Mimic Nature Using Chemotaxis of Ionic Liquid Microdroplets for Drug Delivery Purposes
Abstract
:1. Introduction
2. Ionic Liquids
2.1. Composition and Properties of ILs
2.2. Classification of ILs
2.3. ILs as Promising Alternatives to Traditional Solvents
2.4. Cytotoxicity of ILs
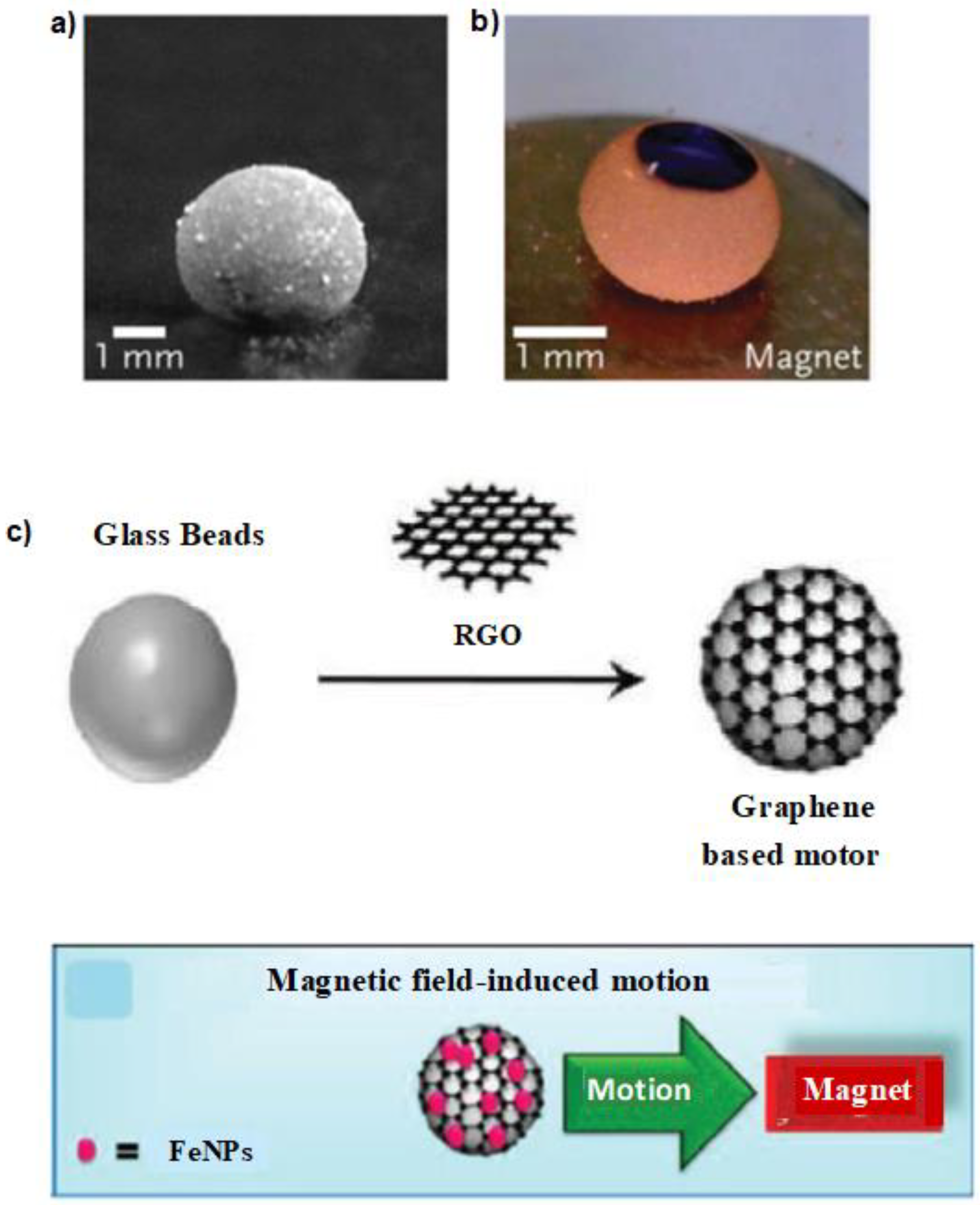
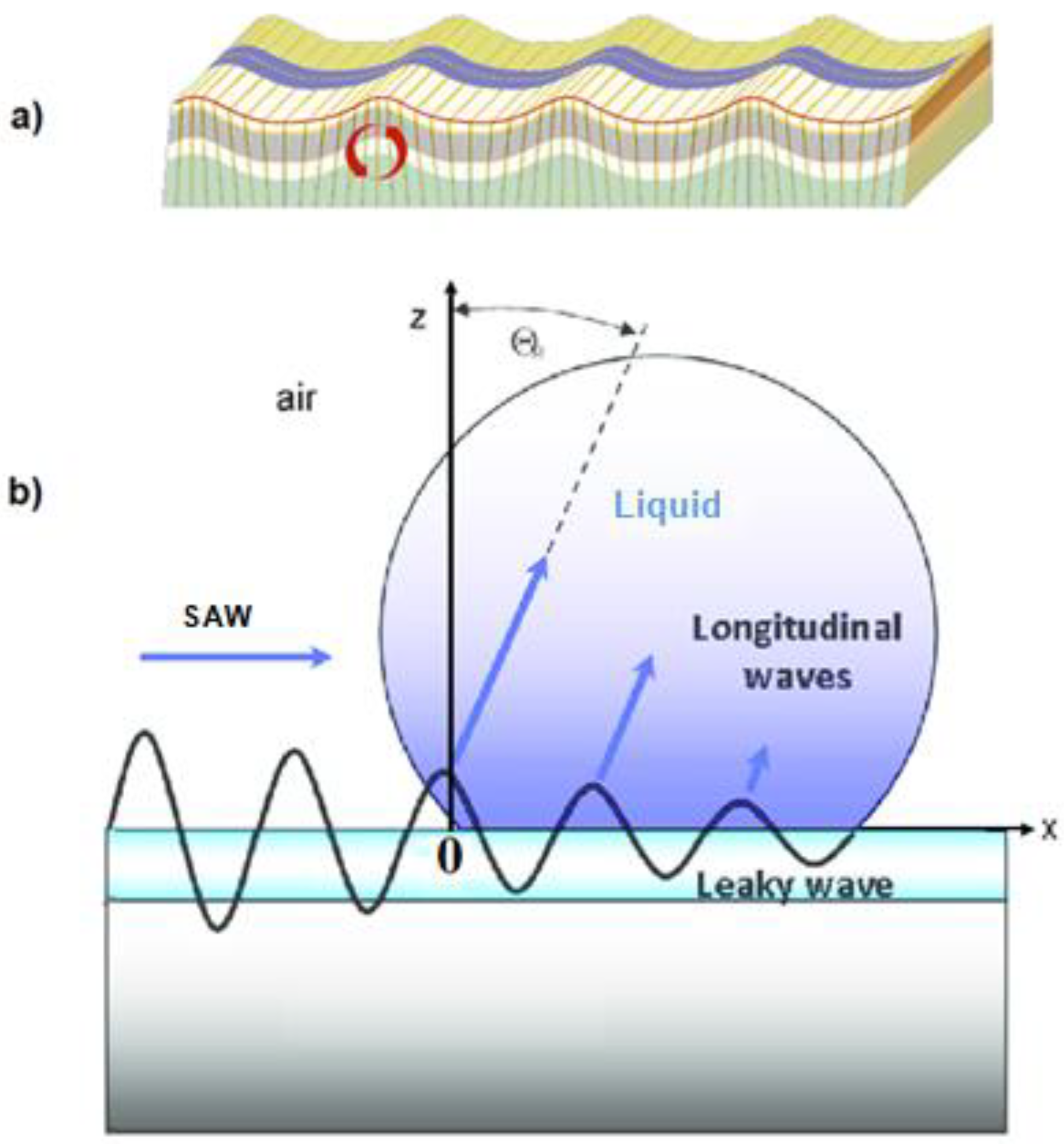
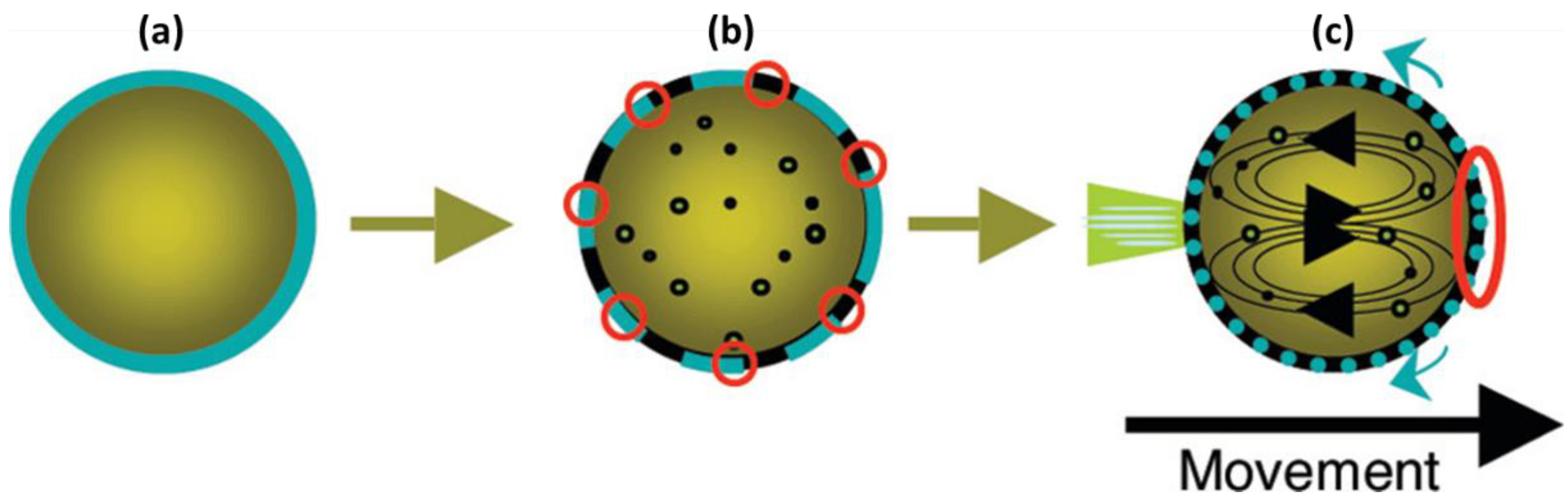
3. Self-Propelled Microdroplets
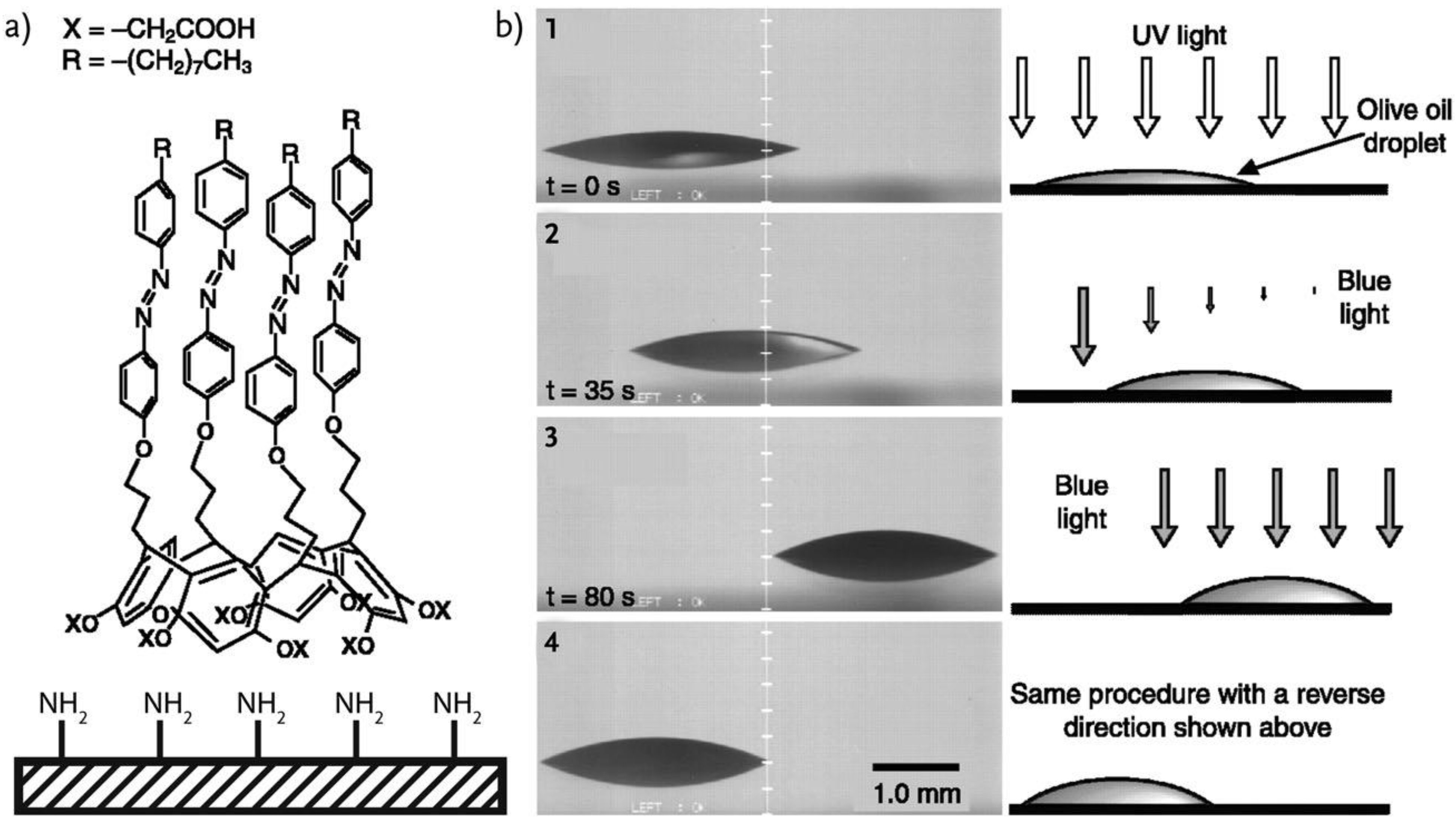
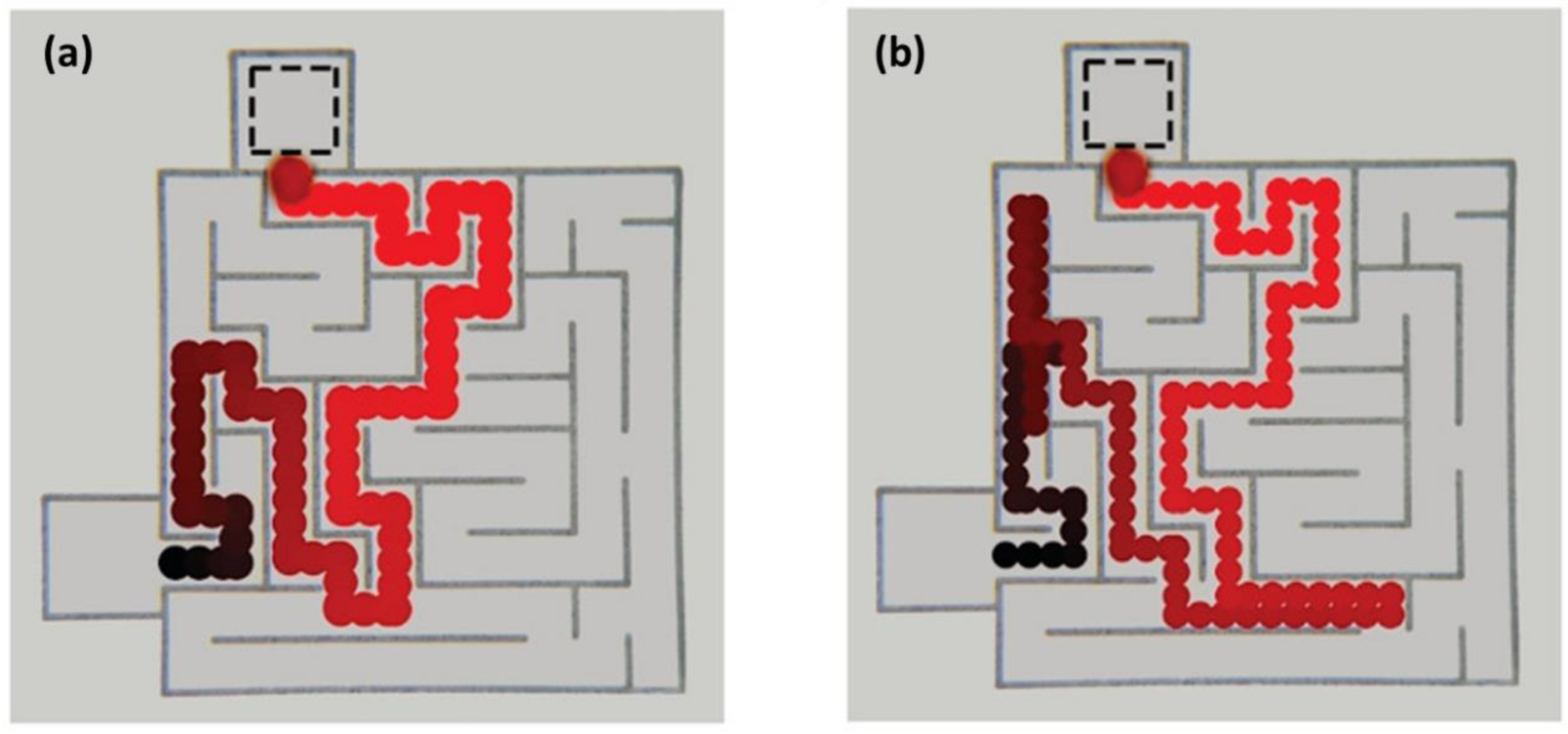
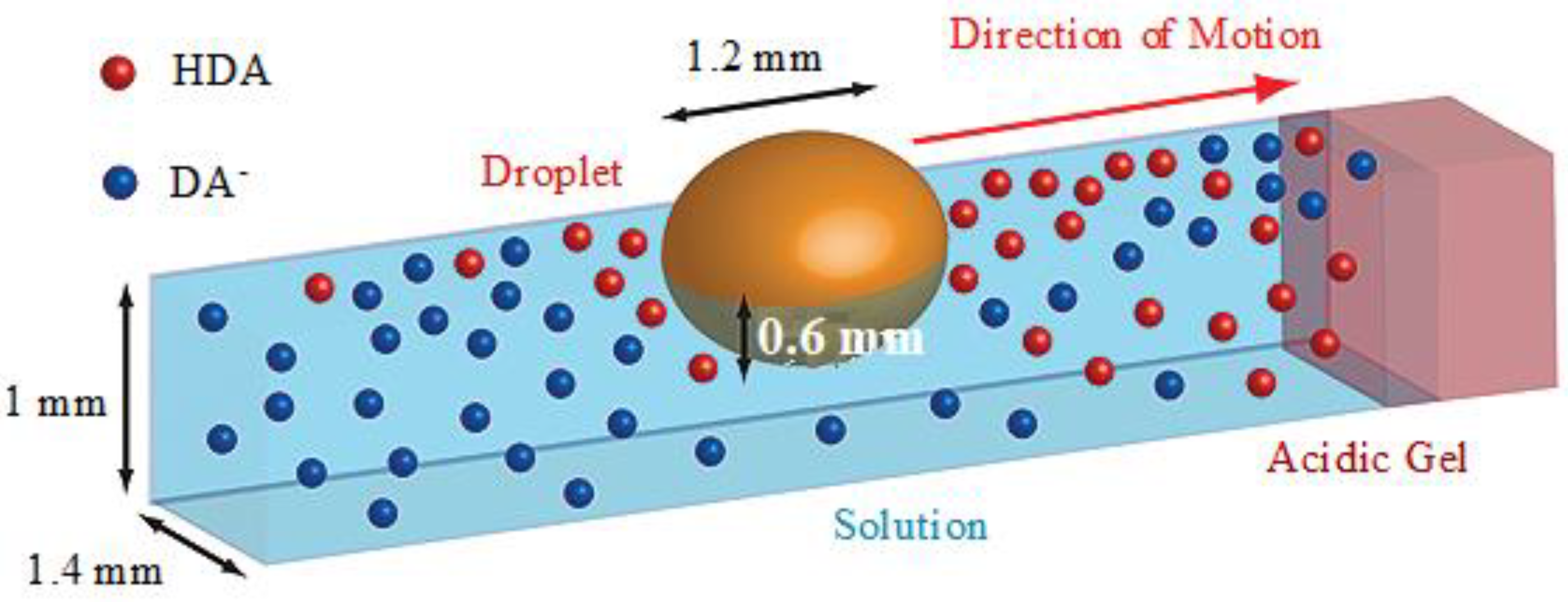
4. IL-Based Chemotaxis
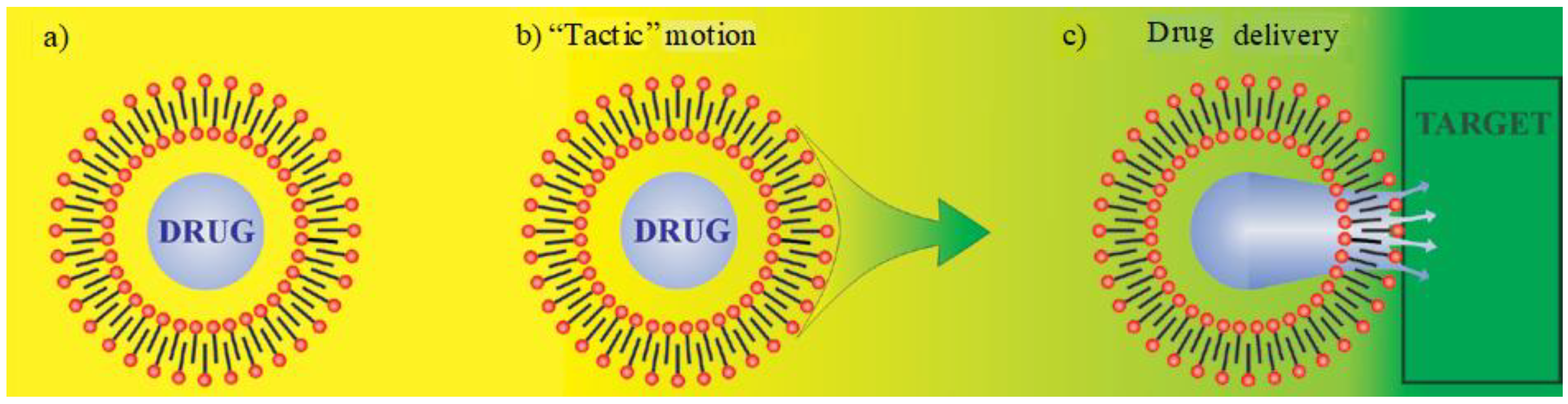
5. Smart Drug Delivery Using ILs
6. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Adler, J. Chemotaxis in bacteria. Science 1966, 153, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Van Haastert, P.J.M.; Devreotes, P.N. Chemotaxis: Signalling the way forward. Nat. Rev. Mol. Cell Biol. 2004, 5, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Spehr, M.; Gisselmann, G.; Poplawski, A.; Riffell Jeffrey, A.; Wetzel Christian, H.; Zimmer Richard, K.; Hatt, H. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science 2003, 299, 2054–2058. [Google Scholar] [CrossRef] [PubMed]
- Funamoto, S.; Meili, R.; Lee, S.; Parry, L.; Firtel, R.A. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell 2002, 109, 611–623. [Google Scholar] [CrossRef] [Green Version]
- Li Jeon, N.; Baskaran, H.; Dertinger, S.K.W.; Whitesides, G.M.; Van De Water, L.; Toner, M. Neutrophil chemotaxis in linear and complex gradients of interleukin-8 formed in a microfabricated device. Nat. Biotechnol. 2002, 20, 826–830. [Google Scholar] [CrossRef]
- Wu, J.Y.; Feng, L.; Park, H.-T.; Havlioglu, N.; Wen, L.; Tang, H.; Bacon, K.B.; Jiang, Z.-h.; Zhang, X.-c.; Rao, Y. The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature 2001, 410, 948–952. [Google Scholar] [CrossRef]
- Chaudhury Manoj, K.; Whitesides George, M. How to make water run uphill. Science 1992, 256, 1539–1541. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Dhiman, R.; Anand, S.; Reza-Garduno, E.; Cohen, R.E.; McKinley, G.H.; Varanasi, K.K. Droplet mobility on lubricant-impregnated surfaces. Soft Matter 2013, 9, 1772–1780. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Kim, C.-J.C. All-electronic droplet generation on-chip with real-time feedback control for EWOD digital microfluidics. Lab Chip 2008, 8, 898–906. [Google Scholar] [CrossRef]
- Wheeler, A.R.; Moon, H.; Bird, C.A.; Ogorzalek Loo, R.R.; Kim, C.-J.; Loo, J.A.; Garrell, R.L. Digital microfluidics with in-line sample purification for proteomics analyses with MALDI-MS. Anal. Chem. 2005, 77, 534–540. [Google Scholar] [CrossRef]
- Brown, P.; Butts, C.P.; Eastoe, J. Stimuli-responsive surfactants. Soft Matter 2013, 9, 2365–2374. [Google Scholar] [CrossRef] [Green Version]
- Darhuber, A.A.; Valentino, J.P.; Troian, S.M.; Wagner, S. Thermocapillary actuation of droplets on chemically patterned surfaces by programmable microheater arrays. J. Microelectromech. Syst. 2003, 12, 873–879. [Google Scholar] [CrossRef] [Green Version]
- Wixforth, A.; Strobl, C.; Gauer, C.; Toegl, A.; Scriba, J.; Guttenberg, Z.V. Acoustic manipulation of small droplets. Anal. Bioanal. Chem. 2004, 379, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fang, J.; Wang, H.; Wang, X.; Lin, T. Magnetic liquid marbles: Manipulation of liquid droplets using highly hydrophobic Fe3O4 nanoparticles. Adv. Mater. 2010, 22, 707–710. [Google Scholar] [CrossRef]
- Chuang, H.-S.; Kumar, A.; Wereley, S.T. Open optoelectrowetting droplet actuation. Appl. Phys. Lett. 2008, 93, 064104. [Google Scholar] [CrossRef] [Green Version]
- Velarde, M.G. Drops, liquid layers and the Marangoni effect. Philos. Trans. R. Soc. A 1998, 356, 829–844. [Google Scholar] [CrossRef]
- Roché, M.; Li, Z.; Griffiths, I.M.; Le Roux, S.; Cantat, I.; Saint-Jalmes, A.; Stone, H.A. Marangoni flow of soluble amphiphiles. Phys. Rev. Lett. 2014, 112, 208302. [Google Scholar] [CrossRef] [Green Version]
- Diguet, A.; Guillermic, R.-M.; Magome, N.; Saint-Jalmes, A.; Chen, Y.; Yoshikawa, K.; Baigl, D. Photomanipulation of a droplet by the chromocapillary effect. Angew. Chem. Int. Ed. 2009, 48, 9281–9284. [Google Scholar] [CrossRef]
- Florea, L.; Wagner, K.; Wagner, P.; Wallace, G.G.; Benito-Lopez, F.; Officer, D.L.; Diamond, D. Photo-chemopropulsion-light-stimulated movement of microdroplets. Adv. Mater. 2014, 26, 7339–7345. [Google Scholar] [CrossRef] [Green Version]
- Lagzi, I.; Soh, S.; Wesson, P.J.; Browne, K.P.; Grzybowski, B.A. Maze solving by chemotactic droplets. J. Am. Chem. Soc. 2010, 132, 1198–1199. [Google Scholar] [CrossRef]
- Petkovic, M.; Seddon, K.R.; Rebelo, L.P.N.; Silva Pereira, C. Ionic liquids: A pathway to environmental acceptability. Chem. Soc. Rev. 2011, 40, 1383–1403. [Google Scholar] [CrossRef] [PubMed]
- Seddon, K.R. Ionic liquids for clean technology. J. Chem. Technol. Biotechnol. 1997, 68, 351–356. [Google Scholar] [CrossRef]
- Ferraz, R.; Branco, L.C.; Prudêncio, C.; Noronha, J.P.; Petrovski, Z. Ionic liquids as active pharmaceutical ingredients. ChemMedChem 2011, 6, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Passos, H.; Freire, M.G.; Coutinho, J.A.P. Ionic liquid solutions as extractive solvents for value-added compounds from biomass. Green Chem. 2014, 16, 4786–4815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawała, J.; Dawidziuk, B.; Dziedzic, D.; Gordon, D.; Popiel, S. Applications of ionic liquids in analytical chemistry with a particular emphasis on their use in solid-phase microextraction. TrAC Trend Anal. Chem. 2018, 105, 18–36. [Google Scholar] [CrossRef]
- Freire, M.G.; Cláudio, A.F.M.; Araújo, J.M.M.; Coutinho, J.A.P.; Marrucho, I.M.; Lopes, J.N.C.; Rebelo, L.P.N. Aqueous biphasic systems: A boost brought about by using ionic liquids. Chem. Soc. Rev. 2012, 41, 4966–4995. [Google Scholar] [CrossRef] [PubMed]
- Shamshina, J.L.; Barber, P.S.; Rogers, R.D. Ionic liquids in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 1367–1381. [Google Scholar] [CrossRef] [PubMed]
- Bica, K.; Shamshina, J.; Hough, W.L.; MacFarlane, D.R.; Rogers, R.D. Liquid forms of pharmaceutical co-crystals: Exploring the boundaries of salt formation. Chem. Commun. 2011, 47, 2267–2269. [Google Scholar] [CrossRef]
- Handy, S. Room temperature ionic liquids: Different classes and physical properties. Curr. Org. Chem. 2005, 9, 959–988. [Google Scholar] [CrossRef]
- Suresh; Sandhu, J.S. Recent advances in ionic liquids: Green unconventional solvents of this century: Part I. Green Chem. Lett. Rev. 2011, 4, 289–310. [Google Scholar] [CrossRef] [Green Version]
- Masri, A.; Abdul Mutalib, M.; Leveque, J. A review on dicationic ionic liquids: Classification and application. Ind. Eng. Manag. 2016, 5, 2–7. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Hal, R.V.; Bösmann, A. 1-n-Butyl-3-methylimidazolium ([bmim]) octylsulfate-an even ‘greener’ ionic liquid. Green Chem. 2002, 4, 400–404. [Google Scholar] [CrossRef]
- Wei, G.-T.; Yang, Z.; Chen, C.-J. Room temperature ionic liquid as a novel medium for liquid/liquid extraction of metal ions. Anal. Chim. Acta 2003, 488, 183–192. [Google Scholar] [CrossRef]
- Paradkar, R.P.; Williams, R.R. Micellar colorimetric determination of dithizone metal chelates. Anal. Chem. 1994, 66, 2752–2756. [Google Scholar] [CrossRef]
- Visser, A.E.; Swatloski, R.P.; Reichert, W.M.; Mayton, R.; Sheff, S.; Wierzbicki, A.; Davis, J.J.H.; Rogers, R.D. Task-specific ionic liquids for the extraction of metal ions from aqueous solutions. Chem. Commun. 2001, 1, 135–136. [Google Scholar] [CrossRef]
- Dubois, P.; Marchand, G.; Fouillet, Y.; Berthier, J.; Douki, T.; Hassine, F.; Gmouh, S.; Vaultier, M. Ionic liquid droplet as e-microreactor. Anal. Chem. 2006, 78, 4909–4917. [Google Scholar] [CrossRef]
- Pu, Y.; Jiang, N.; Ragauskas, A.J. Ionic liquid as a green solvent for lignin. J. Wood Chem. Technol. 2007, 27, 23–33. [Google Scholar] [CrossRef]
- Deng, N.; Li, M.; Zhao, L.; Lu, C.; de Rooy, S.L.; Warner, I.M. Highly efficient extraction of phenolic compounds by use of magnetic room temperature ionic liquids for environmental remediation. J. Hazard. Mater. 2011, 192, 1350–1357. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Lu, Y.; Hu, R.; Chen, J.; Zhang, Z.; Pan, Y. Application of ionic liquids based microwave-assisted extraction of three alkaloids N-nornuciferine, O-nornuciferine, and nuciferine from lotus leaf. Talanta 2010, 80, 1292–1297. [Google Scholar] [CrossRef]
- Xu, W.; Chu, K.; Li, H.; Zhang, Y.; Zheng, H.; Chen, R.; Chen, L. Ionic liquid-based microwave-assisted extraction of flavonoids from Bauhinia championii (Benth.) Benth. Molecules 2012, 17, 14323–14335. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; Wang, Y.; Kong, J.; Nie, C.; Yuan, Y. Ionic liquid-based microwave-assisted extraction of rutin from Chinese medicinal plants. Talanta 2010, 83, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.A.; Vijayaraghavan, R.; MacFarlane, D.R. Distillable ionic liquid extraction of tannins from plant materials. Green Chem. 2010, 12, 1023–1028. [Google Scholar] [CrossRef]
- Usuki, T.; Yasuda, N.; Yoshizawa-Fujita, M.; Rikukawa, M. Extraction and isolation of shikimic acid from Ginkgo biloba leaves utilizing an ionic liquid that dissolves cellulose. Chem. Commun. 2011, 47, 10560–10562. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, Y.; Gao, S.; Zhang, R.; Ren, R.; Li, N.; Zhang, H. Determination of the active constituents in Arnebia euchroma (Royle) Johnst. by ionic liquid-based ultrasonic-assisted extraction high-performance liquid chromatography. J. Chromatogr. B 2011, 879, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Kamiya, N.; Goto, M. Ionic liquid based microemulsion with pharmaceutically accepted components: Formulation and potential applications. J. Colloid Interface Sci. 2010, 352, 136–142. [Google Scholar] [CrossRef]
- Imperato, G.; König, B.; Chiappe, C. Ionic green solvents from renewable resources. Eur. J. Org. Chem. 2007, 2007, 1049–1058. [Google Scholar] [CrossRef]
- Vidiš, A.; Ohlin, C.A.; Laurenczy, G.; Küsters, E.; Sedelmeier, G.; Dyson, P.J. Rationalisation of solvent effects in the Diels-Alder reaction between cyclopentadiene and methyl acrylate in room temperature ionic liquids. Adv. Synth. Catal. 2005, 347, 266–274. [Google Scholar] [CrossRef]
- Morrissey, S.; Pegot, B.; Coleman, D.; Garcia, M.T.; Ferguson, D.; Quilty, B.; Gathergood, N. Biodegradable, non-bactericidal oxygen-functionalised imidazolium esters: A step towards ‘greener’ ionic liquids. Green Chem. 2009, 11, 475–483. [Google Scholar] [CrossRef] [Green Version]
- Afouna, M.I.; Mehta, S.C.; Ghanem, A.-H.; Higuchi, W.I.; Kern, E.R.; De Clercq, E.; El-Shattawy, H.H. Assessment of correlation between skin target site free drug concentration and the in vivo topical antiviral efficacy in hairless mice for (E)-5-(2-bromovinyl)-2′-deoxyuridine and acyclovir formulations. J. Pharm. Sci. 1998, 87, 917–921. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug. Deliv. Rev. 2004, 56, 603–618. [Google Scholar] [CrossRef]
- Alade, S.L.; Brown, R.E.; Paquet, A., Jr. Polysorbate 80 and E-Ferol Toxicity. Pediatrics 1986, 77, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Stolte, S.; Matzke, M.; Arning, J.; Böschen, A.; Pitner, W.-R.; Welz-Biermann, U.; Jastorff, B.; Ranke, J. Effects of different head groups and functionalised side chains on the aquatic toxicity of ionic liquids. Green Chem. 2007, 9, 1170–1179. [Google Scholar] [CrossRef]
- Dorvee, J.R.; Derfus, A.M.; Bhatia, S.N.; Sailor, M.J. Manipulation of liquid droplets using amphiphilic, magnetic one-dimensional photonic crystal chaperones. Nat. Mater. 2004, 3, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Bormashenko, E.; Pogreb, R.; Bormashenko, Y.; Musin, A.; Stein, T. New investigations on ferrofluidics: Ferrofluidic marbles and magnetic-field-driven drops on superhydrophobic surfaces. Langmuir 2008, 24, 12119–12122. [Google Scholar] [CrossRef]
- Ichimura, K.; Oh, S.-K.; Nakagawa, M. Light-driven motion of liquids on a photoresponsive surface. Science 2000, 288, 1624–1626. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, F.A.; Kettunen, M.I.; Day, S.E.; Hu, D.-E.; Ardenkjær-Larsen, J.H.; Zandt, R.i.t.; Jensen, P.R.; Karlsson, M.; Golman, K.; Lerche, M.H.; et al. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature 2008, 453, 940–943. [Google Scholar] [CrossRef]
- Gordon, R.T.; Hines, J.R.; Gordon, D. Intracellular hyperthermia a biophysical approach to cancer treatment via intracellular temperature and biophysical alterations. Med. Hypotheses 1979, 5, 83–102. [Google Scholar] [CrossRef]
- Miura, T.; Oosawa, H.; Sakai, M.; Syundou, Y.; Ban, T.; Shioi, A. Autonomous motion of vesicle via ion exchange. Langmuir 2010, 26, 1610–1618. [Google Scholar] [CrossRef]
- Hanczyc, M.M.; Toyota, T.; Ikegami, T.; Packard, N.; Sugawara, T. Fatty acid chemistry at the oil–water interface: Self-propelled oil droplets. J. Am. Chem. Soc. 2007, 129, 9386–9391. [Google Scholar] [CrossRef]
- Toyota, T.; Maru, N.; Hanczyc, M.M.; Ikegami, T.; Sugawara, T. Self-propelled oil droplets consuming “fuel” surfactant. J. Am. Chem. Soc. 2009, 131, 5012–5013. [Google Scholar] [CrossRef]
- Lagzi, I. Chemical robotics-chemotactic drug carriers. Cent. Eur. J. Med. 2013, 8, 377–382. [Google Scholar] [CrossRef]
- Rösler, A.; Vandermeulen, G.W.M.; Klok, H.-A. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv. Drug. Deliv. Rev. 2012, 64, 270–279. [Google Scholar] [CrossRef]
- Sudimack, J.; Lee, R.J. Targeted drug delivery via the folate receptor. Adv. Drug. Deliv. Rev. 2000, 41, 147–162. [Google Scholar] [CrossRef]
- Szostak, J.W.; Bartel, D.P.; Luisi, P.L. Synthesizing life. Nature 2001, 409, 387–390. [Google Scholar] [CrossRef]
- Francis, W.; Fay, C.; Florea, L.; Diamond, D. Self-propelled chemotactic ionic liquid droplets. Chem. Commun. 2015, 51, 2342–2344. [Google Scholar] [CrossRef] [Green Version]
- Francis, W.; Wagner, K.; Beirne, S.; Officer, D.L.; Wallace, G.G.; Florea, L.; Diamond, D. Electrotactic ionic liquid droplets. Sens. Actuators B Chem. 2017, 239, 1069–1075. [Google Scholar] [CrossRef] [Green Version]
- Byrn, S.; Pfeiffer, R.; Ganey, M.; Hoiberg, C.; Poochikian, G. Pharmaceutical solids: A strategic approach to regulatory considerations. Pharm. Res. 1995, 12, 945–954. [Google Scholar] [CrossRef]
- Giron, D.; Mutz, M.; Garnier, S. Solid-state of pharmaceutical compounds. J. Therm. Anal. Calorim. 2004, 77, 709–747. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Eastoe, J.; Gold, S.; Rogers, S.E.; Paul, A.; Welton, T.; Heenan, R.K.; Grillo, I. Ionic liquid-in-oil microemulsions. J. Am. Chem. Soc. 2005, 127, 7302–7303. [Google Scholar] [CrossRef]
- Araki, S.; Wakabayashi, R.; Moniruzzaman, M.; Kamiya, N.; Goto, M. Ionic liquid-mediated transcutaneous protein delivery with solid-in-oil nanodispersions. MedChemComm 2015, 6, 2124–2128. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Tamura, M.; Tahara, Y.; Kamiya, N.; Goto, M. Ionic liquid-in-oil microemulsion as a potential carrier of sparingly soluble drug: Characterization and cytotoxicity evaluation. Int. J. Pharm. 2010, 400, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Goindi, S.; Arora, P.; Kumar, N.; Puri, A. Development of novel ionic liquid-based microemulsion formulation for dermal delivery of 5-fluorouracil. AAPS PharmSciTech 2014, 15, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Bazylińska, U.; Kulbacka, J.; Wilk, K.A. Dicephalic ionic surfactants in fabrication of biocompatible nanoemulsions: Factors influencing droplet size and stability. Colloids Surf. A Physicochem. Eng. 2014, 460, 312–320. [Google Scholar] [CrossRef]
- Mandal, S.; Ghosh, S.; Banerjee, C.; Kuchlyan, J.; Banik, D.; Sarkar, N. A novel ionic liquid-in-oil microemulsion composed of biologically acceptable components: An excitation wavelength dependent fluorescence resonance energy transfer study. J. Phys. Chem. B 2013, 117, 3221–3231. [Google Scholar] [CrossRef] [PubMed]
- Mat Hadzir, N.; Basri, M.; Abdul Rahman, M.B.; Salleh, A.B.; Raja Abdul Rahman, R.N.Z.; Basri, H. Phase behaviour and formation of fatty acid esters nanoemulsions containing piroxicam. AAPS PharmSciTech 2013, 14, 456–463. [Google Scholar] [CrossRef] [Green Version]
- Mason, T.G.; Wilking, J.N.; Meleson, K.; Chang, C.B.; Graves, S.M. Nanoemulsions: Formation, structure, and physical properties. J. Phys. Condens. Matter 2006, 18, R635–R666. [Google Scholar] [CrossRef] [Green Version]
- Sivakumar, M.; Tang, S.Y.; Tan, K.W. Cavitation technology—A greener processing technique for the generation of pharmaceutical nanoemulsions. Ultrason. Sonochem. 2014, 21, 2069–2083. [Google Scholar] [CrossRef]
- Mashhadi, S.; Javadian, H.; Tyagi, I.; Agarwal, S.; Gupta, V.K. The effect of Na2SO4 concentration in aqueous phase on the phase inversion temperature of lemon oil in water nano-emulsions. J. Mol. Liq. 2016, 215, 454–460. [Google Scholar] [CrossRef]
- Salim, N.; Ahmad, N.; Musa, S.H.; Hashim, R.; Tadros, T.F.; Basri, M. Nanoemulsion as a topical delivery system of antipsoriatic drugs. RSC Adv. 2016, 6, 6234–6250. [Google Scholar] [CrossRef]
- Dalvand, K.; Ghiasvand, A.; Gupta, V.; Paull, B. Chemotaxis-based smart drug delivery of epirubicin using a 3D printed microfluidic chip. J. Chromatogr. B 2021, 1162, 122456. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.; Contini, C.; Cecchin, D.; Nyberg, S.; Ruiz-Perez, L.; Gaitzsch, J.; Fullstone, G.; Tian, X.; Azizi, J.; Preston, J.; et al. Chemotactic synthetic vesicles: Design and applications in blood-brain barrier crossing. Sci. Adv. 2017, 3, e1700362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahamat Nor, S.B.; Woi, P.M.; Ng, S.H. Characterisation of ionic liquids nanoemulsion loaded with piroxicam for drug delivery system. J. Mol. Liq. 2017, 234, 30–39. [Google Scholar] [CrossRef]
- Banerjee, A.; Ibsen, K.; Brown, T.; Chen, R.; Agatemor, C.; Mitragotri, S. Ionic liquids for oral insulin delivery. Proc. Natl. Acad. Sci. USA 2018, 115, 7296. [Google Scholar] [CrossRef] [Green Version]
- Holler, S.; Porcelli, C.; Ieropoulos, I.A.; Hanczyc, M.M. Transport of live cells under sterile conditions using a chemotactic droplet. Sci. Rep. 2018, 8, 8408. [Google Scholar] [CrossRef] [PubMed]
- Bielas, R.; Mielańczyk, A.; Skonieczna, M.; Mielańczyk, Ł.; Neugebauer, D. Choline supported poly(ionic liquid) graft copolymers as novel delivery systems of anionic pharmaceuticals for anti-inflammatory and anti-coagulant therapy. Sci. Rep. 2019, 9, 14410. [Google Scholar] [CrossRef]
- Bielas, R.; Łukowiec, D.; Neugebauer, D. Drug delivery via anion exchange of salicylate decorating poly(meth)acrylates based on a pharmaceutical ionic liquid. New J. Chem. 2017, 41, 12801–12807. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, M. Ionic liquids toxicity-Benefits and threats. Int. J. Mol. Sci. 2020, 21, 6267. [Google Scholar] [CrossRef]
- Thuy Pham, T.P.; Cho, C.-W.; Yun, Y.-S. Environmental fate and toxicity of ionic liquids: A review. Water Res. 2010, 44, 352–372. [Google Scholar] [CrossRef] [PubMed]
- Abramenko, N.; Kustov, L.; Metelytsia, L.; Kovalishyn, V.; Tetko, I.; Peijnenburg, W. A review of recent advances towards the development of QSAR models for toxicity assessment of ionic liquids. J. Hazard. Mater. 2020, 384, 121429. [Google Scholar] [CrossRef]
- Leitch, A.C.; Abdelghany, T.M.; Probert, P.M.; Dunn, M.P.; Meyer, S.K.; Palmer, J.M.; Cooke, M.P.; Blake, L.I.; Morse, K.; Rosenmai, A.K.; et al. The toxicity of the methylimidazolium ionic liquids, with a focus on M8OI and hepatic effects. Food Chem. Toxicol. 2020, 136, 111069. [Google Scholar] [CrossRef] [PubMed]
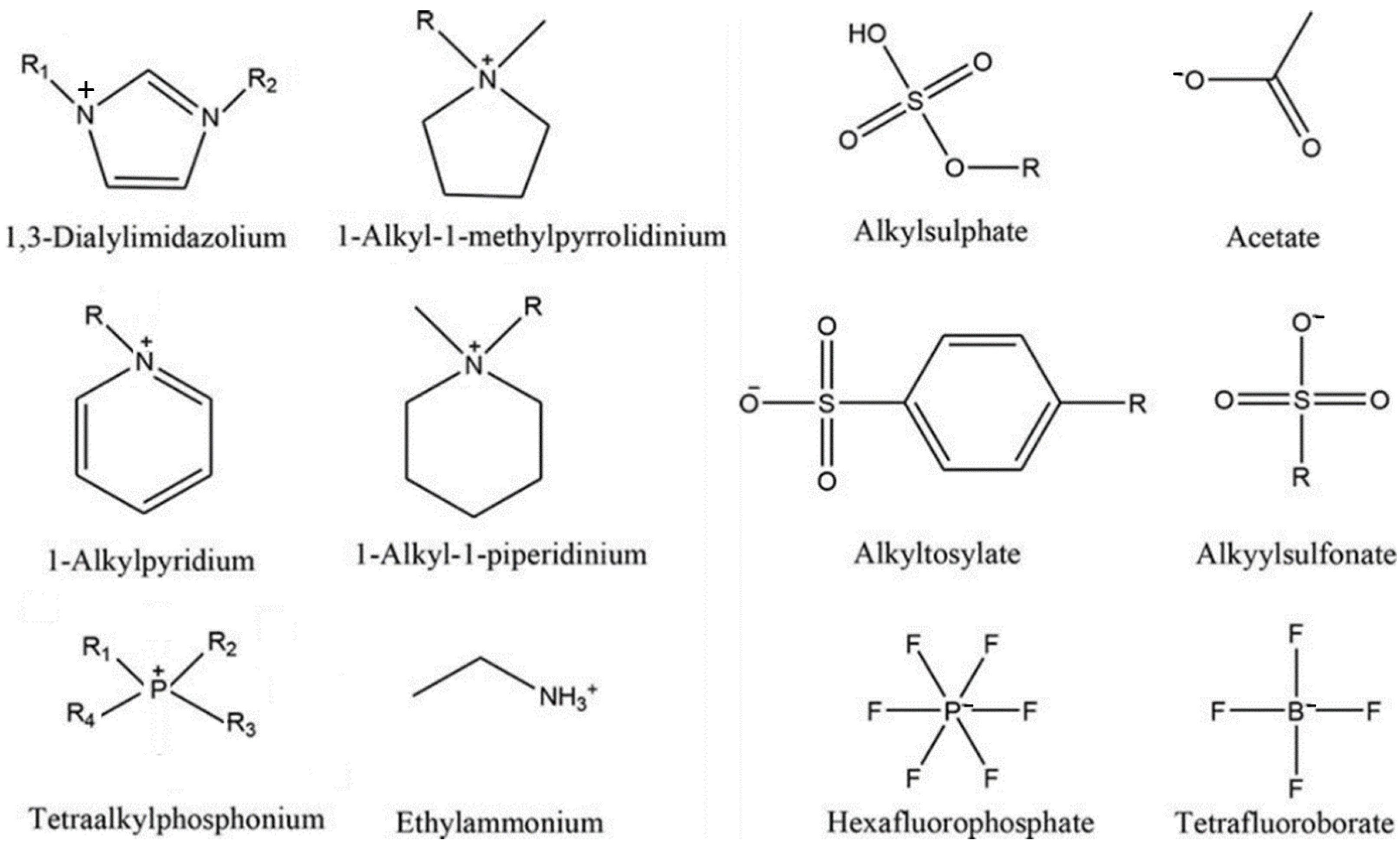
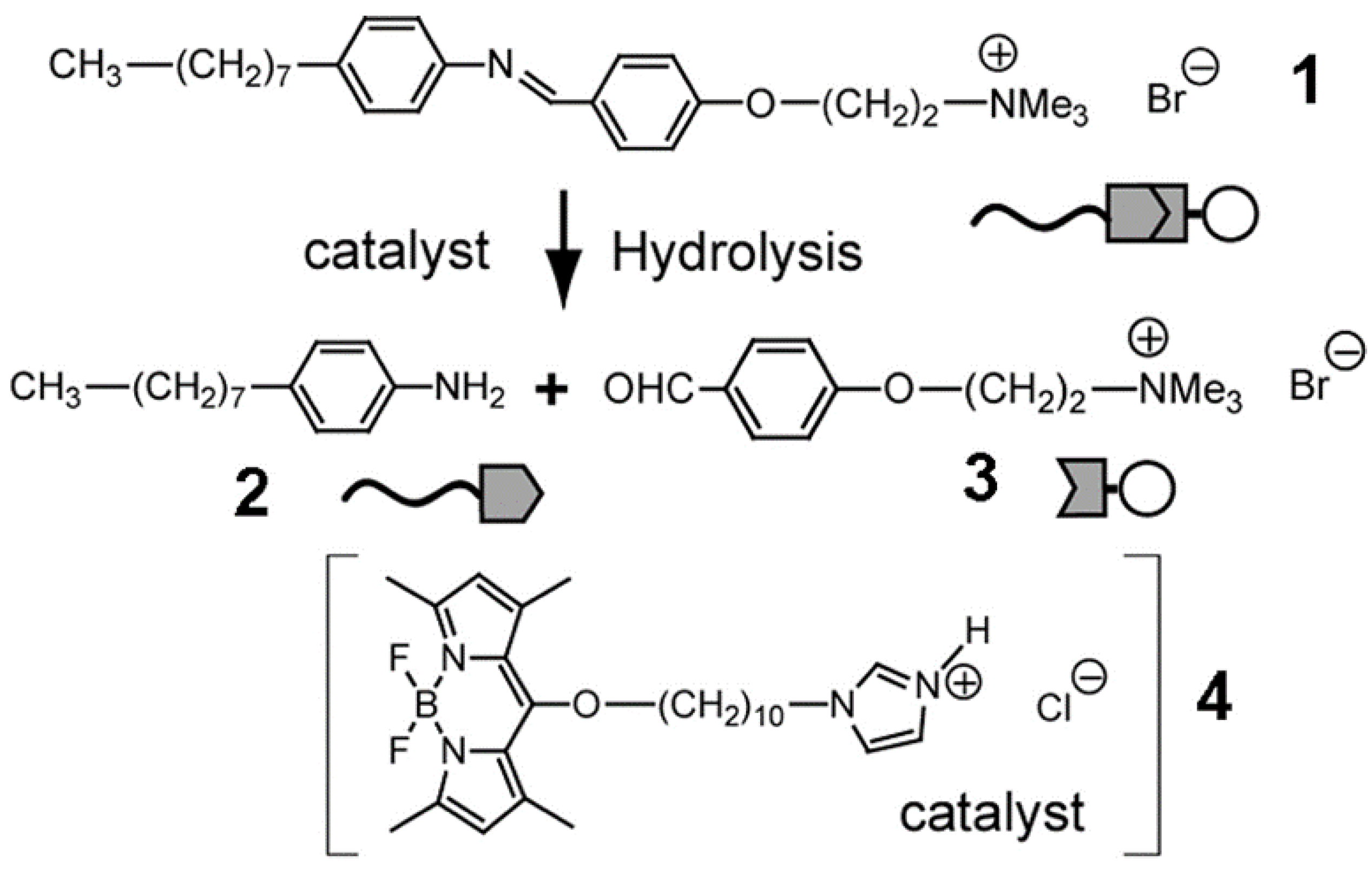
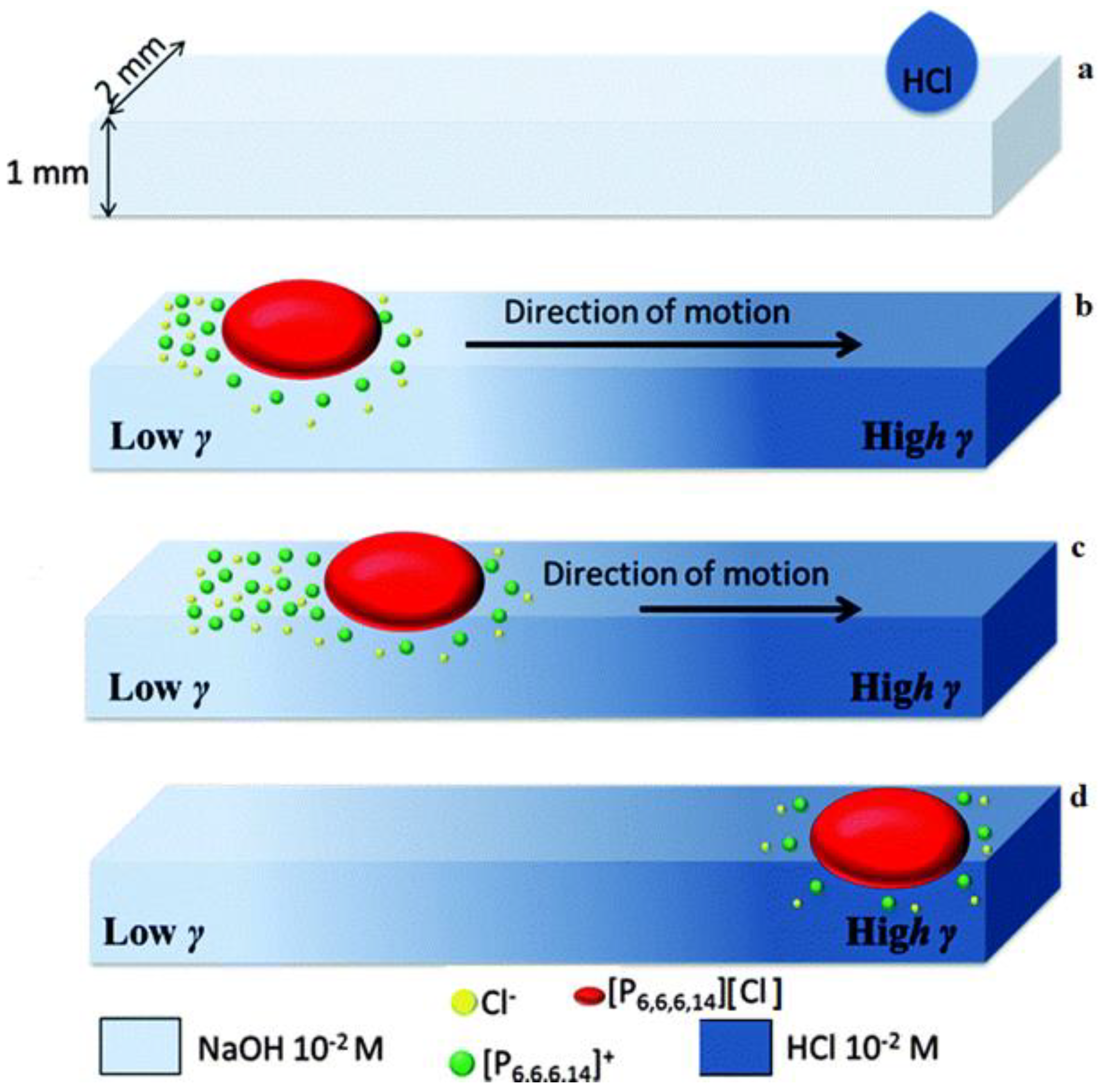
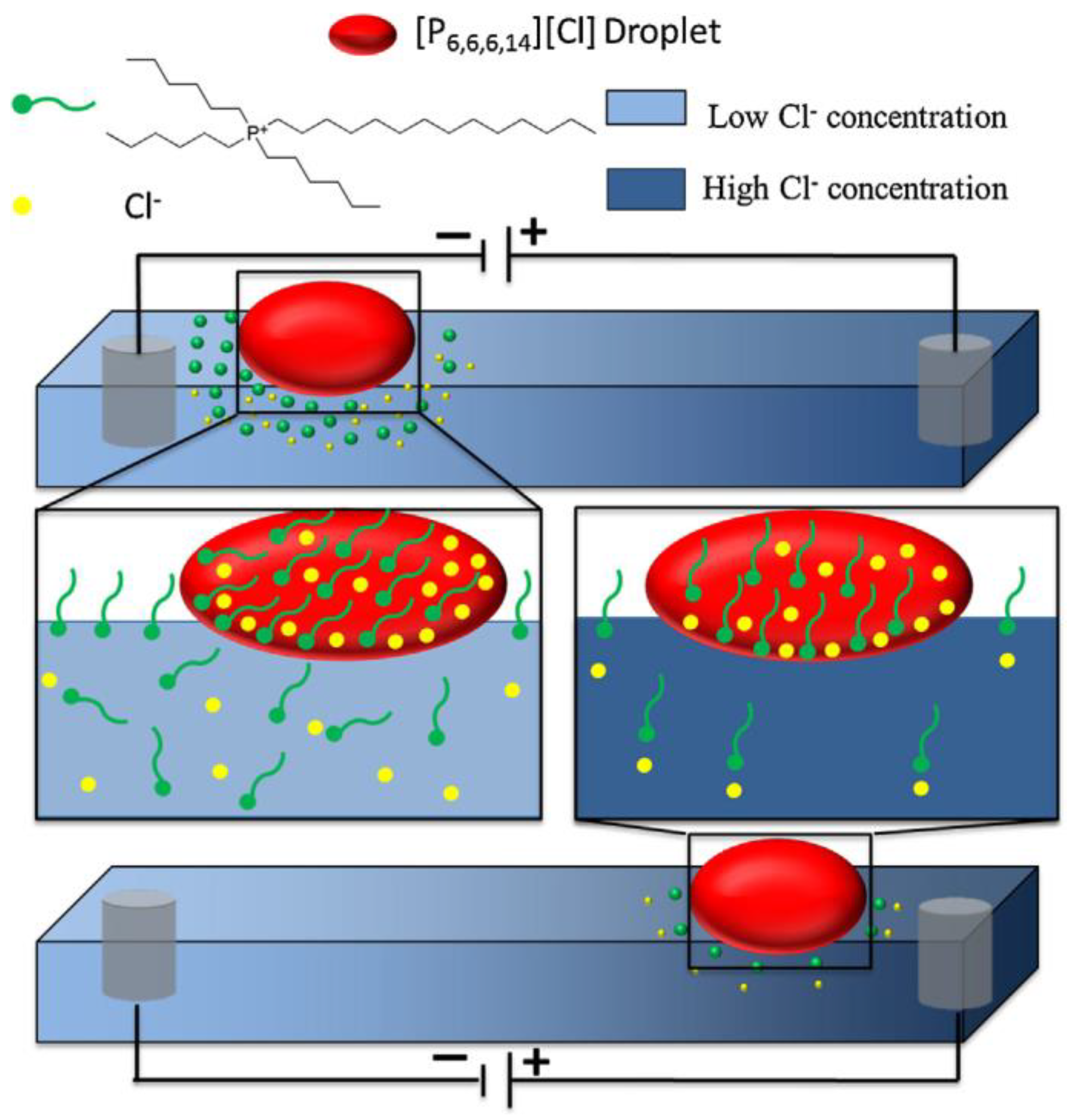
| Name | Abbreviation |
|---|---|
| triisobutyl (methyl) phosphonium tosylate | [P1,4,4,4][Tos] |
| tetrabutylphosphonium dicyanamide | [P4,4,4,4][DCA] |
| trihexyltetradecyl phosphonium dicyanamide | [P6,6,6,14][DCA] |
| trihexyltetradecyl phosphonium bis (trifluoromethanesulfonyl) imide | [P6,6,6,14][Ntf2] |
| trihexyltetradecyl phosphonium dodecylbenzenesulfonate | [P6,6,6,14][DBSA] |
| trihexyltetradecyl phosphonium chloride | [P6,6,6,14][Cl] |
| 1-methyl-3-octylimidazolium tetrafluoroborate | [OMIM][BF4] |
| 1-ethyl-3-methyliidazolium methyl sulphate | [EMIM][MeSO4] |
| 1-ethyl-3-methyl imidazolium ethyl sulfate | [EMIM][EtSO4] |
| 1-butyl-3-methylimidazolium hydrogen sulphate | [BMIM][HSO4] |
| 1-ethyl-3-methyl imidazolium tetrafluoroborate | [EMIM][BF4] |
| 1-ethyl-3-methylimidazolium dicyanamide | [EMIM][DCA] |
| 1-butyl-3-methylimidazolium tetrafluoroborate | [BMIM][BF4] |
| 1-butyl-3-methylimidazolium hexafluorophosphate | [BMIM][PF6] |
| 1-butyl-3-methylimidazolium dodecanesulfonate | [BMIM][DoS] |
| 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide | [BMIM][NTf2] |
| 1-hexyl-3-methylimidazolium bis (trifluoromethanesulfonyl) imide | [HMIM][NTf2] |
| 1-buty1-butyl-4-methylpyridinum tetrafluoroborate | [BMPy][BF4] |
| Application | IL Name | IL Class | Ref. |
|---|---|---|---|
| Separation and extraction of heavy metal ions including Hg2+ and Cd2+ from aqueous solution into [C4mim][PF6] | 1-butyl-3 methylimidazolium hexafluorophosphate, [C4mim][PF6] | RTIL | [33] |
| Extraction and preconcentration of Cd2+ and Hg2+ from aqueous samples into a mixture of different TSILs and a RTIL, [C4mim][PF6] | Combinations of derivatized imidazolium cations with urea, thiourea, and thioether, mixed with 1-butyl-3 methylimidazolium hexafluorophosphate, [C4mim][PF6] | TSILs and RTILs | [35] |
| Synthesis of organic compounds by using ILs as electronic microreactor | 1-butyl-3-methylimidazolium tetrafluoroborate, [bmim][BF4], and 1-butyl-3-methylimidazolium hexafluorophosphate, [bmim][PF6] | RTIL and RTIL | [36] |
| Dissolution of a softwood lignin in different ionic liquids as aprotic green solvents | 1-hexyl-3-methylimidazolium trifluoromethanesulfonate, [hmim][CF3SO3], 1,3-dimethylimidazolium methylsulfate, [mmim][MeSO4], and 1-butyl-3-methylimidazolium methylsulfate [bmim][MeSO4] | TSIL, RTIL, and RTIL | [37] |
| Removal of phenolic compounds such as pentachlorophenol by use of magnetic room-temperature ionic liquid (MRTIL) | trihexyltetradecyl phosphonium etrachloroferrate (III), [3C6PC14][FeCl4] | MRTIL | [38] |
| Extraction of three alkaloids from lotus leaf | 1-hexyl-3-methylimidazolium bromide, ([C(6)MIM]Br) | TSIL | [39] |
| Extraction and determination of flavonoids from Bauhinia championii | 1-butyl-3-methylimidazolium bromide, ([bmim]Br) | TSIL | [40] |
| Effective extraction of rutin from Chinese medicinal plants | 1-butyl-3-methylimidazolium bromide, ([bmim]Br) | TSIL | [41] |
| Separation of tannins from plant materials | N,N-dimethylammonium N′N′-dimethylcarbamate, (DIMCARB) | TSIL | [42] |
| Extraction and isolation of shikimic acid from Ginkgo biloba leaves | 1-butyl-3-methylimidazolium chloride, ([bmim]Cl) | TSIL | [43] |
| Extraction of Shikonin and β,β’-dimethylacrylshikonin in Arnebia euchroma (Royle) Johnst | 1-hexyl-3-methylimidazolium tetrafluoroborate, [C(6)MIM][BF4] | RTIL | [44] |
| Drug Delivery Application | Chemotactic System (IL Name) | Ref. |
|---|---|---|
| Transdermal delivery of Acyclovir (to treat infections caused by certain types of viruses such as cold sores around the mouth) | dimethylimidazolium dimethylphosphate [C1mim][(MeO)2PO2] | [72] |
| Targeted delivery of Epirubicin (anticancer drug) | trihexyltetradecyl phosphonium chloride ([P6,6,6,14][Cl]) | [81] |
| Encapsulating of glucose oxidase alone or in combination with catalase into biocompatible nanoscopic asymmetric polymer vesicles (polymersomes), applications in blood–brain barrier crossing | asymmetric polymersomes: poly [(2-methacryloyl) ethyl phosphorylcholine]–poly[2-(diisopropylamino) ethyl methacrylate] (PMPC-PDPA) and poly[oligo (ethylene glycol) methyl methacrylate] (POEGMA-PDPA) | [82] |
| Delivery of Piroxicam (a nonsteroidal anti-inflammatory drug), which is sparingly soluble in water | 1-hexyl-3-methylimidazolium chloride [Hmim][Cl] and 1-butyl-3-methylimidazolium hexafluorophosphate [Bmim][PF6] | [83] |
| Development of a highly effective oral insulin formulation and delivery | choline and geranate (CAGE) ionic liquid | [84] |
| Transport live cells protected in alginate capsules as a protective unit along chemical gradients | 1-decanol chemotactic droplets in an aqueous medium containing decanoate at high pH by chemical gradient in the external aqueous environment | [85] |
| Delivery of biologically active anionic pharmaceuticals for anti-inflammatory and anti-coagulant therapy | salicylate decorating poly (2-(trimethylammonium) ethyl methacrylate based on a pharmaceutical ionic liquid | [86,87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khodarahmian, K.; Ghiasvand, A. Mimic Nature Using Chemotaxis of Ionic Liquid Microdroplets for Drug Delivery Purposes. Molecules 2022, 27, 786. https://doi.org/10.3390/molecules27030786
Khodarahmian K, Ghiasvand A. Mimic Nature Using Chemotaxis of Ionic Liquid Microdroplets for Drug Delivery Purposes. Molecules. 2022; 27(3):786. https://doi.org/10.3390/molecules27030786
Chicago/Turabian StyleKhodarahmian, Kobra, and Alireza Ghiasvand. 2022. "Mimic Nature Using Chemotaxis of Ionic Liquid Microdroplets for Drug Delivery Purposes" Molecules 27, no. 3: 786. https://doi.org/10.3390/molecules27030786







