Domínguez-Avila, J.A.; Segura-Carretero, A.; González-Aguilar, G.A. Evaluation of metabolic changes in liver and serum of streptozotocin-induced diabetic rats after Mango diet supplementation. J. Funct. Foods 2020, 64. [Google Scholar] [CrossRef]
- Qin, Z.; Li, S.; Yao, Z.; Hong, X.; Xu, J.; Lin, P.; Zhao, G.; Gonzalez, F.J.; Yao, X. Metabolic profiling of corylin in vivo and in vitro. J. Pharm. Biomed. Anal. 2018, 155, 157–168. [Google Scholar] [CrossRef] [PubMed]
- López-Yerena, A.; Pérez, M.; Vallverdú-Queralt, A.; Miliarakis, E.; Lamuela-Raventós, R.M.; Escribano-Ferrer, E. Oleacein intestinal permeation and metabolism in rats using an in situ perfusion technique. Pharmaceutics 2021, 13, 719. [Google Scholar] [CrossRef]
- López-yerena, A.; Vallverdú-queralt, A.; Mols, R.; Augustijns, P.; Lamuela-raventós, R.M.; Escribano-ferrer, E. Absorption and Intestinal Metabolic Profile of Oleocanthal in Rats. Pharmaceutics 2020, 12, 134, Erratum in Pharmaceutics 2020, 12, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Ochoa, Á.; Borrás-Linares, I.; Pérez-Sánchez, A.; Barrajón-Catalán, E.; González-Álvarez, I.; Arráez-Román, D.; Micol, V.; Segura-Carretero, A. Phenolic compounds in rosemary as potential source of bioactive compounds against colorectal cancer: In situ absorption and metabolism study. J. Funct. Foods 2017, 33. [Google Scholar] [CrossRef]
- Sun, Y.H.; He, X.; Yang, X.L.; Dong, C.L.; Zhang, C.F.; Song, Z.J.; Lu, M.X.; Yang, Z.L.; Li, P. Absorption characteristics of the total alkaloids from Mahonia bealei in an in situ single-pass intestinal perfusion assay. Chin. J. Nat. Med. 2014, 12, 554–560. [Google Scholar] [CrossRef]
- Jesudoss, V.A.S.; Victor Antony Santiago, S.; Venkatachalam, K.; Subramanian, P. Zingerone (Ginger Extract): Antioxidant Potential for Efficacy in Gastrointestinal and Liver Disease. In Gastrointestinal Tissue: Oxidative Stress and Dietary Antioxidants; Academic Press: Cambridge, MA, USA, 2017; pp. 289–297. ISBN 9780128093009. [Google Scholar]
- Musther, H.; Olivares-Morales, A.; Hatley, O.J.D.; Liu, B.; Rostami Hodjegan, A. Animal versus human oral drug bioavailability: Do they correlate? Eur. J. Pharm. Sci. 2014, 57, 280–291. [Google Scholar] [CrossRef]
- Correddu, F.; Lunesu, M.F.; Buffa, G.; Atzori, A.S.; Nudda, A.; Battacone, G.; Pulina, G. Can agro-industrial by-products rich in polyphenols be advantageously used in the feeding and nutrition of dairy small ruminants? Animals 2020, 10, 131. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Ochoa, Á.; Leyva-Jiménez, F.J.; Pimentel-Moral, S.; del Carmen Villegas-Aguilar, M.; Alañón, M.E.; Segura-Carretero, A.; de la Luz Cádiz-Gurrea, M. Revalorisation of Agro-Industrial Wastes into High Value-Added Products. Adv. Sci. Technol. Innov. 2021, 229–245. [Google Scholar] [CrossRef]
- Gómez-Juaristi, M.; Sarria, B.; Martínez-López, S.; Clemente, L.B.; Mateos, R. Flavanol bioavailability in two cocoa products with different phenolic content. A comparative study in humans. Nutrients 2019, 11, 1441. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Mateos, A.; Feliciano, R.P.; Cifuentes-Gomez, T.; Spencer, J.P.E. Bioavailability of wild blueberry (poly)phenols at different levels of intake. J. Berry Res. 2016, 6, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Richling, E. Human intervention study to investigate the intestinal accessibility and bioavailability of anthocyanins from bilberries. Food Chem. 2017, 231, 275–286. [Google Scholar] [CrossRef]
- Gómez-Juaristi, M.; Martínez-López, S.; Sarria, B.; Bravo, L.; Mateos, R. Absorption and metabolism of yerba mate phenolic compounds in humans. Food Chem. 2018, 240, 1028–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motilva, M.J.; Macià, A.; Romero, M.P.; Rubió, L.; Mercader, M.; González-Ferrero, C. Human bioavailability and metabolism of phenolic compounds from red wine enriched with free or nano-encapsulated phenolic extract. J. Funct. Foods 2016, 25, 80–93. [Google Scholar] [CrossRef]
- Baron, G.; Altomare, A.; Regazzoni, L.; Fumagalli, L.; Artasensi, A.; Borghi, E.; Ottaviano, E.; Del Bo, C.; Riso, P.; Allegrini, P.; et al. Profiling Vaccinium macrocarpon components and metabolites in human urine and the urine ex-vivo effect on Candida albicans adhesion and biofilm-formation. Biochem. Pharmacol. 2020, 173, 113726. [Google Scholar] [CrossRef]
- Hakeem Said, I.; Truex, J.D.; Heidorn, C.; Retta, M.B.; Petrov, D.D.; Haka, S.; Kuhnert, N. LC-MS/MS based molecular networking approach for the identification of cocoa phenolic metabolites in human urine. Food Res. Int. 2020, 132, 109119. [Google Scholar] [CrossRef]
- Sasot, G.; Martínez-Huélamo, M.; Vallverdú-Queralt, A.; Mercader-Martí, M.; Estruch, R.; Lamuela-Raventós, R.M. Identification of phenolic metabolites in human urine after the intake of a functional food made from grape extract by a high resolution LTQ-Orbitrap-MS approach. Food Res. Int. 2017, 100, 435–444. [Google Scholar] [CrossRef]
- Ramírez-Garza, S.L.; Laveriano-Santos, E.P.; Marhuenda-Muñoz, M.; Storniolo, C.E.; Tresserra-Rimbau, A.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Health Effects of Resveratrol: Results from Human Intervention Trials. Nutrients 2018, 10, 1892. [Google Scholar] [CrossRef] [Green Version]
- Turner, A.L.; Michaelson, L.V.; Shewry, P.R.; Lovegrove, A.; Spencer, J.P.E. Increased bioavailability of: A randomized, controlled, single blind, crossover human intervention trial. Clin. Nutr. 2021, 40, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, A.; Romasco, T.; D’Ovidio, C.; Rosato, E.; Ulusoy, H.I.; Furton, K.G.; Kabir, A.; Locatelli, M. Determination of phenolic compounds in human saliva after oral administration of red wine by high performance liquid chromatography. J. Pharm. Biomed. Anal. 2022, 209, 114486. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Guo, Z.; Jia, X.; Liu, H.; Ma, L.; Xue, L. The key points in the pre-analytical procedures of blood and urine samples in metabolomics studies. Metabolomics 2020, 16, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Villaret-Cazadamont, J.; Claus, S.P.; Canlet, C.; Guillou, H.; Cabaton, N.J.; Ellero-Simatos, S. Important Considerations for Sample Collection in Metabolomics Studies with a Special Focus on Applications to Liver Functions. Metabolites 2020, 10, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pimpão, R.C.; Ventura, M.R.; Ferreira, R.B.; Williamson, G.; Santos, C.N. Phenolic sulfates as new and highly abundant metabolites in human plasma after ingestion of a mixed berry fruit purée. Br. J. Nutr. 2015, 113, 454–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Juaristi, M.; Martínez-López, S.; Sarria, B.; Bravo, L.; Mateos, R. Bioavailability of hydroxycinnamates in an instant green/roasted coffee blend in humans. Identification of novel colonic metabolites. Food Funct. 2018, 9, 331–343. [Google Scholar] [CrossRef] [Green Version]
- Mecha, E.; Feliciano, R.P.; Rodriguez-Mateos, A.; Silva, S.D.; Figueira, M.E.; Vaz Patto, M.C.; Bronze, M.R. Human bioavailability of phenolic compounds found in common beans: The use of high-resolution MS to evaluate inter-individual variability. Br. J. Nutr. 2020, 123, 273–292. [Google Scholar] [CrossRef]
- Ordóñez, J.L.; Pereira-Caro, G.; Ludwig, I.; Muñoz-Redondo, J.M.; Ruiz-Moreno, M.J.; Crozier, A.; Moreno-Rojas, J.M. A critical evaluation of the use of gas chromatography- and high performance liquid chromatography-mass spectrometry techniques for the analysis of microbial metabolites in human urine after consumption of orange juice. J. Chromatogr. A 2018, 1575, 100–112. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Ordóñez, J.L.; Ludwig, I.; Gaillet, S.; Mena, P.; Del Rio, D.; Rouanet, J.M.; Bindon, K.A.; Moreno-Rojas, J.M.; Crozier, A. Development and validation of an UHPLC-HRMS protocol for the analysis of flavan-3-ol metabolites and catabolites in urine, plasma and feces of rats fed a red wine proanthocyanidin extract. Food Chem. 2018, 252, 49–60. [Google Scholar] [CrossRef]
- Perez-Ternero, C.; Macià, A.; De Sotomayor, M.A.; Parrado, J.; Motilva, M.J.; Herrera, M.D. Bioavailability of the ferulic acid-derived phenolic compounds of a rice bran enzymatic extract and their activity against superoxide production. Food Funct. 2017, 8, 2165–2174. [Google Scholar] [CrossRef]
- Gagnebin, Y.; Tonoli, D.; Lescuyer, P.; Ponte, B.; de Seigneux, S.; Martin, P.-Y.; Schappler, J.; Boccard, J.; Rudaz, S. Metabolomic analysis of urine samples by UHPLC-QTOF-MS: Impact of normalization strategies. Anal. Chim. Acta 2017, 955, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Wiese, S.; Esatbeyoglu, T.; Winterhalter, P.; Kruse, H.P.; Winkler, S.; Bub, A.; Kulling, S.E. Comparative biokinetics and metabolism of pure monomeric, dimeric, and polymeric flavan-3-ols: A randomized cross-over study in humans. Mol. Nutr. Food Res. 2015, 59, 610–621. [Google Scholar] [CrossRef]
- Mosele, J.I.; Martín-Peláez, S.; Macià, A.; Farràs, M.; Valls, R.M.; Catalán, Ú.; Motilva, M.J. Faecal microbial metabolism of olive oil phenolic compounds: In vitro and in vivo approaches. Mol. Nutr. Food Res. 2014, 58, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- Danneskiold-Samsøe, N.B.; Dias de Freitas Queiroz Barros, H.; Santos, R.; Bicas, J.L.; Cazarin, C.B.B.; Madsen, L.; Kristiansen, K.; Pastore, G.M.; Brix, S.; Maróstica Júnior, M.R. Interplay between food and gut microbiota in health and disease. Food Res. Int. 2019, 115, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Durack, J.; Lynch, S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 2019, 216, 20. [Google Scholar] [CrossRef] [Green Version]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Stasiak, M.; Oniszczuk, A. Beneficial Effects of Phenolic Compounds on Gut Microbiota and Metabolic Syndrome. Int. J. Mol. Sci. 2021, 22, 3715. [Google Scholar] [CrossRef]
- Trakooncharoenvit, A.; Tanaka, S.; Mizuta, E.; Hira, T.; Hara, H. Water-soluble dietary fibers enhance bioavailability of quercetin and a fiber derived from soybean is most effective after long-term feeding in rats. Eur. J. Nutr. 2020, 59, 1389–1398. [Google Scholar] [CrossRef]
- McKay, D.L.; Chen, C.Y.O.; Zampariello, C.A.; Blumberg, J.B. Flavonoids and phenolic acids from cranberry juice are bioavailable and bioactive in healthy older adults. Food Chem. 2015, 168, 233–240. [Google Scholar] [CrossRef]
- Emwas, A.H.M. The strengths and weaknesses of NMR spectroscopy and mass spectrometry with particular focus on metabolomics research. Methods Mol. Biol. 2015, 1277, 161–193. [Google Scholar] [CrossRef]
- Gharari, Z.; Bagheri, K.; Derakhshani, B.; Sharafi, A. HPLC-DAD-ESI/MSn analysis of phenolic components of Scutellaria araxensis, S. bornmuelleri and S. orientalis. Nat. Prod. Res. 2020, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Jeong, H.Y.; Jin, M.R.; Lee, H.J.; Cho, J.Y.; Moon, J.H. Metabolism and antioxidant effect of malaxinic acid and its corresponding aglycone in rat blood plasma. Free Radic. Biol. Med. 2017, 110, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, L.; Pastene, E.; Duran-Sandoval, D.; Vergara, C.; Von Baer, D.; Mardones, C. Pharmacokinetics of low molecular weight phenolic compounds in gerbil plasma after the consumption of calafate berry (Berberis microphylla) extract. Food Chem. 2018, 268, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Carry, E.; Zhao, D.; Mogno, I.; Faith, J.; Ho, L.; Villani, T.; Patel, H.; Pasinetti, G.M.; Simon, J.E.; Wu, Q. Targeted analysis of microbial-generated phenolic acid metabolites derived from grape flavanols by gas chromatography-triple quadrupole mass spectrometry. J. Pharm. Biomed. Anal. 2018, 159, 374–383. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Duan, F.; Liu, A.; Li, S.; Zhong, W.; Sheng, W.; Chen, J.; Xu, J.; Xiao, S. Prebiotics enhance the biotransformation and bioavailability of ginsenosides in rats by modulating gut microbiota. J. Ginseng Res. 2021, 45, 334–343. [Google Scholar] [CrossRef]
- Rathod, R.H.; Chaudhari, S.R.; Patil, A.S.; Shirkhedkar, A.A. Ultra-high performance liquid chromatography-MS/MS (UHPLC-MS/MS) in practice: Analysis of drugs and pharmaceutical formulations. Futur. J. Pharm. Sci. 2019, 5. [Google Scholar] [CrossRef] [Green Version]
- Kumar, B.R. Application of HPLC and ESI-MS techniques in the analysis of phenolic acids and flavonoids from green leafy vegetables (GLVs). J. Pharm. Anal. 2017, 7, 349–364. [Google Scholar] [CrossRef]
- Castello, F.; Fernández-Pachón, M.S.; Cerrillo, I.; Escudero-López, B.; Ortega, Á.; Rosi, A.; Bresciani, L.; Del Rio, D.; Mena, P. Absorption, metabolism, and excretion of orange juice (poly)phenols in humans: The effect of a controlled alcoholic fermentation. Arch. Biochem. Biophys. 2020, 695, 108627. [Google Scholar] [CrossRef]
- Iglesias-Carres, L.; Mas-Capdevila, A.; Bravo, F.I.; Aragonès, G.; Arola-Arnal, A.; Muguerza, B. A comparative study on the bioavailability of phenolic compounds from organic and nonorganic red grapes. Food Chem. 2019, 299, 125092. [Google Scholar] [CrossRef]
- Iglesias-Carres, L.; Mas-Capdevila, A.; Bravo, F.I.; Arola, L.; Muguerza, B.; Arola-Arnal, A. Exposure of Fischer 344 rats to distinct photoperiods nfluences the bioavailability of red grape polyphenols. J. Photochem. Photobiol. B Biol. 2019, 199, 111623. [Google Scholar] [CrossRef]
- Agulló, V.; García-Viguera, C.; Domínguez-Perles, R. Beverages Based on Second Quality Citrus Fruits and Maqui Berry, a Source of Bioactive (Poly)phenols: Sorting Out Urine Metabolites upon a Longitudinal Study. Nutrients 2021, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Perles, R.; Auñón, D.; Ferreres, F.; Gil-Izquierdo, A. Gender differences in plasma and urine metabolites from Sprague–Dawley rats after oral administration of normal and high doses of hydroxytyrosol, hydroxytyrosol acetate, and DOPAC. Eur. J. Nutr. 2017, 56, 215–224. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Tomás-Barberán, F.A.; Fança-Berthon, P.; Roller, M.; Zafrilla, P.; Issaly, N.; García-Conesa, M.T.; Combet, E. Targeted and untargeted metabolomics to explore the bioavailability of the secoiridoids from a seed/fruit extract (fraxinus angustifolia vahl) in human healthy volunteers: A preliminary study. Molecules 2015, 20, 22202–22219. [Google Scholar] [CrossRef] [Green Version]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies—Challenges and Emerging Directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef] [Green Version]
- Achour, M.; Bravo, L.; Sarriá, B.; Ben Fredj, M.; Nouira, M.; Mtiraoui, A.; Saguem, S.; Mateos, R. Bioavailability and nutrikinetics of rosemary tea phenolic compounds in humans. Food Res. Int. 2021, 139, 109815. [Google Scholar] [CrossRef]
- Castello, F.; Costabile, G.; Bresciani, L.; Tassotti, M.; Naviglio, D.; Luongo, D.; Ciciola, P.; Vitale, M.; Vetrani, C.; Galaverna, G.; et al. Bioavailability and pharmacokinetic profile of grape pomace phenolic compounds in humans. Arch. Biochem. Biophys. 2018, 646, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]
- Brindani, N.; Mena, P.; Calani, L.; Benzie, I.; Choi, S.W.; Brighenti, F.; Zanardi, F.; Curti, C.; Del Rio, D. Synthetic and analytical strategies for the quantification of phenyl-γ-valerolactone conjugated metabolites in human urine. Mol. Nutr. Food Res. 2017, 61, 6–10. [Google Scholar] [CrossRef]
- Kundisová, I.; Juan, M.E.; Planas, J.M. Simultaneous Determination of Phenolic Compounds in Plasma by LC-ESI-MS/MS and Their Bioavailability after the Ingestion of Table Olives. J. Agric. Food Chem. 2020, 68, 10213–10222. [Google Scholar] [CrossRef]
- Magnusson, B.; Örnemark, U. Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 2nd ed.; Eurachem: Teddington, UK, 2014; ISBN 978-91-87461-59-0. [Google Scholar]
- Kaza, M.; Karaźniewicz-Łada, M.; Kosicka, K.; Siemiątkowska, A.; Rudzki, P.J. Bioanalytical method validation: New FDA guidance vs. EMA guideline. Better or worse? J. Pharm. Biomed. Anal. 2019, 165, 381–385. [Google Scholar] [CrossRef]
- Pluskal, T.; Korf, A.; Smirnov, A.; Schmid, R.; Fallon, T.R.; Du, X.; Weng, J.K. Metabolomics Data Analysis Using MZmine. In Processing Metabolomics and Proteomics Data with Open Software: A Practical Guide; Royal Society of Chemistry: London, UK, 2020; pp. 232–254. [Google Scholar]
- Fernández-Ochoa, Á.; Quirantes-Piné, R.; Borrás-Linares, I.; Cádiz-Gurrea, M.d.l.L.; Alarcón Riquelme, M.E.; Brunius, C.; Segura-Carretero, A. A Case Report of Switching from Specific Vendor-Based to R-Based Pipelines for Untargeted LC-MS Metabolomics. Metabolites 2020, 10, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aron, A.T.; Gentry, E.C.; McPhail, K.L.; Nothias, L.F.; Nothias-Esposito, M.; Bouslimani, A.; Petras, D.; Gauglitz, J.M.; Sikora, N.; Vargas, F.; et al. Reproducible molecular networking of untargeted mass spectrometry data using GNPS. Nat. Protoc. 2020, 15, 1954–1991. [Google Scholar] [CrossRef] [PubMed]
- Hakeem Said, I.; Heidorn, C.; Petrov, D.D.; Retta, M.B.; Truex, J.D.; Haka, S.; Ullrich, M.S.; Kuhnert, N. LC-MS based metabolomic approach for the efficient identification and relative quantification of bioavailable cocoa phenolics in human urine. Food Chem. 2021, 364, 130198. [Google Scholar] [CrossRef] [PubMed]
- Bilal Hussain, M.; Hassan, S.; Waheed, M.; Javed, A.; Adil Farooq, M.; Tahir, A. Bioavailability and Metabolic Pathway of Phenolic Compounds. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019; ISBN 978-1-78984-034-6. [Google Scholar]
- Chacar, S.; Tarighi, M.; Fares, N.; Faivre, J.F.; Louka, N.; Maroun, R.G. Identification of phenolic compounds-rich grape pomace extracts urine metabolites and correlation with gut microbiota modulation. Antioxidants 2018, 7, 75. [Google Scholar] [CrossRef] [Green Version]
- Baldrick, F.R.; McFadden, K.; Ibars, M.; Sung, C.; Moffatt, T.; Megarry, K.; Thomas, K.; Mitchell, P.; Wallace, J.M.W.; Pourshahidi, L.K.; et al. Impact of a (poly)phenol-rich extract from the brown algae Ascophyllum nodosum on DNA damage and antioxidant activity in an overweight or obese population: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 688–700. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [Green Version]
- McClements, D.J. Recent developments in encapsulation and release of functional food ingredients: Delivery by design. Curr. Opin. Food Sci. 2018, 23, 80–84. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the encapsulation in bioavailability of phenolic compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 203. [Google Scholar]
- Zhang, X.; Quinn, K.; Cruickshank-Quinn, C.; Reisdorph, R.; Reisdorph, N. The application of ion mobility mass spectrometry to metabolomics. Curr. Opin. Chem. Biol. 2018, 42, 60–66. [Google Scholar] [CrossRef]
- Levy, A.J.; Oranzi, N.R.; Ahmadireskety, A.; Kemperman, R.H.J.; Wei, M.S.; Yost, R.A. Recent progress in metabolomics using ion mobility-mass spectrometry. TrAC Trends Anal. Chem. 2019, 116, 274–281. [Google Scholar] [CrossRef]
- Song, X.C.; Canellas, E.; Dreolin, N.; Nerin, C.; Goshawk, J. Discovery and Characterization of Phenolic Compounds in Bearberry (Arctostaphylos uva-ursi) Leaves Using Liquid Chromatography-Ion Mobility-High-Resolution Mass Spectrometry. J. Agric. Food Chem. 2021, 69, 10856–10868. [Google Scholar] [CrossRef]
- Montero, L.; Schmitz, O.J.; Meckelmann, S.W. Chemical characterization of eight herbal liqueurs by means of liquid chromatography coupled with ion mobility quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2020, 1631. [Google Scholar] [CrossRef]
- Masike, K.; De Villiers, A.; Hoffman, E.W.; Brand, D.J.; Causon, T.; Stander, M.A. Detailed Phenolic Characterization of Protea Pure and Hybrid Cultivars by Liquid Chromatography-Ion Mobility-High Resolution Mass Spectrometry (LC-IM-HR-MS). J. Agric. Food Chem. 2020, 68, 485–502. [Google Scholar] [CrossRef]
- Delgado-Povedano, M.D.M.; de Villiers, A.; Hann, S.; Causon, T. Identity confirmation of anthocyanins in berries by LC–DAD–IM-QTOFMS. Electrophoresis 2021, 42, 473–481. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Ochoa, Á.; Cádiz-Gurrea, M.d.l.L.; Fernández-Moreno, P.; Rojas-García, A.; Arráez-Román, D.; Segura-Carretero, A. Recent Analytical Approaches for the Study of Bioavailability and Metabolism of Bioactive Phenolic Compounds. Molecules 2022, 27, 777. https://doi.org/10.3390/molecules27030777
Fernández-Ochoa Á, Cádiz-Gurrea MdlL, Fernández-Moreno P, Rojas-García A, Arráez-Román D, Segura-Carretero A. Recent Analytical Approaches for the Study of Bioavailability and Metabolism of Bioactive Phenolic Compounds. Molecules. 2022; 27(3):777. https://doi.org/10.3390/molecules27030777
Chicago/Turabian StyleFernández-Ochoa, Álvaro, María de la Luz Cádiz-Gurrea, Patricia Fernández-Moreno, Alejandro Rojas-García, David Arráez-Román, and Antonio Segura-Carretero. 2022. "Recent Analytical Approaches for the Study of Bioavailability and Metabolism of Bioactive Phenolic Compounds" Molecules 27, no. 3: 777. https://doi.org/10.3390/molecules27030777
APA StyleFernández-Ochoa, Á., Cádiz-Gurrea, M. d. l. L., Fernández-Moreno, P., Rojas-García, A., Arráez-Román, D., & Segura-Carretero, A. (2022). Recent Analytical Approaches for the Study of Bioavailability and Metabolism of Bioactive Phenolic Compounds. Molecules, 27(3), 777. https://doi.org/10.3390/molecules27030777











