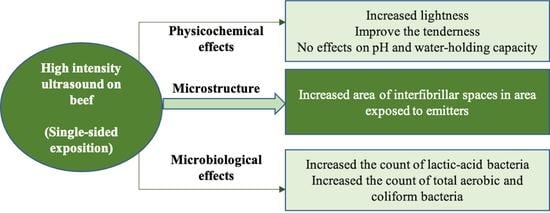
The Physicochemical, Microbiological, and Structural Changes in Beef Are Dependent on the Ultrasound System, Time, and One-Side Exposition
Abstract
:1. Introduction
2. Results and Discussion
2.1. CIE L*a*b* Color
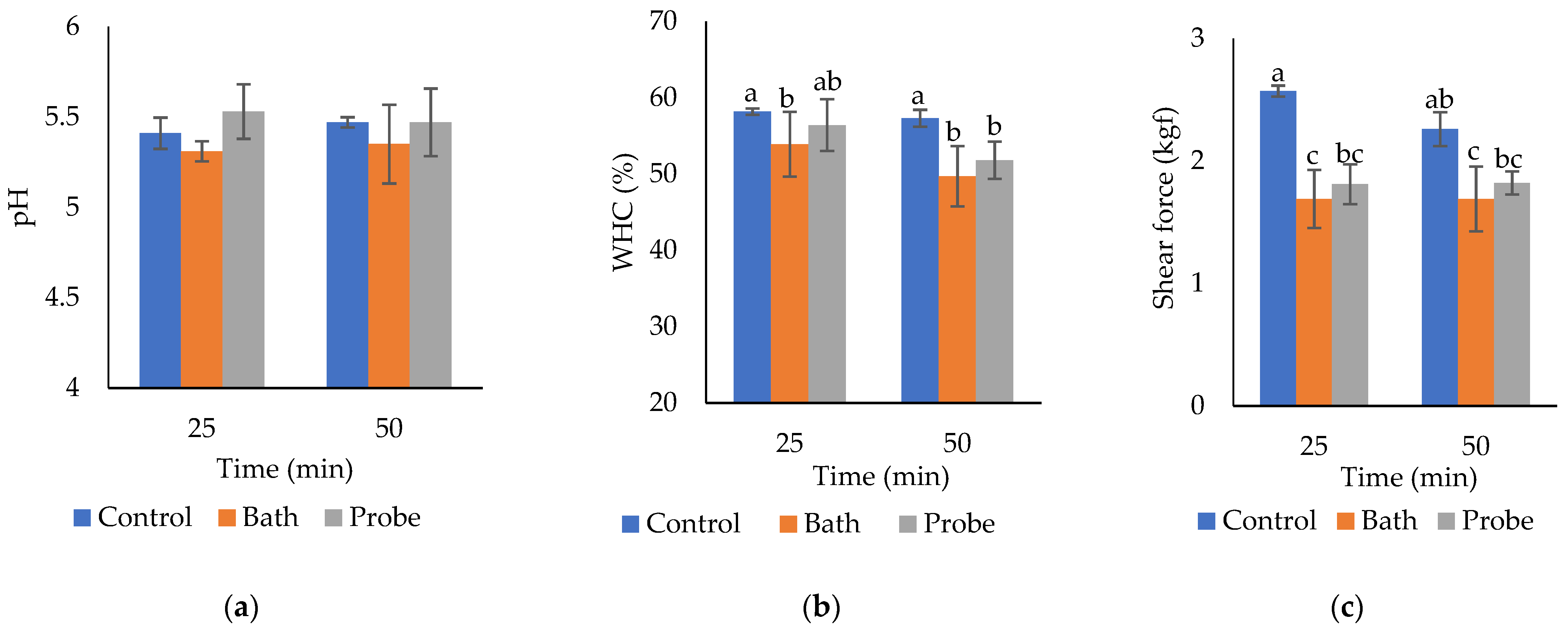
2.2. PH
2.3. Water Holding Capacity (WHC)
2.4. Shear Force
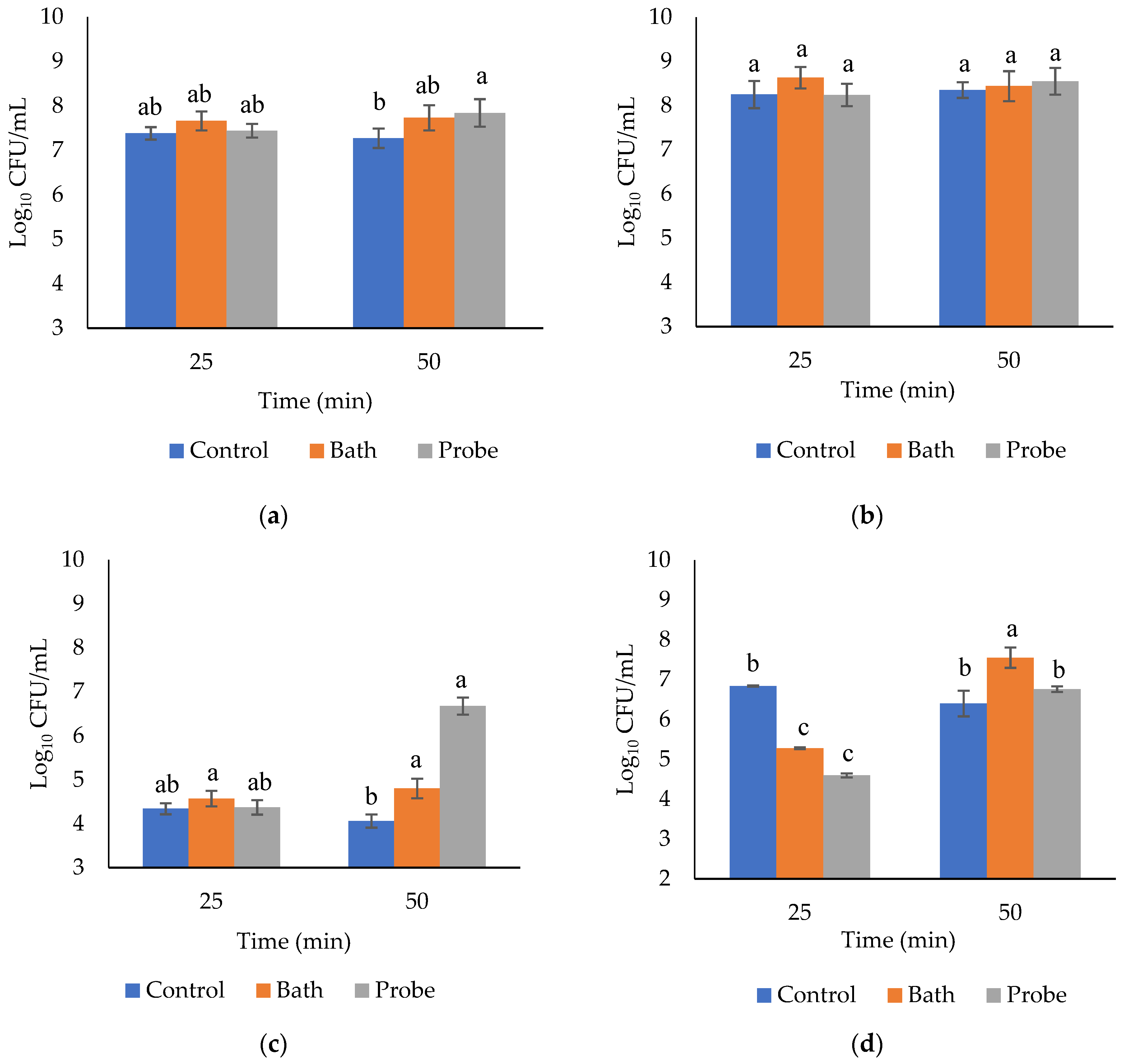
2.5. Microbiological Evaluations
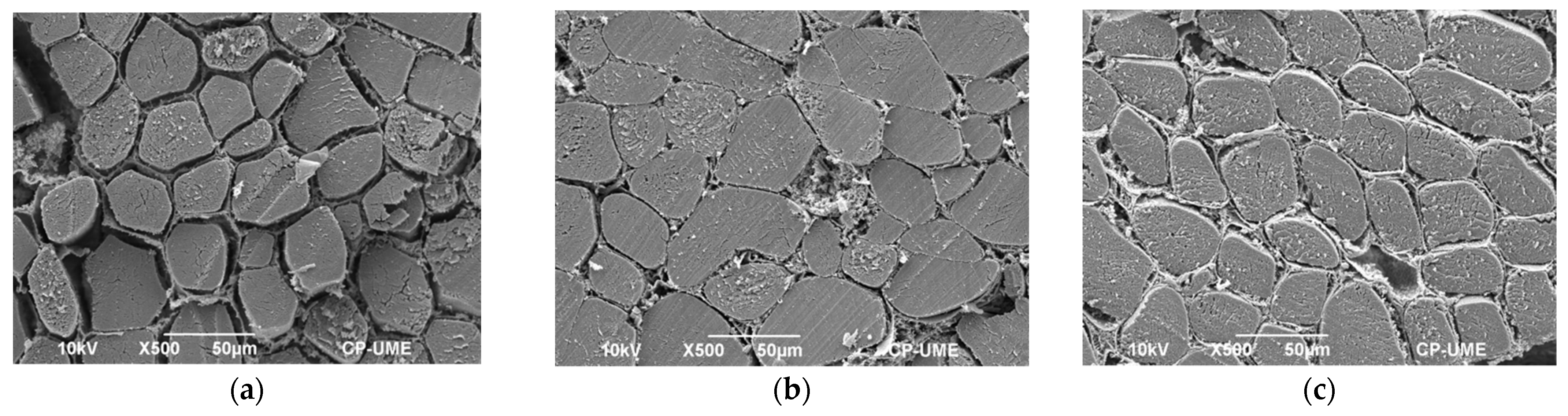
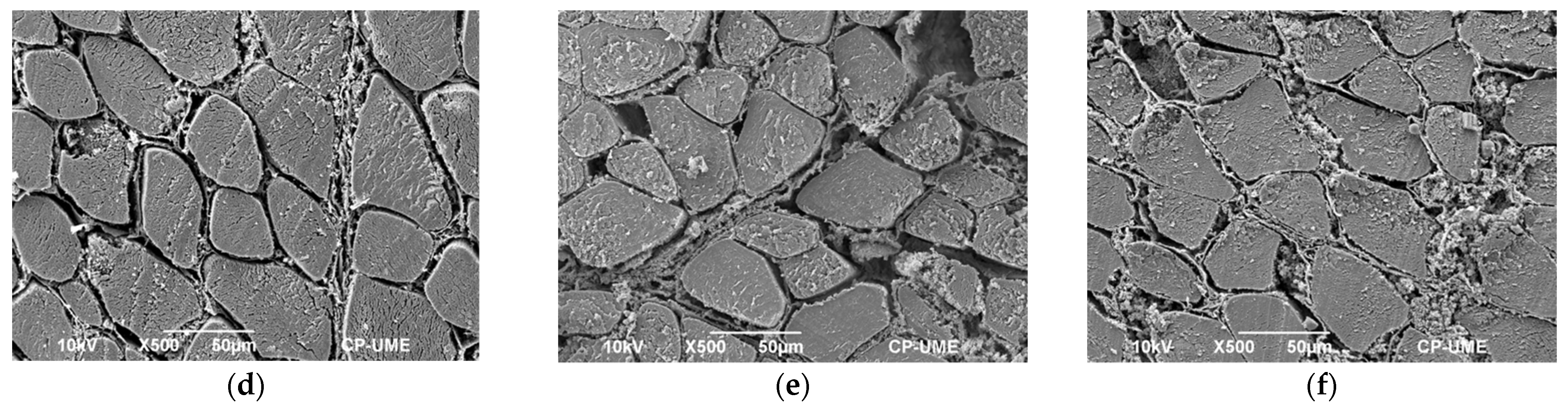
2.6. Microstructural Studies
3. Materials and Methods
3.1. Description of the Sample and Assignment of Treatments
3.2. Ultrasonic Treatment
3.3. Physicochemical Evaluations
3.4. Microstructural Analysis
3.5. Microbiological Analysis
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Alarcon-Rojo, A.D.; Carrillo-Lopez, L.M.; Reyes-Villagrana, R.; Huerta-Jimenez, M.; Garcia-Galicia, I.A. Ultrasound and meat quality: A review. Ultrason. Sonochem. 2019, 55, 369–382. [Google Scholar] [CrossRef]
- Chemat, F.; Zill-E-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Alarcon-Rojo, A.D.; Janacua, H.; Rodriguez, J.C.; Paniwnyk, L.; Mason, T.J. Power ultrasound in meat processing. Meat Sci. 2015, 107, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Awad, T.S.; Moharram, H.A.; Shaltout, O.E.; Asker, D.; Youssef, M.M. Applications of ultrasound in analysis, processing and quality control of food: A review. Food Res. Int. 2012, 48, 410–427. [Google Scholar] [CrossRef]
- Chemat, F.; Ashokkumar, M. Preface: Ultrasound in the processing of liquid foods, beverages and alcoholic drinks. Ultrason. Sonochem. 2017, 38, 753. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Lopez, L.M.; Huerta-Jimenez, M.; Garcia-Galicia, I.A.; Alarcon-Rojo, A.D. Bacterial control and structural and physicochemical modification of bovine Longissimus dorsi by ultrasound. Ultrason. Sonochem. 2019, 58, 104608. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Wang, A.; Zhou, G.; Zhang, W.; Xu, S.; Guo, G. Power ultrasonic on mass transport of beef: Effects of ultrasound intensity and NaCl concentration. Innov. Food Sci. Emerg. Technol. 2016, 35, 36–44. [Google Scholar] [CrossRef]
- Kang, D.; Gao, X.; Ge, Q.; Zhou, G.; Zhang, W. Effects of ultrasound on the beef structure and water distribution during curing through protein degradation and modification. Ultrason. Sonochem. 2017, 38, 317–325. [Google Scholar] [CrossRef]
- Ojha, K.S.; Keenan, D.F.; Bright, A.; Kerry, J.P.; Tiwari, B.K. Ultrasound assisted diffusion of sodium salt replacer and effect on physicochemical properties of pork meat. Int. J. Food Sci. Technol. 2016, 51, 37–45. [Google Scholar] [CrossRef]
- Barekat, S.; Soltanizadeh, N. Improvement of meat tenderness by simultaneous application of high-intensity ultrasonic radiation and papain treatment. Innov. Food Sci. Emerg. Technol. 2017, 39, 223–229. [Google Scholar] [CrossRef]
- Barekat, S.; Soltanizadeh, N. Effects of ultrasound on microstructure and enzyme penetration in beef longissimus lumborum muscle. Food Bioprocess Technol. 2018, 11, 680–693. [Google Scholar] [CrossRef]
- Wang, A.; Kang, D.; Zhang, W.; Zhang, C.; Zou, Y.; Zhou, G. Changes in calpain activity, protein degradation and microstructure of beef M. semitendinosus by the application of ultrasound. Food Chem. 2018, 245, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.L.; Rampelotto, C.; Silva, M.S.; De Moura, H.C.; Durante, E.C.; Mello, R.O.; Menezes, C.R.; Barin, J.S.; Campagnol, P.C.B.; Cichoski, A.J. The effect of cold storage on physicochemical and microbiological properties of beef Semitendinosus muscle subjected to ultrasonic treatment in different systems (bath or probe). Int. Food Res. J. 2018, 25, 504–514. [Google Scholar]
- Asadi, A.; Pourfattah, F.; Szilágyi, I.M.; Afrand, M.; Zyla, G.; Ahn, H.S.; Wongwises, S.; Nguyen, H.M.; Arabkoohsar, A.; Mahian, O. Effect of sonication characteristics on stability, thermophysical properties, and heat transfer of nanofluids: A comprehensive review. Ultrason. Sonochem. 2019, 58, 104701. [Google Scholar] [CrossRef]
- Xue, S.; Xu, X.; Shan, H.; Wang, H.; Yang, J.; Zhou, G. Effects of high-intensity ultrasound, high-pressure processing, and high-pressure homogenization on the physicochemical and functional properties of myofibrillar proteins. Innov. Food Sci. Emerg. Technol. 2018, 45, 354–360. [Google Scholar] [CrossRef]
- Dhanalakshmi, N.P.; Nagarajan, R. Ultrasonic intensification of the chemical degradation of methyl violet: An exper mental study. Int. J. Chem. Mol. Eng. 2011, 5, 1019–1024. [Google Scholar]
- Peña-González, E.M.; Alarcón-Rojo, A.D.; Rentería, A.; García, I.; Santellano, E.; Quintero, A.; Luna, L. Quality and sensory profile of ultrasound-treated beef. Ital. J. Food Sci. 2017, 29, 463–475. [Google Scholar] [CrossRef]
- Chang, H.-J.; Wang, Q.; Tang, C.-H.; Zhou, G.-H. Effects of ultrasound treatment on connective tissue collagen and meat quality of beef semitendinosus muscle. J. Food Qual. 2015, 38, 256–267. [Google Scholar] [CrossRef]
- Got, F.; Culioli, J.; Berge, P.; Vignon, X.; Astruc, T.; Quideau, J.M.; Lethiecq, M. Effects of high-intensity high-frequency ultrasound on ageing rate, ultrastructure and some physico-chemical properties of beef. Meat Sci. 1999, 51, 35–42. [Google Scholar] [CrossRef]
- Ristic, V.M. Principles of Acoustic Devices, 2nd ed.; Wiley-Interscience: New York, NY, USA, 1983; pp. 3–45. [Google Scholar]
- Caraveo, O.; Alarcon-Rojo, A.D.; Renteria, A.; Santellano, E.; Paniwnyk, L. Physicochemical and microbiological characteristics of beef treated with high-intensity ultrasound and stored at 4 °C. J. Sci. Food Agric. 2015, 95, 2487–2493. [Google Scholar] [CrossRef] [PubMed]
- Jayasooriya, S.D.; Torley, P.J.; D’Arcy, B.R.; Bhandari, B.R. Effect of high-power ultrasound and ageing on the physical properties of bovine Semitendinosus and Longissimus muscles. Meat Sci. 2007, 75, 628–639. [Google Scholar] [CrossRef]
- Diaz-Almanza, S.; Reyes-Villagrana, R.; Alarcon-Rojo, A.D.; Huerta-Jimenez, M.; Carrillo-Lopez, L.M.; Estepp, C.; Urbina-Perez, J.; Garcia-Galicia, I. Time matters when ultrasonicating beef: The best time for tenderness is not the best for reducing microbial counts. J. Food Process Eng. 2019, 42, e13210. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, W.; Kang, D.; Zhou, G. Improvement of tenderness and water holding capacity of spiced beef by the application of ultrasound during cooking. Int. J. Food Sci. Technol. 2018, 53, 828–836. [Google Scholar] [CrossRef]
- McDonnell, C.K.; Allen, P.; Morin, C.; Lyng, J.G. The effect of ultrasonic salting on protein and water–protein interactions in meat. Food Chem. 2014, 147, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Stadnik, J.; Dolatowski, Z.J. Influence of sonication on Warner-Bratzler shear force, colour and myoglobin of beef (m. semimembranosus). Eur. Food Res. Technol. 2011, 233, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Xiong, G.Y.; Zhang, L.L.; Zhang, W.; Wu, J. Influence of ultrasound and proteolytic enzyme inhibitors on muscle degradation, tenderness, and cooking loss of hens during aging. Czech J. Food Sci. 2012, 30, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Siró, I.; Vén, C.; Balla, C.; Jónás, G.; Zeke, I.; Friedrich, L. Application of an ultrasonic assisted curing technique for improving the diffusion of sodium chloride in porcine meat. J. Food Eng. 2009, 91, 353–362. [Google Scholar] [CrossRef]
- Garcia-Galicia, I.A.; Huerta-Jimenez, M.; Morales-Piñon, C.; Diaz-Almanza, S.; Carrillo-Lopez, L.M.; Reyes-Villagrana, R.; Estepp, C.; Alarcon-Rojo, A.D. The impact of ultrasound and vacuum pack on quality properties of beef after modified atmosphere on display. J. Food Process Eng. 2020, 43, e13044. [Google Scholar] [CrossRef]
- Piñon, M.I.; Alarcon-Rojo, A.D.; Renteria, A.L.; Carrillo-Lopez, L.M. Microbiological properties of poultry breast meat treated with high-intensity ultrasound. Ultrasonics 2020, 102, 105680. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Ramaswamy, R. Application of emerging technologies to control Salmonella in foods: A review. Food Res. Int. 2012, 45, 666–677. [Google Scholar] [CrossRef]
- Dolatowski, Z.; Stasiak, D. Bacterial contamination of meat and meat products after ultrasound treatment. Acta Sci. Pol. 2002, 1, 55–65. [Google Scholar] [CrossRef]
- Haughton, P.N.; Lyng, J.G.; Morgan, D.J.; Cronin, D.A.; Noci, F.; Fanning, S.; Whyte, P. An evaluation of the potential of high-intensity ultrasound for improving the microbial safety of poultry. Food Bioprocess Technol. 2012, 5, 992–998. [Google Scholar] [CrossRef]
- Woraprayote, W.; Malila, Y.; Sorapukdee, S.; Swetwiwathana, A.; Benjakul, S.; Visessanguan, W. Bacteriocins from lactic acid bacteria and their applications in meat and meat products. Meat Sci. 2016, 120, 118–132. [Google Scholar] [CrossRef] [PubMed]
- de Lima Alves, L.; da Silva, M.S.; Flores, D.R.M.; Athayde, D.R.; Ruviaro, A.R.; Brum, D.D.S.; Batista, V.S.F.; Mello, R.D.O.; de Menezes, C.R.; Campagnol, P.C.B.; et al. Effect of ultrasound on the physicochemical and microbiological characteristics of Italian salami. Food Res. Int. 2018, 106, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Aguirre, D.; Mobbs, T.; Barbosa-Cánovas, G.V. Ultrasound applications in food processing. In Ultrasound Technologies for Food and Bioprocessing, 1st ed.; Feng, H., Barbosa-Cánovas, G.V., Weiss, J., Eds.; Springer: New York, NY, USA, 2011; p. 678. [Google Scholar]
- Barukčić, I.; Lisak Jakopović, K.; Herceg, Z.; Karlović, S.; Božanić, R. Influence of high intensity ultrasound on microbial reduction, physico-chemical characteristics and fermentation of sweet whey. Innov. Food Sci. Emerg. Technol. 2015, 27, 94–101. [Google Scholar] [CrossRef]
- Yeo, S.-K.; Liong, M.-T. Effect of ultrasound on bioconversion of isoflavones and probiotic properties of parent organisms and subsequent passages of Lactobacillus. LWT-Food Sci. Technol. 2013, 51, 289–295. [Google Scholar] [CrossRef]
- American Meat Science Association, Meat Color Measurement Guidelines. Available online: https://meatscience.org/publications-resources/printed-publications/amsa-meat-color-measurement-guidelines (accessed on 15 May 2020).
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Tsai, T.; Ockerman, H. Water binding measurement of meat. J. Food Sci. 1981, 46, 697–701. [Google Scholar] [CrossRef]
- American Meat Science Association, Research Guidelines for Cookery, Sensory Evaluation, and Instrumental Tenderness Measurements of Meat. Available online: https://meatscience.org/publications-resources/printed-publications/sensory-and-tenderness-evaluation-guidelines (accessed on 15 May 2020).
- Secretaría De Salud, Norma Oficial Mexicana Nom-092-Ssa1-1994, Bienes Y Servicios, Método Para La Cuenta De Bacterias Aerobias En Placa. Available online: http://www.salud.gob.mx/unidades/cdi/nom/092ssa14.html (accessed on 15 May 2020).
- FDA. Bacteriological Analytical Manual on Line, 8th ed.; AOAC International: Arlington, MA, USA, 2004; Chapter 4.
| Treatment | CIE L*a*b* | ||||
|---|---|---|---|---|---|
| HIU system | L* | a* | b* | Hue | Chroma |
| Control without HIU | 37.9 ± 1.9 b | 19.1 ± 1.2 a | 8.5 ± 1.5 a | 23.5 ± 2.6 a | 20.9 ± 1.7 a |
| HIU bath | 40.6 ± 1.6 a | 20.3 ± 1.2 a | 8.6 ± 1.0 a | 23.0 ± 1.9 a | 22.0 ± 1.4 a |
| HIU probe (100% amplitude) | 39.8 ± 1.5 b | 20.1 ± 1.4 a | 8.8 ± 1.2 a | 23.5 ± 1.7 a | 21.9 ± 1.7 a |
| HIU time (min) | L* | a* | b* | Hue | Chroma |
| 25 | 39.5 ± 1.9 a | 19.5 ± 1.5 a | 8.4 ± 0.8 a | 23.0 ± 1.4 a | 21.2 ± 1.6 a |
| 50 | 39.3 ± 2.1 a | 20.1 ± 1.2 a | 8.9 ± 1.5 a | 23.6 ± 2.5 a | 22.0 ± 1.6 a |
| HIU system*HIU time | L* | a* | b* | Hue | Chroma |
| Control | |||||
| 25 min | 38.1 ± 1.6 a | 18.3 ± 0.2 a | 7.9 ± 0.4 a | 22.8 ± 1.1 a | 19.9 ± 1.1 a |
| 50 min | 37.7 ± 2.3 a | 19.8 ± 1.1 a | 9.0 ± 2.1 a | 24.1 ± 3.7 a | 21.8 ± 1.8 a |
| Bath | |||||
| 25 min | 40.7 ± 1.3 a | 20.4 ± 1.4 a | 8.5 ± 0.9 a | 22.7 ± 1.3 a | 22.1 ± 1.6 a |
| 50 min | 40.4 ± 2.1 a | 20.1 ± 1.1 a | 8.7 ± 1.3 a | 23.4 ± 2.5 a | 21.9 ± 1.4 a |
| Probe | |||||
| 25 min | 39.6 ± 1.9 a | 19.9 ± 1.3 a | 8.7 ± 1.1 a | 23.6 ± 2.1 a | 21.7 ± 1.5 a |
| 50 min | 39.9 ± 1.2 a | 20.3 ± 1.6 a | 8.8 ± 1.4 a | 23.4 ± 1.7 a | 22.2 ± 2.0 a |
| Treatment | Physicochemical Variables | ||
|---|---|---|---|
| HIU system | pH | WHC (%) | Shear force (kgf) |
| Control without HIU | 5.54 ± 0.07 | 57.73 ± 0.89 a | 2.41 ± 0.19 a |
| HIU bath | 5.33 ± 0.15 | 51.77 ± 4.32 b | 1.69 ± 0.22 b |
| HIU probe (100% amplitude) | 5.50 ± 0.16 | 54.1 ± 3.67 b | 1.82 ± 0.12 b |
| HIU time (min) | pH | WHC (%) | Shear force (kgf) |
| 25 | 5.41 ± 0.14 | 56.15 ± 3.31 a | 2.02 ± 0.44 a |
| 50 | 5.43 ± 0.16 | 52.90 ± 4.16 b | 1.92 ± 0.29 a |
| a* | b* | C* | Hue | pH | WHC | SF | Meso | Psi | LAB | Coli | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | Pearson C. | 0.320 | 0.260 | 0.380 | −0.297 | −0.553 | −0.457 | −0.505 | 0.533 | 0.381 | −0.076 | 0.462 |
| Sig. | 0.195 | 0.297 | 0.120 | 0.231 | 0.017 | 0.056 | 0.033 | 0.023 | 0.119 | 0.766 | 0.054 | |
| a* | Pearson C. | 0.120 | 0.985 | −0.028 | −0.069 | −0.221 | −0.567 | 0.505 | 0.568 | −0.004 | 0.328 | |
| Sig. | 0.634 | 0.000 | 0.913 | 0.785 | 0.378 | 0.014 | 0.033 | 0.014 | 0.987 | 0.184 | ||
| b* | Pearson C. | 0.190 | 0.375 | −0.165 | 0.061 | −0.245 | 0.294 | 0.424 | −0.033 | 0.049 | ||
| Sig. | 0.450 | 0.125 | 0.514 | 0.810 | 0.328 | 0.237 | 0.079 | 0.896 | 0.847 | |||
| C* | Pearson C. | 0.009 | −0.112 | −0.181 | −0.503 | 0.500 | 0.603 | 0.042 | 0.312 | |||
| Sig. | 0.971 | 0.657 | 0.472 | 0.033 | 0.035 | 0.008 | 0.868 | 0.207 | ||||
| Hue | Pearson C. | 0.153 | 0.049 | 0.088 | −0.413 | −0.186 | 0.076 | −0.467 | ||||
| Sig. | 0.545 | 0.847 | 0.730 | 0.088 | 0.459 | 0.763 | 0.051 | |||||
| pH | Pearson C. | 0.328 | 0.122 | −0.409 | −0.491 | −0.428 | −0.317 | |||||
| Sig. | 0.184 | 0.628 | 0.092 | 0.039 | 0.076 | 0.201 | ||||||
| WHC | Pearson C. | 0.670 | −0.474 | −0.050 | −0.306 | −0.679 | ||||||
| Sig. | 0.002 | 0.047 | 0.843 | 0.217 | 0.002 | |||||||
| SF | Pearson C. | −0.571 | −0.332 | 0.245 | −0.578 | |||||||
| Sig. | 0.013 | 0.179 | 0.328 | 0.012 | ||||||||
| Meso | Pearson C. | 0.745 | 0.169 | 0.845 | ||||||||
| Sig. | 0.000 | 0.503 | 0.000 | |||||||||
| Psi | Pearson C. | 0.095 | 0.474 | |||||||||
| Sig. | 0.707 | 0.047 | ||||||||||
| LAB | Pearson C. | 0.258 | ||||||||||
| Sig. | 0.301 | |||||||||||
| Treatment | Microbiological Count (log10 CFU/mL) | |||
|---|---|---|---|---|
| HIU system | Mesophiles | Psychrophiles | Coliforms | Lactic acid bacteria |
| Control without HIU | 7.32 ± 0.17 b | 8.03 ± 0.23 a | 4.20 ± 0.19 b | 6.62 ± 0.31 a |
| HIU bath | 7.70 ± 0.23 a | 8.53 ± 0.29 a | 4.68 ± 0.21 a | 6.42 ± 1.25 a |
| HIU probe (100% amplitude) | 7.64 ± 0.31 a | 8.39 ± 0.30 a | 4.52 ± 0.23 a | 5.68 ± 1.18 b |
| HIU time (min) | Mesophiles | Psychrophiles | Coliforms | Lactic acid bacteria |
| 25 | 7.49 ± 0.20 a | 8.37 ± 0.30 a | 4.43 ± 0.17 a | 5.57 ± 0.99 b |
| 50 | 7.61 ± 0.35 a | 8.45 ± 0.26 a | 4.51 ± 0.37 a | 6.9 ± 0.54 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrillo-Lopez, L.M.; Cruz-Garibaldi, B.Y.; Huerta-Jimenez, M.; Garcia-Galicia, I.A.; Alarcon-Rojo, A.D. The Physicochemical, Microbiological, and Structural Changes in Beef Are Dependent on the Ultrasound System, Time, and One-Side Exposition. Molecules 2022, 27, 541. https://doi.org/10.3390/molecules27020541
Carrillo-Lopez LM, Cruz-Garibaldi BY, Huerta-Jimenez M, Garcia-Galicia IA, Alarcon-Rojo AD. The Physicochemical, Microbiological, and Structural Changes in Beef Are Dependent on the Ultrasound System, Time, and One-Side Exposition. Molecules. 2022; 27(2):541. https://doi.org/10.3390/molecules27020541
Chicago/Turabian StyleCarrillo-Lopez, Luis M., Bianka Y. Cruz-Garibaldi, Mariana Huerta-Jimenez, Ivan A. Garcia-Galicia, and Alma D. Alarcon-Rojo. 2022. "The Physicochemical, Microbiological, and Structural Changes in Beef Are Dependent on the Ultrasound System, Time, and One-Side Exposition" Molecules 27, no. 2: 541. https://doi.org/10.3390/molecules27020541
APA StyleCarrillo-Lopez, L. M., Cruz-Garibaldi, B. Y., Huerta-Jimenez, M., Garcia-Galicia, I. A., & Alarcon-Rojo, A. D. (2022). The Physicochemical, Microbiological, and Structural Changes in Beef Are Dependent on the Ultrasound System, Time, and One-Side Exposition. Molecules, 27(2), 541. https://doi.org/10.3390/molecules27020541