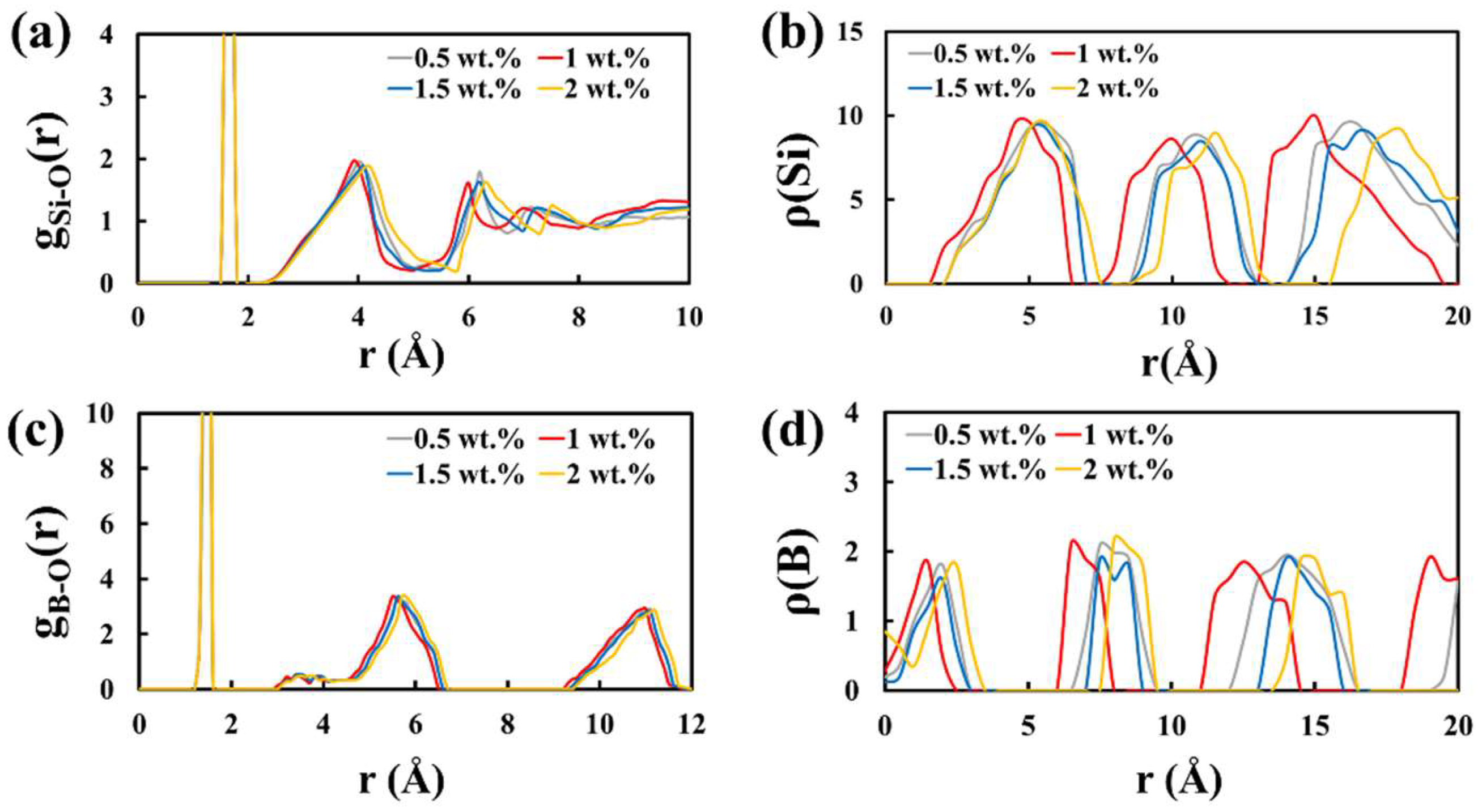
All the experimental raw materials and reagents are industrially pure and commercially available. Basic raw chemicals used in this study included H
3BO
3 (Tianjin Yishang Group Co., Ltd., Tianjin, China), SiO
2 (quartz-phase, Dahan Minerals (Xinyi) Co., Ltd., Xuzhou, China), Na
2CO
3, K
2CO
3, and Al
2O
3 (Hehai Science Technology & Engineering Co., Ltd.). These raw chemicals were well blended as a mixture with a basic stochiometric ratio of H
3BO
3 13 wt.%, SiO
2 60 wt.%, Na
2CO
3 17 wt.%, K
2CO
3 5 wt.%, Al
2O
3 5 wt.%. This basic composition was fixed throughout the entire study and experimental variables were given as follows: foaming agents included carbon black (Tianjin Chemical Reagents Co., Tianjin, China), graphite (Beijing Hanjie Science & Technology Development Co., Ltd., Beijing, China), dextrin (Tianjin Chemical Reagents Co.), and other addition materials of CaSO
4 and Na
2HPO
4 (Tianjin University Kewei, Tianjin, China). The reagent was sulfuric acid (Tianjin Chemical Reagents Co., pH = 1 and pH = 3). The experimental facilities included electronic scales (PL203, METTLER TOLEDO Group), an electrically heated thermostatic drying oven (DH-204, Tianjin Middle Ring Experiment Electric Cooker Co., Ltd., Tianjin, China), a ball mill (DH48S, Xinling Electrical Co., Ltd., Wenzhou, China), a cased resistance-heated furnace (SSX-8–16, Shanghai Shiyan Electric Furnace Co., Ltd., Shanghai, China), a thermal expansion tester (Germany NETZSCH DIL-402C, Selb, Germany), quartz mortar, saw, abrasive papers, 250-mesh sieve, vernier caliper, graphite crucible, spoon, and brush. The experimental procedures, in order, involved: raw materials scaling, blending, wet grinding, drying, dry grinding, sieving, molding, heating, sintering, annealing, and characterizing. The detailed heat treatment consisted of the following: After the molding of the mixtures in the graphite crucible, the mixtures were preheated at 400 °C at a rate of 3 °C/min in a furnace for 30 min to allow for sufficient heating. Then, the mixture was heated to 775/780 °C at a rate of 4 °C/min, and preserved at this temperature for 30 min. Next, the mixture was annealed to 600
at a rate of 3 °C/min, and preserved at this temperature for 30 min. Finally, the mixture was naturally cooled for at least 24 h to room temperature, before the characterization of the products. Following the abovementioned procedures, the obtained products were confirmed to be glass by XRD patterns [
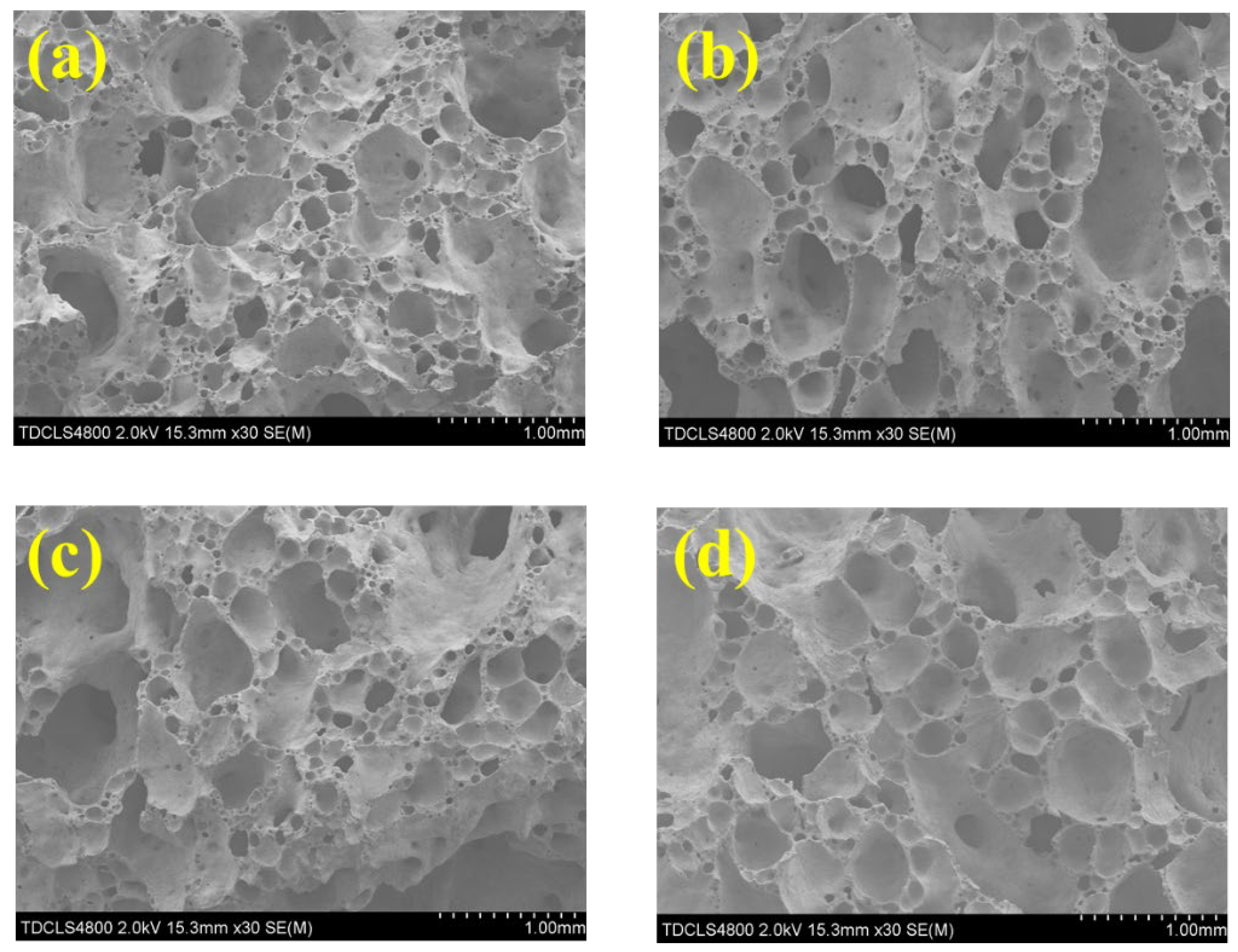
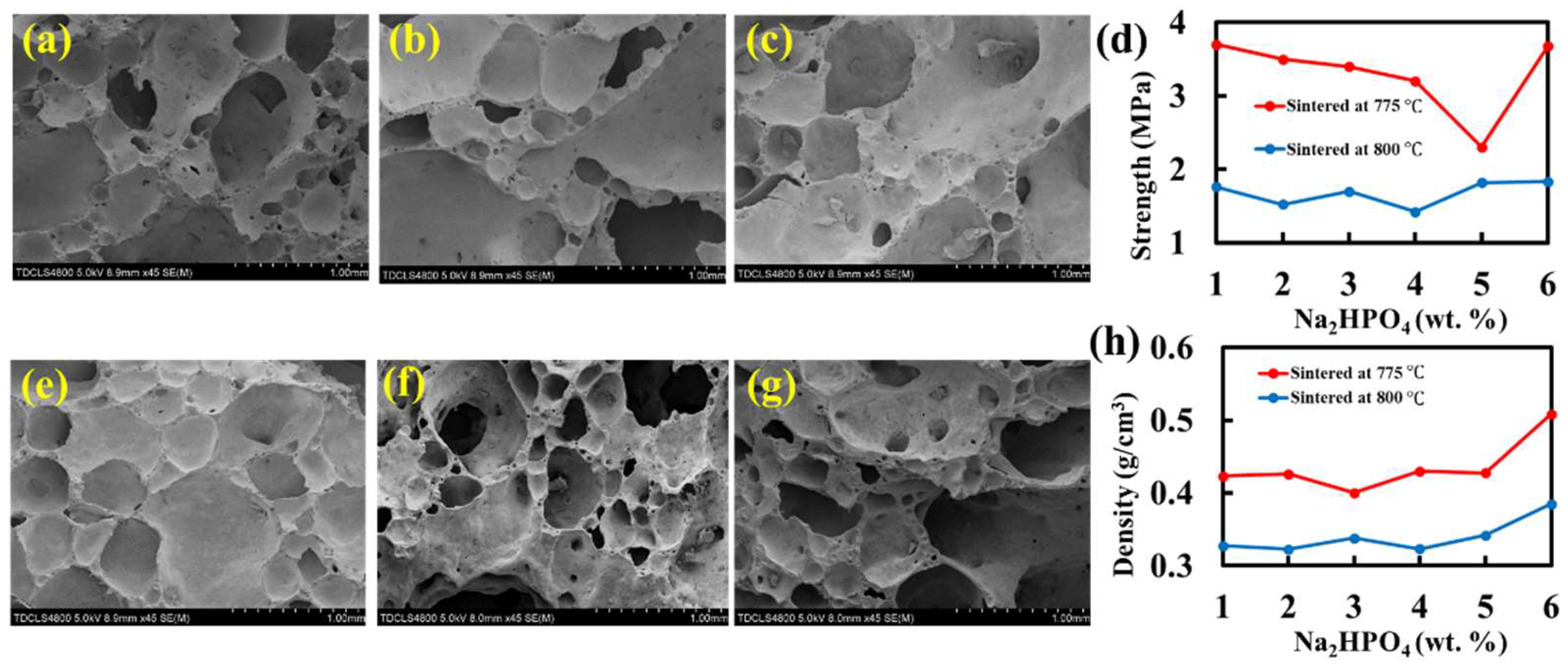
17]. Thermogravimetric and differential scanning calorimetry (TG-DSC, STA449C, Netzsch Gerätebau, Bavaria, Germany) were used to measure the thermal behaviors of the foam glass samples. Scanning electron microscopy (SEM, S-4800, Hitachi, Tokyo, Japan) and optical microscopy were used to characterize the surface morphology of the foam glass samples.
After removing the foam glass samples from the furnace, a saw and abrasive papers were used to cut and polish the samples into cubic shapes with a side length of 1 cm. Then, we used a vernier caliper to measure the length, width, and height of the cubic, 10 times, to obtain the average volume
V. The dried samples were put onto an electronic scales to obtain a weight
. Next, the samples were soaked in deionized water for 2 h at room temperature. After 2 h, the wet sample surfaces were dried with water-absorbing papers and put on the electronic scales again to obtain a weight
. The measurement of weight change in the sulfuric acid and deionized water for testing chemical stability was obtained similarly to these procedures, except that the soaking time was much longer. Finally, the volume-absorption rate can be calculated as follows:
where
and
are weights before and after soaking, respectively, in grams;
is the volume of the absorbed water of the fully soaked samples, in cm
3; and
V is the average volume, in cm
3. Considering that water has a density of 1 g/cm
3, the water volume change is equal to its weight change in magnitude (Equation (1)). Bulk density is calculated to be the weight in a unit volume:
where
is the apparent bulk density in g/cm
3. The true density,
, is defined as the weight of the fully ground and dried samples of a unit volume without pores inside. Porosity is an important metric for measuring the compactness of the samples and is categorized into opened, closed, and total porosity. Total porosity is defined as:
where
is the true density, in g/cm
3.
A universal tester was used to perform the compressive strength test of the foam glass samples. The stressed area
S was readily available by virtue of the cubic shape of the samples. A low strain rate of 1 mm/min with the universal tester was used and a maximum compressive force
P was obtained before fracture. Therefore, the compressive strength is calculated as:
where
P is the maximum compressive force, in a unit of N;
S is the stressed area, in a unit of mm
2; and
is the compressive strength, in a unit of MPa. All the test results were averaged by repeatedly testing 10 samples under the same condition. A thermal expansion tester was used with a temperature heating rate of 5 °C/min. The thermal coefficient of linear expansion is calculated as:
where
L is the length of the sample at a temperature
T, in a unit of mm;
dL is the length change corresponding to a temperature change of
dT; and
is in a unit of 10
−6/°C.