The Impact of Foaming Effect on the Physical and Mechanical Properties of Foam Glasses with Molecular-Level Insights
Abstract
:1. Introduction
2. Results
2.1. Foaming Effects of Dextrin and Different Carbon Allotropes with Molecular-Level Insights
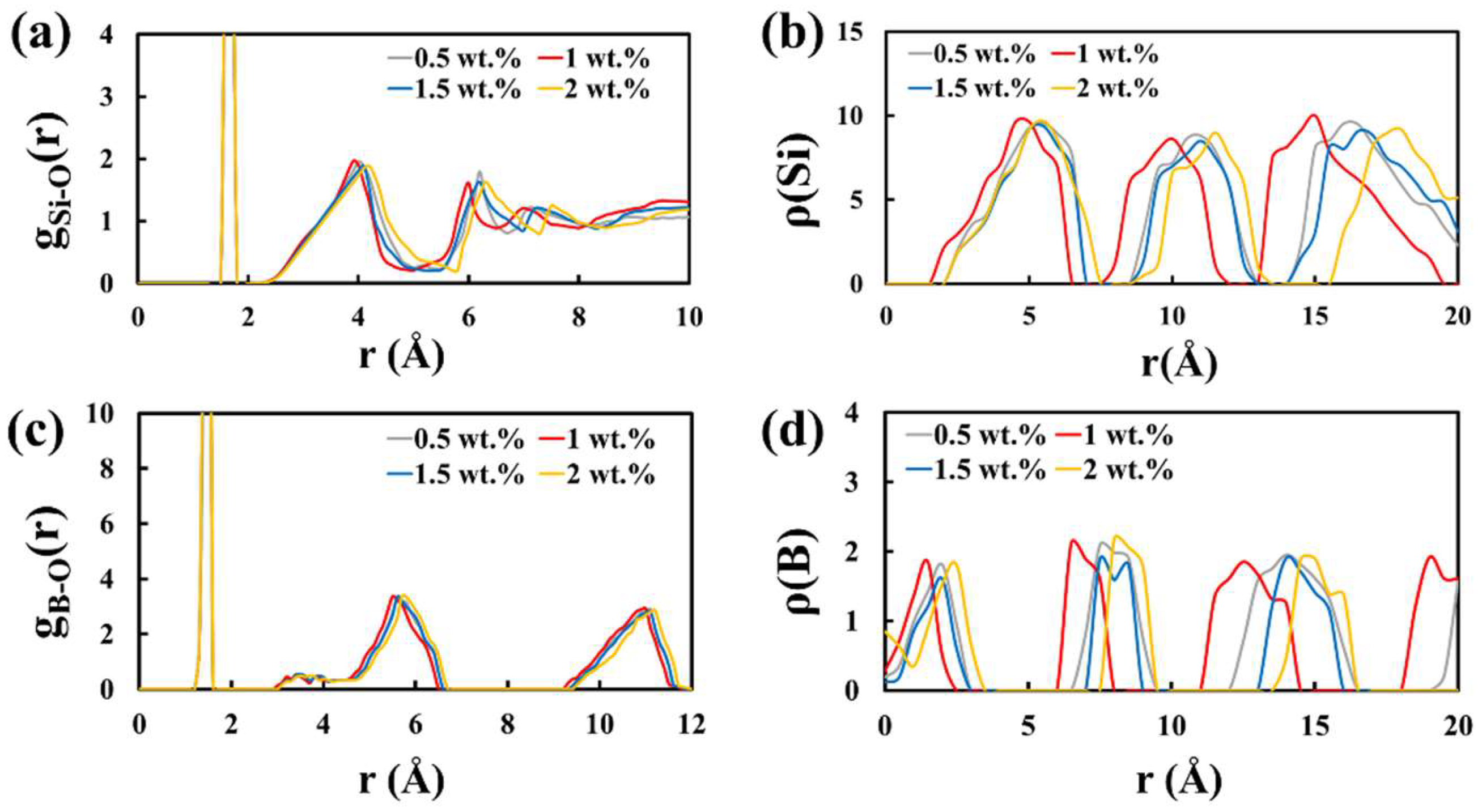
2.2. Chemical and Thermal Stability of the Foam Glass Samples with Carbon as Foaming Agents
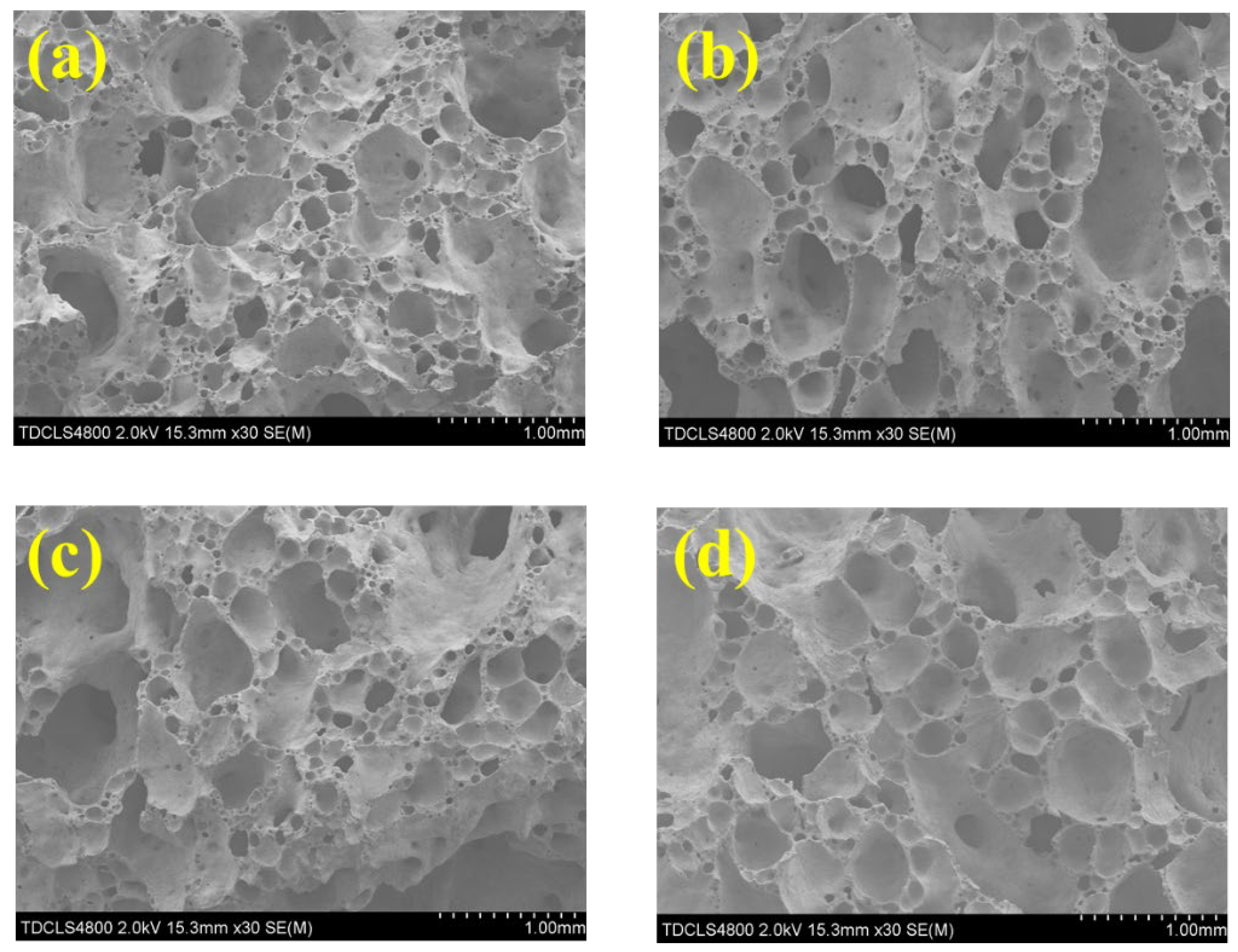
2.3. Foaming Effect of CaSO4 and Its Impact on the Physical and Thermal Properties of the Foam Glass Samples
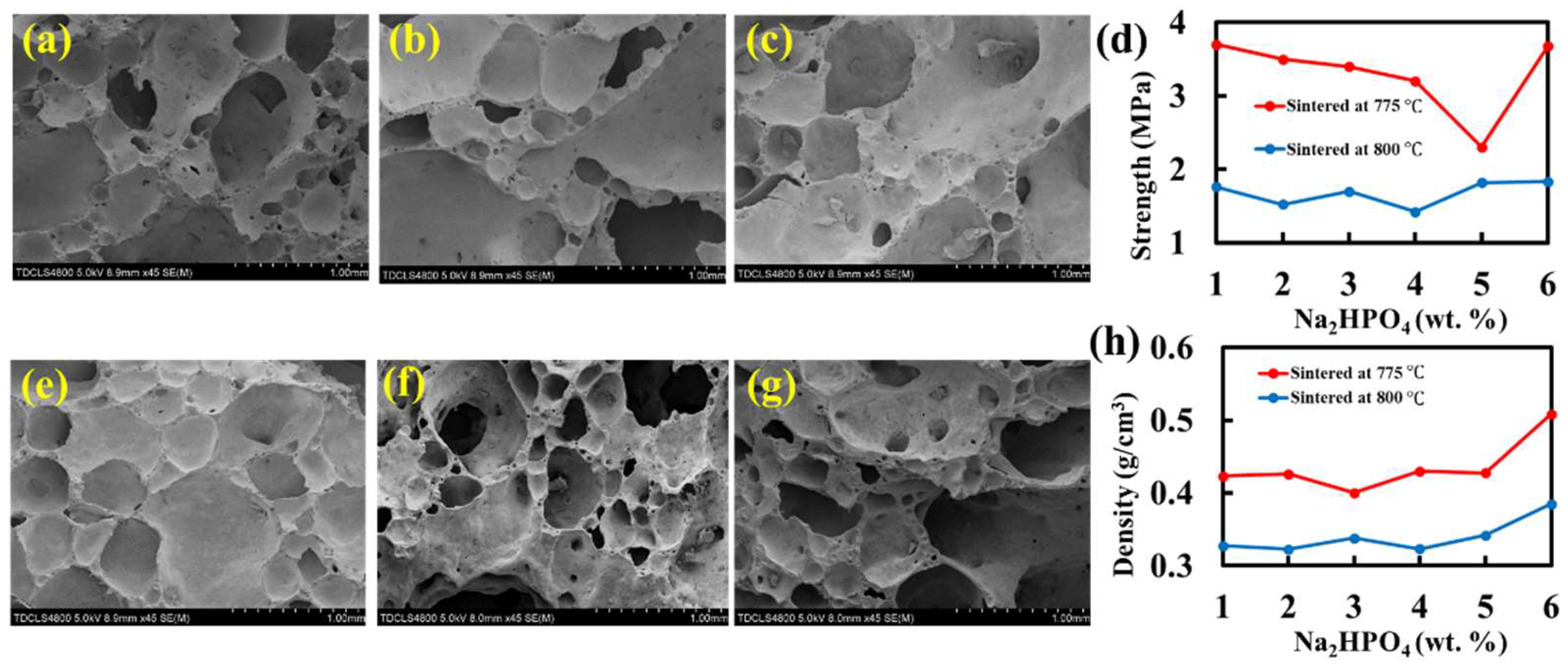
2.4. Foaming Effect of Na2HPO4 and Its Impact on the Physical and Mechanical Properties of the Foam Glass Samples
3. Discussion
4. Materials and Methods
4.1. Experimental Methods
4.2. Computational Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Bu, F.; Zhou, C.; Zhou, Q.; Wei, T.; Liu, J.; Zhai, W. Bonding performance and mechanism of a heat-resistant composite precursor adhesive (RT-1000 °C) for TC4 titanium alloy. J. Micromechanics Mol. Phys. 2020, 5, 2050016. [Google Scholar] [CrossRef]
- Wang, M.; Liu, J.; Chen, Z.; Hu, X.; Zhai, W.; Tao, X.; Liu, J. Joining zirconia with nickel-based superalloys for extreme applications by using a pressure-free high-temperature resistant adhesive. Ceram. Int. 2021. [Google Scholar] [CrossRef]
- Guo, H.; Wang, X.; Gong, Y.; Liu, X.; Gao, D. Improved mechanical property of foam glass composites toughened by glass fiber. Mater. Lett. 2010, 64, 2725–2727. [Google Scholar] [CrossRef]
- Zhai, C.; Zhong, Y.; Li, Z.; Wang, X.; Zhao, L.; Pan, L.; Zhang, J. Preparation and characterization of mechanical properties of foam glass for artificial floating island carrier. Adv. Mech. Eng. 2015, 7, 1–6. [Google Scholar] [CrossRef]
- Kuznetsova, V.O.; Yatsenko, N.D.; Subbotin, A.I.; Klimenko, M.Y. Resource-Saving Technologies in the Ther-mal Insulation-Structural Foam Glass Production. Mater. Sci. Forum 2020, 974, 356–361. [Google Scholar] [CrossRef]
- Lakov, L.; Toncheva, K.; Staneva, A.; Ilcheva, Z.; Simeonova, T. Composition, Synthesis and Properties of Color Architecture Building Foam Glass Obtained from Waste Packing Glass. J. Chem. Technol. Metall. 2013, 48, 130–135. [Google Scholar]
- Fernandes, H.; Tulyaganov, D.; Ferreira, J. Preparation and characterization of foams from sheet glass and fly ash using carbonates as foaming agents. Ceram. Int. 2009, 35, 229–235. [Google Scholar] [CrossRef]
- Kurtulus, C.; Kurtulus, R.; Kavas, T. Foam glass derived from ferrochrome slag and waste container glass: Synthesis and extensive characterizations. Ceram. Int. 2021, 47, 24997–25008. [Google Scholar] [CrossRef]
- Méar, F.; Yot, P.; Cambon, M.; Ribes, M. Elaboration and characterisation of foam glass from cathode ray tubes. Adv. Appl. Ceram. 2005, 104, 123–130. [Google Scholar] [CrossRef]
- Chen, B.; Wang, K.; Chen, X.; Lu, A. Study of Foam Glass with High Content of Fly Ash Using Calcium Carbonate as Foaming Agent. Mater. Lett. 2012, 79, 263–265. [Google Scholar] [CrossRef]
- Koudelka, L.; Šubčík, J.; Mošner, P.; Montagne, L.; Delevoye, L. Structure and properties of Sb2O3-containing zinc borophosphate glasses. J. Non Cryst. Solids 2007, 353, 1828–1833. [Google Scholar] [CrossRef]
- Liao, Y.-C.; Huang, C.-Y. Glass foam from the mixture of reservoir sediment and Na2CO3. Ceram. Int. 2012, 38, 4415–4420. [Google Scholar] [CrossRef]
- Tulyaganov, D.U.; Fernandes, H.R.; Agathopoulos, S.; Ferreira, J.M.F. Preparation and characterization of high compressive strength foams from sheet glass. J. Porous Mater. 2006, 13, 133–139. [Google Scholar] [CrossRef]
- Méar, F.; Pascal, Y.; Michel, R. Effects of Temperature, Reaction Time and Reducing Agent Content on the Synthesis of Macroporous Foam Glasses from Waste Funnel Glasses. Mater. Lett. 2006, 60, 929–934. [Google Scholar] [CrossRef]
- Zhai, C.; Li, Z.; Zhu, Y.; Zhang, J.; Wang, X.; Zhao, L.; Pan, L.; Wang, P. Effects of Sb2O3 on the Mechanical Properties of the Borosilicate Foam Glasses Sintered at Low Temperature. Adv. Mater. Sci. Eng. 2014, 2014, 703194. [Google Scholar] [CrossRef] [Green Version]
- Du, J. Study on Preparation Technique and Performance of Borosilicate Foam Glass; Tianjin University: Tianjin, China, 2007. [Google Scholar]
- Zhai, C.; Zhong, Y.; Liu, J.; Zhang, J.; Zhu, Y.; Wang, M.; Yeo, J. Customizing the Properties of Borosilicate Foam Glasses Via Additions under Low Sintering Temperatures with Insights from Molecular Dynamics Simulations. J. Non Cryst. Solids 2022, 576, 121273. [Google Scholar] [CrossRef]
- Zhai, C.; Zhou, H.; Gao, T.; Zhao, L.; Lin, S. Electrostatically Tuned Micro-domain Morphology and Phase-Dependent Ion Transport Anisotropy in Single-Ion Conducting Block Copoly-electrolytes. Macromolecules 2018, 51, 4471–4483. [Google Scholar] [CrossRef]
- Zhai, C.; Lin, S.; Wang, M.; Zhou, H.; Stanisauskis, E.; Cai, Z.; Yeo, J.; Oates, W. Conformational Freedom-Enhanced Optomechanical Energy Conversion Efficiency in Bulk Azo-Polyimides. Adv. Funct. Mater. 2021, 31, 2104414. [Google Scholar] [CrossRef]
- Zhai, C.; Li, T.; Shi, H.; Yeo, J. Discovery and design of soft polymeric bio-inspired materials with multiscale simulations and artificial intelligence. J. Mater. Chem. B 2020, 8, 6562–6587. [Google Scholar] [CrossRef]
- Lin, S.; Cai, Z.; Wang, Y.; Zhao, L.; Zhai, C. Tailored morphology and highly enhanced phonon transport in polymer fibers: A multiscale computational framework. NPJ Comput. Mater. 2019, 5, 126. [Google Scholar] [CrossRef]
- Isard, J. The mixed alkali effect in glass. J. Non-Cryst. Solids 1969, 1, 235–261. [Google Scholar] [CrossRef]
- Chen, B.; Luo, Z.; Lu, A. Preparation of sintered foam glass with high fly ash content. Mater. Lett. 2011, 65, 3555–3558. [Google Scholar] [CrossRef]
- Saparuddin, D.I.; Hisham, N.A.N.; Ab Aziz, S.; Matori, K.A.; Honda, S.; Iwamoto, Y.; Zaid, M.H.M. Effect of sintering temperature on the crystal growth, microstructure and mechanical strength of foam glass-ceramic from waste materials. J. Mater. Res. Technol. 2020, 9, 5640–5647. [Google Scholar] [CrossRef]
- da Silva, R.C.; Puglieri, F.N.; Chiroli, D.M.D.G.; Bartmeyer, G.A.; Kubaski, E.T.; Tebcherani, S.M. Recycling of glass waste into foam glass boards: A comparison of cradle-to-gate life cycles of boards with different foaming agents. Sci. Total Environ. 2021, 771, 145276. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Feng, Z.; Zhai, C.; Zhou, Q.; Wei, T.; Liu, J. Chromium carbide micro-whiskers: Preparation and strengthening effects in extreme conditions with experiments and molecular dynamics simulations. J. Solid State Chem. 2020, 291, 121598. [Google Scholar] [CrossRef]
- Wang, M.; Krishnan, N.A.; Wang, B.; Smedskjaer, M.M.; Mauro, J.C.; Bauchy, M. A new transferable interatomic potential for molecular dynamics simulations of borosilicate glasses. J. Non Cryst. Solids 2018, 498, 294–304. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, G.; Sator, N. A Computer Simulation Study of Natural Silicate Melts. Part I: Low Pres-sure Properties. Geochim. Cosmochim. Acta 2007, 71, 1249–1265. [Google Scholar]
- Zhang, Z.; Duan, Z. An optimized molecular potential for carbon dioxide. J. Chem. Phys. 2005, 122, 214507. [Google Scholar] [CrossRef]
- Markus, D.; Holm, C. How to Mesh up Ewald Sums. Ii. An Accurate Error Estimate for the Particle–Particle–Particle-Mesh Algorithm. J. Chem. Phys. 1998, 109, 7694–7701. [Google Scholar]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Thompson, P.A.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. Lammps-a Flexible Simulation Tool for Particle-Based Materials Modeling at the Atomic, Meso, and Continuum Scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]

| Foaming Agents (wt.%) | Strength (MPa) | Density (g/cm3) | Porosity | Volume-Absorption Rate |
|---|---|---|---|---|
| Dextrin (2.5) | 2.254 | 0.306 | 85.42% | 1.15% |
| Dextrin (0.5) and carbon black (1.75) | 2.854 | 0.339 | 82.52% | 2.15% |
| Foaming Agents | Strength (MPa) | Density (g/cm3) | Porosity |
|---|---|---|---|
| Graphite (1 wt.%) | 5.12 | 0.536 | 74.47% |
| Carbon Black (1 wt.%) | 3.24 | 0.346 | 83.52% |
| pH of the Solution | 15 Days | 30 Days | 45 Days | 60 Days |
|---|---|---|---|---|
| 1 | −0.30% | −0.50% | −0.60% | −0.60% |
| 3 | −0.30% | −0.50% | −0.57% | −0.60% |
| 7 | +0.45% | +0.55% | +0.70% | +0.70% |
| Temperature Ranges (Celsius) | Averaged Thermal Coefficient (10−6/°C) |
|---|---|
| 27–50 | 10.63 |
| 50–75 | 11.55 |
| 75–100 | 11.03 |
| Temperature Ranges (Celsius) | Averaged Thermal Coefficient (10−6/°C) |
|---|---|
| 30–100 | 9.56 |
| 100–150 | 8.78 |
| 150–200 | 12.21 |
| 200–300 | 12.21 |
| 30–300 | 10.91 |
| Bond | |||
|---|---|---|---|
| B–O | 206,941.81 | 0.124 | 35.0018 |
| Si–O | 50,306.10 | 0.161 | 46.2978 |
| O–O | 9022.79 | 0.265 | 85.0921 |
| B–B | 484.40 | 0.35 | 0 |
| B–Si | 337.70 | 0.29 | 0 |
| Na–O | 120,303.80 | 0.17 | 0 |
| Ca–O | 155,667.70 | 0.178 | 42.2597 |
| K–O | 2284.77 | 0.29 | 0 |
| Al–O | 28,538.42 | 0.172 | 34.5778 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, C.; Yu, Y.; Zhu, Y.; Zhang, J.; Zhong, Y.; Yeo, J.; Wang, M. The Impact of Foaming Effect on the Physical and Mechanical Properties of Foam Glasses with Molecular-Level Insights. Molecules 2022, 27, 876. https://doi.org/10.3390/molecules27030876
Zhai C, Yu Y, Zhu Y, Zhang J, Zhong Y, Yeo J, Wang M. The Impact of Foaming Effect on the Physical and Mechanical Properties of Foam Glasses with Molecular-Level Insights. Molecules. 2022; 27(3):876. https://doi.org/10.3390/molecules27030876
Chicago/Turabian StyleZhai, Chenxi, Yang Yu, Yumei Zhu, Jing Zhang, Ying Zhong, Jingjie Yeo, and Mingchao Wang. 2022. "The Impact of Foaming Effect on the Physical and Mechanical Properties of Foam Glasses with Molecular-Level Insights" Molecules 27, no. 3: 876. https://doi.org/10.3390/molecules27030876
APA StyleZhai, C., Yu, Y., Zhu, Y., Zhang, J., Zhong, Y., Yeo, J., & Wang, M. (2022). The Impact of Foaming Effect on the Physical and Mechanical Properties of Foam Glasses with Molecular-Level Insights. Molecules, 27(3), 876. https://doi.org/10.3390/molecules27030876








