Preparation of Losartan Potassium Controlled Release Matrices and In-Vitro Investigation Using Rate Controlling Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Tablets’ Fabrication
2.3. Flow Properties
2.4. Tablets’ Preparation
2.5. Physical Characteristic
2.6. Dissolution
2.7. Drug Release Mechanisms
2.8. Difference and Similarity Factors
3. Results and Discussion
3.1. Flow Properties
3.2. Physical Characteristics
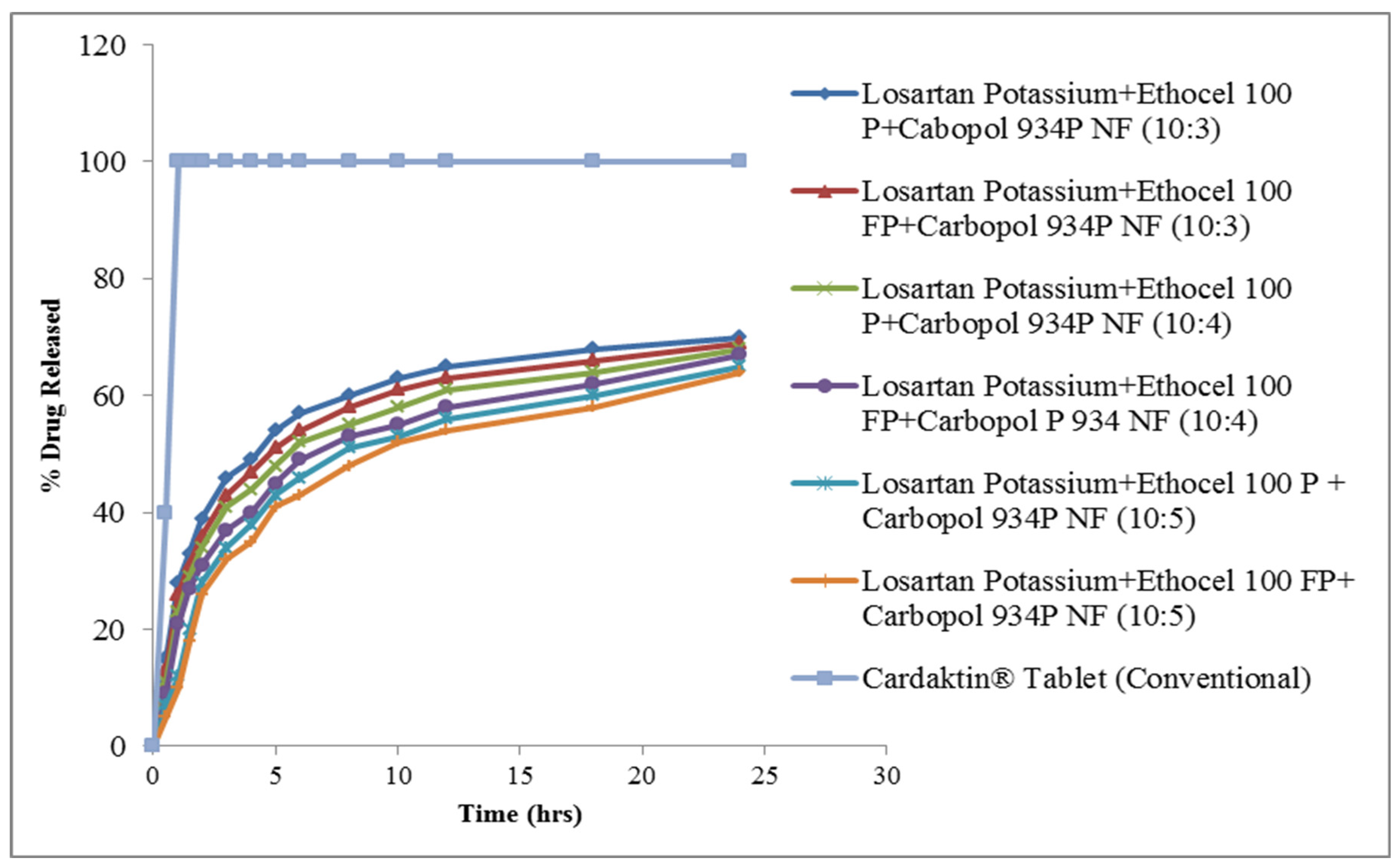
3.3. Drug Release from Tablets
3.4. Content Uniformity
3.5. Kinetics of Drug Release and Dissolution Comparison
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Drug Delivery. Available online: http://en.wikipedia.org/wiki/Drug%20delivery (accessed on 20 April 2012).
- Parashar, T.; Singh, V.; Singh, G.; Tyagi, S.; Patel, C.; Gupta, A. Novel Oral Sustained Release Technology: A Concise Review. Int. J. Res. Dev. Pharm. Life Sci. 2013, 2, 262–269. [Google Scholar]
- Khan, K.A.; Khan, G.M.; Jan, A.U.; Mehsud, S. Formulation and in vitro evaluation of directly compressed controlled release tablets designed from the Co-precipitates. Pak. J. Pharm. Sci. 2018, 31, 455–461. [Google Scholar] [PubMed]
- Varma, M.V.S.; Kaushal, A.M.; Garg, A.; Garg, S. Factors Affecting Mechanism and Kinetics of Drug Release from Matrix Based Oral Controlled Drug Delivery Systems. Am. J. Drug Deliv. 2004, 2, 43–57. [Google Scholar] [CrossRef]
- Kumar, K.P.S.; Bhowmik, D.; Dutta, A.; Paswan, S.; Deb, L. Recent Trends in Scope and Opportunities of Control Release Oral Drug Delivery Systems. Crit. Rev. Pharm. Sci. 2012, 1, 21–33. [Google Scholar]
- Uma, U.; Rathore, K.S. Formulation and Evaluation of Sustained Release Matrix Tablets of Metformin Hydrochloride. Pharm. Chem. J. 2014, 1, 5–13. [Google Scholar]
- Bakhsh, S.; Khan, G.M.; Menaa, F.; Khan, B.A. Design and Evaluation of Matrix Based Controlled Release Tablets of Flurbiprofen. Lat. Am. J. Pharm. 2014, 33, 1297–1304. [Google Scholar]
- Swaleh, M.M.; Syed, I.A.Z.; Maqsood, M.A.; Shehnaz, S. A Detailed Review on Oral Controlled Release Matrix Tablets. Int. J. Pharm. Sci. Rev. Res. 2020, 64, 27–38. [Google Scholar]
- Ali, W.; Badawi, A.A.; Mahdy, M.A.; El-Nahas, H.M. Formulation and Evaluation of Carbamazepine 200 mg Controlled Release Tablets Using Different HPMC Grades. Br. J. Pharm. Res. 2013, 3, 632–647. [Google Scholar] [CrossRef]
- Ige, P.; Swami, B.; Patil, T.; Pradhan, J.; Patil, P.; Nerkar, P.; Surana, S.J. Design and Development of Sustained Release Swelling Matrix Tablets of Glipizide for Type II Diabetes Mellitus. Farmacia 2013, 61, 883–900. [Google Scholar]
- Khairnar, G.; Naik, J.; Mokale, V. A Statistical study on the development of micro particulate sustained drug delivery system for Losartan potassium by 32 factorial design approach. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Khan, K.A.; Khan, G.M.; Danish, M.Z.; Khan, H.; Rehman, F.; Mehsud, S. Formulation and in-vitro evaluation of directly compressed controlled release matrices of Losartan Potassium using Ethocel Grade 100 as rate retarding agent. Int. J. Pharm. 2015, 496, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Gollapudi, R.; Javvaji, H.; Tadikonda, R.R.; Arpineni, V. Formulation and In-vitro Evaluation of Sustained Release Matrix Tablets of Losartan Potassium. Pharmanest 2011, 2, 31–36. [Google Scholar]
- Muralidharan, S.; Meyyanathan, S.N.; Kumar, J.; Dhanaraj, S.A. In-Vivo Evaluation of Newly Developed Losartan Potassium Sustained Release Dosage form Using Healthy Male Indian Volunteers. Int. J. Drug Deliv. 2012, 4, 31–43. [Google Scholar]
- Azharuddin, M.; Kamath, K.; Panneerselvam, T.; Pillai, S.S.; Shabaraya, A.R. Formulation and Evaluation of Controlled Release Matrix Tablets of Antihypertensive Drug Using Natural and Synthetic Hydrophilic Polymers. Res. Biotechnol. 2011, 2, 26–32. [Google Scholar]
- Ahad, H.A.; Kumar, C.S.; Yesupadam, P.; Harika, B.; Deepika, D.; Leela, L.V.; Chandra, S.A. Formulation and Evaluation of Once-Daily Sustained Release Aceclofenac Prosophis Juliflora Gum Matrix Tablets. Int. J. Pharm. Sci. Rev. Res. 2010, 1, 23–28. [Google Scholar]
- Shanmugam, S.; Chakrahari, R.; Sundaramoorthy, K.; Ayyappan, T.; Vetrichelvan, T. Formulation and Evaluation of Sustained Release Matrix Tablets of Losartan potassium. Int. J. PharmTech Res. 2011, 3, 526–534. [Google Scholar]
- Nayak, N.V.K.; Kotade, K.B.; Gaware, V.M.; Dolas, R.T.; Dhamak, K.B.; Somwanshi, S.B.; Khadse, A.N.; Kashid, V.A. Eudragit A Versatile Polymer: A Review. Pharmacol. Online 2011, 1, 152–164. [Google Scholar]
- Khan, K.A.; Khan, G.M.; Shah, K.U.; Niazi, Z.R.; Khan, H.; Ahmad, A.; Jan, S.U. Design, Preparation and evaluation of various parameters of controlled release matrices of losartan potassium using polymers combination. Pak. J. Pharm. Sci. 2020, 33 (Suppl. 5), 2231–2237. [Google Scholar]
- USP. <1174> Powder Flow. USP30 NF 25 (2007).
- Shah, S.U.; Shah, K.U.; Jan, S.U.; Ahmad, K.; Rehman, A.U.; Hussain, A.; Khan, G.M. Formulation and In Vitro Evaluation of Ofloxacin-Ethocel Controlled Release Matrix Tablets Prepared by Wet Granulation Method: Influence of Co-Excipients on Drug Release Rates. Pak. J. Pharm. Sci. 2011, 24, 255–261. [Google Scholar]
- Anees, M.; Chowdhary, F.; Masood, M.I.; Javeed, A.; Ilyas, M. Formulation and In-Vitro Evaluation of Polymers Blend Based Diclofenac Sodium Microparticles for Sustained Release Drug Delivery. RADS J. Pharmacy Pharm. Sci. 2018, 6, 171–177. [Google Scholar]
- Nagra, U.; Adnan, S.; Shafqat, S.; Shabbir, M.; Ali, S.; Zafar, A. Effect of Sustained Release Polymers on Drug Release Profile of Aceclofenac Tablets Physicochemical Properties, Analysis of Kinetics and Fit Factor. Indian J. Pharm. Educ. Res. 2019, 53 (Suppl. 2), 58–65. [Google Scholar] [CrossRef] [Green Version]
- Gopikrishna, A.; Ramu, B.; Srikanth, G.; Rajkamal, B. Formulation of isoniazide sustained release formulation by using carbopol 934 P. Int. J. Appl. Pharm. Sci. Res. 2016, 1, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Razaque, G.; Khan, G.M.; Danish, M.Z.; Muhammad, S.; Shahwani, N.A.; Khan, K.A.; Younis, M. Formulation and In-Vitro Evaluation of Controlled Release Matrix Tablets of Diltiazem Hydrochloride Using Different Rate Controlling Polymers. Open Conf. Proc. J. 2016, 7, 114–125. [Google Scholar] [CrossRef] [Green Version]
- USP. Drug Information for Losartan Potassium from United States Pharmacopoeia; USP29-NF 24:1280; USP: Rockville, MD, USA, 2007. [Google Scholar]
- Akhlaq, M.; Khan, G.M.; Wahab, A.; Hussain, A.; Khan, A.; Nawaz, A.; Shah, K.U. Formulation and In-Vitro Evaluation of Flurbiprofen Controlled Release Matrix Tablets Using Cellulose Derivative Polymers. Pak. J. Pharm. Sci. 2010, 23, 23–29. [Google Scholar]
- Venkataswamy, M.; Santhoshini, M.; Priyanka, J.P.; Prathyusha, K.; Manasareddy, J.G.; Alluri, R. Preparation and Evaluation of Biphasic Bilayered Buccal Tablet Containing Ketorolac Immediate Release Layer and Domperidone Maleate Sustained Release Layer. World J. Pharm. Res. 2018, 7, 905–949. [Google Scholar]
- Shah, S.U.; Khan, G.M.; Jan, S.U.; Shah, K.U.; Hussain, A.; Khan, H.; Khan, K.A. Development of Novel Diclofenac Potassium Controlled Release Tablets by Wet Granulation Technique and the Effect of Co-Excipients on In Vitro Drug Release Rates. Pak. J. Pharm. Sci. 2012, 25, 161–168. [Google Scholar]
- Guerra-Ponce, W.L.; Gracia-Vásquez, S.L.; González-Barranco, P.; Camacho-Mora, I.A.; Gracia-Vásquez, Y.A.; Orozco-Beltrán, E.; Felton, L.A. In vitro evaluation of sustained released matrix tablets containing ibuprofen: A model poorly water-soluble drug. Braz. J. Pharm. Sci. 2016, 52, 751–759. [Google Scholar] [CrossRef]

| Losartan Potassium CR Tablets | |||||
|---|---|---|---|---|---|
| D:P | Drug (mg) | Polymers’ Combination (Ethocel 100 Premium + Carbopol 934P NF and Ethocel 100 FP Premium + Carbopol 934P NF) (mg) | Filler (mg) | Magnesium Stearate 0.5% (mg) | |
| 10:3 | 100 | 30 | 69 | 1.0 | |
| 10:4 | 100 | 40 | 59 | 1.0 | |
| 10:5 | 100 | 50 | 49 | 1.0 | |
| Losartan Potassium CR tablets with Co-excipients | |||||
| D:P | Drug (mg) | Polymeric combination (mg) | Filler (mg) | Lubricant (0.5%) | Co-excipient (10% of filler of HPMC or CMC or Starch) |
| 10:5 | 100 | 50 | 44.1 | 1.0 | 4.9 mg |
| Drug: Losartan potassium Filler: Spray dried lactose | |||||
| Formulations | Angle of Repose (n = 3, mean ± SD) | Carr’s Index (n = 3, mean ± SD) | Hausner’s Ratio (n = 3, mean ± SD) |
|---|---|---|---|
| Ethocel 100P + Carbopol 934P NF (10:3) | 30.24 ± 0.65 | 11.64 ± 0.49 | 1.16 ± 0.51 |
| Ethocel 100FP + Carbopol 934P NF (10:3) | 33.61 ± 0.53 | 14.04 ± 0.44 | 1.17 ± 0.82 |
| Ethocel 100P + Carbopol 934P NF (10:4) | 29.59 ± 0.62 | 9.12 ± 0.28 | 1.10 ± 0.69 |
| Ethocel 100FP + Carbopol 934P NF (10:4) | 25.41 ± 0.30 | 9.1 ± 0.23 | 1.02 ± 0.81 |
| Ethocel 100P + Carbopol 934P NF (10:5) | 26.16 ± 0.06 | 10.04 ± 0.09 | 1.0 ± 0.11 |
| Ethocel 100FP + Carbopol 934P NF (10:5) | 32.35 ± 0.24 | 13.96 ± 0.03 | 1.15 ± 0.06 |
| Ethocel 100P (10:5) + Carbopol 934P NF with HPMC | 31.12 ± 0.06 | 12.73 ± 0.72 | 1.14 ± 0.75 |
| Ethocel 100FP (10:5) + Carbopol 934P NF with HPMC | 34.18 ± 0.09 | 14.78 ± 0.41 | 1.16 ± 0.67 |
| Ethocel 100P (10:5) + Carbopol 934P NF with CMC | 30.33 ± 0.15 | 11.71 ± 0.63 | 1.13 ± 0.19 |
| Ethocel 100FP (10:5) + Carbopol 934P NF with CMC | 28.26 ± 0.23 | 9.58 ± 0.70 | 1.09 ± 0.33 |
| Ethocel 100P (10:5) + Carbopol 934P NF with Starch | 33.23 ± 0.45 | 14.83 ± 0.09 | 1.17 ± 0.39 |
| Ethocel 100FP + Carbopol 934P NF (10:5) with Starch | 30.82 ± 0.68 | 11.45 ± 0.06 | 1.14 ± 0.03 |
| Formulations | Thickness (mm, n = 10, Acceptable Limit 2–4 nm) | Diameter (mm, n = 10, Acceptable Limit 4–13 nm) | Friability (%, n = 20, Acceptable limit < 0.8%) | Hardness (kg/cm2, n = 10, Acceptable Limit 5–10 kg/cm2) | Weight Variation (mg, n = 20, 130–324 Acceptable Variation ± 7.5%) |
|---|---|---|---|---|---|
| Ethocel 100P + Carbopol 934P NF (10:3) | 2.5 ± 0.15 | 8.0 ± 0.36 | 0.05 ± 0.40 | 8.2 ± 0.61 | 203 ± 0.38 |
| Ethocel 100FP + Carbopol 934P NF (10:3) | 2.4 ± 0.08 | 8.0 ± 0.08 | 0.08 ± 0.15 | 9.2 ± 0.19 | 202 ± 0.12 |
| Ethocel 100P + Carbopol 934P NF (10:4) | 2.5 ± 0.33 | 8.0 ± 0.47 | 0.13 ± 0.09 | 8.4 ± 0.25 | 200 ± 0.32 |
| Ethocel 100FP + Carbopol 934P NF (10:4) | 2.4 ± 0.27 | 8.0 ± 0.23 | 0.23 ± 0.35 | 9.6 ± 0.03 | 200 ± 0.21 |
| Ethocel 100P + Carbopol 934P NF (10:5) | 2.5 ± 0.49 | 8.0 ± 0.46 | 0.34 ± 0.16 | 8.5 ± 0.35 | 202 ± 0.33 |
| Ethocel 100FP + Carbopol 934P NF (10:5) | 2.4 ± 0.65 | 8.0 ± 0.23 | 0.13 ± 0.32 | 9.8 ± 0.06 | 199 ± 0.44 |
| Ethocel 100P + Carbopol P934 NF (10:5) with HPMC | 2.5 ± 0.19 | 8.0 ± 0.44 | 0.02 ± 0.28 | 7.7 ± 0.21 | 202 ± 0.16 |
| Ethocel 100FP + Carbopol P934 NF (10:5) with HPMC | 2.4 ± 0.05 | 8.0 ± 0.99 | 0.22 ± 0.31 | 9.4 ± 0.03 | 201 ± 0.25 |
| Ethocel 100P + Carbopol P934 NF (10:5) with CMC | 2.5 ± 0.07 | 8.0 ± 0.68 | 0.08 ± 0.49 | 9.4 ± 0.27 | 200 ± 0.28 |
| Ethocel 100FP + Carbopol P934 NF (10:5) with CMC | 2.4 ± 0.03 | 8.0 ± 0.39 | 0.19 ± 0.05 | 9.9 ± 0.17 | 199 ± 0.34 |
| Ethocel 100 P + Carbopol P934 NF (10:5) with Starch | 2.5 ± 0.14 | 8.0 ± 0.42 | 0.15 ± 0.43 | 8.5 ± 0.16 | 201 ± 0.53 |
| Ethocel 100FP + Carbopol P934 NF (10:5) with Starch | 2.4 ± 0.12 | 8.0 ± 0.28 | 0.07 ± 0.26 | 8.6 ± 0.13 | 200 ± 0.81 |
| Formulations | Content Uniformity (%, n = 10) |
|---|---|
| Ethocel 100P + Carbopol 934P NF (10:3) | 98.94 |
| Ethocel 100FP + Carbopol 934P NF (10:3) | 99.32 |
| Ethocel 100P + Carbopol 934P NF (10:4) | 98.79 |
| Ethocel 100FP + Carbopol 934P NF (10:4) | 98.56 |
| Ethocel 100P + Carbopol 934P NF (10:5) | 99.09 |
| Ethocel 100FP + Carbopol 934P NF (10:5) | 98.52 |
| Ethocel 100P + Carbopol 934P NF (10:5) with HPMC | 98.90 |
| Ethocel 100FP + Carbopol 934P NF (10:5) with HPMC | 99.00 |
| Ethocel 100P + Carbopol 934P NF (10:5) with CMC | 98.70 |
| Ethocel 100FP + Carbopol 934P NF (10:5) with CMC | 98.61 |
| Ethocel 100P + Carbopol 934P NF (10:5) with Starch | 99.43 |
| Ethocel 100FP + Carbopol 934P NF (10:5) with Starch | 99.08 |
| Ist-Order Kinetic | Zero-Order Kinetic | Hixon Crowell’s Erosion Model | Highuchi Diffusion Model | Power Law | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k1 ± SD | r2 | k2 ± SD | r2 | k3 ± SD | r2 | k4 ± SD | r2 | k5 ± SD | r2 | N |
| Losartan Potassium + Ethocel 100P + Carbopol 934P NF (10:3) Controlled Release Matrices | ||||||||||
| −0.354 ± 0.43 | 0.884 | 7.387 ± 0.37 | 0.986 | 0.356 ± 0.43 | 0.942 | 7.654 ± 0.54 | 0.987 | 0.012 ± 0.03 | 0.973 | 0.682 |
| Losartan Potassium + Ethocel 100FP + Carbopol 934P NF (10:3) Controlled Release Matrices | ||||||||||
| −0.395 ± 0.28 | 0.871 | 8.525 ± 0.31 | 0.990 | 0. 457 ± 0.28 | 0.897 | 7.786 ± 0.58 | 0.992 | 0.013 ± 0.44 | 0.945 | 0.633 |
| Losartan Potassium + Ethocel 100P + Carbopol 934P NF (10:4) Controlled Release Matrices | ||||||||||
| −0.351 ± 0.37 | 0.873 | 7.783 ± 0.23 | 0.994 | 0.293 ± 0.26 | 0.883 | 6.758 ± 0.69 | 0.991 | 0.016 ± 0.08 | 0.939 | 0.626 |
| Losartan Potassium + Ethocel 100FP + Carbopol 934P NF (10:4) Controlled Release Matrices | ||||||||||
| −0.393 ± 0.38 | 0.789 | 7.988 ± 0.66 | 0.992 | 0.289 ± 0.26 | 0.845 | 6.657 ± 0.55 | 0.994 | 0.018 ± 0.26 | 0.942 | 0.734 |
| Losartan Potassium + Ethocel 100P + Carbopol 934P NF (10:5) Controlled Release Matrices | ||||||||||
| −0.379 ± 0.30 | 0.863 | 8.355 ± 0.34 | 0.986 | 0.276 ± 0.53 | 0.990 | 7.769 ± 0.365 | 0.983 | 0.028 ± 0.18 | 0.988 | 0.957 |
| Losartan Potassium + Ethocel 100FP + Carbopol 934P NF (10:5) Controlled Release Matrices | ||||||||||
| −0.186 ± 0.31 | 0.681 | 8.768 ± 0.54 | 0.988 | 0.264 ± 0.59 | 0.978 | 7.786 ± 0.53 | 0.986 | 0.073 ± 0.29 | 0.986 | 0.895 |
| Losartan Potassium and Ethocel 100 Premium + Carbopol 934P NF (10:5) Controlled Release Matrices with HPMC | ||||||||||
| −0.131 ± 0.16 | 0.776 | 2.347 ± 0.11 | 0.782 | 0.116 ± 0.13 | 0.728 | 2.276 ± 0.75 | 0.789 | 0.039 ± 0.01 | 0.875 | 0.758 |
| Losartan Potassium and Ethocel 100FP Premium + Carbopol P934 NF (10:5) Controlled Release Matrices with HPMC | ||||||||||
| −0.173 ± 0.12 | 0.889 | 3.269 ± 0.66 | 0.898 | 0.198 ± 0.17 | 0.862 | 2.382 ± 0.63 | 0.894 | 0.054 ± 0.04 | 0.849 | 0.766 |
| Losartan Potassium and Ethocel 100 Premium + Carbopol 934P NF (10:5) Controlled Release Matrices with CMC | ||||||||||
| −0.145 ± 0.16 | 0.888 | 4.354 ± 0.34 | 0.968 | 0.235 ± 0.25 | 0.881 | 3.661 ± 0.28 | 0.956 | 0.016 ± 0.02 | 0.987 | 0.694 |
| Losartan Potassium and Ethocel 100FP Premium + Carbopol 934P NF (10:5) Controlled Release Matrices with CMC | ||||||||||
| −02873 ± 0.16 | 0.769 | 3.775 ± 0.36 | 0.982 | 0.143 ± 0.11 | 0.987 | 3.268 ± 0.76 | 0.984 | 0.041 ± 0.01 | 0.989 | 0.779 |
| Losartan Potassium and Ethocel 100 Premium + Carbopol 934P NF (10:5) Controlled Release Matrices with Starch | ||||||||||
| −0.298 ± 0.13 | 0.879 | 2.354 ± 0.55 | 0.980 | 0.176 ± 0.12 | 0.985 | 3.721 ± 0.27 | 0.979 | 0.045 ± 0.01 | 0.976 | 0.737 |
| Losartan Potassium and Ethocel 100FP Premium + Carbopol 934P NF (10:5) Controlled Release Matrices with Starch | ||||||||||
| −0.292 ± 0.78 | 0.889 | 4.359 ± 0.22 | 0.985 | 0.154 ± 0.11 | 0.978 | 3.481 ± 0.25 | 0.972 | 0.049 ± 0.18 | 0.984 | 0.854 |
| Test Formulation versus Reference Cardaktin® Tablet | f1 Values (Acceptable Limit 1–15) | f2 Values (Acceptable Limit 50–50) |
|---|---|---|
| Ethocel 100P + Carbopol 934P NF (10:3) CR Matrices versus Cardaktin® Tablet | 42.73 | 17.99 |
| Ethocel 100FP + Carbopol 934P NF (10:3) CR Matrices versus Cardaktin® Tablet | 52.64 | 13.47 |
| Ethocel 100P + Carbopol 934P NF (10:4) CR Matrices versus Cardaktin® Tablet | 53. 22 | 12.38 |
| Ethocel 100FP + Carbopol 934P NF (10:4) CR Matrices versus Cardaktin® Tablet | 56. 48 | 10.16 |
| Ethocel 100P + Carbopol 934P NF (10:5) CR Matrices versus Cardaktin® Tablet | 38.16 | 20.76 |
| Ethocel 100FP + Carbopol 934P NF (10:5) CR Matrices versus Cardaktin® Tablet | 41.69 | 17.78 |
| Ethocel 100P + Carbopol 934P NF (10:5) with HPMC CR Matrices versus Cardaktin® Tablet | 46.53 | 14.06 |
| Ethocel 100FP + Carbopol 934P NF (10:5) with HPMC CR Matrices versus Cardaktin® Tablet | 48.54 | 13.22 |
| Ethocel 100P + Carbopol 934P NF (10:5) with CMC CR Matrices versus Cardaktin® Tablet | 56.48 | 11.36 |
| Ethocel 100FP + Carbopol 934P NF (10:5) with CMC CR Matrices versus Cardaktin® Tablet | 47.78 | 14.98 |
| Ethocel 100P + Carbopol 934P NF (10:5) with Starch CR Matrices versus Cardaktin® Tablet | 45.34 | 14.04 |
| Ethocel 100FP + Carbopol 934P NF (10:5) with Starch CR Matrices versus Cardaktin® Tablet | 48.76 | 13.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, K.A.; Khan, G.M.; Muzammal, M.; Al Mohaini, M.; Alsalman, A.J.; Al Hawaj, M.A.; Ahmad, A.; Niazi, Z.R.; Shah, K.U.; Farid, A. Preparation of Losartan Potassium Controlled Release Matrices and In-Vitro Investigation Using Rate Controlling Agents. Molecules 2022, 27, 864. https://doi.org/10.3390/molecules27030864
Khan KA, Khan GM, Muzammal M, Al Mohaini M, Alsalman AJ, Al Hawaj MA, Ahmad A, Niazi ZR, Shah KU, Farid A. Preparation of Losartan Potassium Controlled Release Matrices and In-Vitro Investigation Using Rate Controlling Agents. Molecules. 2022; 27(3):864. https://doi.org/10.3390/molecules27030864
Chicago/Turabian StyleKhan, Kamran Ahmad, Gul Majid Khan, Muhammad Muzammal, Mohammed Al Mohaini, Abdulkhaliq J. Alsalman, Maitham A. Al Hawaj, Ashfaq Ahmad, Zahid Rasul Niazi, Kifayat Ullah Shah, and Arshad Farid. 2022. "Preparation of Losartan Potassium Controlled Release Matrices and In-Vitro Investigation Using Rate Controlling Agents" Molecules 27, no. 3: 864. https://doi.org/10.3390/molecules27030864
APA StyleKhan, K. A., Khan, G. M., Muzammal, M., Al Mohaini, M., Alsalman, A. J., Al Hawaj, M. A., Ahmad, A., Niazi, Z. R., Shah, K. U., & Farid, A. (2022). Preparation of Losartan Potassium Controlled Release Matrices and In-Vitro Investigation Using Rate Controlling Agents. Molecules, 27(3), 864. https://doi.org/10.3390/molecules27030864








