Screening Wild Yeast Isolated from Cocoa Bean Fermentation Using Volatile Compounds Profile
Abstract
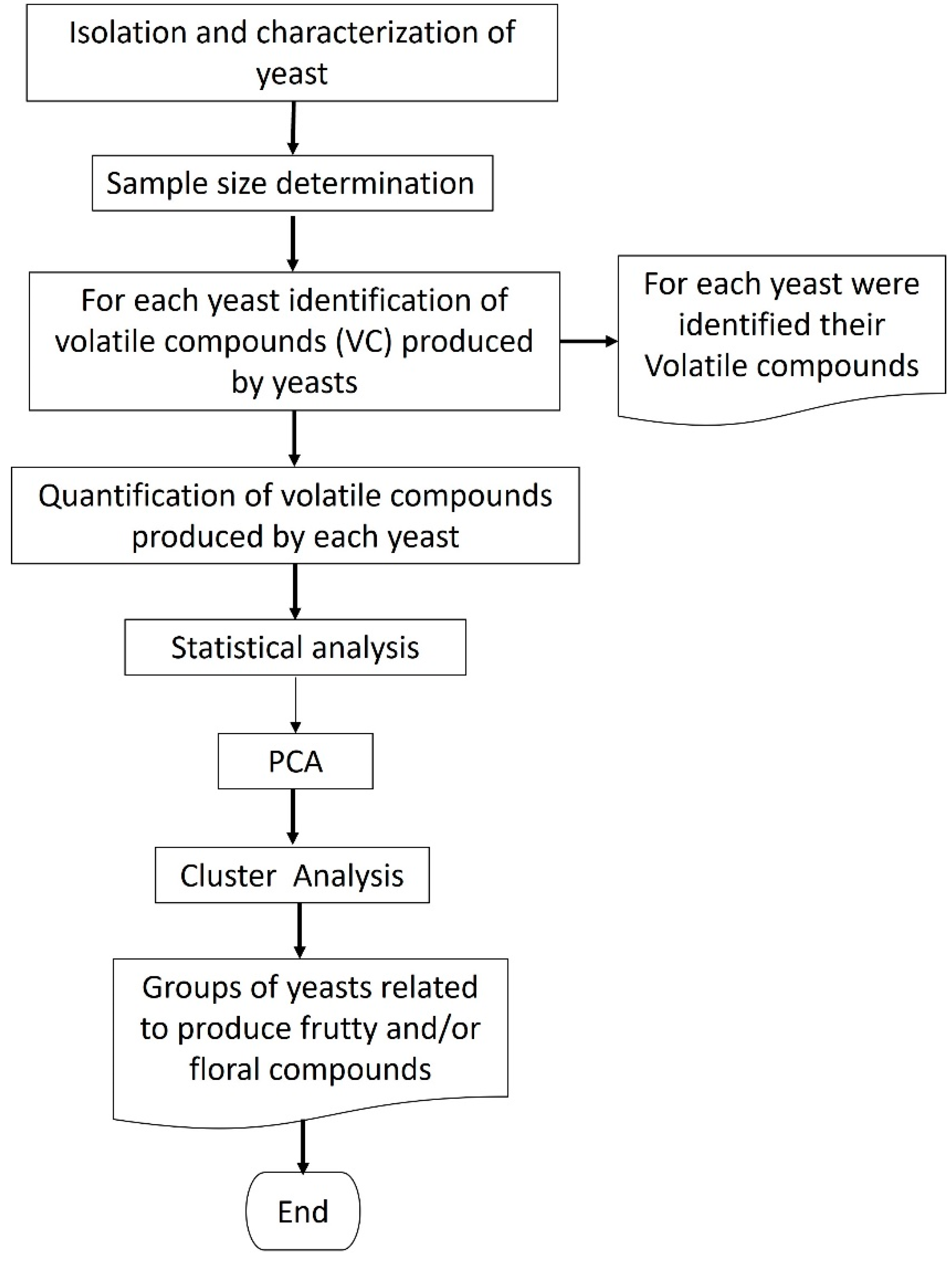
:1. Introduction
2. Results and Discussion
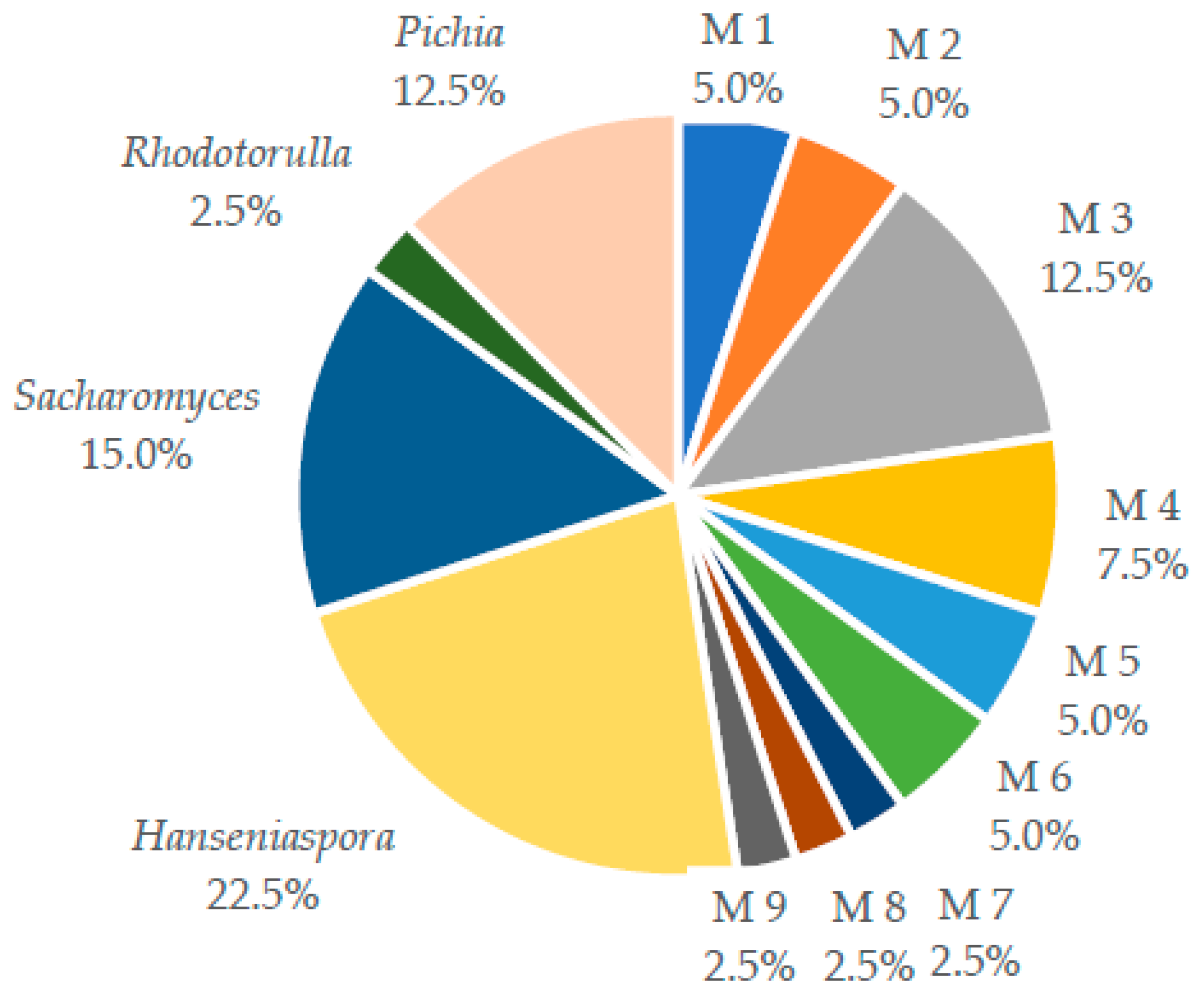
2.1. Yeast Isolation
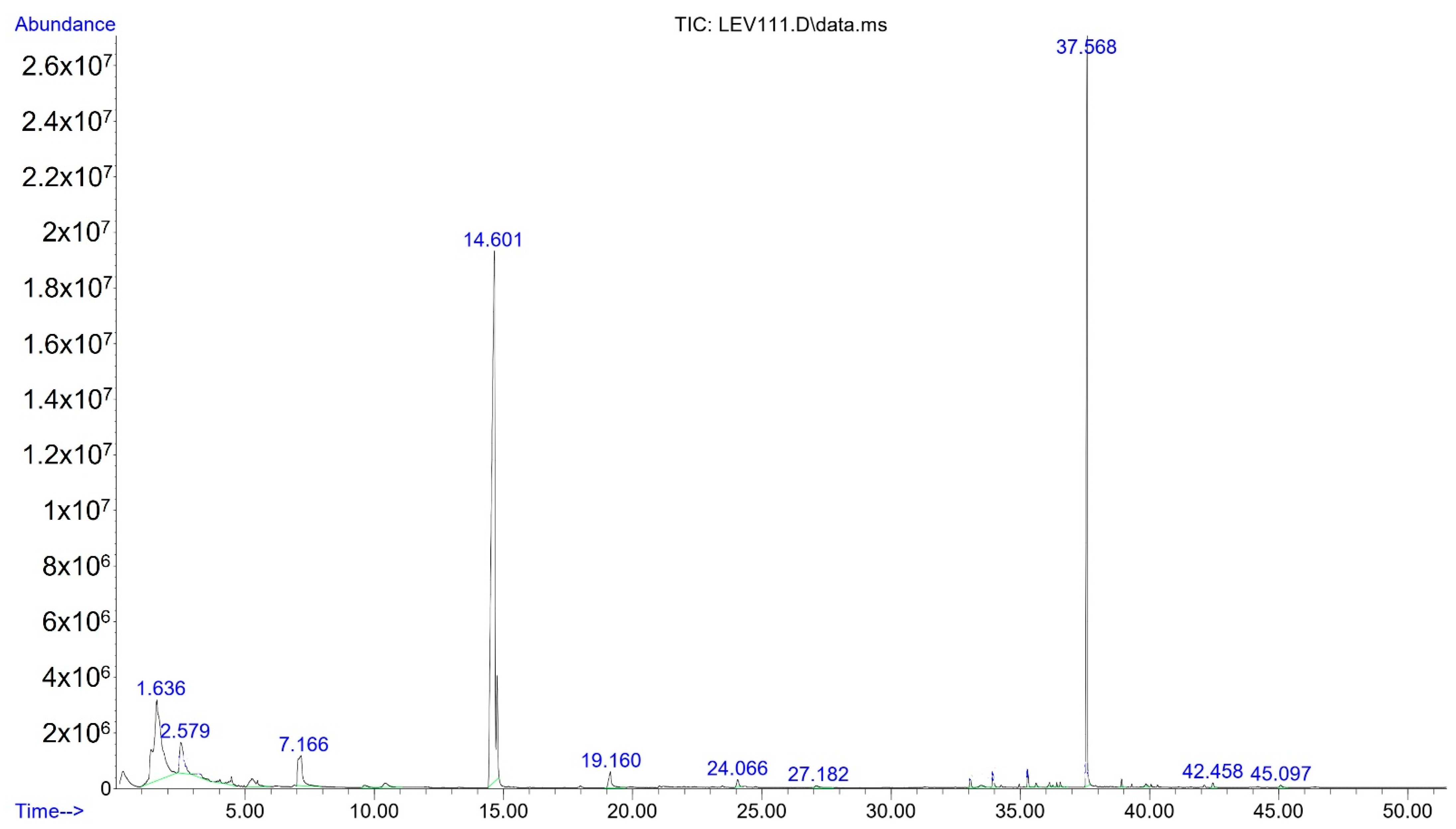
2.2. Identification and Quantification for Volatile Compounds (VC’s)
| Families Compounds | Compound | Concentration [mg/kg] | OTV * [mg/kg] | |
|---|---|---|---|---|
| Min | Max | |||
| alcohols | ethanol | 1.9755 | 974.8454 | 40 |
| 3-methylbuthanol | 0.1687 | 65.4272 | 3 | |
| 2-pentanol | 0.0813 | 10.2901 | 4 | |
| 2-phenylethanol | 0.0759 | 51.4509 | 14 | |
| alpha-terpinol | 0.0034 | 2.4455 | 0.33 | |
| 2,4-DI-tert-butylphenol | 0.1968 | 6.3304 | - | |
| esters | Ethyl acetate | 0.0141 | 102.0740 | 5 |
| 2-penthyl acetate | 0.2473 | 14.5378 | 0.005 | |
| 3-methylbuthyl acetate | 0.0314 | 317.1846 | 0.16 | |
| methylpropil acetate | 0.0315 | 25.1614 | 1.6 | |
| ethyl benzoate | 0.0002 | 17.3732 | 0.06 | |
| di-ethyl butanodiate | 0.0245 | 3.8429 | 200 | |
| ethyl octanoate | 0.0094 | 12.9963 | 0.58 | |
| 2-phenylethyl acetate | 0.0081 | 276.7966 | 0.65 | |
| ethyl decanoate | 0.0104 | 14.3622 | 0.2 | |
| ethyl 3-phenylpropanoate | 0.0547 | 12.0306 | 0.0016 | |
| ethyl 3-phenyl-2-propenoate | 0.0355 | 6.9843 | 0.0011 | |
| ethyl dodecanoate | 0.0150 | 10.4593 | 1.5 | |
| aldehydes | 3-methylbutanal | 0.0069 | 18.6677 | 0.001 |
| phenylacetaldehyde | 0.0054 | 54.0838 | 0.004 | |
| 2-phenyl-2-butenal | 0.0107 | 0.1917 | 1.7 | |
| 4-methyl-2-phenyl-2-pentenal | 0.0007 | 2.9953 | - | |
| 5-methyl-2-phneyl-2-hexenal | 0.0029 | 3.0785 | - | |
| ketones | 3-hydroxi-2-butanone | 0.0013 | 0.0603 | 150 |
| 2-heptanone | 0.0125 | 1.6152 | 0.14 | |
| 2-octanone | 0.0077 | 0.0254 | 0.05 | |
| 2-nonanone | 0.0158 | 0.1291 | 0.2 | |
| 6-methyl-3.5-dihydroxi-2,3-dihydro-4h-pyran-4-one | 0.0001 | 0.3478 | 0.25 | |
| acids | acetic acid | 0.0079 | 76.8848 | 33 |
| 2 methyl propanoic acid | 0.0027 | 0.0861 | 2.3 | |
| 3-methylbutanoic acid | 0.0009 | 13.0664 | 0.022 | |
| 2-methylbutanoic Acid | 0.0125 | 0.6547 | 0.05 | |
| decanoic acid | 0.4681 | 3.0440 | 10 | |
| dodecanoic acid | 0.0447 | 0.3476 | - | |
| pyrazines | 2-methylpyrazine | 6.4451 | 6.4451 | 2.5 |
| 2,3,5-trimethylpyrazine | 0.0178 | 0.2596 | 1.8 | |
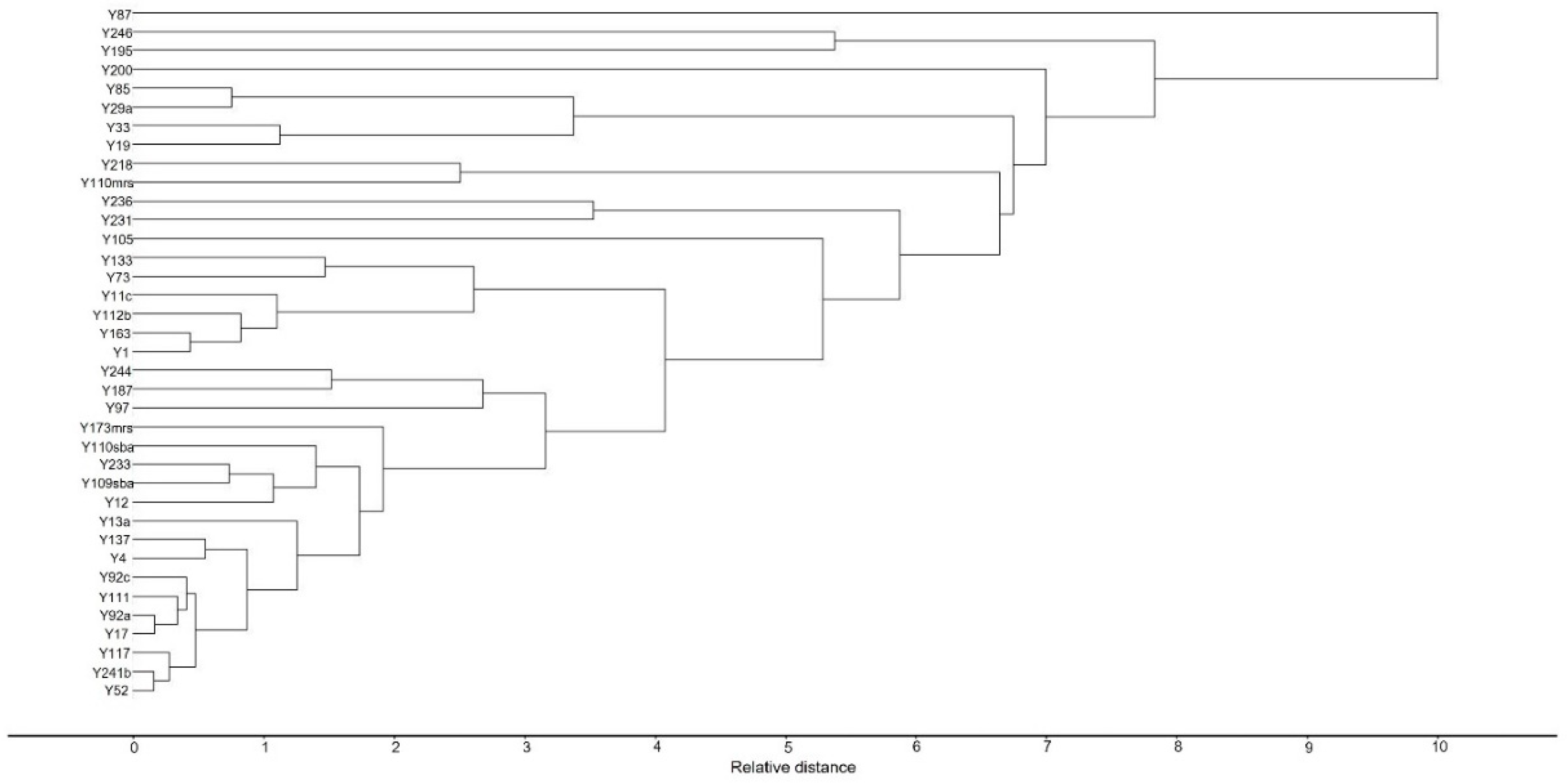
2.3. Principal Component Analysis (PCA)
3. Materials and Methods
3.1. Spontaneous Cocoa Beans Fermentations
3.2. Sampling
3.3. Culture-Dependent Microbiological Analysis
3.4. Yeast Identification
3.5. Screening of Yeast Isolates
3.5.1. Extraction of Volatile Compounds
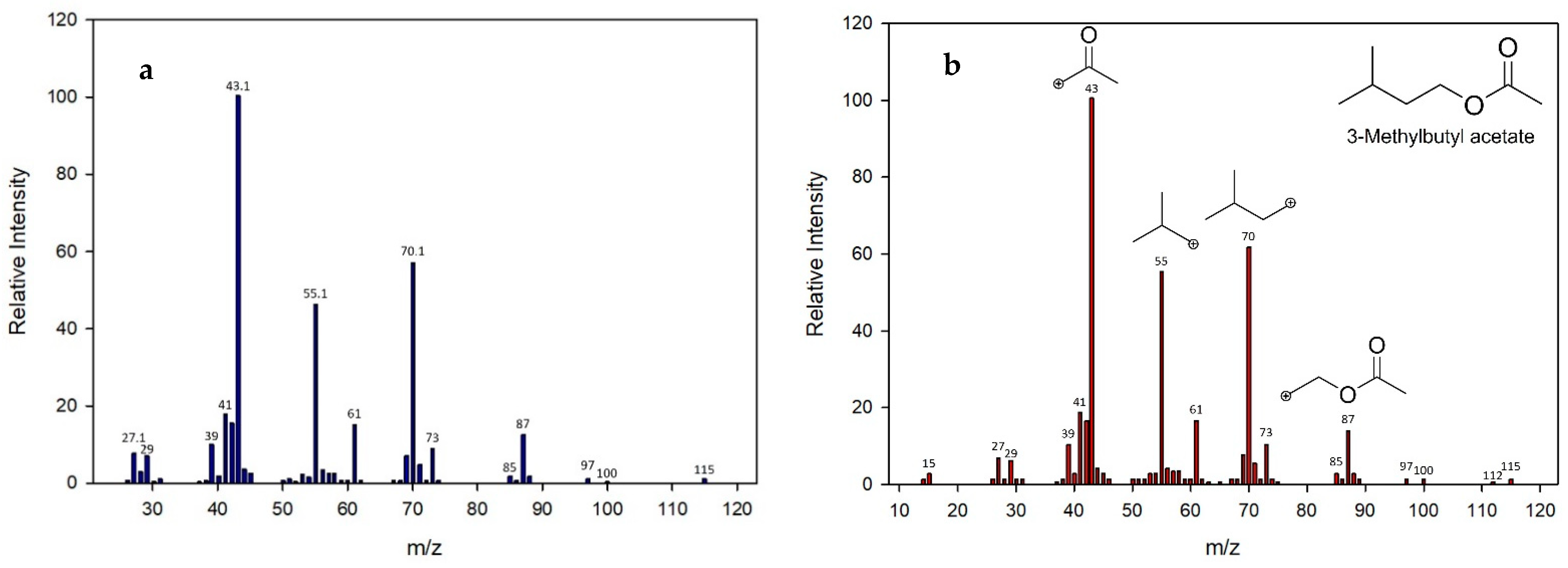
3.5.2. Identification of Volatile Compounds
3.5.3. Quantification of Volatile Compounds Produced by Each Yeast
3.6. Statistical Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- ICCO—International Cocoa Organization. Quartely Bulletin of Cocoa Statistics, Vol. XLVII, N°.2, Cocoa Year 2020/2021. Available online: https://www.icco.org/statistics/ (accessed on 10 August 2021).
- FEDECACAO—Federación Nacional de Cacaoteros. Producción Anual 2011–2020. Available online: https://www.fedecacao.com.co/economianacional (accessed on 15 September 2021).
- ICCO—International Cocoa Organization. Fine or Flavour Cocoa. 2021. Available online: https://www.icco.org/fine-or-flavor-cocoa/ (accessed on 17 September 2021).
- Escobar, S.; Santander, M.; Zuluaga, M.; Chacón, I.; Rodríguez, J.; Vaillant, F. Fine cocoa beans production: Tracking aroma precursors through a comprehensive analysis of flavor attributes formation. Food Chem. 2021, 365, 130627. [Google Scholar] [CrossRef] [PubMed]
- Trade Statistics for International Business Development, “Trade Map”. 2021. Available online: https://www.trademap.org/Index.aspx (accessed on 3 November 2021).
- Santander Muñoz, M.; Rodríguez Cortina, J.; Vaillant, F.E.; Escobar Parra, S. An overview of the physical and biochemical transformation of cocoa seeds to beans and to chocolate: Flavor formation. Crit. Rev. Food Sci. Nutr. 2020, 60, 1593–1613. [Google Scholar] [CrossRef] [PubMed]
- ICCO—International Cocoa Organization. Revision of Annex ‘c’ of the International Cocoa Agreement (ICA). 2010–34th Special Session of the International Cocoa Council. no. December, 2020. Available online: https://www.icco.org/revision-of-annex-c-of-the-international-cocoa-agreement-ica-2010/ (accessed on 17 September 2021).
- Ordoñez-Araque, R.H.; Landines-Vera, E.F.; Urresto-Villegas, J.C.; Caicedo-Jaramillo, C.F. Microorganisms during cocoa fermentation: Systematic review. Foods Raw Mater. 2020, 8, 155–162. [Google Scholar] [CrossRef]
- Dulce, V.R.; Anne, G.; Manuel, K.; Carlos, A.A.; Jacobo, R.C.; Sergio de Jesús, C.E.; Eugenia, L.C. Cocoa bean turning as a method for redirecting the aroma compound profile in artisanal cocoa fermentation. Heliyon 2021, 7, e07694. [Google Scholar] [CrossRef]
- Cubillos, G.; Correa, E.; Merizalde, G.J. Manual de Beneficio del Cacao. In Secretaria de Agricultura de Antioquia, Compañía Nacional de Chocolates S.A, Corporación para Investigaciones Biológicas (CIB); Grupo GIEM Universidad de Antioquia: Medellín, Colombia, 2008. [Google Scholar]
- Fang, Y.; Li, R.; Chu, Z.; Zhu, K.; Gu, F.; Zhang, Y. Chemical and flavor profile changes of cocoa beans (Theobroma cacao L.) during primary fermentation. Food Sci. Nutr. 2020, 8, 4121–4133. [Google Scholar] [CrossRef]
- Sarbu, I.; Csutak, O. The Microbiology of Cocoa Fermentation; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128158647. [Google Scholar]
- Koné, M.K.; Guéhi, S.T.; Durand, N.; Ban-Koffi, L.; Berthiot, L.; Tachon, A.F.; Brou, K.; Boulanger, R.; Montet, D. Contribution of predominant yeasts to the occurrence of aroma compounds during cocoa bean fermentation. Food Res. Int. 2016, 89, 910–917. [Google Scholar] [CrossRef]
- Vinicius De Melo Pereira, G.; De Carvalho Neto, D.P.; Junqueira, A.C.D.O.; Karp, S.G.; Letti, L.A.J.; Magalhães Júnior, A.I.; Soccol, C.R. A Review of Selection Criteria for Starter Culture Development in the Food Fermentation Industry. Food Rev. Int. 2020, 36, 135–167. [Google Scholar] [CrossRef]
- Samagaci, L.; Ouattara, H.G.; Goualié, B.G.; Niamke, S.L. Growth capacity of yeasts potential starter strains under cocoa fermentation stress conditions in Ivory Coast. Emirates J. Food Agric. 2014, 26, 861–870. [Google Scholar] [CrossRef]
- Koffi, O.; Samagaci, L.; Goualie, B.; Niamke, S. Screening of potential yeast starters with high ethanol production for small-scale cocoa fermentation in Ivory Coast. Food Environ. Saf. 2018, 17, 113–130. [Google Scholar]
- Meersman, E.; Steensels, J.; Struyf, N.; Paulus, T.; Saels, V.; Mathawan, M.; Allegaert, L.; Vrancken, G.; Verstrepen, K.J. Tuning Chocolate Flavor through Development of Thermotolerant Saccharomyces cerevisiae Starter Cultures with Increased Acetate Ester Production. Appl. Environ. Microbiol. 2016, 82, 732–746. [Google Scholar] [CrossRef] [Green Version]
- Palencia-Blanco, C.; Gualdrón-Zambrano, A.; Guarín-Henao, I.; Ojeda-Galeano, Y.; Villamizar-Jaimes, A.; Zárate-Caicedo, D.A.; Coronado-Silva, R.A.; Rodríguez-Silva, L.; López-Giraldo, L.J. Proposal for a semi-quantitative method for the determination of volatile compounds in cocoa liquors. Respuestas 2020, 25, 60–69. [Google Scholar] [CrossRef]
- Lozano Tovar, M.D.; Tibasosa, G.; González, C.M.; Ballestas Alvarez, K.; Lopez Hernandez, M.D.P.; Rodríguez Villamizar, F. Isolation and identification of microbial species found in cocoa fermentation as microbial starter culture candidates for cocoa bean fermentation in Colombia. Pelita Perkeb. 2020, 36, 236–248. [Google Scholar] [CrossRef]
- Magalhães da Veiga Moreira, I.; de Figueiredo Vilela, L.; da Cruz Pedroso Miguel, M.G.; Santos, C.; Lima, N.; Freitas Schwan, R. Impact of a Microbial Cocktail Used as a Starter Culture on Cocoa Fermentation and Chocolate Flavor. Molecules 2017, 22, 766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jjunju, F.P.M.; Giannoukos, S.; Marshall, A.; Taylor, S. In-Situ Analysis of Essential Fragrant Oils Using a Portable Mass Spectrometer. Int. J. Anal. Chem. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assi-Clair, B.J.; Koné, M.K.; Kouamé, K.; Lahon, M.C.; Berthiot, L.; Durand, N.; Lebrun, M.; Julien-Ortiz, A.; Maraval, I.; Boulanger, R.; et al. Effect of aroma potential of Saccharomyces cerevisiae fermentation on the volatile profile of raw cocoa and sensory attributes of chocolate produced thereof. Eur. Food Res. Technol. 2019, 245, 1459–1471. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Lu, X.; Zong, H.; Zhuge, B. Advances in 2-phenylethanol production from engineered microorganisms. Biotechnol. Adv. 2019, 37, 403–409. [Google Scholar] [CrossRef]
- de Lima, L.A.; Diniz, R.H.S.; de Queiroz, M.V.; Fietto, L.G.; da Silveira, W.B. Screening of Yeasts Isolated from Brazilian Environments for the 2-Phenylethanol (2-PE) Production. Biotechnol. Bioprocess. Eng. 2018, 23, 326–332. [Google Scholar] [CrossRef]
- Martínez-Avila, O.; Sánchez, A.; Font, X.; Barrena, R. Bioprocesses for 2-phenylethanol and 2-phenylethyl acetate production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 9991–10004. [Google Scholar] [CrossRef]
- Mendoza Salazar, M.M.; Martínez Álvarez, O.L.; Ardila Castañeda, M.P.; Lizarazo Medina, P.X. Bioprospecting of indigenous yeasts involved in cocoa fermentation using sensory and chemical strategies for selecting a starter inoculum. Food Microbiol. 2022, 101, 103896. [Google Scholar] [CrossRef]
- Frauendorfer, F.; Schieberle, P. Key aroma compounds in fermented Forastero cocoa beans and changes induced by roasting. Eur. Food Res. Technol. 2019, 245, 1907–1915. [Google Scholar] [CrossRef]
- Kampranis, S.C.; Makris, A.M. Developing a yeast cell factory for the production of terpenoids. Comput. Struct. Biotechnol. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, A.D.J.R. Biotransformation of monoterpene alcohols bySaccharomyces cerevisiae, Torulaspora delbrueckii andKluyveromyces lactis. Yeast 2000, 16, 499–506. [Google Scholar] [CrossRef]
- Aznar, M.; López, R.; Cacho, J.; Ferreira, V. Prediction of aged red wine aroma properties from aroma chemical composition. Partial least squares regression models. J. Agric. Food Chem. 2003, 51, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Cordero, C.; Guglielmetti, A.; Sgorbini, B.; Bicchi, C.; Allegrucci, E.; Gobino, G.; Baroux, L.; Merle, P. Odorants quantitation in high-quality cocoa by multiple headspace solid phase micro-extraction: Adoption of FID-predicted response factors to extend method capabilities and information potential. Anal. Chim. Acta 2019, 1052, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Glory, L.; Ortiz-Vazquez, E.; Sauri-Duch, E.; Pino, J. Characterization of aroma-active compounds in sugar apple (Annona squamosa L.). Acta Aliment. 2013, 42, 102–108. [Google Scholar] [CrossRef]
- Culleré, L.; Escudero, A.; Cacho, J.; Ferreira, V. Gas Chromatography-Olfactometry and Chemical Quantitative Study of the Aroma of Six Premium Quality Spanish Aged Red Wines. J. Agric. Food Chem. 2004, 52, 1653–1660. [Google Scholar] [CrossRef]
- Feng, Y.; Cai, Y.; Fu, X.; Zheng, L.; Xiao, Z.; Zhao, M. Comparison of aroma-active compounds in broiler broth and native chicken broth by aroma extract dilution analysis (AEDA), odor activity value (OAV) and omission experiment. Food Chem. 2018, 265, 274–280. [Google Scholar] [CrossRef]
- Jetti, R.R.; Yang, E.; Kurnianta, A.; Finn, C.; Qian, M.C. Quantification of selected aroma-active compounds in strawberries by headspace solid-phase microextraction gas chromatography and correlation with sensory descriptive analysis. J. Food Sci. 2007, 72, S487–S496. [Google Scholar] [CrossRef]
- Liu, T.; Feng, T.; Chen, W. Identification of volatile flavour components of tuber melanosporum using simultaneous distillation-extraction. Czech J. Food Sci. 2017, 35, 483–487. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, X.; Yuan, G.; Ren, J.; Wang, L.; Wang, M.; Li, Y.; Zhang, B.; Zhu, B. Aromatic compounds and organoleptic features of fermented wolfberry wine: Effects of maceration time. Int. J. Food Prop. 2017, 20, 2234–2248. [Google Scholar] [CrossRef]
- Qian, M.C.; Wang, Y. Seasonal variation of volatile composition and odor activity value of “Marion” (Rubus spp. hyb) and “Thornless Evergreen” (R. laciniatus L.) blackberries. J. Food Sci. 2005, 70, 13–20. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T.; Robert, V. Methods for Isolation, Phenotypic Characterization and Maintenance of Yeasts; Elsevier: Amsterdam, The Netherlands, 2011; Volume 1, ISBN 9780444521491. [Google Scholar]
- Bustamante Gladys, M.C. Aproximación al muestreo estadístico en investigación científicas. Rev. Actual. Clín. 2011, 10, 476–480. Available online: https://docplayer.es/80117374-Revista-de-actualizacion-clinica-volumen.html (accessed on 17 September 2021).

| Family | Compounds | Attributed Aroma | |
|---|---|---|---|
| alcohols | C1 | ethanol | alcoholic |
| C2 | 3-methyl butanol Isoamyl alcohol | balsamic fruit | |
| C3 | 2-pentanol | green, fruity, sweet, pungent, plastic–floral | |
| C4 | 2-phenyl ethanol Phenylethyl alcohol | flowery, rose, spicy, honey-like, caramel | |
| C5 | alpha-terpineol | floral, lilac | |
| esters | C6 | ethyl acetate | pineapple |
| C7 | 3-methyl butyl acetate Isoamyl acetate | sweet fruit banana solvent, pear | |
| C8 | methyl propyl acetate | banana, apple | |
| C9 | ethyl Benzoate | fruity, sweet, medicinal, cherry, grape | |
| C10 | ethyl Octanoate | fruity, sweet, musty, floral, pineapple | |
| C11 | 2-phenyl ethyl acetate | flowery (rose), honeyfruity, Sweet | |
| C12 | ethyl decanoate | fruity, sweet, pear, grape, brandy | |
| C13 | ethyl 3-phenylpropanoate | fruity | |
| C14 | ethyl 3-phenyl-2-propenoate | fruity, cinnamon | |
| C15 | ethyl dodecanoate | fruity floral, sweet | |
| aldehydes | C16 | 3-methyl butanal | malty, sweet, chocolate |
| C17 | phenylacetaldehyde | nutty, honey-like | |
| acids | C18 | acetic acid | acid, vinegar |
| C19 | 3-methyl butanoic acid | sweet, rancid |
| Farm | Approximate Content * | Opening Pods | Boxes Recubering | Turning | Fermentation Time | Drying Time | Height (msnm) | Relative Humidity (%) | Temperature (°C) |
|---|---|---|---|---|---|---|---|---|---|
| Villa Mónica 1 | 500 kg | 10–12 h before start of fermentation | burlap bags | turned daily, starting at 48 h | 6 days | 6 days | 879 | 77.2 | 22.4 |
| El Paraiso 1 | 500 kg | 10–12 h before start of fermentation | burlap bags | turned daily, starting at 48 h | 7 days | 6 days | 1140 | 77 | 23 |
| La Meseta 1 | 400 kg | 2 days before start of fermentation | burlap bags | turned daily, starting at 48 h | 6 days | 6 days | 715 | 77 | 26 |
| Parcela 7 2 | 800 kg | 2 days before start of fermentation | burlap bags | turned daily, starting at 48 h | 7 days | 6 days | 325 | 58 | 30 |
| El Dique 3 | 200 kg | 2 days before start of fermentation | burlap bags | turned daily, starting at 48 h | 8 days | 4 days | 1039 | 80 | 32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandoval-Lozano, C.J.; Caballero-Torres, D.; López-Giraldo, L.J. Screening Wild Yeast Isolated from Cocoa Bean Fermentation Using Volatile Compounds Profile. Molecules 2022, 27, 902. https://doi.org/10.3390/molecules27030902
Sandoval-Lozano CJ, Caballero-Torres D, López-Giraldo LJ. Screening Wild Yeast Isolated from Cocoa Bean Fermentation Using Volatile Compounds Profile. Molecules. 2022; 27(3):902. https://doi.org/10.3390/molecules27030902
Chicago/Turabian StyleSandoval-Lozano, Claudia Johanna, David Caballero-Torres, and Luis Javier López-Giraldo. 2022. "Screening Wild Yeast Isolated from Cocoa Bean Fermentation Using Volatile Compounds Profile" Molecules 27, no. 3: 902. https://doi.org/10.3390/molecules27030902
APA StyleSandoval-Lozano, C. J., Caballero-Torres, D., & López-Giraldo, L. J. (2022). Screening Wild Yeast Isolated from Cocoa Bean Fermentation Using Volatile Compounds Profile. Molecules, 27(3), 902. https://doi.org/10.3390/molecules27030902






