Dittrichia graveolens (L.) Greuter, a Rapidly Spreading Invasive Plant: Chemistry and Bioactivity
Abstract
:1. Introduction
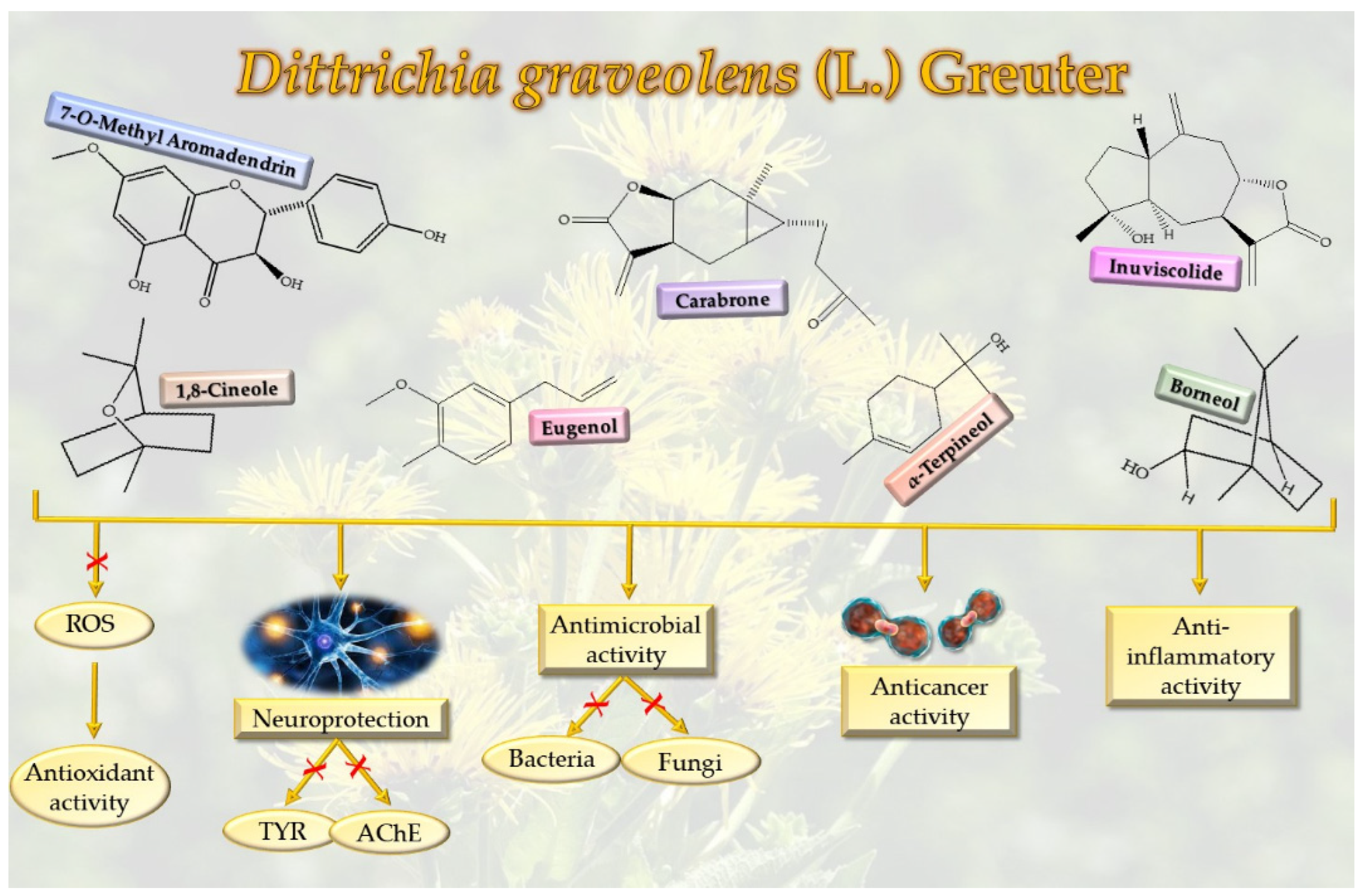
2. Phytochemistry
Chemical Characterization of D. graveolens
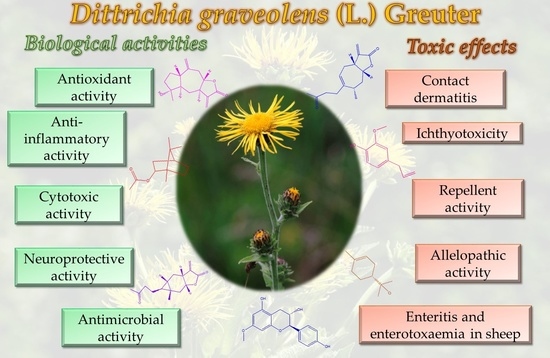
3. Biological Activities
3.1. Antioxidant Activity
3.2. Antitumor Activity
3.3. Antimicrobial Activity
3.4. Anti-Inflammatory Activity
3.5. Anti-Cholinesterase and Anti-Tyrosinase Activity
4. Toxicology
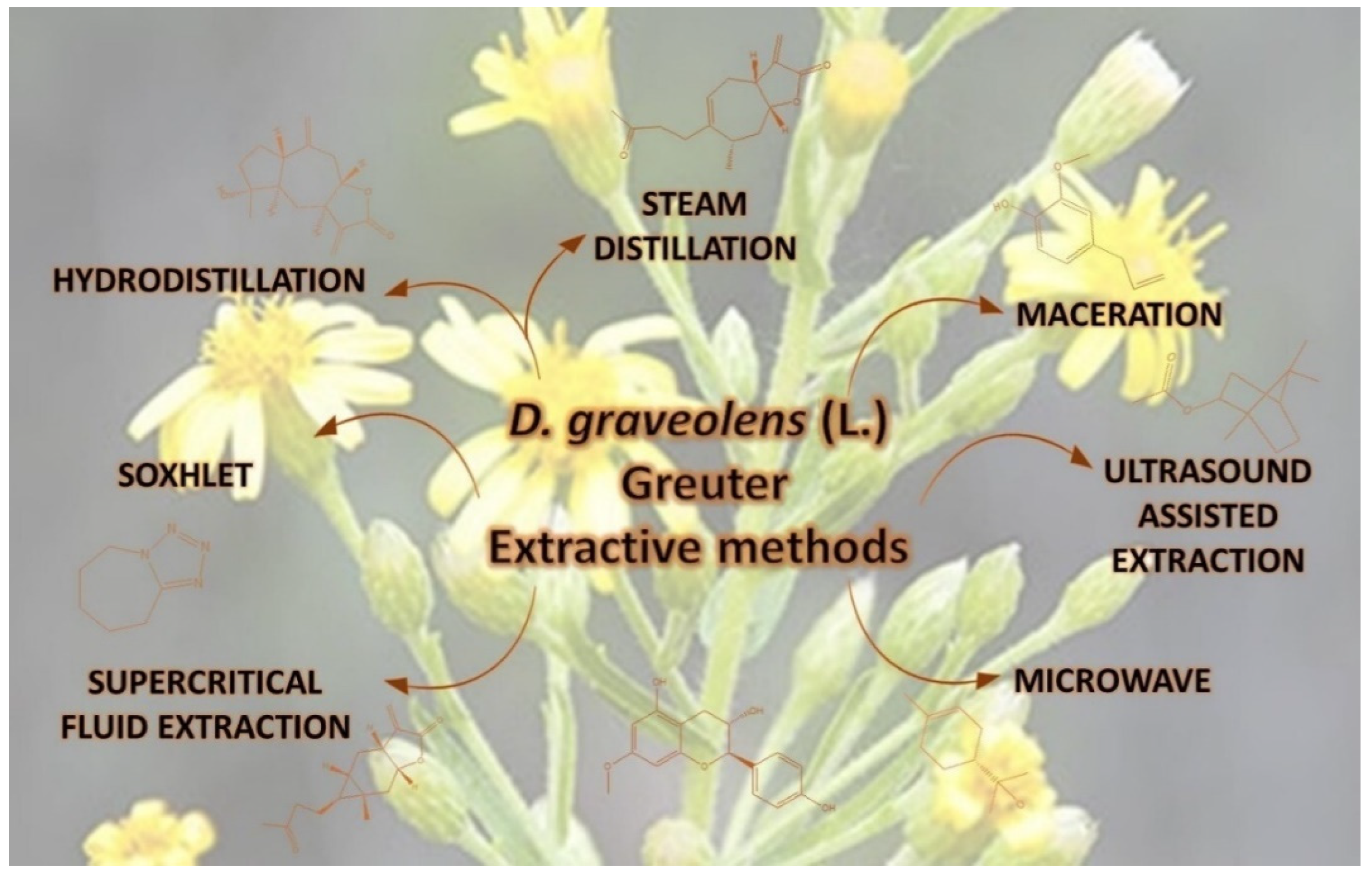
5. Materials and Methods
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A549 | Human lung carcinoma |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid |
| AChE | Acetylcholinesterase |
| BCB | β-carotene bleaching tests |
| BHT | butylhydroxytoluene |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EIMS | Electron Ionization Mass Spectroscopy |
| GC-FID | Gas chromatograph-flame ionization detection |
| GC-MS | Gas chromatography-mass spectrometer |
| FL | Human amnion cells |
| HeLa | Human cervix carcinoma |
| HPLC | High-performance liquid chromatography |
| HT29 | Human colon carcinoma |
| KB-3 | Nasopharyngeal carcinoma |
| KB-Vl | Vinblastine resistant |
| LC-MS | Liquid chromatography-mass spectrometry |
| MAE | Microwave-assisted extraction |
| MBC | Minimum bactericidal concentration |
| MCF7 | Human breast adenocarcinoma |
| MIC | Minimum inhibitory concentration |
| NMR | Nuclear magnetic resonance |
| ORAC | quenching peroxyl radicals |
| P-388 | Murine lymphocytic leukemia |
| PCL | Polycaprolactone |
| ROS | Reactive oxygen species |
| SFE | Supercritical CO2 extraction |
| TLC | Thin-layer chromatography |
| UAE | Ultrasound-assisted extraction |
References
- Brullo, S.; de Marco, G. Taxonomical revision of the genus Dittrichia (Asteraceae). Port. Acta Biol. 2000, 19, 341–354. [Google Scholar]
- Parsons, W.T.; Cuthbertson, E. Noxious Weeds of Australia; CSIRO Publishing: Clayton, Australia, 2001. [Google Scholar]
- Brownsey, R.N.; Kyser, G.B.; DiTomaso, J.M. Seed and germination biology of Dittrichia graveolens (Stinkwort). Invasive Plant. Sci. Manag. 2013, 6, 371–380. [Google Scholar] [CrossRef]
- Lustenhouwer, N.; Wilschut, R.A.; Williams, J.L.; van der Putten, W.H.; Levine, J.M. Rapid evolution of phenology during range expansion with recent climate change. Glob. Chang. Biol. 2018, 24, e534–e544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parson, W.T.; Cuthbertson, E. Noxiuos Weeds of Australia; CSIRO Publishing: Clayton, Australia, 1992; Volume 1, pp. 281–283. ISBN 9780909605810. [Google Scholar]
- Parolin, P.; Scotta, M.I.; Bresch, C. Biology of Dittrichia viscosa, a Mediterranean ruderal plant: A review. Phyton Int. J. Exp. Bot. 2014, 83, 251–262. [Google Scholar]
- Brownsey, R.N.; Kyser, G.B.; DiTomaso, J.M. Growth and phenology of Dittrichia graveolens, a rapidly spreading invasive plant in California. Biol. Invasions 2014, 16, 43–52. [Google Scholar] [CrossRef]
- Roy, D.; Alderman, D.; Anastasiu, P.; Arianoutsou, M.; Augustin, S.; Bacher, S.; Başnou, C.; Beisel, J.; Bertolino, S.; Bonesi, L.; et al. DAISIE Inventory of Alien Invasive Species in Europe; Version 1.7.; Research Institute for Nature and Forest (INBO): Brussels, Belgium, 2020. [Google Scholar] [CrossRef]
- Pieroni, A.; Giusti, M.E.; De Pasquale, C.; Lenzarini, C.; Censorii, E.; Gonzáles-Tejero, M.R.; Sánchez-Rojas, C.P.; Ramiro-Gutiérrez, J.M.; Skoula, M.; Johnson, C. Circum-Mediterranean cultural heritage and medicinal plant uses in traditional animal healthcare: A field survey in eight selected areas within the RUBIA project. J. Ethnobiol. Ethnomed. 2006, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Aghel, N.; Mahmoudabadi, A.Z.; Darvishi, L. Volatile constituents and anti candida activity of the aerial parts essential oil of Dittrichia graveolens (L.) Greuter grown in Iran. Afr. J. Pharm. Pharmacol. 2011, 5, 772–775. [Google Scholar] [CrossRef] [Green Version]
- Gharred, N.; Baaka, N.; Bettache, N.; Hamdi, A.; Dbeibia, A.; Dhaouadi, H.; Morere, A.; Menut, C.; Dridi-Dhaouadi, S. Wastewater to Ecological dyeing process and bioactive compounds resources: Case study of Dittrichia graveolens hydrodistillation aqueous residue. Waste Biomass Valorization 2021, 12, 5065–5077. [Google Scholar] [CrossRef]
- Abu-Dahab, R.; Afifi, F. Antiproliferative activity of selected medicinal plants of Jordan against a breast adenocarcinoma cell line (MCF7). Sci. Pharm. 2007, 75, 121–146. [Google Scholar] [CrossRef] [Green Version]
- Abou-Douh, A.M. New eudesmane derivatives and other sesquiterpenes from the epigeal parts of Dittrichia graveolens. Chem. Pharm. Bull. 2008, 56, 1535–1545. [Google Scholar] [CrossRef] [Green Version]
- Lanzetta, R.; Lama, G.; Mauriello, G.; Parrilli, M.; Racioppi, R.; Sodano, G. Ichthyotoxic sesquiterpenes and xanthanolides from Dittrichia graveolens. Phytochemistry 1991, 30, 1121–1124. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Jakupovic, J.; Chau-Thi, T.; Bohlmann, F.; Sadjadi, A. Further sesquiterpene lactones from the genus Dittrichia. Phytochemistry 1987, 26, 2603–2606. [Google Scholar] [CrossRef]
- Sarkar, B.; Alam, S.; Rajib, T.K.; Islam, S.S.; Araf, Y.; Ullah, M.A. Identification of the most potent acetylcholinesterase inhibitors from plants for possible treatment of Alzheimer’s disease: A computational approach. Egypt. J. Med. Hum. Genet. 2021, 22, 1–20. [Google Scholar] [CrossRef]
- Sevil, O. A eudesmanolide and other constituents from Inula graveolens. Phytochemistry 1992, 31, 195–197. [Google Scholar]
- Baldovini, N.; Tomi, F.; Casanova, J. Enantiomeric differentiation of bornyl acetate by 13C-NMR using a chiral lanthanide shift reagent. Phytochem. Anal. 2003, 14, 241–244. [Google Scholar] [CrossRef]
- Blanc, M.C.; Muselli, A.; Bradesi, P.; Casanova, J. Chemical composition and variability of the essential oil of Inula graveolens from Corsica. Flavour Fragr. J. 2004, 19, 314. [Google Scholar] [CrossRef]
- Boudouda, H.B.; Kabouche, A.; Aburjai, T.; Kabouche, Z. GC-MS analysis of Inula graveolens (L.) Desf. from Algeria. J. Essent. Oil Bear. Plants 2013, 16, 651–654. [Google Scholar] [CrossRef]
- Djenane, D.; Yangueela, J.; Gomez, D.; Roncales, P. Perspectives on the use of essential oils as antimicrobials against Campylobacter jejuni CECT 7572 in retail chicken meats packaged in microaerobic atmosphere. J. Food Saf. 2012, 32, 37–47. [Google Scholar] [CrossRef]
- Dohi, S.; Terasaki, M.; Makino, M. Acetylcholinesterase inhibitory activity and chemical composition of commercial essential oils. J. Agric. Food Chem. 2009, 57, 4313–4318. [Google Scholar] [CrossRef]
- Ghosn, M.W.; Chemali, C.B.; Zaknoun, F.I.; Saliba, N.A. Chemical profle of the Dittrichia graveolens (Desf.) greuter essential oil of Lebanese origin. J. Essent. Oil Res. 2006, 18, 443–444. [Google Scholar] [CrossRef]
- Karan, T.; Yildiz, I.; Aydin, A.; Erenler, R. Inhibition of various cancer cells proliferation of bornyl acetate and essential oil from Inula graveolens (Linnaeus) Desf. Rec. Nat. Prod. 2018, 12, 273–283. [Google Scholar] [CrossRef]
- Marongiu, B.; Piras, A.; Pani, F.; Porcedda, S.; Ballero, M. Extraction, separation and isolation of essential oils from natural matrices by supercritical CO2. Flavour Fragr. J. 2003, 18, 505–509. [Google Scholar] [CrossRef]
- Miladinović, D.L.; Ilić, B.S.; Kocić, B.D.; Marković, M.S.; Miladinović, L.C. In vitro trials of Dittrichia graveolens essential oil combined with antibiotics. Nat. Prod. Commun. 2016, 11, 1934578X1601100642. [Google Scholar] [CrossRef] [Green Version]
- Mirza, M.; Ahmadi, L. Composition of the essential oil of Dittrichia graveolens (L.) Greuter. J. Essent. Oil Res. 2000, 12, 507–508. [Google Scholar] [CrossRef]
- Mitic, V.; Stankov Jovanovic, V.; Ilic, M.; Jovanovic, O.; Djordjevic, A.; Stojanovic, G. Dittrichia graveolens (L.) Greuter essential oil: Chemical composition, multivariate analysis, and antimicrobial activity. Chem. Biodivers. 2016, 13, 85–90. [Google Scholar] [CrossRef]
- Petropoulou, A.; Tzakou, O.; Verykokidou, E. Volatile constituents of Dittrichia graveolens (L.) Greuter from Greece. J. Essent. Oil Res. 2004, 16, 400–401. [Google Scholar] [CrossRef]
- Sellem, I.; Chakchouk-Mtibaa, A.; Zaghden, H.; Smaoui, S.; Ennouri, K.; Mellouli, L. Harvesting season dependent variation in chemical composition and biological activities of the essential oil obtained from Inula graveolens (L.) grown in Chebba (Tunisia) salt marsh. Arab. J. Chem. 2020, 13, 4835–4845. [Google Scholar] [CrossRef]
- Souri, M.; Shakeri, A. Optimization of total phenol and tannin content and biological activity of Dittrichia graveolens (L.) GREUTER. Curr. Bioact. Compd. 2020, 16, 124–132. [Google Scholar] [CrossRef]
- Silinsin, M.; Bursal, E. UHPLC-MS/MS phenolic profiling and in vitro antioxidant activities of Inula graveolens (L.) Desf. Nat. Prod. Res. 2018, 32, 1467–1471. [Google Scholar] [CrossRef]
- Topçu, G.; Öksüz, S.; Shieh, H.-L.; Cordell, G.A.; Pezzuto, J.M.; Bozok-Johansson, C. Cytotoxic and antibacterial sesquiterpenes from Inula graveolens. Phytochemistry 1993, 33, 407–410. [Google Scholar] [CrossRef]
- Sinisgalli, C.; Faraone, I.; Vassallo, A.; Caddeo, C.; Bisaccia, F.; Armentano, M.F.; Milella, L.; Ostuni, A. Phytochemical profile of Capsicum annuum L. cv Senise, incorporation into liposomes, and evaluation of cellular antioxidant activity. Antioxidants 2020, 9, 428. [Google Scholar] [CrossRef] [PubMed]
- Vinatoru, M.; Mason, T.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Russo, D.; Faraone, I.; Labanca, F.; Sinisgalli, C.; Bartolo, M.; Andrade, P.B.; Valentao, P.; Milella, L. Comparison of different green-extraction techniques and determination of the phytochemical profile and antioxidant activity of Echinacea angustifolia L. extracts. Phytochem. Anal. 2019, 30, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Al-Fartosy, A.J. Antioxidant properties of methanolic extract from Inula graveolens L. Turk. J. Agric. For. 2011, 35, 591–596. [Google Scholar]
- Boudkhili, M.; Greche, H.; Bouhdid, S.; Zerargui, F.; Aarab, L. In vitro antioxidant and antimicrobial properties of some Moroccan’s medicinal. Int. J. PharmTech Res. 2012, 4, 637–642. [Google Scholar]
- Spatuzzi, R.; Giulietti, M.V.; Ricciuti, M.; Merico, F.; Fabbietti, P.; Raucci, L.; Bilancia, D.; Cormio, C.; Vespa, A. Exploring the associations between spiritual well-being, burden, and quality of life in family caregivers of cancer patients. Palliat. Support. Care 2019, 17, 294–299. [Google Scholar] [CrossRef]
- Khan, H.; Labanca, F.; Ullah, H.; Hussain, Y.; Tzvetkov, N.T.; Akkol, E.K.; Milella, L.J.P.R. Advances and challenges in cancer treatment and nutraceutical prevention: The possible role of dietary phenols in BRCA regulation. Phytochem. Rev. 2021, 1–16. [Google Scholar] [CrossRef]
- Al-Kaabi, W.J.; Albukhaty, S.; Al-Fartosy, A.J.; Al-Karagoly, H.K.; Al-Musawi, S.; Sulaiman, G.M.; Dewir, Y.H.; Alwahibi, M.S.; Soliman, D.A. Development of Inula graveolens (L.) plant extract electrospun/polycaprolactone nanofibers: A novel material for biomedical application. Appl. Sci. 2021, 11, 828. [Google Scholar] [CrossRef]
- Silva, B.I.; Nascimento, E.A.; Silva, C.J.; Silva, T.G.; Aguiar, J.S. Anticancer activity of monoterpenes: A systematic review. Mol. Biol. Rep. 2021, 48, 5775–5785. [Google Scholar] [CrossRef]
- Han, Z.; Liu, F.-y.; Lin, S.-q.; Zhang, C.-y.; Ma, J.-h.; Guo, C.; Jia, F.-j.; Zhang, Q.; Xie, W.-d.; Li, X.J.M. Ivalin Induces Mitochondria-Mediated Apoptosis Associated with the NF-κB Activation in Human Hepatocellular Carcinoma SMMC-7721 Cells. Molecules 2019, 24, 3809. [Google Scholar] [CrossRef] [Green Version]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 1–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guinoiseau, E.; Luciani, A.; Rossi, P.; Quilichini, Y.; Ternengo, S.; Bradesi, P.; Berti, L. Cellular effects induced by Inula graveolens and Santolina corsica essential oils on Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 873–879. [Google Scholar] [CrossRef] [Green Version]
- Bamuamba, K.; Gammon, D.W.; Meyers, P.; Dijoux-Franca, M.-G.; Scott, G. Anti-mycobacterial activity of five plant species used as traditional medicines in the Western Cape Province (South Africa). J. Ethnopharmacol. 2008, 117, 385–390. [Google Scholar] [CrossRef]
- Benbelaïd, F.; Khadir, A.; Abdoune, M.A.; Bendahou, M.; Muselli, A.; Costa, J. Antimicrobial activity of some essential oils against oral multidrug–resistant Enterococcus faecalis in both planktonic and biofilm state. Asian Pac. J. Trop. Biomed. 2014, 4, 463–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Dorman, H.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid. -Based Complementary Altern. Med. 2016, 2016, 3012462 . [Google Scholar] [CrossRef]
- Kim, T.; Song, B.; Cho, K.S.; Lee, I. Therapeutic potential of volatile terpenes and terpenoids from forests for inflammatory diseases. Int. J. Mol. Sci. 2020, 21, 2187. [Google Scholar] [CrossRef] [Green Version]
- Barrero, A.F.; Herrador, M.M.; Arteaga, P.; Catalán, J.V. Dittrichia viscosa L. Greuter: Phytochemistry and biological activity. Nat. Prod. Commun. 2008, 3, 1934578X0800301110. [Google Scholar] [CrossRef] [Green Version]
- Labanca, F.; Faraone, I.; Nolè, M.R.; Hornedo-Ortega, R.; Russo, D.; García-Parrilla, M.C.; Chiummiento, L.; Bonomo, M.G.; Milella, L. New Insights into the exploitation of Vitis vinifera L. cv. Aglianico leaf extracts for nutraceutical purposes. Antioxidants 2020, 9, 708. [Google Scholar] [CrossRef] [PubMed]
- Hajimehdipoor, H.; Mosaddegh, M.; Naghibi, F.; Haeri, A.; Hamzeloo-Moghadam, M.J. Natural sesquiterpen lactones as acetylcholinesterase inhibitors. An. Acad. Bras. Ciências 2014, 86, 801–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choo, S.-J.; Ryoo, I.-J.; Kim, K.C.; Na, M.; Jang, J.-H.; Ahn, J.S.; Yoo, I.-D. Hypo-pigmenting effect of sesquiterpenes from Inula britannica in B16 melanoma cells. Arch. Pharmacal Res. 2014, 37, 567–574. [Google Scholar] [CrossRef]
- Fujita, H.; Motokawa, T.; Katagiri, T.; Yokota, S.; Yamamoto, A.; Himeno, M.; Tanaka, Y. Inulavosin, a melanogenesis inhibitor, leads to mistargeting of tyrosinase to lysosomes and accelerates its degradation. J. Investig. Dermatol. 2009, 129, 1489–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceylan, R.; Zengin, G.; Mahomoodally, M.F.; Sinan, K.I.; Ak, G.; Jugreet, S.; Cakır, O.; Ouelbani, R.; Paksoy, M.Y.; Yılmaz, M.A. Enzyme inhibition and antioxidant functionality of eleven Inula species based on chemical components and chemometric insights. Biochem. Syst. Ecol. 2021, 95, 104225. [Google Scholar] [CrossRef]
- Maxia, A.; Lancioni, M.C.; Balia, A.N.; Alborghetti, R.; Pieroni, A.; Loi, M.C. Medical ethnobotany of the Tabarkins, a Northern Italian (Ligurian) minority in south-western Sardinia. Genet. Resour. Crop. Evol. 2008, 55, 911–924. [Google Scholar] [CrossRef]
- Nortje, J.; Van Wyk, B.-E. Medicinal plants of the kamiesberg, namaqualand, South Africa. J. Ethnopharmacol. 2015, 171, 205–222. [Google Scholar] [CrossRef]
- Thong, H.Y.; Yokota, M.; Kardassakis, D.; Maibach, H.I. Allergic contact dermatitis from Dittrichia graveolens (L.) Greuter (stinkwort). Contact Dermat. 2008, 58, 51–53. [Google Scholar] [CrossRef]
- Philbey, A.; Morton, A. Pyogranulomatous enteritis in sheep due to penetrating seed heads of Dittrichia graveolens. Aust. Vet. J. 2000, 78, 858–860. [Google Scholar] [CrossRef]
- Schneider, D.; Du Plessis, J. Enteritis in sheep due to the ingestion of Inula graveolens Desf (Cape Khakiweed). J. S. Afr. Vet. Assoc. 1980, 51, 159–161. [Google Scholar]
- Seddon, H.; Carne, H. Feeding tests with stinkwort (Inula graveolens). NSW Dept Agric. Sci Bull. 1927, 29, 46–47. [Google Scholar]
- Drapeau, J.; Fröhler, C.; Touraud, D.; Kröckel, U.; Geier, M.; Rose, A.; Kunz, W. Repellent studies with Aedes aegypti mosquitoes and human olfactory tests on 19 essential oils from Corsica, France. Flavour Fragr. J. 2009, 24, 160–169. [Google Scholar] [CrossRef]
- Omezzine, F.; Ladhari, A.; Rinez, A.; Haouala, R. Allelopathic potential of Inula graveolens on crops and weeds. Allelopath. J. 2011, 28, 63–76. [Google Scholar]
- Abu Irmaileh, B.E.; Al-Aboudi, A.M.; Abu Zarga, M.H.; Awwadi, F.; Haddad, S.F. Selective phytotoxic activity of 2,3,11β,13-tetrahydroaromaticin and ilicic acid isolated from Inula graveolens. Nat. Prod. Res. 2015, 29, 893–898. [Google Scholar] [CrossRef]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef]
- Chengxu, W.; Mingxing, Z.; Xuhui, C.; Bo, Q. Review on allelopathy of exotic invasive plants. Procedia Eng. 2011, 18, 240–246. [Google Scholar] [CrossRef] [Green Version]
- Hierro, J.L.; Callaway, R.M. Allelopathy and exotic plant invasion. Plant. Soil 2003, 256, 29–39. [Google Scholar] [CrossRef]


| Bioactive Compounds | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound | Formula | Structure | Analyzed Sample | Extraction Method | Chemical Composition Analysis | Quantitative | Origin of Plant | Reference |
| Eugenol (9) | C10H12O2 | 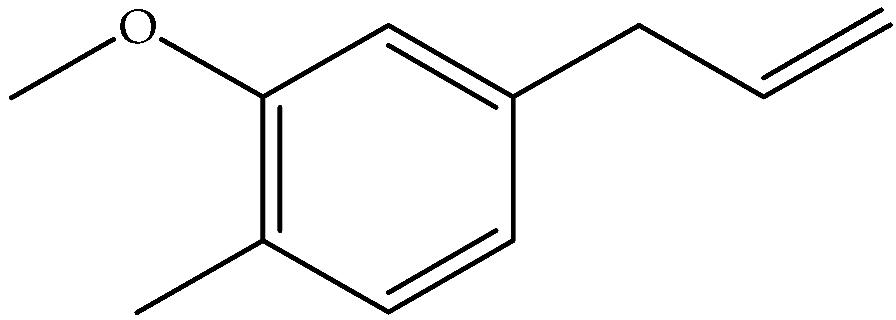 | Essential oil | Steam-distilled | GC-MS | Trace | France | [16,22] |
| Aromadendrin, 7-O-methyl (18) | C16H14O6 |  | Air-dried and powdered aerial parts | Maceration with petrol- Et2O-MeOH (1:1:1), then with MeOH | Sephadex LH-20 columns, prep. TLC, EIMS, NMR | 470 mg/32 g of the residue of extract treated with MeOH/1 Kg of initial aerial parts | Aydos Dag (Istanbul) | [17] |
| Quercetin (28) | C15H10O7 |  | Aqueous residue of dried leaves and flowers hydrodistillation | Hydrodistillation | HPLC UV-vis | 4 mg/g of extract | Monastir (Tunisia) | [11] |
| Dried leaves | Maceration with with MeOH | UHPLC-MS/MS | 118 ± 8 μg/kg extract | Bingol (Turkey) | [32] | |||
| Borneol (40) | C10H18O |  | Essential oil | Hydrodistillation of air-dried aerial parts | GC-MS | 5.44% | Shush (Khuzestan Province, Iran) | [10] |
| Vapour distillation of aerial parts | GC-MS | 7.6% | Ajaccio (Corsica) | [19] | ||||
| Hydrodistillation of fresh aerial parts | GC-MS | 2.74–12.40% | Bekaa and Sannine (Lebanon) | [23] | ||||
| Hydrodistillation of dried aerial parts | GC-MS | 11.34% | Tokat Turkey Provence | [24] | ||||
| Steam distillation of air-dried aerial parts | GC-MS | 60.7% | Gorgan province (Iran) | [27] | ||||
| Hydrodistillation of air-dried aerial parts | GC-MS | 12.8% | Attiki County (Greece) | [29] | ||||
| Steam distillation of fresh plant | GC-MS | 23.65–37.29% | Chebba (Mahdia, Tunisia) | [30] | ||||
| Steam hydrodistillation of dried leaves | GC-MS | 21.04% | Tizi-Ouzou province (Algeria) | [21] | ||||
| Steam-distilled | GC-MS | 20 ± 0.35% | France | [22] | ||||
| Hydrodistillation of dried aerial parts | GC-MS | 18.7% | Stara planina mountain (Serbia) | [26] | ||||
| Hydrodistillation of dried flowering aerial parts | GC-MS and GC-FID | 43.6% | Village Vladimirovci (Montenegro) | [28] | ||||
| Hydrodistillation of the fresh aerial parts | GC-MS | 18.3% | Constantine (North Easter Algerian) | [20] | ||||
| Bornyl acetate (41) | C12H20O2 | 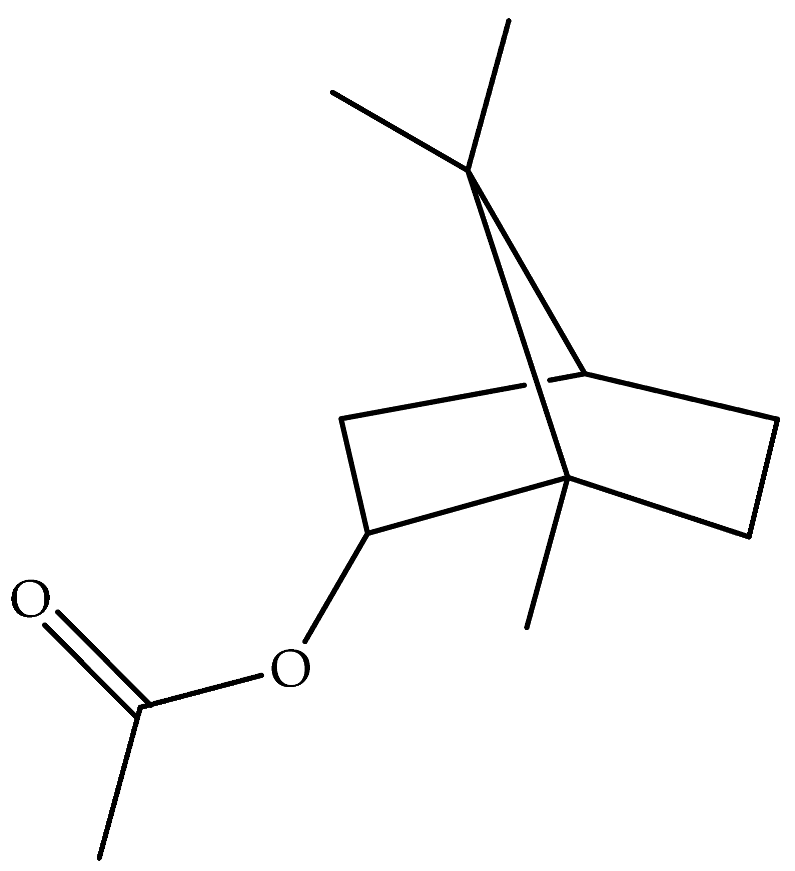 | Essential oil | Hydrodistillation of air-dried aerial parts | GC-MS | 0.24% | Shush (Khuzestan Province, Iran) | [10] |
| Vapour distillation of aerial parts | GC-MS | 56.8% | Ajaccio (Corsica) | [19] | ||||
| Hydrodistillation of fresh aerial parts | GC-MS | 70.64–72.31% | Bekaa and Sannine (Lebanon) | [23] | ||||
| Hydrodistillation of dried aerial parts | GC-MS | 60.43% | Tokat Turkey Provence | [24] | ||||
| Steam distillation of air-dried aerial parts | GC-MS | 6.8% | Gorgan province (Iran) | [27] | ||||
| Hydrodistillation of air-dried aerial parts | GC-MS | 25.4% | Attiki County (Greece) | [29] | ||||
| Steam distillation of fresh plant | GC-MS | 40.16–45.34% | Chebba (Mahdia, Tunisia) | [30] | ||||
| Steam hydrodistillation of dried leaves | GC-MS | 40.85% | Tizi-Ouzou province (Algeria) | [21] | ||||
| Steam-distilled | GC-MS | 54 ± 2.0% | France | [22] | ||||
| Hydrodistillation of dried aerial parts | GC-MS | 21.7% | Stara planina mountain (Serbia) | [26] | ||||
| Hydrodistillation of dried flowering aerial parts | GC-MS and GC-FID | 38.3% | Village Vladimirovci (Montenegro) | [28] | ||||
| Distillation | NMR | 57.4% | - | [18] | ||||
| Cineole, 1,8 (syn. Eucalyptol) (55) | C10H18O | 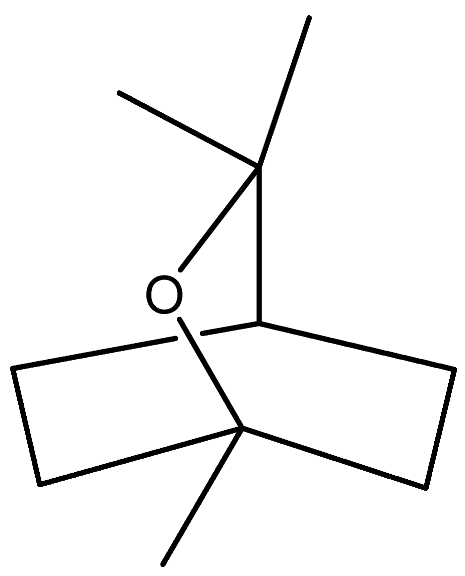 | Essential oil | Hydrodistillation of air-dried aerial parts | GC-MS | 54.89% | Shush (Khuzestan Province, Iran) | [10] |
| Steam distillation of fresh plant | GC-MS | Trace—0.22% | Chebba (Mahdia, Tunisia) | [30] | ||||
| Steam hydrodistillation of dried leaves | GC-MS | 2.41% | Tizi-Ouzou province (Algeria) | [21] | ||||
| Hydrodistillation of the fresh aerial parts | GC-MS | 0.9% | Constantine (North Easter Algerian) | [20] | ||||
| Steam-distilled | GC-MS | Trace | France | [16,22] | ||||
| Supercritical fluid extract | Supercritical fluid extraction (SFE) of air-dried aerial parts | GC-MS | Trace | Monserrato (southern Sardinia) | [25] | |||
| p-Mentha-1(7),2-dien-8-ol (β-phellandren-8-ol) (76) | C10H16O | 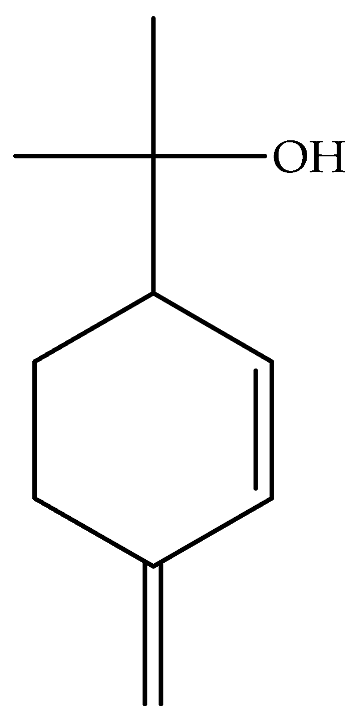 | Essential oil | Vapour distillation of aerial parts | GC-MS | 0.3% | Ajaccio (Corsica) | [19] |
| α-Pinene (86) | C10H16 | 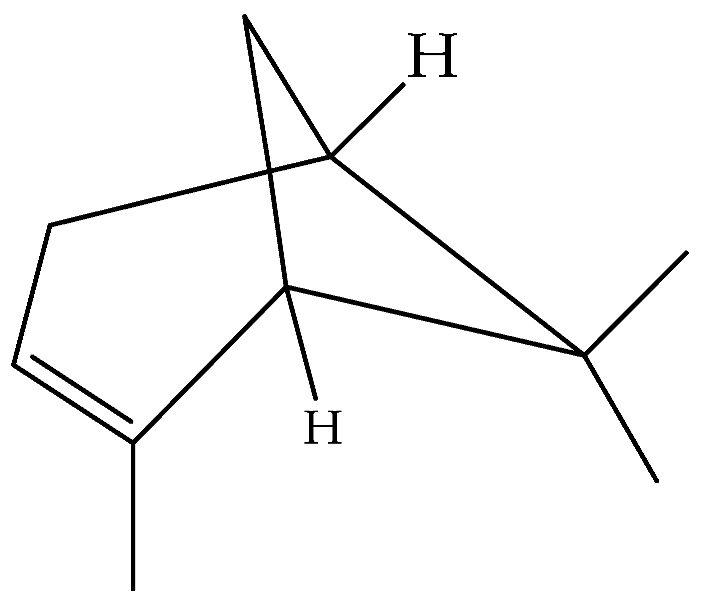 | Essential oil | Hydrodistillation of air-dried aerial parts | GC-MS | 3.21% | Shush (Khuzestan Province, Iran) | [10] |
| Vapour distillation of aerial parts | GC-MS | 0.3% | Ajaccio (Corsica) | [19] | ||||
| Hydrodistillation of fresh aerial parts | GC-MS | 0.02–0.03% | Bekaa and Sannine (Lebanon) | [23] | ||||
| Hydrodistillation of dried aerial parts | GC-MS | 0.16% | Tokat Turkey Provence | [24] | ||||
| Steam distillation of air-dried aerial parts | GC-MS | 0.2% | Gorgan province (Iran) | [27] | ||||
| Hydrodistillation of air-dried aerial parts | GC-MS | Trace | Attiki County (Greece) | [29] | ||||
| Steam distillation of fresh plant | GC-MS | Trace–0.85% | Chebba (Mahdia, Tunisia) | [30] | ||||
| Steam-distilled | GC-MS | 0.21 ± 0.0050% | France | [22] | ||||
| Hydrodistillation of dried aerial parts | GC-MS | 1.2% | Stara planina mountain (Serbia) | [26] | ||||
| Hydrodistillation of dried flowering aerial parts | GC-MS and GC-FID | Trace | Village Vladimirovci (Montenegro) | [28] | ||||
| α-Terpineol (95) | C10H18O | 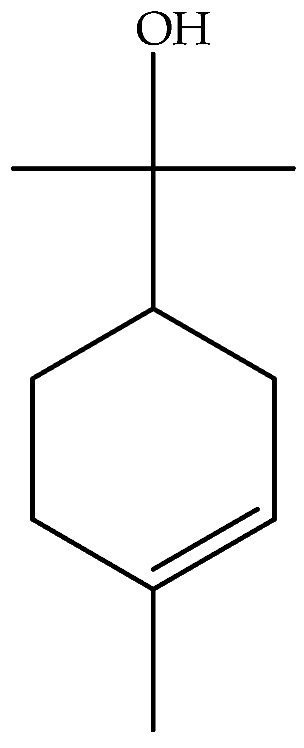 | Essential oil | Hydrodistillation of air-dried aerial parts | GC-MS | 1.31% | Shush (Khuzestan Province, Iran) | [10] |
| Vapour distillation of aerial parts | GC-MS | 0.4% | Ajaccio (Corsica) | [19] | ||||
| Steam distillation of fresh plant | GC-MS | Trace–0.85% | Chebba (Mahdia, Tunisia) | [30] | ||||
| Steam hydrodistillation of dried leaves | GC-MS | 1.52% | Tizi-Ouzou province (Algeria) | [21] | ||||
| Steam-distilled | GC-MS | 1.6 ± 0.062% | France | [16,22] | ||||
| Hydrodistillation of dried flowering aerial parts | GC-MS and GC-FID | Trace | Village Vladimirovci (Montenegro) | [28] | ||||
| Carabrone (122) | C15H20O3 |  | Air-dried and powdered aerial parts | Maceration with petrol- Et2O-MeOH (1:1:1), then with MeOH | Sephadex LH-20 columns, prep. TLC, EIMS, NMR | 18 mg/32 g of residue of extract treated with MeOH/1 Kg of initial aerial parts | Aydos Dag (Istanbul) | [17] |
| Eudesma, 12-carboxy,3,11(13)-diene (148) | C15H22O2 | 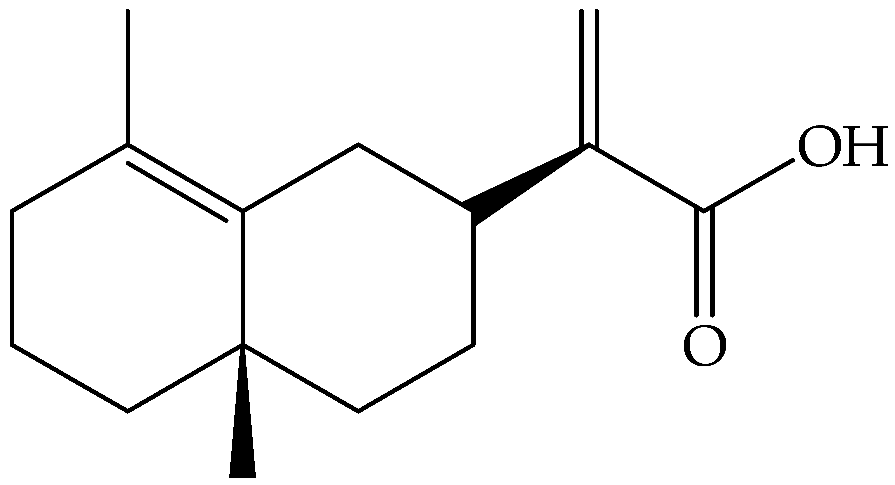 | Fresh leaves | Maceration with Me2CO, then partition with different solvents | LC-MS and NMR | 340 mg/1.3 g of petrol extract | Mediterranean area | [14] |
| Ilicic acid (169) | C15H24O3 | 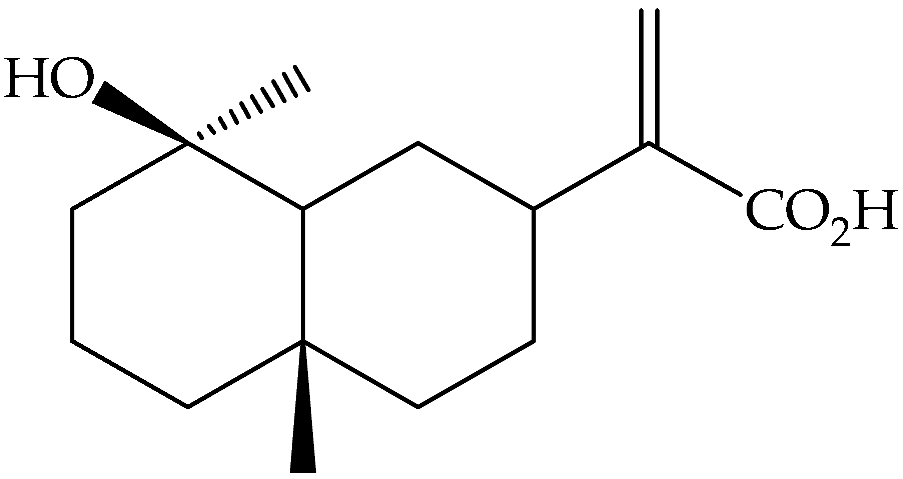 | Air-dried and powdered aerial parts | Maceration with petrol- Et2O-MeOH (1:1:1), then with MeOH | Sephadex LH-20 columns, prep. TLC, EIMS, NMR | 4800 mg/32 g of the residue of extract treated with MeOH/1 Kg of initial aerial parts | Aydos Dag (Istanbul) | [17] |
| Air-dried epigeal parts extracts | Exhaustive maceration with CH2Cl2/MeOH followed by 80% MeOH | NMR | 0.33% | Coastal regions of north-western Egypt | [13] | |||
| Fresh leaves | Maceration with Me2CO, then partition with different solvents | LC-MS and NMR | Mediterranean area | [14] | ||||
| Ivalin (177) | C15H20O3 | 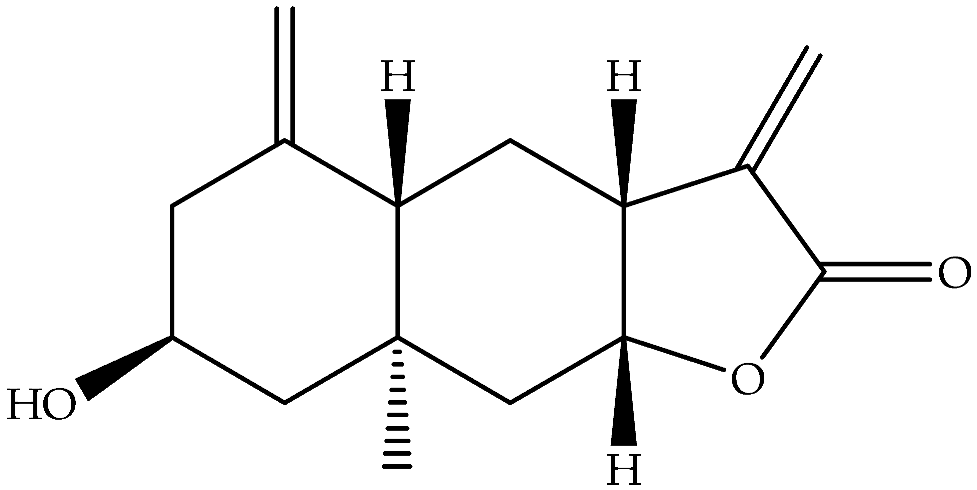 | Air-dried aerial parts | Maceration with petrol-Et2O-MeOH | NMR | Aydos Mountain (Istanbul) | [33] | |
| Ivalin, acetate (syn. Acetylivalin) (178) | C17H22O4 | 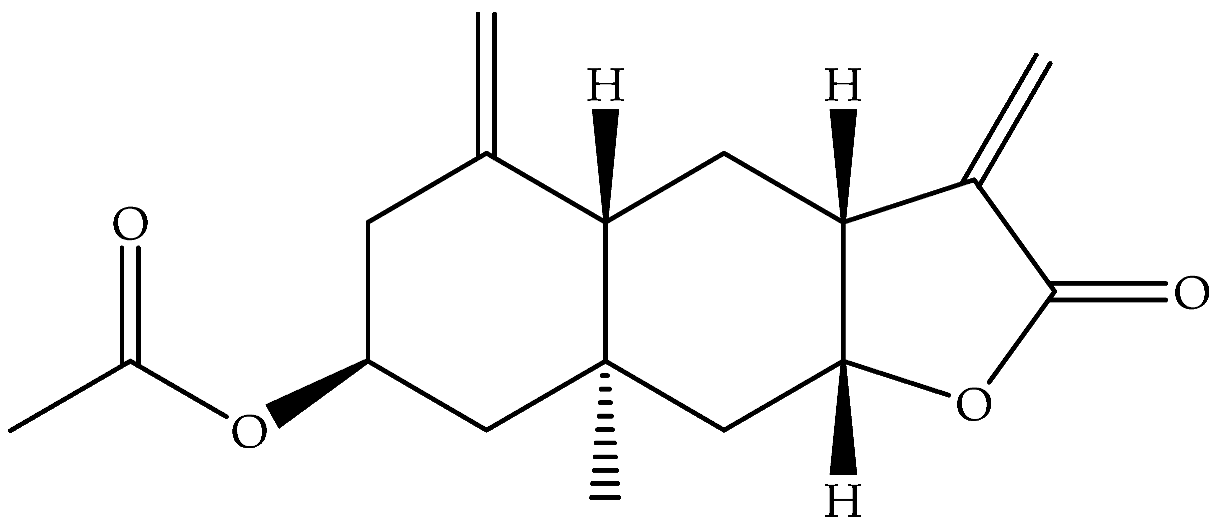 | Aerial parts | Maceration with petrol-Et2O-MeOH | NMR | Aydos Mountain (Istanbul) | [33] | |
| Sample | Test/Model | Concentration/Dosage Tested | Effect | Reference |
|---|---|---|---|---|
| Essential oil from aerial parts | DPPH ABTS BCB | 500; 250; 125; 60.25; 30.125 µg/mL | Antioxidant activity | [30] |
| S. aureus M. luteus C. albicans | Antimicrobial activity | [10,30,45] | ||
| C. jejuni | 32 μL/mL to 0.3125 μL/mL | Antimicrobial activity | [21] | |
| Enzymatic assay | 1 mg/mL to 0.0048 mg/mL | Acetylcholinesterase inhibition Tyrosinase inhibition | [22,30] | |
| HeLa, HT29, A549, MCF-7 cancer cells | 0 μg/mL to 200 μg/mL | Antitumoral activity | [24] | |
| Essential oil from leaves and flowers | DPPH ORAC | 0 mg/mL to 0.12 mg/mL 0.01 mg/mL to 0.06 mg/mL | Antioxidant activity | [11] |
| V. alginolyticus, V. parahaemolyticus S. epidermidis | 10 mg/mL | Antibacterial activity | [11,47] | |
| E. faecalis | 10 μL | |||
| Swiss mice | 5 mg/kg and 10 mg/kg | Anti-inflammatory activity | [11] | |
| Flowers essential oil | S. aureus B.s subtilis | 10 mg | Antibacterial activity | [28] |
| Aerial parts extract | DPPH | Antioxidant activity | [31] | |
| B. subtilis E. faecalis S. typhi E. aerogenes | 1.25 mg | Antibacterial activity | [31] | |
| B. cereus S. brevicaulis | 200 μg/mL | Antibacterial activity | [13] | |
| Fibroblasts’ (rat dermal) cell line | 5 wt.% of extract inserted on a scaffold made with polycaprolactone (PCL) | Cell proliferation promotion | [41] | |
| Male albino rats | 1 mL/100 g body weight | Anti-inflammatory and antipyretic activity | [13] | |
| Whole plant extract | BCB Reducing power Ferrous ion chelating ability Superoxide radical scavenging activity Hydroxyl radical scavenging activity | 0 mg/mL to 20 mg/mL | Antioxidant activity | [37,38] |
| B. subtilis, Escherichia coli, Micrococcus luteus, Salmonella spp., Staphylococcus spp. | 10 mg/mL | No Antimicrobial activity | [38] | |
| S. aureus | 62.5 μg/mL; 125 μg/mL; 250 μg/mL; 500 μg/mL; 1 mg/mL; 2 mg/mL | Antibacterial activity | [46] | |
| Leaf extract | DPPH | 30 μg/mL | Antioxidant activity | [32] |
| Flowers extract | MCF7 cell line | 0.1 μg/mL to 100 μg/mL | Antiproliferative activity | [12] |
| Isolated compounds | P-338, KB-3, KB-V1 cell line | 5 different concentrations not specified | Cytotoxic activity | [33] |
| S. epidermidis B. subtilis | Antimicrobial activity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponticelli, M.; Lela, L.; Russo, D.; Faraone, I.; Sinisgalli, C.; Mustapha, M.B.; Esposito, G.; Jannet, H.B.; Costantino, V.; Milella, L. Dittrichia graveolens (L.) Greuter, a Rapidly Spreading Invasive Plant: Chemistry and Bioactivity. Molecules 2022, 27, 895. https://doi.org/10.3390/molecules27030895
Ponticelli M, Lela L, Russo D, Faraone I, Sinisgalli C, Mustapha MB, Esposito G, Jannet HB, Costantino V, Milella L. Dittrichia graveolens (L.) Greuter, a Rapidly Spreading Invasive Plant: Chemistry and Bioactivity. Molecules. 2022; 27(3):895. https://doi.org/10.3390/molecules27030895
Chicago/Turabian StylePonticelli, Maria, Ludovica Lela, Daniela Russo, Immacolata Faraone, Chiara Sinisgalli, Mayssa Ben Mustapha, Germana Esposito, Hichem Ben Jannet, Valeria Costantino, and Luigi Milella. 2022. "Dittrichia graveolens (L.) Greuter, a Rapidly Spreading Invasive Plant: Chemistry and Bioactivity" Molecules 27, no. 3: 895. https://doi.org/10.3390/molecules27030895
APA StylePonticelli, M., Lela, L., Russo, D., Faraone, I., Sinisgalli, C., Mustapha, M. B., Esposito, G., Jannet, H. B., Costantino, V., & Milella, L. (2022). Dittrichia graveolens (L.) Greuter, a Rapidly Spreading Invasive Plant: Chemistry and Bioactivity. Molecules, 27(3), 895. https://doi.org/10.3390/molecules27030895