Phenylalanine-Based AMPA Receptor Antagonist as the Anticonvulsant Agent with Neuroprotective Activity—In Vitro and In Vivo Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. In Vitro Assays
2.1.1. Antioxidant Activity
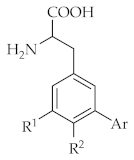
| Cmpd. | R 1 | R 2 | Ar | FRAP (%AAA) 1 | ORAC (TE) 2 | [3H]AMPA Ki (μM) 3 |
|---|---|---|---|---|---|---|
| 1 | NO2 | Cl | 2′,5′-diOH Ph | 748.49 | 3.46 | 0.92 |
| 2 | NO2 | Cl | 5′-OH-3-pyridyl | 691.21 | 3.15 | 2.0 |
| 3 | Cl | OH | 3′-OH Ph | 311.56 | 4.61 | 7.9 |
| 4 | Cl | Cl | 3′,4′-diOH Ph | 177.23 | 4.02 | 12 4 |
| 5 | NO2 | H | 4′-OH Ph | 96.32 | 4.32 | 21 |
| 6 | H | NH2 | 3′-OH Ph | 67.35 | 6.09 | 9.1 |
| 7 | Cl | H | 4′-OH Ph | 49.39 | 3.29 | 46 |
| 8 | Cl | NH2 | 4′-OH Ph | 48.19 | 7.56 | >100 |
| 9 | NO2 | H | 3′-OH Ph | 43.43 | 4.67 | 8.2 |
| 10 | H | NO2 | 3′-OH Ph | 32.04 | 1.43 | 21 |
| 11 | Cl | Cl | 3′,5′-diOH Ph | 28.90 | 4.18 | 16 4 |
| 12 | NO2 | NH2 | 3′-OH Ph | 24.93 | 4.27 | 14 |
| 13 | H | Cl | 3′-OH Ph | 24.08 | 2.86 | 9.2 |
| 14 | Cl | NH2 | 3′-OH Ph | 23.80 | 4.75 | 10 |
| 15 | Cl | Cl | 4′-OH Ph | 13.70 | 1.45 | 33 4 |
| 16 | Cl | Cl | 2′-OH Ph | 10.32 | 1.54 | 85 4 |
| 17 | H | Cl | 4′-OH Ph | 9.13 | 1.96 | 65 |
| 18 | NO2 | Cl | 3′-OH Ph | 6.29 | 1.34 | 3.1 |
| 19 | H | H | 3′-OH Ph | 4.94 | 1.96 | 9.9 4 |
| 20 | H | NO2 | 4′-OH Ph | 4.41 | 1.62 | 64 |
| 21 | NO2 | Cl | 4′-OH Ph | 2.76 | 2.56 | 23 |
| 22 | Cl | OCH3 | 3′-OH Ph | 2.76 | 1.38 | >100 |
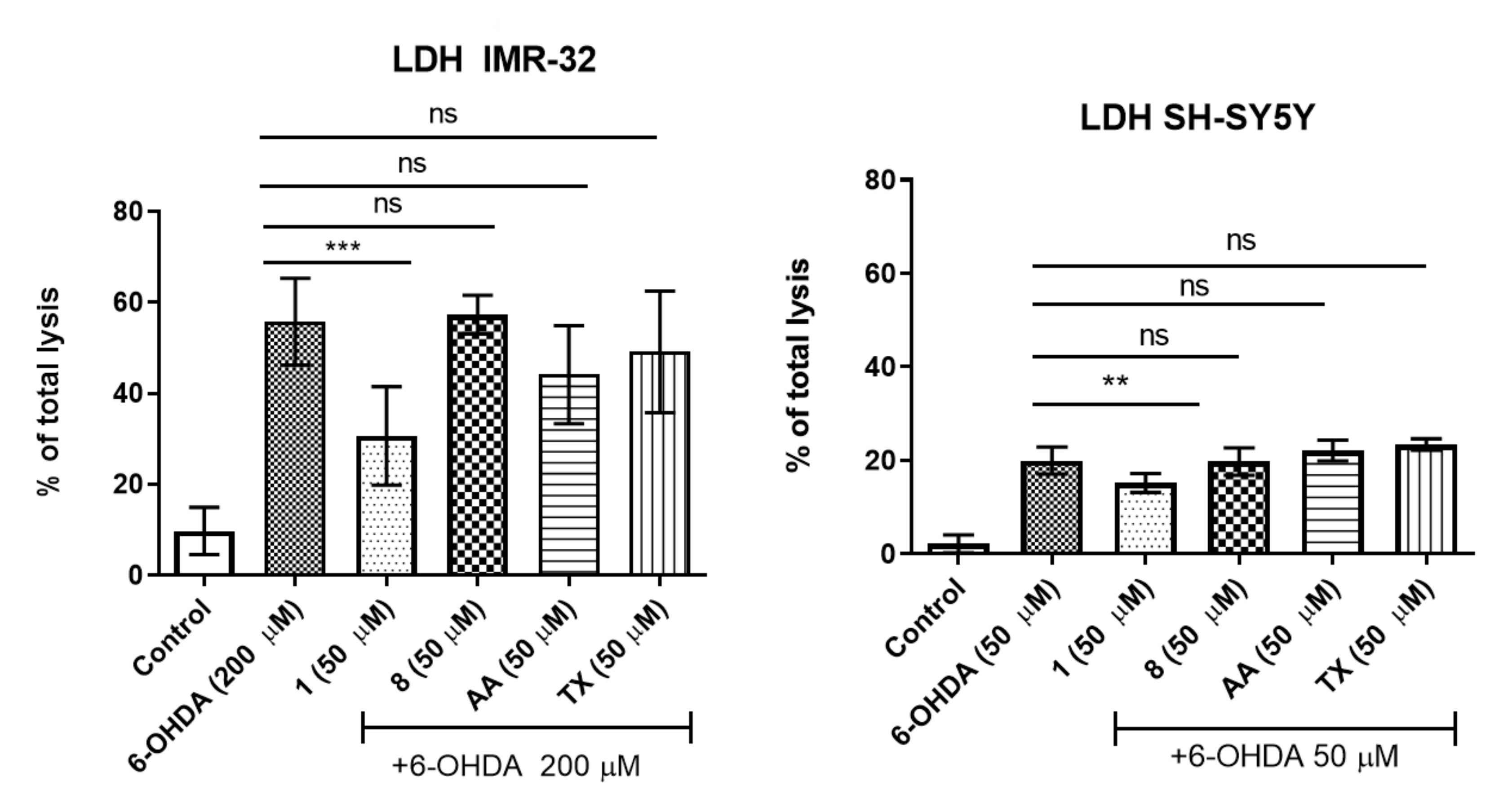
2.1.2. Neuroprotection
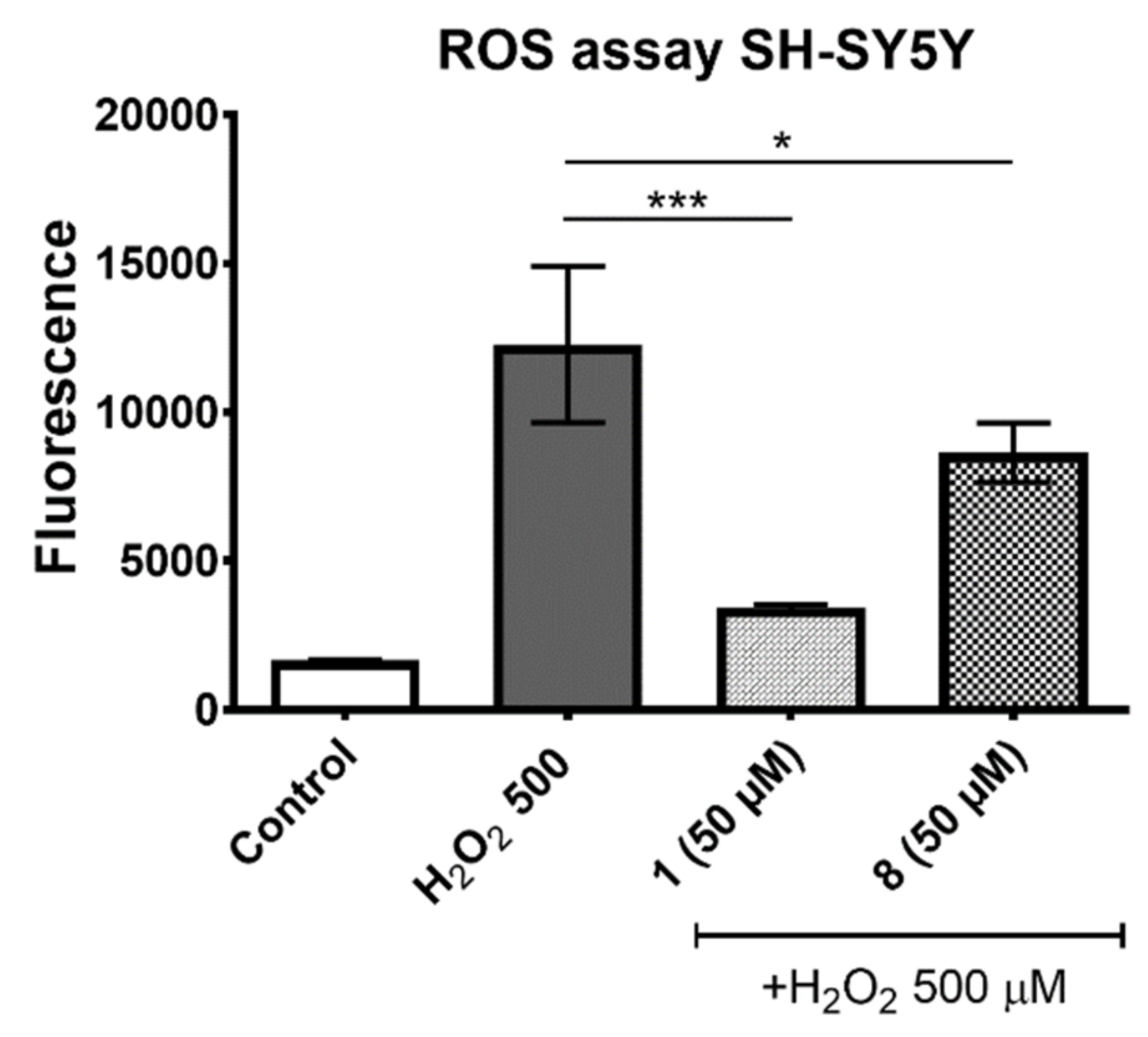
2.1.3. ROS Assay
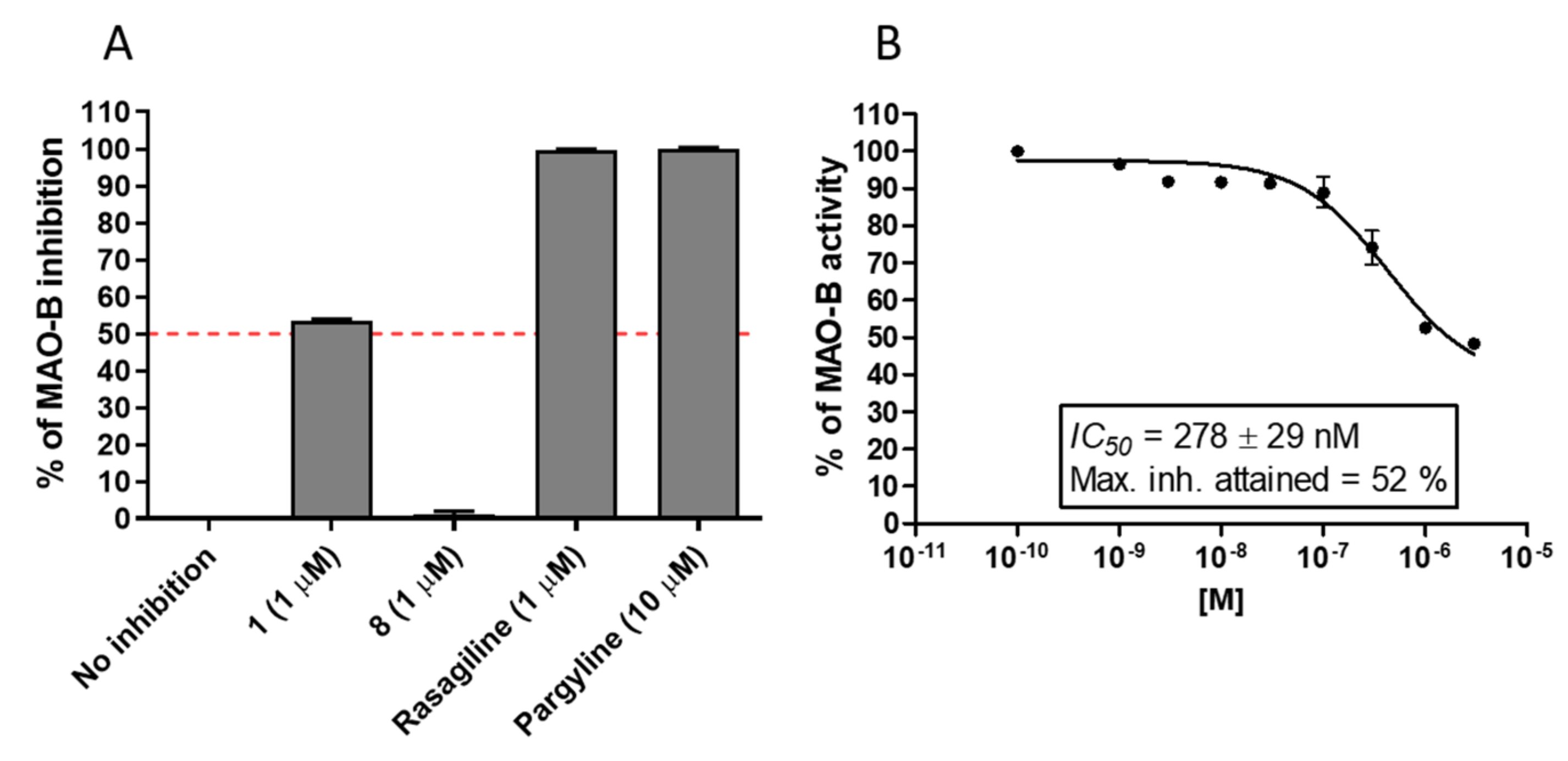
2.1.4. MAO-B Inhibition
2.2. In Vivo Assays
2.2.1. Anticonvulsant Activity in Seizure Models
- MES Test
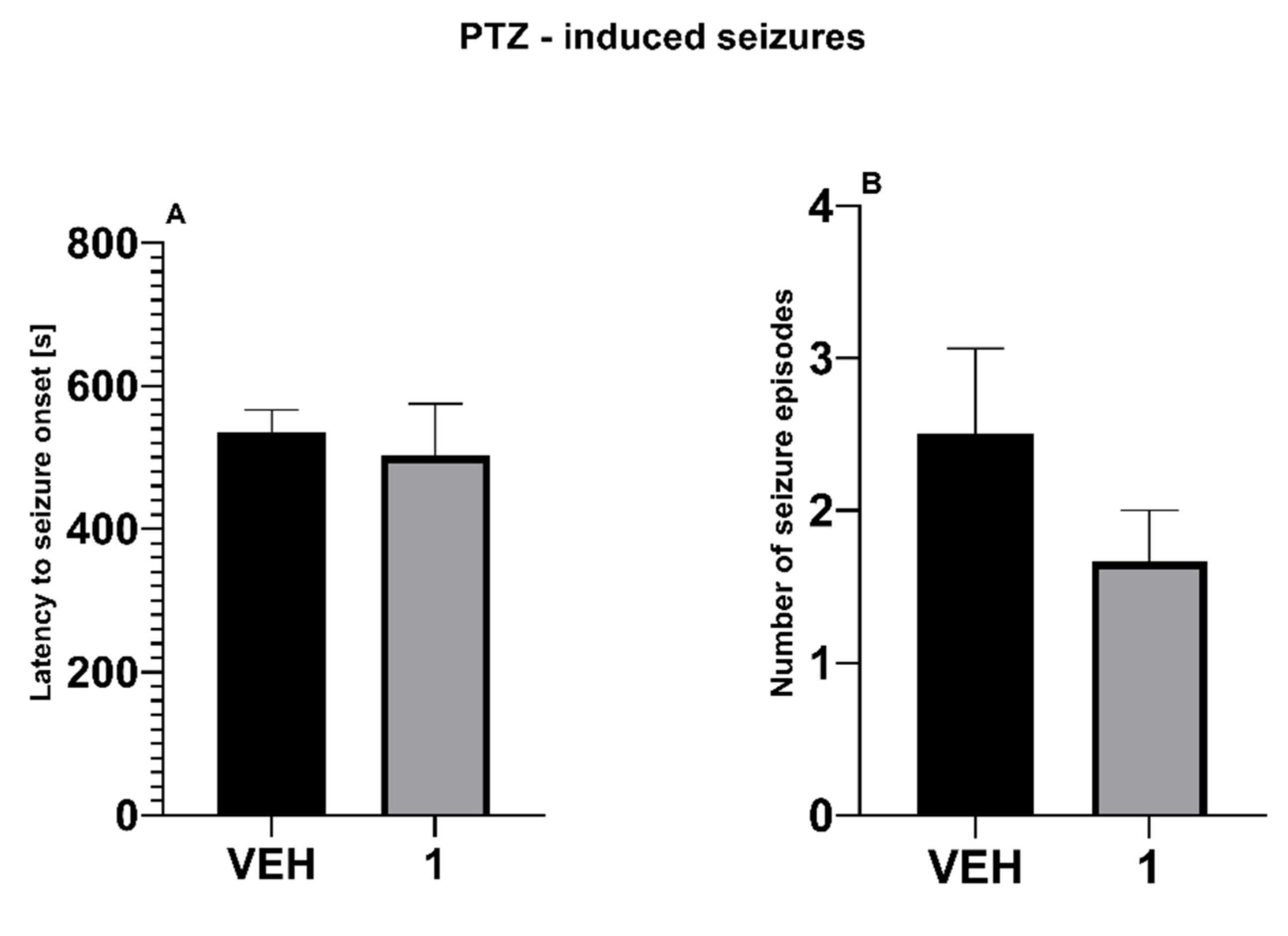
- PTZ Seizure Test
- 6-Hz Test
2.2.2. Antiallodynic and Antihyperalgesic Activity in Neuropathic Pain Models
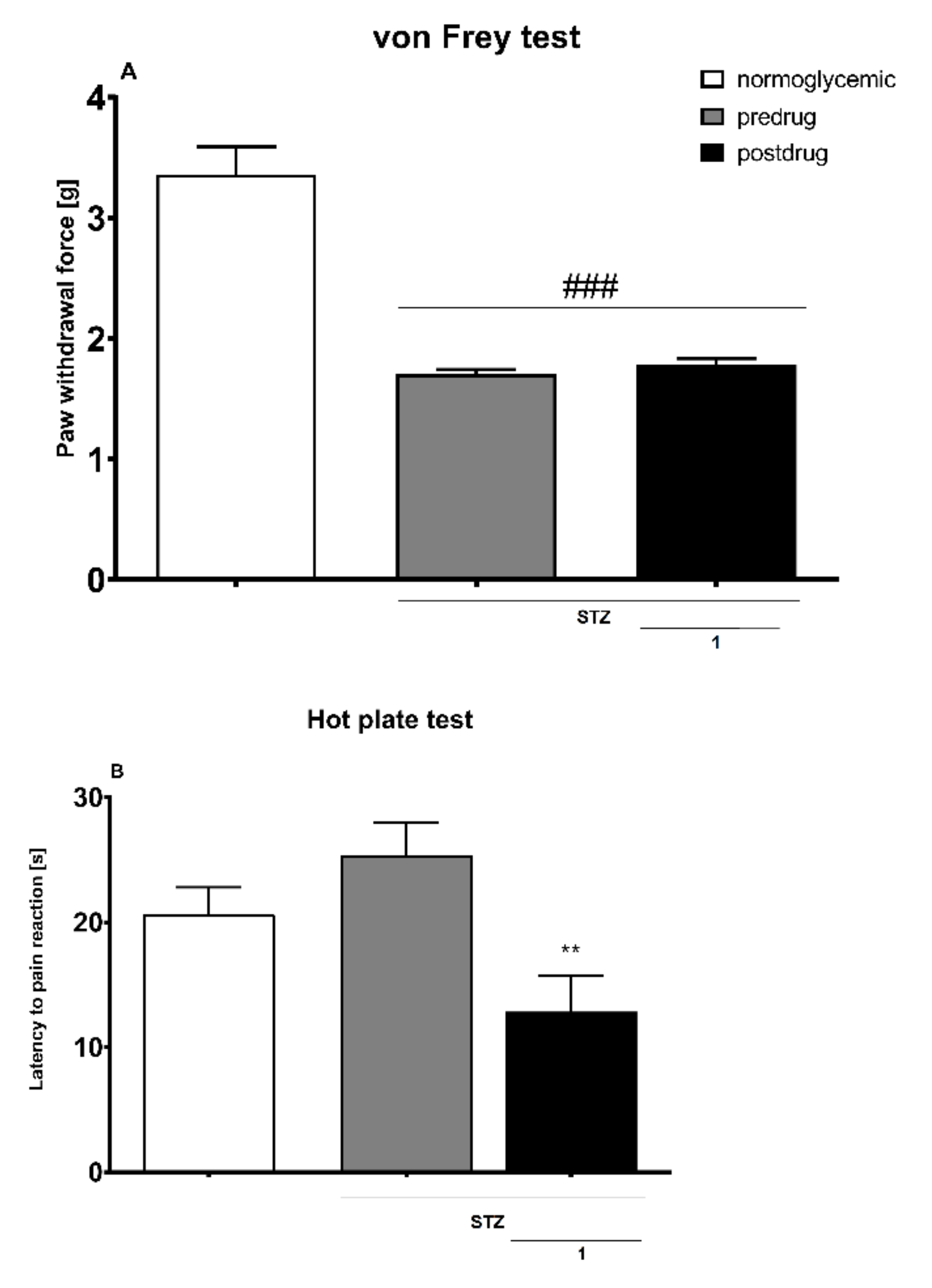
Diabetic Neuropathic Pain Model
- Effect on Tactile Allodynia—Von Frey Test
- Effect on Heat Hyperalgesia—Hot Plate Test
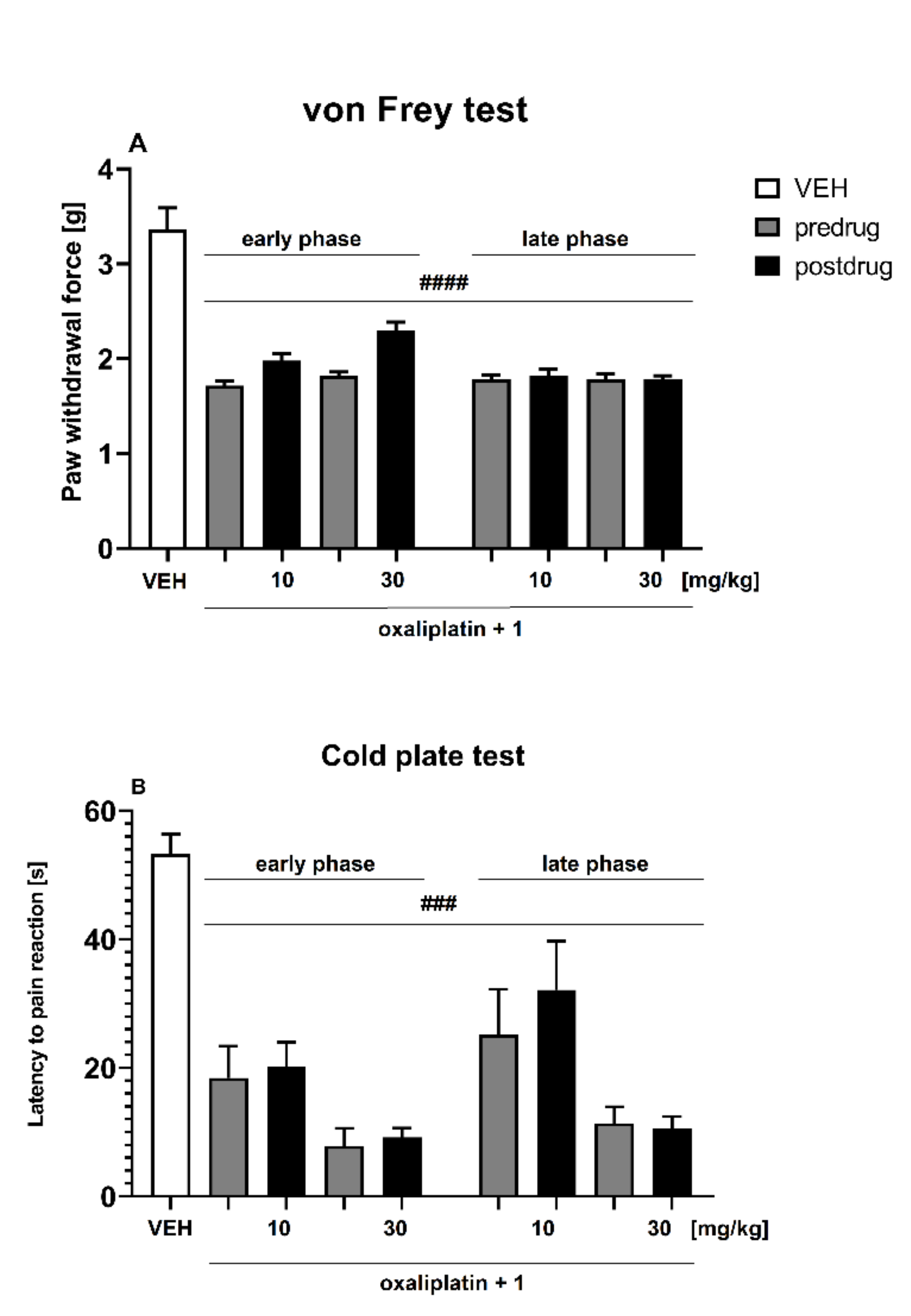
Oxaliplatin-Induced Neuropathic Pain Model
- Effect on Tactile Allodynia—Von Frey Test
- Effect on Cold Hyperalgesia—Cold Plate Test
3. Conclusions
4. Materials and Methods
4.1. In Vitro Assays
4.1.1. Antioxidant Activity
- FRAP
- ORAC-FL
4.1.2. Neuroprotection
- Cell Viability
- Lactate Dehydrogenase Test
- ROS Assay
4.1.3. MAO-B Inhibition
4.2. In Vivo Assays
4.2.1. Animals and Housing Conditions
4.2.2. Chemicals
4.2.3. Assessment of Anticonvulsant Activity
- Maximal Electroshock Test (MES Test)
- PTZ Test
- 6-Hz Test
4.2.4. Assessment of Antiallodynic and Antihyperalgesic Properties in Neuropathic Pain Models
Streptozotocin-Induced Painful Diabetic Neuropathy
- Induction of Diabetes
- Influence on Tactile Allodynia in Diabetic Mice—Von Frey Test
- Influence on Heat Hyperalgesia in Diabetic Mice—Hot Plate Test
Oxaliplatin-Induced Peripheral Neuropathy
- Induction of Neuropathy
- Influence on Tactile Allodynia in Oxaliplatin-Treated Mice—Von Frey Test
- Influence on Cold Hyperalgesia in Oxaliplatin-Treated Mice—Cold Plate Test
4.2.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chang, P.K.Y.; Verbich, D.; McKinney, R.A. AMPA receptors as drug targets in neurological disease–advantages, caveats, and future outlook. Eur. J. Neurosci. 2012, 35, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, D.; Jiang, R.; Taly, A.; Chataigneau, T.; Specht, A.; Grutter, T. Ligand-gated Ion channels: New insights into neurological disorders and ligand recognition. Chem. Rev. 2012, 112, 6285–6318. [Google Scholar] [CrossRef] [PubMed]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate Receptor Ion Channels: Structure, Regulation, and Function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, K.A.; Conn, P.J.; Niswender, C.M. Glutamate Receptors as Therapeutic Targets for Parkinsons Disease. CNS Neurol. Disord.-Drug Targets 2009, 8, 475–491. [Google Scholar] [CrossRef]
- Bleakman, D.; Alt, A.; Nisenbaum, E.S. Glutamate receptors and pain. Semin. Cell Dev. Biol. 2006, 17, 592–604. [Google Scholar] [CrossRef]
- Gui, Y.; Chen, L.; Duan, S.; Li, G.; Tang, J.; Li, A. Methyl cinnamate alleviated CCI-induced upregualtion of spinal AMPA receptors and pain hypersensitivity by targeting AMPK. Eur. J. Pharmacol. 2018, 833, 183–189. [Google Scholar] [CrossRef]
- Xie, X.; Ma, L.; Xi, K.; Zhang, W.; Fan, D. MicroRNA-183 suppresses neuropathic pain and expression of AMPA receptors by targeting mTOR/VEGF signaling pathway. Cell. Physiol. Biochem. 2017, 41, 181–192. [Google Scholar] [CrossRef]
- Bleakman, D.; Alt, A.; Witkin, J.M. AMPA Receptors in the Therapeutic Management of Depression. CNS Neurol. Disord.-Drug Targets 2007, 6, 117–126. [Google Scholar] [CrossRef]
- Alt, A.; Witkin, J.M.; Bleakman, D. AMPA Receptor Potentiators as Novel Antidepressants. Curr. Pharm. Des. 2005, 11, 1511–1527. [Google Scholar] [CrossRef]
- Russo, E.; Gitto, R.; Citraro, R.; Chimirri, A.; De Sarro, G. New AMPA antagonists in epilepsy. Expert Opin. Investig. Drugs 2012, 21, 1371–1389. [Google Scholar] [CrossRef]
- Rogawski, M.A. Revisiting AMPA Receptors as an Antiepileptic Drug Target. Epilepsy Curr. 2011, 11, 56–63. [Google Scholar] [CrossRef]
- Di Bonaventura, C.; Labate, A.; Maschio, M.; Meletti, S.; Russo, E. AMPA receptors and perampanel behind selected epilepsies: Current evidence and future perspectives. Expert Opin. Pharmacother. 2017, 18, 1751–1764. [Google Scholar] [CrossRef]
- Leo, A.; Giovannini, G.; Russo, E.; Meletti, S. The role of AMPA receptors and their antagonists in status epilepticus. Epilepsia 2018, 59, 1098–1108. [Google Scholar] [CrossRef] [Green Version]
- Hanada, T. Ionotropic Glutamate Receptors in Epilepsy: A Review Focusing on AMPA and NMDA Receptors. Biomolecules 2020, 10, 464. [Google Scholar] [CrossRef] [Green Version]
- Traynor, K. Perampanel approved for epilepsy. Am. J. Health Pharm. 2012, 69, 2024. [Google Scholar] [CrossRef]
- Cavalli, A.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V.; Recanatini, M.; Melchiorre, C. Multi-target-directed ligands to combat neurodegenerative diseases. J. Med. Chem. 2008, 51, 347–372. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Waldbaum, S.; Patel, M. Mitochondrial dysfunction and oxidative stress: A contributing link to acquired epilepsy? J. Bioenerg. Biomembr. 2010, 42, 449–455. [Google Scholar] [CrossRef] [Green Version]
- Youdim, M.B.H.; Bakhle, Y.S. Monoamine oxidase: Isoforms and inhibitors in Parkinson’s disease and depressive illness. Br. J. Pharmacol. 2006, 147, S287–S296. [Google Scholar] [CrossRef] [Green Version]
- Szymańska, E.; Pickering, D.S.; Nielsen, B.; Johansen, T. 3-Substituted phenylalanines as selective AMPA- and kainate receptor ligands. Bioorganic Med. Chem. 2009, 17, 6390–6401. [Google Scholar] [CrossRef]
- Szymanska, E.; Frydenvang, K.; Pickering, D.S.; Krintel, C.; Nielsen, B.; Kooshki, A.; Zachariassen, L.G.; Olsen, L.; Kastrup, J.S.; Johansen, T. Studies on Aryl-Substituted Phenylalanines: Synthesis, Activity, and Different Binding Modes at AMPA Receptors. J. Med. Chem. 2015, 59, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Nielsen, B.; Johansen, T.N.; Cuñado Moral, A.M.; Pickering, D.S.; Szczepańska, K.; Mickowska, A.; Kieć-Kononowicz, K. Pharmacological characterization and binding modes of novel racemic and optically active phenylala-nine-based antagonists of AMPA receptors. Eur. J. Med. Chem. 2017, 138, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Ma, Y.-Y. Calcium Permeable-AMPA Receptors and Excitotoxicity in Neurological Disorders. Front. Neural Circuits 2021, 15, 82. [Google Scholar] [CrossRef]
- Potschka, H.; Trinka, E. Perampanel: Does it have broad-spectrum potential? Epilepsia 2019, 60, 22–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnet, C.S.; Williams, A.S.; Gilbert, S.J.; Harvey, A.K.; Evans, B.A.; Mason, D.J. AMPA/kainate glutamate receptors contribute to inflammation, degeneration and pain related behaviour in inflammatory stages of arthritis. Ann. Rheum. Dis. 2013, 74, 242–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Caro, C.; Cristiano, C.; Avagliano, C.; Cuozzo, M.; La Rana, G.; Aviello, G.; De Sarro, G.; Calignano, A.; Russo, E.; Russo, R. Analgesic and anti-inflammatory effects of Perampanel in acute and chronic pain models in mice: Interaction with the can-nabinergic system. Front. Pharmacol. 2021, 11, 2230. [Google Scholar] [CrossRef] [PubMed]
- Kopach, O.; Krotov, V.; Goncharenko, J.; Voitenko, N. Inhibition of Spinal Ca2+-Permeable AMPA Receptors with Dicationic Compounds Alleviates Persistent Inflammatory Pain without Adverse Effects. Front. Cell. Neurosci. 2016, 10, 50. [Google Scholar] [CrossRef] [Green Version]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Wade, L.A.; Katzman, R. Synthetic amino acids and the nature of L-DOPA transport at the blood-brain barrier. J. Neurochem. 1975, 25, 837–842. [Google Scholar] [CrossRef]
- Kageyama, T.; Nakamura, M.; Matsuo, A.; Yamasaki, Y.; Takakura, Y.; Hashida, M.; Kanai, Y.; Naito, M.; Tsuruo, T.; Minato, N.; et al. The 4F2hc/LAT1 complex transports l-DOPA across the blood–brain barrier. Brain Res. 2000, 879, 115–121. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood-Brain Barrier and Delivery of Protein and Gene Therapeutics to Brain. Front. Aging Neurosci. 2020, 11, 373. [Google Scholar] [CrossRef]
- Szymańska, E.; Frydenvang, K.; Contreras-Sanz, A.; Pickering, D.S.; Frola, E.; Serafimoska, Z.; Nielsen, B.; Kastrup, J.S.; Johansen, T.N. A New Phenylalanine Derivative Acts as an Antagonist at the AMPA Receptor GluA2 and Introduces Partial Domain Closure: Synthesis, Resolution, Pharmacology, and Crystal Structure. J. Med. Chem. 2011, 54, 7289–7298. [Google Scholar] [CrossRef]
- Szymańska, E.; Chałupnik, P.; Johansen, T.; Nielsen, B.; Moral, A.M.C.; Pickering, D.S.; Więckowska, A.; Kiec-Kononowicz, K. Aryl- and heteroaryl-substituted phenylalanines as AMPA receptor ligands. Chem. Biol. Drug Des. 2017, 90, 1271–1281. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘‘antioxidant power’’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.R.; Hu, L.S.; Li, G.Y. SH-SY5Y human neuroblastoma cell line: In vitro cell model of dopaminergic neurons in Par-kinson’s disease. Chin. Med. J. 2010, 123, 1086–1092. [Google Scholar]
- Neill, D.; Hughes, D.; Edwardson, J.A.; Rima, B.K.; Allsop, D. Human IMR-32 neuroblastoma cells as a model cell line in Alzheimer’s disease research. J. Neurosci. Res. 1994, 39, 482–493. [Google Scholar] [CrossRef]
- Schober, A. Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res. 2004, 318, 215–224. [Google Scholar] [CrossRef]
- Blum, D.; Torch, S.; Lambeng, N.; Nissou, M.F.; Benabid, A.L.; Sadoul, R.; Verna, J.M. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson’s disease. Prog. Neurobiol. 2001, 65, 135–172. [Google Scholar] [CrossRef]
- Rodriguez-Pallares, J.; Parga, J.A.; Muñoz, A.; Rey, P.; Guerra, M.J.; Labandeira-Garcia, J.L. Mechanism of 6-hydroxydopamine neurotoxicity: The role of NADPH oxidase and microglial activation in 6-hydroxydopamine-induced degeneration of dopaminergic neurons. J. Neurochem. 2007, 103, 145–156. [Google Scholar] [CrossRef]
- Simola, N.; Morelli, M.; Carta, A.R. The 6-hydroxydopamine model of Parkinson’s disease. Neurotox. Res. 2007, 11, 151–167. [Google Scholar] [CrossRef]
- Zhou, M.J.; Diwu, Z.J.; PanchukVoloshina, N.; Haugland, R.P. A stable nonfluorescent derivative of resorufin for the fluo-rometric determination of trace hydrogen peroxide: Applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal. Biochem. 1997, 253, 162–168. [Google Scholar] [CrossRef]
- Ażewska, D.; Olejarz-Maciej, A.; Kaleta, M.; Bajda, M.; Siwek, A.; Karcz, T.; Doroz-Płonka, A.; Cichoń, U.; Kuder, K.; Kieć-Kononowicz, K. 4-Tert-pentylphenoxyalkyl derivatives–histamine H3 receptor ligands and monoamine oxidase B inhibitors. Bioorg. Med. Chem. Lett. 2018, 28, 3596–3600. [Google Scholar] [CrossRef]
- Ho, Y.-H.; Lin, Y.-T.; Wu, C.-W.J.; Chao, Y.-M.; Chang, A.Y.W.; Chan, J.Y.H. Peripheral inflammation increases seizure susceptibility via the induction of neuroinflammation and oxidative stress in the hippocampus. J. Biomed. Sci. 2015, 22, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Zuo, Z.; Yan, W.; Liu, W.; Zheng, Q.; Liu, X. Berberine ameliorates diabetic neuropathic pain in a rat model: Involvement of oxidative stress, inflammation, and μ-opioid receptors. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 2019, 392, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Sałat, K.; Gawlik, K.; Witalis, J.; Pawlica-Gosiewska, D.; Filipek, B.; Solnica, B.; Więckowski, K.; Malawska, B. Evaluation of antinociceptive and antioxidant properties of 3-[4-(3-trifluoromethyl-phenyl)-piperazin-1-yl]-dihydrofuran-2-one in mice. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 2013, 386, 493–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, F.; Wang, R.; Cui, G.; Li, X.; Wang, T.; Li, X. Engagement of MicroRNA-155 in Exaggerated Oxidative Stress Signal and TRPA1 in the Dorsal Horn of the Spinal Cord and Neuropathic Pain During Chemotherapeutic Oxaliplatin. Neurotox. Res. 2019, 36, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Connolly, M.B. Dravet Syndrome: Diagnosis and Long-Term Course. Can. J. Neurol. Sci. 2016, 43, S3–S8. [Google Scholar] [CrossRef] [Green Version]
- Haoudy, S.; Jonveaux, T.; Aron, O.; Puisieux, S.; Hopes, L.; Roudil, J.; Tyvaert, L. Epilepsy in early onset Alzheimer’s disease. Eur. J. Neurol. 2020, 27, e037573. [Google Scholar] [CrossRef]
- Cravello, L.; Di Santo, S.; Varrassi, G.; Benincasa, D.; Marchettini, P.; De Tommaso, M.; Shofany, J.; Assogna, F.; Perotta, D.; Palmer, K.; et al. Chronic Pain in the Elderly with Cognitive Decline: A Narrative Review. Pain Ther. 2019, 8, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Letro, G.H.; Quagliato, E.M.A.B.; Viana, M.A. Pain in Parkinson’s disease. Arq. Neuropsiquiatr. 2009, 67, 591–594. [Google Scholar] [CrossRef] [Green Version]
- Lopez, J.; Gamache, K.; Schneider, R.; Nader, K. Memory Retrieval Requires Ongoing Protein Synthesis and NMDA Receptor Activity-Mediated AMPA Receptor Trafficking. J. Neurosci. 2015, 35, 2465–2475. [Google Scholar] [CrossRef] [Green Version]
- Alt, A.; Nisenbaum, E.S.; Bleakman, D.; Witkin, J.M. A role for AMPA receptors in mood disorders. Biochem. Pharmacol. 2006, 71, 1273–1288. [Google Scholar] [CrossRef]
- Löscher, W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure 2011, 20, 359–368. [Google Scholar] [CrossRef] [Green Version]
- Löscher, W.; Schmidt, D. Modern antiepileptic drug development has failed to deliver: Ways out of the current dilemma. Epilepsia 2011, 52, 657–678. [Google Scholar] [CrossRef]
- Bankstahl, M.; Bankstahl, J.P.; Löscher, W. Pilocarpine-induced epilepsy in mice alters seizure thresholds and the efficacy of antiepileptic drugs in the 6-Hertz psychomotor seizure model. Epilepsy Res. 2013, 107, 205–216. [Google Scholar] [CrossRef]
- Brodie, M.J.; Covanis, A.; Gil-Nagel, A.; Lerche, H.; Perucca, E.; Sills, G.J.; White, H.S. Antiepileptic drug therapy: Does mechanism of action matter? Epilepsy Behav. 2011, 21, 331–341. [Google Scholar] [CrossRef]
- Pedre, L.L.; Gallardo, J.M.; Chacón, L.M.M.; García, A.V.; Flores-Mendoza, M.; Neri-Gomez, T.; Díaz, B.E.; Cruz-Xenes, R.M.; Fuentes, N.P.; Orozco-Suárez, S. Oxidative Stress in Patients with Drug Resistant Partial Complex Seizure. Behav. Sci. 2018, 8, 59. [Google Scholar] [CrossRef] [Green Version]
- Nieoczym, D.; Socala, K.; Wlaź, P. Assessment of the Anticonvulsant Potency of Ursolic Acid in Seizure Threshold Tests in Mice. Neurochem. Res. 2018, 43, 995–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vamecq, J.; Maurois, P.; Pages, N.; Bac, P.; Stables, J.P.; Gressens, P.; Stanicki, D.; Eynde, J.J.V. 1,2-Ethane bis-1-amino-4-benzamidine is active against several brain insult and seizure challenges through anti-NMDA mechanisms targeting the 3H-TCP binding site and antioxidant action. Eur. J. Med. Chem. 2010, 45, 3101–3110. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Zanardelli, M.; Failli, P.; Ghelardini, C. Oxaliplatin-induced neuropathy: Oxidative stress as patho-logical mechanism. Protective effect of silibinin. J. Pain 2012, 13, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Di Cesare Mannelli, L.; Zanardelli, M.; Failli, P.; Ghelardini, C. Oxaliplatin-induced oxidative stress in nervous system-derived cellular models: Could it correlate with in vivo neuropathy? Free Radic. Biol. Med. 2013, 61, 143–150. [Google Scholar] [CrossRef]
- Mannelli, L.D.C.; Zanardelli, M.; Landini, I.; Pacini, A.; Ghelardini, C.; Mini, E.; Bencini, A.; Valtancoli, B.; Failli, P. Effect of the SOD mimetic MnL4 on in vitro and in vivo oxaliplatin toxicity: Possible aid in chemotherapy induced neuropathy. Free Radic. Biol. Med. 2016, 93, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Kurnik-Lucka, M.; Latacz, G.; Martyniak, A.; Bugajski, A.; Kiec-Kononowicz, K.; Gil, K. Salsolinol-neurotoxic or neuropro-tective? Neurotox. Res. 2020, 37, 286–297. [Google Scholar] [CrossRef] [Green Version]
- Ażewska, D.; Olejarz-Maciej, A.; Reiner, D.; Kaleta, M.; Latacz, G.; Zygmunt, M.; Doroz-Płonka, A.; Karcz, T.; Frank, A.; Stark, H.; et al. Dual target ligands with 4-tert-butylphenoxy scaffold as histamine H-3 receptor antagonists and monoamine oxidase B inhibitors. Int. J. Mol. Sci. 2020, 21, 3411. [Google Scholar] [CrossRef]
- Sałat, K.; Podkowa, A.; Kowalczyk, P.; Kulig, K.; Dziubina, A.; Filipek, B.; Librowski, T. Anticonvulsant active inhibitor of GABA transporter subtype 1, tiagabine, with activity in mouse models of anxiety, pain and depression. Pharmacol. Rep. 2015, 67, 465–472. [Google Scholar] [CrossRef]
- Barton, M.E.; Klein, B.D.; Wolf, H.H.; White, H.S. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001, 47, 217–227. [Google Scholar] [CrossRef]
- Łączkowski, K.Z.; Konklewska, N.; Biernasiuk, A.; Malm, A.; Sałat, K.; Furgała-Wojas, A.; Dzitko, K.; Bekier, A.; Baranowska-Łączkowska, A.; Paneth, A. Thiazoles with cyclopropyl fragment as antifungal, anticonvulsant, and anti-Toxoplasma gondii agents: Synthesis, toxicity evaluation, and molecular docking study. Med. Chem. Res. 2018, 27, 2125–2140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sałat, K.; Kolaczkowski, M.; Furgała-Wojas, A.; Rojek, A.; Śniecikowska, J.; Varney, M.A.; Newman-Tancredi, A. Antinociceptive, antiallodynic and antihyperalgesic effects of the 5-HT1A receptor selective agonist, NLX-112 in mouse models of pain. Neuropharmacology 2017, 125, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Furgała, A.; Sałat, R.; Sałat, K. Acute cold allodynia induced by oxaliplatin is attenuated by amitriptyline. Acta Neurobiol. Exp. 2018, 78, 315–321. [Google Scholar] [CrossRef] [Green Version]
- Sałat, K.; Furgała, A.; Malikowska-Racia, N. Searching for analgesic drug candidates alleviating oxaliplatin-induced cold hypersensitivity in mice. Chem. Biol. Drug Des. 2019, 93, 1061–1072. [Google Scholar] [CrossRef]

| Compound | Dose [mg/kg] | X/Y 1 | Mortality Rate (%) |
|---|---|---|---|
| Vehicle | - | 1/5 | 20 |
| 1 | 100 | 0/4 | 25 |
| Compound | Dose [mg/kg] | X/Y 1 | Mortality rate (%) |
|---|---|---|---|
| Vehicle | - | 0/3 | 0 |
| 1 | 100 | 3/4 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latacz, G.; Sałat, K.; Furgała-Wojas, A.; Martyniak, A.; Olejarz-Maciej, A.; Honkisz-Orzechowska, E.; Szymańska, E. Phenylalanine-Based AMPA Receptor Antagonist as the Anticonvulsant Agent with Neuroprotective Activity—In Vitro and In Vivo Studies. Molecules 2022, 27, 875. https://doi.org/10.3390/molecules27030875
Latacz G, Sałat K, Furgała-Wojas A, Martyniak A, Olejarz-Maciej A, Honkisz-Orzechowska E, Szymańska E. Phenylalanine-Based AMPA Receptor Antagonist as the Anticonvulsant Agent with Neuroprotective Activity—In Vitro and In Vivo Studies. Molecules. 2022; 27(3):875. https://doi.org/10.3390/molecules27030875
Chicago/Turabian StyleLatacz, Gniewomir, Kinga Sałat, Anna Furgała-Wojas, Adrian Martyniak, Agnieszka Olejarz-Maciej, Ewelina Honkisz-Orzechowska, and Ewa Szymańska. 2022. "Phenylalanine-Based AMPA Receptor Antagonist as the Anticonvulsant Agent with Neuroprotective Activity—In Vitro and In Vivo Studies" Molecules 27, no. 3: 875. https://doi.org/10.3390/molecules27030875
APA StyleLatacz, G., Sałat, K., Furgała-Wojas, A., Martyniak, A., Olejarz-Maciej, A., Honkisz-Orzechowska, E., & Szymańska, E. (2022). Phenylalanine-Based AMPA Receptor Antagonist as the Anticonvulsant Agent with Neuroprotective Activity—In Vitro and In Vivo Studies. Molecules, 27(3), 875. https://doi.org/10.3390/molecules27030875






