Progress in Colloid Delivery Systems for Protection and Delivery of Phenolic Bioactive Compounds: Two Study Cases—Hydroxytyrosol and Curcumin
Abstract
:1. Introduction
2. Colloid-Mediated Delivery Systems
2.1. Conventional Emulsions
2.2. Multilayer Emulsions
2.3. Multiple Emulsions
2.4. Pickering Emulsions
2.5. Gelled Emulsions
2.6. Liposomes
2.7. Solid Particles
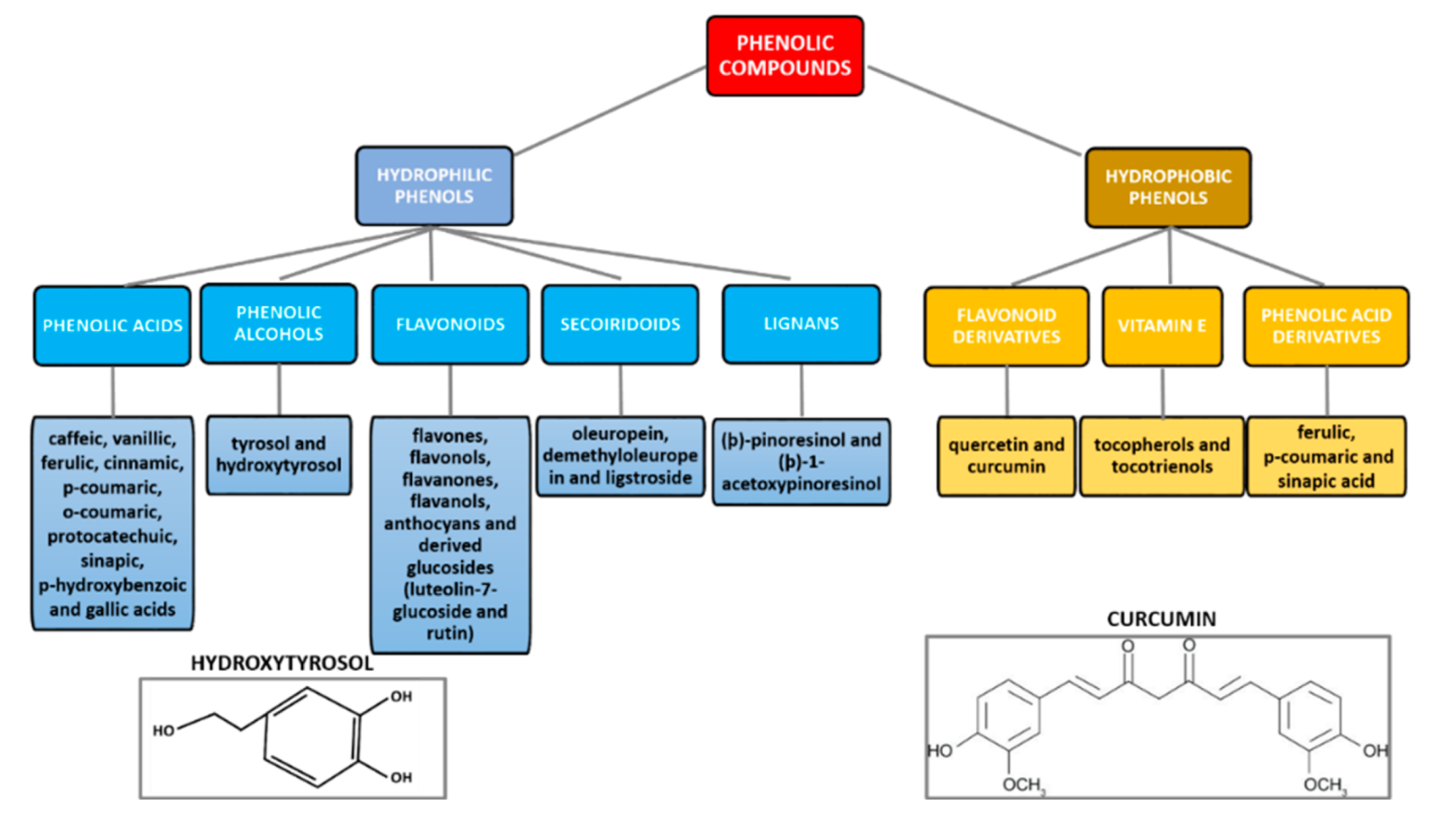
3. Phenolic Compounds
3.1. Hydrophilic Phenols: The Case of Hydroxytyrosol
3.1.1. Macro and Multiple Emulsions for HYT
3.1.2. Gelled Emulsions for HYT
3.1.3. Liposomes for HYT
3.1.4. Solid Particles for HYT
3.2. Hydrophobic Phenols: The Case of Curcumin
3.2.1. Macroemulsions for CUR
3.2.2. Nanoemulsions for CUR
3.2.3. Pickering Emulsions (PE) for CUR
3.2.4. Multilayer Emulsions for CUR
3.2.5. Liposomes for CUR
3.2.6. Solid Particles for CUR
4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gul, K.; Singh, A.K.; Jabeen, R. Nutraceuticals and Functional Foods: The Foods for the Future World. Crit. Rev. Food Sci. Nutr. 2016, 56, 2617–2627. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Nanoparticle-and Microparticle-Based Delivery Systems: Encapsulation, Protection and Release of Active Compounds; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Cinelli, G.; Sbrocchi, G.; Iacovino, S.; Ambrosone, L.; Ceglie, A.; Lopez, F.; Cuomo, F. Red Wine-Enriched Olive Oil Emulsions: Role of Wine Polyphenols in the Oxidative Stability. Colloids Surf. 2019, 3, 59. [Google Scholar] [CrossRef] [Green Version]
- Horbańczuk, O.K.; Kurek, M.A.; Atanasov, A.G.; Brnčić, M.; Brnčić, S.R. The effect of natural antioxidants on quality and shelf life of beef and beef products. Food Technol. Biotechnol. 2019, 57, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Oswell, N.J.; Thippareddi, H.; Pegg, R.B. Practical use of natural antioxidants in meat products in the U.S.: A review. Meat Sci. 2018, 145, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Barba, F.J.; Domínguez, R.; Sant’Ana, A.S.; Mousavi Khaneghah, A.; Gavahian, M.; Gómez, B.; Lorenzo, J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, F.; Iacovino, S.; Messia, M.C.; Sacco, P.; Lopez, F. Protective action of lemongrass essential oil on mucilage from chia (Salvia hispanica) seeds. Food Hydrocoll. 2020, 105, 105860. [Google Scholar] [CrossRef]
- Cinelli, G.; Cuomo, F.; Hochkoeppler, A.; Ceglie, A.; Lopez, F. Use of Rhodotorula minuta Live Cells Hosted in Water-in-Oil Macroemulsion for Biotrasformation Reaction. Biotechnol. Prog. 2006, 22, 689–695. [Google Scholar] [CrossRef]
- Cuomo, F.; Cinelli, G.; Chirascu, C.; Marconi, E.; Lopez, F. Antioxidant effect of vitamins in olive oil emulsion. Colloids Interfaces 2020, 4, 23. [Google Scholar] [CrossRef]
- Patravale, V.B.; Date, A.A.; Kulkarni, R.M. Nanosuspensions: A promising drug delivery strategy. J. Pharm. Pharmacol. 2010, 56, 827–840. [Google Scholar] [CrossRef] [Green Version]
- Perugini, L.; Cinelli, G.; Cofelice, M.; Ceglie, A.; Lopez, F.; Cuomo, F. Effect of the coexistence of sodium caseinate and Tween 20 as stabilizers of food emulsions at acidic pH. Colloids Surf. B Biointerfaces 2018, 168, 163–168. [Google Scholar] [CrossRef]
- Baldauf, L.M.; Schechter, R.S.; Wade, W.H.; Graciaa, A. The relationship between surfactant phase behavior and the creaming and coalescence of macroemulsions. J. Colloid Interface Sci. 1982, 85, 187–197. [Google Scholar] [CrossRef]
- Kralova, I.; Sjöblom, J. Surfactants Used in Food Industry: A Review. J. Dispers. Sci. Technol. 2009, 30, 1363–1383. [Google Scholar] [CrossRef]
- Dickinson, E. Food emulsions and foams: Stabilization by particles. Curr. Opin. Colloid Interface Sci. 2010, 15, 40–49. [Google Scholar] [CrossRef]
- De Leonardis, A.; Macciola, V.; Iacovino, S. Delivery systems for hydroxytyrosol supplementation: State of the art. Colloids Interfaces 2020, 4, 25. [Google Scholar] [CrossRef]
- Mosca, M.; Diantom, A.; Lopez, F.; Ambrosone, L.; Ceglie, A. Impact of antioxidants dispersions on the stability and oxidation of water-in-olive-oil emulsions. Eur. Food Res. Technol. 2013, 236, 319–328. [Google Scholar] [CrossRef]
- Kohajdová, Z.; Karovičová, J. Application of hydrocolloids as baking improvers. Chem. Pap. 2009, 63, 26–38. [Google Scholar] [CrossRef]
- Pegg, A.M. 8-The application of natural hydrocolloids to foods and beverages. In Natural Food Additives, Ingredients and Flavourings; Baines, D., Seal, R., Eds.; Woodhead Publishing: Philadelphia, PA, USA, 2012; pp. 175–196. [Google Scholar]
- Ting, Y.; Jiang, Y.; Ho, C.-T.; Huang, Q. Common delivery systems for enhancing in vivo bioavailability and biological efficacy of nutraceuticals. J. Funct. Foods 2014, 7, 112–128. [Google Scholar] [CrossRef]
- Aditya, N.P.; Espinosa, Y.G.; Norton, I.T. Encapsulation systems for the delivery of hydrophilic nutraceuticals: Food application. Biotechnol. Adv. 2017, 35, 450–457. [Google Scholar] [CrossRef] [Green Version]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [Green Version]
- Obied, H.K.; Bedgood Jr, D.R.; Prenzler, P.D.; Robards, K. Chemical screening of olive biophenol extracts by hyphenated liquid chromatography. Anal. Chim. Acta 2007, 603, 176–189. [Google Scholar] [CrossRef]
- Briante, R.; Patumi, M.; Febbraio, F.; Nucci, R. Production of highly purified hydroxytyrosol from Olea europaea leaf extract biotransformed by hyperthermophilic β-glycosidase. J. Biotechnol. 2004, 111, 67–77. [Google Scholar] [CrossRef] [PubMed]
- De Leonardis, A.; Macciola, V.; Cuomo, F.; Lopez, F. Evidence of oleuropein degradation by olive leaf protein extract. Food Chem. 2015, 175, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Lombardi, S.J.; Macciola, V.; Testa, B.; Lustrato, G.; Lopez, F.; De Leonardis, A. Technological potential of lactobacillus strains isolated from fermented green olives: In vitro studies with emphasis on oleuropein-degrading capability. Sci. World J. 2016, 2016, 1917592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzuca, S.; Spadafora, A.; Innocenti, A.M. Cell and tissue localization of β-glucosidase during the ripening of olive fruit (Olea europaea) by in situ activity assay. Plant Sci. 2006, 171, 726–733. [Google Scholar] [CrossRef]
- Wang, W.; Li, C.; Hu, X. Developmental expression of β-glucosidase in olive leaves. Biol. Plant. 2009, 53, 138–140. [Google Scholar] [CrossRef]
- Esti, M.; Cinquanta, L.; La Notte, E. Phenolic compounds in different olive varieties. J. Agric. Food Chem. 1998, 46, 32–35. [Google Scholar] [CrossRef]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar]
- Kunchandy, E.; Rao, M.N.A. Oxygen radical scavenging activity of curcumin. Int. J. Pharm. 1990, 58, 237–240. [Google Scholar] [CrossRef]
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple biological activities of curcumin: A short review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.E.; Benya, R.V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens, F.L.; et al. Phase IIa Clinical Trial of Curcumin for the Prevention of Colorectal Neoplasia. Cancer Prev. Res. 2011, 4, 354–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II Trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef] [Green Version]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer 2011, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of curcumin: From mechanism to biological implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Gao, R.; Cao, Y.; Guo, M.; Wei, Z.; Zhou, E.; Li, Y.; Yao, M.; Yang, Z.; Zhang, N. Curcumin attenuates inflammatory responses by suppressing TLR4-mediated NF-κB signaling pathway in lipopolysaccharide-induced mastitis in mice. Int. Immunopharmacol. 2014, 20, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Jitoe-Masuda, A.; Fujimoto, A.; Masuda, T. Curcumin: From chemistry to chemistry-based functions. Curr. Pharm. Des. 2013, 19, 2084–2092. [Google Scholar]
- Heger, M.; van Golen, R.F.; Broekgaarden, M.; Michel, M.C. The Molecular Basis for the Pharmacokinetics and Pharmacodynamics of Curcumin and Its Metabolites in Relation to Cancer. Pharmacol. Rev. 2014, 66, 222–307. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.R.; Li, Y.; Jin, W.; An, Y.; He, L.; Li, Z.; Xu, W.; Li, B. Preparation and optimization of Pickering emulsion stabilized by chitosan-tripolyphosphate nanoparticles for curcumin encapsulation. Food Hydrocoll. 2016, 52, 369–377. [Google Scholar] [CrossRef]
- Chen, X.; Zou, L.-Q.; Niu, J.; Liu, W.; Peng, S.-F.; Liu, C.-M. The Stability, Sustained Release and Cellular Antioxidant Activity of Curcumin Nanoliposomes. Molecules 2015, 20, 14293–14311. [Google Scholar] [CrossRef] [Green Version]
- Sari, T.P.; Mann, B.; Kumar, R.; Singh, R.R.B.; Sharma, R.; Bhardwaj, M.; Athira, S. Preparation and characterization of nanoemulsion encapsulating curcumin. Food Hydrocoll. 2015, 43, 540–546. [Google Scholar] [CrossRef]
- Ahmed, K.; Li, Y.; McClements, D.J.; Xiao, H. Nanoemulsion- and emulsion-based delivery systems for curcumin: Encapsulation and release properties. Food Chem. 2012, 132, 799–807. [Google Scholar] [CrossRef]
- Patel, A.; Velikov, K.P. Colloidal delivery systems in foods: A general comparison with oral drug delivery. LWT-Food Sci. Technol. 2011, 44, 1958–1964. [Google Scholar] [CrossRef]
- Hu, K.; Huang, X.; Gao, Y.; Huang, X.; Xiao, H.; McClements, D.J. Core–shell biopolymer nanoparticle delivery systems: Synthesis and characterization of curcumin fortified zein–pectin nanoparticles. Food Chem. 2015, 182, 275–281. [Google Scholar] [CrossRef]
- Xiao, J.; Nian, S.; Huang, Q. Assembly of kafirin/carboxymethyl chitosan nanoparticles to enhance the cellular uptake of curcumin. Food Hydrocoll. 2015, 51, 166–175. [Google Scholar] [CrossRef]
- Sagalowicz, L.; Leser, M. Delivery Systems for Liquid Food Products. Curr. Opin. Colloid Interface Sci. 2010, 15, 61–72. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on Flavouring Group Evaluation 7, Revision 4 (FGE.07Rev4): Saturated and unsaturated aliphatic secondary alcohols, ketones and esters of secondary alcohols and saturated linear or branched-chain carboxylic acids from chemical group 5. EFSA J. 2012, 10, 2899. [Google Scholar]
- Matalanis, A.; Jones, O.G.; McClements, D.J. Structured biopolymer-based delivery systems for encapsulation, protection, and release of lipophilic compounds. Food Hydrocoll. 2011, 25, 1865–1880. [Google Scholar] [CrossRef] [Green Version]
- Cinelli, G.; Cofelice, M.; Venditti, F. Veiled Extra Virgin Olive Oils: Role of Emulsion, Water and Antioxidants. Colloids Interfaces 2020, 4, 38. [Google Scholar] [CrossRef]
- Kalaitzaki, A.; Emo, M.; Stébé, M.J.; Xenakis, A.; Papadimitriou, V. Biocompatible nanodispersions as delivery systems of food additives: A structural study. Food Res. Int. 2013, 54, 1448–1454. [Google Scholar] [CrossRef]
- Danielsson, I.; Lindman, B. The definition of microemulsion. Colloids Surf. 1981, 3, 391–392. [Google Scholar] [CrossRef]
- Mao, L.; Roos, Y.H.; Biliaderis, C.G.; Miao, S. Food emulsions as delivery systems for flavor compounds: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3173–3187. [Google Scholar] [CrossRef] [PubMed]
- Faridi Esfanjani, A.; Jafari, S.M.; Assadpour, E. Preparation of a multiple emulsion based on pectin-whey protein complex for encapsulation of saffron extract nanodroplets. Food Chem. 2017, 221, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-S.; Liu, T.-T.; Hu, T.-F. Effects of Lecithin and Pectin on Riboflavin-Photosensitized Oxidation of Orange Oil in a Multilayered Oil-in-Water Emulsion. J. Agric. Food Chem. 2011, 59, 9344–9350. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, O.; Silcock, P.; Beauchamp, J.; Buettner, A.; Everett, D.W. Volatile release and structural stability of β-lactoglobulin primary and multilayer emulsions under simulated oral conditions. Food Chem. 2013, 140, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Bou, R.; Cofrades, S.; Jiménez-Colmenero, F. Physicochemical properties and riboflavin encapsulation in double emulsions with different lipid sources. LWT-Food Sci. Technol. 2014, 59, 621–628. [Google Scholar] [CrossRef] [Green Version]
- Suganya, V.; Anuradha, V. Microencapsulation and nanoencapsulation: A review. Int. J. Pharm. Clin. Res. 2017, 9, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Colmenero, F. Potential applications of multiple emulsions in the development of healthy and functional foods. Food Res. Int. 2013, 52, 64–74. [Google Scholar] [CrossRef]
- Spyropoulos, F.; Duffus, L.J.; Smith, P.; Norton, I.T. Impact of Pickering Intervention on the Stability of W1/O/W2 Double Emulsions of Relevance to Foods. Langmuir 2019, 35, 15137–15150. [Google Scholar] [CrossRef]
- Aveyard, R.; Binks, B.P.; Clint, J.H. Emulsions stabilised solely by colloidal particles. Adv. Colloid Interface Sci. 2003, 100–102, 503–546. [Google Scholar] [CrossRef]
- Yusoff, A.; Murray, B.S. Modified starch granules as particle-stabilizers of oil-in-water emulsions. Food Hydrocoll. 2011, 25, 42–55. [Google Scholar] [CrossRef]
- Tzoumaki, M.V.; Moschakis, T.; Kiosseoglou, V.; Biliaderis, C.G. Oil-in-water emulsions stabilized by chitin nanocrystal particles. Food Hydrocoll. 2011, 25, 1521–1529. [Google Scholar] [CrossRef]
- Estrada-Fernández, A.G.; Román-Guerrero, A.; Jiménez-Alvarado, R.; Lobato-Calleros, C.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. Stabilization of oil-in-water-in-oil (O1/W/O2) Pickering double emulsions by soluble and insoluble whey protein concentrate-gum Arabic complexes used as inner and outer interfaces. J. Food Eng. 2018, 221, 35–44. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, W.; Cao, J.; Liu, W.; Zhu, S. Preparation of core–shell CaCO3 capsules via Pickering emulsion templates. J. Colloid Interface Sci. 2012, 372, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rodriguez, P.E.; Meshulam, D.; Lesmes, U.J.F.B. Characterization of Pickering O/W Emulsions Stabilized by Silica Nanoparticles and Their Responsiveness to In vitro Digestion Conditions. Food Biophys. 2014, 9, 406–415. [Google Scholar] [CrossRef]
- Eskandar, N.G.; Simovic, S.; Prestidge, C.A. Chemical stability and phase distribution of all-trans-retinol in nanoparticle-coated emulsions. Int. J. Pharm. 2009, 376, 186–194. [Google Scholar] [CrossRef]
- Schröder, A.; Laguerre, M.; Sprakel, J.; Schroën, K.; Berton-Carabin, C.C. Pickering particles as interfacial reservoirs of antioxidants. J. Colloid Interface Sci. 2020, 575, 489–498. [Google Scholar] [CrossRef]
- Serdaroglu, M.; Öztürk Kerimoğlu, B.; Urgu, M. Emulsion characteristics, chemical and textural properties of meat systems produced with double emulsions as beef fat replacers. Meat Sci. 2016, 117, 187–195. [Google Scholar] [CrossRef]
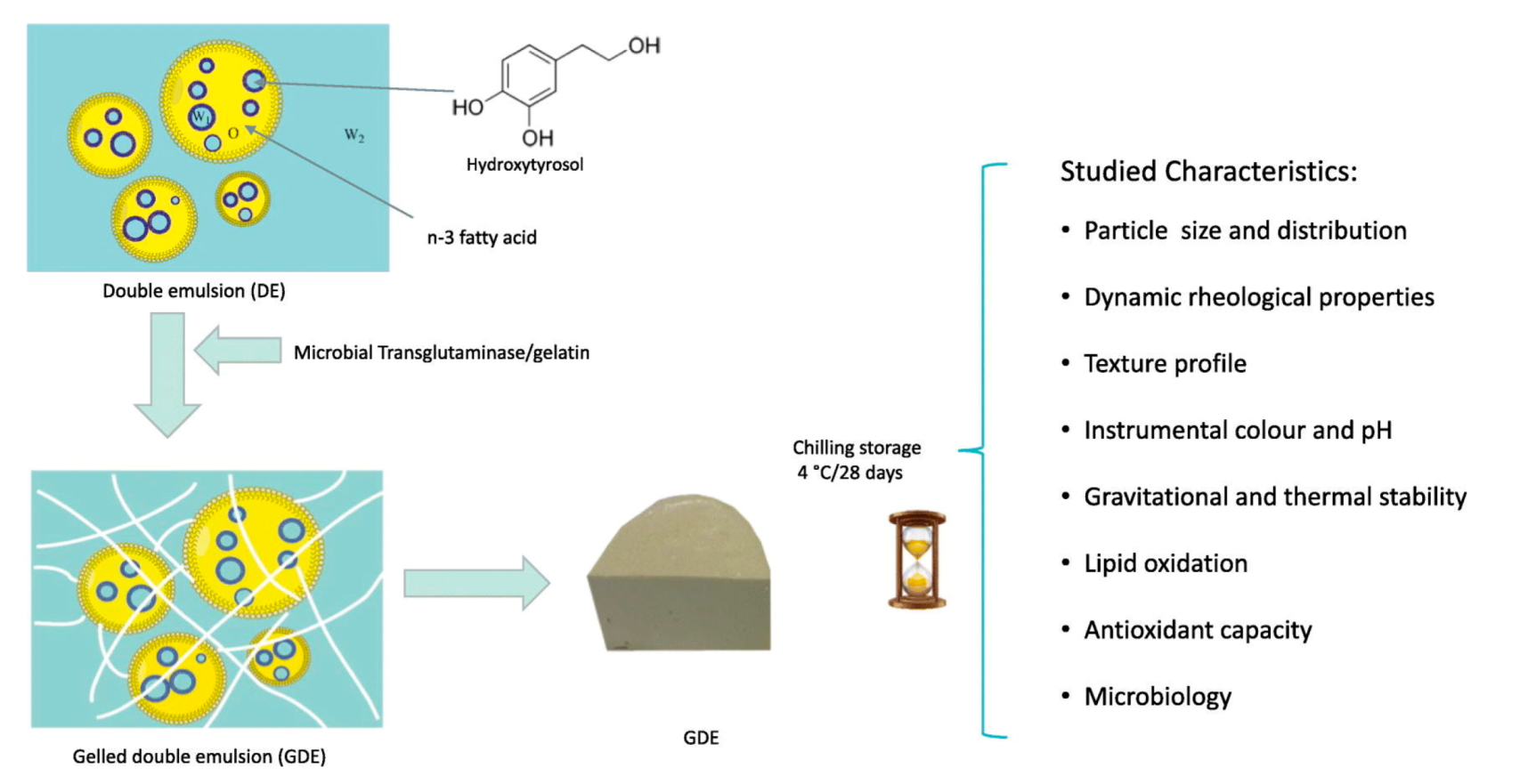
- Freire, M.; Cofrades, S.; Serrano-Casas, V.; Pintado, T.; Jiménez, M.J.; Jimenez-Colmenero, F. Gelled double emulsions as delivery systems for hydroxytyrosol and n-3 fatty acids in healthy pork patties. J. Food Sci. Technol. 2017, 54, 3959–3968. [Google Scholar] [CrossRef]
- Bonechi, C.; Donati, A.; Tamasi, G.; Pardini, A.; Rostom, H.; Leone, G.; Lamponi, S.; Consumi, M.; Magnani, A.; Rossi, C. Chemical characterization of liposomes containing nutraceutical compounds: Tyrosol, hydroxytyrosol and oleuropein. Biophys. Chem. 2019, 246, 25–34. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Cuomo, F.; Ceglie, S.; Miguel, M.; Lindman, B.; Lopez, F. Oral delivery of all-trans retinoic acid mediated by liposome carriers. Colloids Surf. B Biointerfaces 2021, 201, 111655. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, J.; Costa, R.; Rocha, F.; Estevinho, B.N.; Santos, L. Design of microparticles containing natural antioxidants: Preparation, characterization and controlled release studies. Powder Technol. 2017, 313, 287–292. [Google Scholar] [CrossRef]
- Duan, Y.; Sun, X.; Gong, T.; Wang, Q.; Zhang, Z. Preparation of DHAQ-loaded mPEG-PLGA-mPEG nanoparticles and evaluation of drug release behaviors in vitro/in vivo. J. Mater. Sci. Mater. Med. 2006, 17, 509–516. [Google Scholar] [CrossRef]
- Couvreur, P.; Dubernet, C.; Puisieux, F. Controlled drug delivery with nanoparticles: Current possibilities and future trends. Eur. J. Pharm. Biopharm. 1995, 41, 2–13. [Google Scholar]
- Paulo, F.; Santos, L. Design of experiments for microencapsulation applications: A review. Mater. Sci. Eng. C 2017, 77, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Baena-Aristizábal, C.M.; Fessi, H.; Elaissari, A.; Mora-Huertas, C.E. Biodegradable microparticles preparation by double emulsification—Solvent extraction method: A Systematic study. Colloids Surf. A Physicochem. Eng. Asp. 2016, 492, 213–229. [Google Scholar] [CrossRef]
- Barichello, J.M.; Morishita, M.; Takayama, K.; Nagai, T. Encapsulation of Hydrophilic and Lipophilic Drugs in PLGA Nanoparticles by the Nanoprecipitation Method. Drug Dev. Ind. Pharm. 1999, 25, 471–476. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Zou, D.; Hui, Y.; Nigam, K.; Middelberg, A.P.J.; Zhao, C.-X. Formulation of Nanoparticles Using Mixing-Induced Nanoprecipitation for Drug Delivery. Ind. Eng. Chem. Res. 2020, 59, 4134–4149. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Quideau, S.; Treutter, D. Plant phenolics—Secondary metabolites with diverse functions. Recent Adv. Polyphen. Res. 2008, 1, 1–35. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Rodríguez-Morató, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Perez-Mana, C.; Khymenets, O.; Fito, M.; Farre, M. Metabolic disposition and biological significance of simple phenols of dietary origin: Hydroxytyrosol and tyrosol. Drug Metab. Rev. 2016, 48, 218–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, C.-T. Phenolic Compounds in Food. In Phenolic Compounds in Food and Their Effects on Health II; American Chemical Society: Washington, DC, USA, 1992; Volume 507, pp. 2–7. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayres, D.C.; Ayres, D.C.; Loike, J.D. Lignans: Chemical, Biological and Clinical Properties; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Materska, M. Evaluation of the lipophilicity and stability of phenolic compounds in herbal extracts. Acta Sci. Pol. Technol. Aliment. 2010, 9, 61–69. [Google Scholar]
- Stone, W.L.; Papas, A. Tocopherols, tocotrienols and vitamin E. In Lipids for Functional Foods and Nutraceuticals; Gunstone, F.D., Ed.; Woodhead Publishing: Bridgwater, UK, 2003; pp. 53–72. [Google Scholar]
- Silva, A.F.; Resende, D.; Monteiro, M.; Coimbra, M.A.; Silva, A.; Cardoso, S.M. Application of Hydroxytyrosol in the Functional Foods Field: From Ingredient to Dietary Supplements. Antioxidants 2020, 9, 1246. [Google Scholar] [CrossRef]
- Chatzidaki, M.D.; Arik, N.; Monteil, J.; Papadimitriou, V.; Leal-Calderon, F.; Xenakis, A. Microemulsion versus emulsion as effective carrier of hydroxytyrosol. Colloids Surf. B Biointerfaces 2016, 137, 146–151. [Google Scholar] [CrossRef]
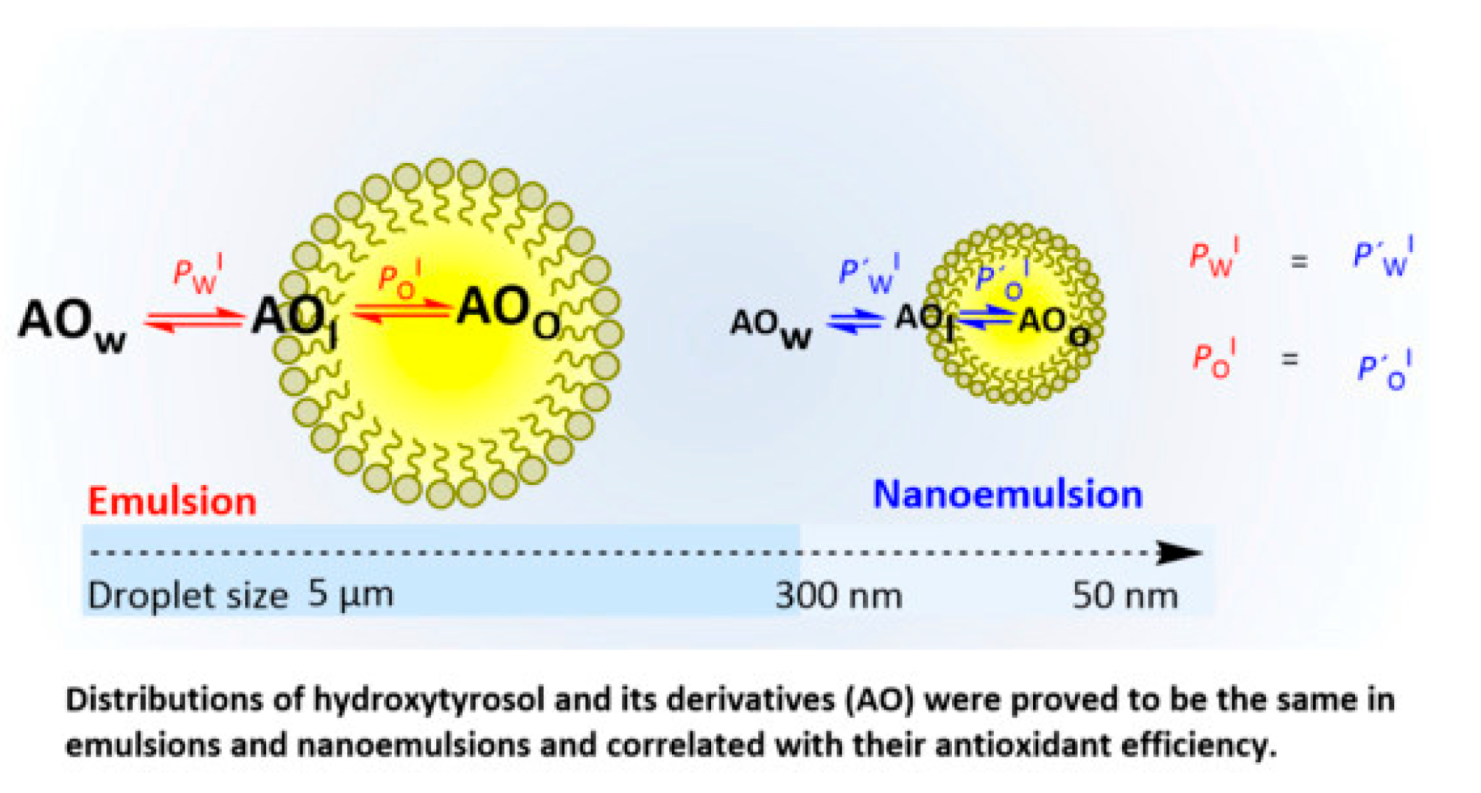
- Costa, M.; Losada-Barreiro, S.; Bravo-Díaz, C.; S Monteiro, L.; Paiva-Martins, F. Interfacial Concentrations of Hydroxytyrosol Derivatives in Fish Oil-in-Water Emulsions and Nanoemulsions and Its Influence on Their Lipid Oxidation: Droplet Size Effects. Foods 2020, 9, 1897. [Google Scholar] [CrossRef]
- Flaiz, L.; Freire, M.; Cofrades, S.; Mateos, R.; Weiss, J.; Jiménez-Colmenero, F.; Bou, R. Comparison of simple, double and gelled double emulsions as hydroxytyrosol and n-3 fatty acid delivery systems. Food Chem. 2016, 213, 49–57. [Google Scholar] [CrossRef]
- Cofrades, S.; Jiménez-Colmenero, F.; Sánchez-Muniz, F.J.; Benedí, J.; Santos-López, J.A.; Freire, M. Oxidative stability of meat systems made with W1/O/W2 emulsions prepared with hydroxytyrosol and chia oil as lipid phase. Lebenson Wiss Technol. 2014, 59, 941–947. [Google Scholar] [CrossRef]
- Almeida, J.; Losada-Barreiro, S.; Costa, M.; Paiva-Martins, F.; Bravo-Díaz, C.; Romsted, L.S. Interfacial Concentrations of Hydroxytyrosol and Its Lipophilic Esters in Intact Olive Oil-in-Water Emulsions: Effects of Antioxidant Hydrophobicity, Surfactant Concentration, and the Oil-to-Water Ratio on the Oxidative Stability of the Emulsions. J. Agric. Food Chem. 2016, 64, 5274–5283. [Google Scholar] [CrossRef]
- Cofrades, S.; Bou, R.; Flaiz, L.; Garcimartín, A.; Benedí, J.; Mateos, R.; Sánchez-Muniz, F.J.; Olivero-David, R.; Jiménez-Colmenero, F. Bioaccessibility of hydroxytyrosol and n-3 fatty acids as affected by the delivery system: Simple, double and gelled double emulsions. J. Food Sci. Technol. 2017, 54, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.; Bou, R.; Cofrades, S.; Jiménez-Colmenero, F. Technological characteristics of cold-set gelled double emulsion enriched with n-3 fatty acids: Effect of hydroxytyrosol addition and chilling storage. Food Res. Int. 2017, 100, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-González, I.; Ruiz-Capillas, C.; Salvador, M.; Herrero, A.M. Emulsion gels as delivery systems for phenolic compounds: Nutritional, technological and structural properties. Food Chem. 2021, 339, 128049. [Google Scholar] [CrossRef] [PubMed]
- Balducci, V.; Incerpi, S.; Stano, P.; Tofani, D. Antioxidant activity of hydroxytyrosyl esters studied in liposome models. Biochim. Biophys. Acta (BBA)-Biomembr. 2018, 1860, 600–610. [Google Scholar] [CrossRef]
- Evans, K.O.; Compton, D.L. Phosphatidyl-hydroxytyrosol and phosphatidyl-tyrosol bilayer properties. Chem. Phys. Lipids 2017, 202, 69–76. [Google Scholar] [CrossRef]
- Evans, K.O.; Compton, D.L.; Appell, M. Determination of pH Effects on Phosphatidyl-Hydroxytyrosol and Phosphatidyl-Tyrosol Bilayer Behavior. Methods Protoc 2018, 1, 41. [Google Scholar] [CrossRef] [Green Version]
- Evans, K.O.; Laszlo, J.A.; Compton, D.L. Hydroxytyrosol and tyrosol esters partitioning into, location within, and effect on DOPC liposome bilayer behavior. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 1175–1182. [Google Scholar] [CrossRef] [Green Version]
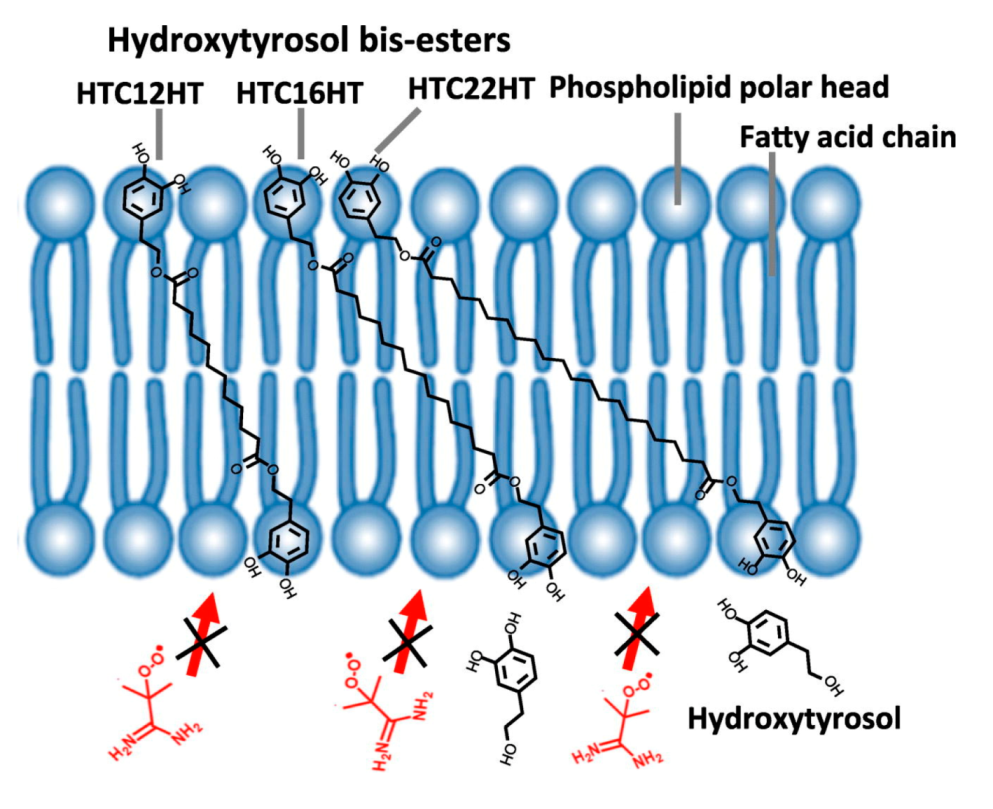
- Lopes, R.; Costa, M.; Ferreira, M.; Gameiro, P.; Paiva-Martins, F. A new family of hydroxytyrosol phenolipids for the antioxidant protection of liposomal systems. Biochim. Biophys. Acta (BBA)-Biomembr. 2021, 1863, 183505. [Google Scholar] [CrossRef]
- Yuan, J.-J.; Qin, F.G.; Tu, J.-L.; Li, B. Preparation, characterization, and antioxidant activity evaluation of liposomes containing water-soluble hydroxytyrosol from olive. Molecules 2017, 22, 870. [Google Scholar] [CrossRef] [Green Version]
- Bazzarelli, F.; Piacentini, E.; Giorno, L. Biophenols-loaded solid lipid particles (SLPs) development by membrane emulsification. J. Membr. Sci. 2017, 541, 587–594. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Lama-Muñoz, A.; Fernández-Bolaños, J. Complexation of hydroxytyrosol and 3,4-dihydroxyphenylglycol with pectin and their potential use for colon targeting. Carbohydr. Polym. 2017, 163, 292–300. [Google Scholar] [CrossRef]
- Guan, Q.; Sun, S.; Li, X.; Lv, S.; Xu, T.; Sun, J.; Feng, W.; Zhang, L.; Li, Y. Preparation, in vitro and in vivo evaluation of mPEG-PLGA nanoparticles co-loaded with syringopicroside and hydroxytyrosol. J. Mater. Sci. Mater. Med. 2016, 27, 24. [Google Scholar] [CrossRef] [PubMed]
- Malapert, A.; Reboul, E.; Tourbin, M.; Dangles, O.; Thiéry, A.; Ziarelli, F.; Tomao, V. Characterization of hydroxytyrosol-β-cyclodextrin complexes in solution and in the solid state, a potential bioactive ingredient. LWT-Food Sci. Technol. 2019, 102, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Paulo, F.; Santos, L. Inclusion of hydroxytyrosol in ethyl cellulose microparticles: In vitro release studies under digestion conditions. Food Hydrocoll. 2018, 84, 104–116. [Google Scholar] [CrossRef]
- Pham, L.B.; Wang, B.; Zisu, B.; Adhikari, B. Complexation between flaxseed protein isolate and phenolic compounds: Effects on interfacial, emulsifying and antioxidant properties of emulsions. Food Hydrocoll. 2019, 94, 20–29. [Google Scholar] [CrossRef]
- Chainani-Wu, N. Safety and anti-inflammatory activity of curcumin: A component of tumeric (Curcuma longa). J. Altern. Complementary Med. 2003, 9, 161–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, B.; Peng, S.; Zhang, X.; McClements, D.J. Impact of delivery system type on curcumin bioaccessibility: Comparison of curcumin-loaded nanoemulsions with commercial curcumin supplements. J. Agric. Food Chem. 2018, 66, 10816–10826. [Google Scholar] [CrossRef]
- Cheng, C.; Wu, Z.; Wang, Y.; Chen, J.; Zhong, Y.; Liang, R.; Peng, S.; McClements, D.J.; Liu, W. Tunable high internal phase emulsions (HIPEs) formulated using lactoferrin-gum Arabic complexes. Food Hydrocoll. 2020, 113, 106445. [Google Scholar] [CrossRef]
- Feng, T.; Hu, Z.; Wang, K.; Zhu, X.; Chen, D.; Zhuang, H.; Yao, L.; Song, S.; Wang, H.; Sun, M. Emulsion-based delivery systems for curcumin: Encapsulation and interaction mechanism between debranched starch and curcumin. Int. J. Biol. Macromol. 2020, 161, 746–754. [Google Scholar] [CrossRef]
- Hamad, A.; Suriyarak, S.; Devahastin, S.; Borompichaichartkul, C. A novel approach to develop spray-dried encapsulated curcumin powder from oil-in-water emulsions stabilized by combined surfactants and chitosan. J. Food Sci. 2020, 85, 3874–3884. [Google Scholar] [CrossRef]
- Kharat, M.; Aberg, J.; Dai, T.; McClements, D.J. Comparison of Emulsion and Nanoemulsion Delivery Systems: The Chemical Stability of Curcumin Decreases as Oil Droplet Size Decreases. J. Agric. Food Chem. 2020, 68, 9205–9212. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yu, Y.; Peng, J.; Dai, Z.; Wu, J.; Wang, Z. Effects of the degree of substitution of OSA on the properties of starch microparticle-stabilized emulsions. Carbohydr. Polym. 2021, 255, 117546. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Zhang, Z.; Chen, F.; Luo, X.; McClements, D.J. Impact of delivery system type on curcumin stability: Comparison of curcumin degradation in aqueous solutions, emulsions, and hydrogel beads. Food Hydrocoll. 2017, 71, 187–197. [Google Scholar] [CrossRef]
- Zou, L.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Utilizing Food Matrix Effects to Enhance Nutraceutical Bioavailability: Increase of Curcumin Bioaccessibility Using Excipient Emulsions. J. Agric. Food Chem. 2015, 63, 2052–2062. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Designing excipient emulsions to increase nutraceutical bioavailability: Emulsifier type influences curcumin stability and bioaccessibility by altering gastrointestinal fate. Food Funct. 2015, 6, 2475–2486. [Google Scholar] [CrossRef]
- Zou, L.; Zheng, B.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Enhancing nutraceutical bioavailability using excipient emulsions: Influence of lipid droplet size on solubility and bioaccessibility of powdered curcumin. J. Funct. Foods 2015, 15, 72–83. [Google Scholar] [CrossRef]
- Artiga-Artigas, M.; Lanjari-Pérez, Y.; Martín-Belloso, O. Curcumin-loaded nanoemulsions stability as affected by the nature and concentration of surfactant. Food Chem. 2018, 266, 466–474. [Google Scholar] [CrossRef]
- Cuomo, F.; Perugini, L.; Marconi, E.; Messia, M.C.; Lopez, F. Enhanced Curcumin Bioavailability through Nonionic Surfactant/Caseinate Mixed Nanoemulsions. J. Food Sci. 2019, 84, 2584–2591. [Google Scholar] [CrossRef]
- Esperón-Rojas, A.A.; Baeza-Jiménez, R.; Santos-Luna, D.; Velasco-Rodríguez, L.D.C.; Ochoa-Rodríguez, L.R.; García, H.S. Bioavailability of curcumin in nanoemulsions stabilized with mono- and diacylglycerols structured with conjugated linoleic acid and n-3 fatty acids. Biocatal. Agric. Biotechnol. 2020, 26, 101638. [Google Scholar] [CrossRef]
- Kharat, M.; Skrzynski, M.; Decker, E.A.; McClements, D.J. Enhancement of chemical stability of curcumin-enriched oil-in-water emulsions: Impact of antioxidant type and concentration. Food Chem. 2020, 320, 126653. [Google Scholar] [CrossRef]
- Espinosa-Andrews, H.; Páez-Hernández, G. Optimization of ultrasonication curcumin-hydroxylated lecithin nanoemulsions using response surface methodology. J. Food Sci. Technol. 2020, 57, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Asabuwa Ngwabebhoh, F.; Ilkar Erdagi, S.; Yildiz, U. Pickering emulsions stabilized nanocellulosic-based nanoparticles for coumarin and curcumin nanoencapsulations: In vitro release, anticancer and antimicrobial activities. Carbohydr. Polym. 2018, 201, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hwang, I.-C.; Chen, X.; Park, H.J. Effects of chitosan coating on curcumin loaded nano-emulsion: Study on stability and in vitro digestibility. Food Hydrocoll. 2016, 60, 138–147. [Google Scholar] [CrossRef]
- Richa, R.; Roy Choudhury, A. Exploration of polysaccharide based nanoemulsions for stabilization and entrapment of curcumin. Int. J. Biol. Macromol. 2020, 156, 1287–1296. [Google Scholar] [CrossRef]
- Han, J.; Chen, F.; Gao, C.; Zhang, Y.; Tang, X. Environmental stability and curcumin release properties of Pickering emulsion stabilized by chitosan/gum arabic nanoparticles. Int. J. Biol. Macromol. 2020, 157, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Huang, Q. Stability and in vitro digestion study of curcumin-encapsulated in different milled cellulose particle stabilized Pickering emulsions. Food Funct. 2020, 11, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Wang, D.; Dai, L.; Wu, X.; Gao, Y.; Yuan, F. Pickering emulsion gels stabilized by high hydrostatic pressure-induced whey protein isolate gel particles: Characterization and encapsulation of curcumin. Food Res. Int. 2020, 132, 109032. [Google Scholar] [CrossRef]
- Marefati, A.; Bertrand, M.; Sjöö, M.; Dejmek, P.; Rayner, M. Storage and digestion stability of encapsulated curcumin in emulsions based on starch granule Pickering stabilization. Food Hydrocoll. 2017, 63, 309–320. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, Q. Development of high internal phase Pickering emulsions stabilised by ovotransferrin–gum arabic particles as curcumin delivery vehicles. Int. J. Food Sci. Technol. 2020, 55, 1891–1899. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, J.; Hu, Y.; Zhang, N.; Fu, Y.; Chen, X. Tuning complexation of carboxymethyl cellulose/ cationic chitosan to stabilize Pickering emulsion for curcumin encapsulation. Food Hydrocoll. 2021, 110, 106135. [Google Scholar] [CrossRef]
- Leiva-Vega, J.; Villalobos-Carvajal, R.; Ferrari, G.; Donsì, F.; Zúñiga, R.N.; Shene, C.; Beldarraín-Iznaga, T. Influence of interfacial structure on physical stability and antioxidant activity of curcumin multilayer emulsions. Food Bioprod. Processing 2020, 121, 65–75. [Google Scholar] [CrossRef]
- Sabet, S.; Rashidinejad, A.; Qazi, H.J.; McGillivray, D.J. An efficient small intestine-targeted curcumin delivery system based on the positive-negative-negative colloidal interactions. Food Hydrocoll. 2021, 111, 106375. [Google Scholar] [CrossRef]
- Silva, H.D.; Beldíková, E.; Poejo, J.; Abrunhosa, L.; Serra, A.T.; Duarte, C.M.M.; Brányik, T.; Cerqueira, M.A.; Pinheiro, A.C.; Vicente, A.A. Evaluating the effect of chitosan layer on bioaccessibility and cellular uptake of curcumin nanoemulsions. J. Food Eng. 2019, 243, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Silva, H.D.; Cerqueira, M.A.; Donsì, F.; Pinheiro, A.C.; Ferrari, G.; Vicente, A.A. Development and Characterization of Lipid-Based Nanosystems: Effect of Interfacial Composition on Nanoemulsion Behavior. Food Bioprocess Technol. 2020, 13, 67–87. [Google Scholar] [CrossRef] [Green Version]
- Silva, H.D.; Poejo, J.; Pinheiro, A.C.; Donsì, F.; Serra, A.T.; Duarte, C.M.M.; Ferrari, G.; Cerqueira, M.A.; Vicente, A.A. Evaluating the behaviour of curcumin nanoemulsions and multilayer nanoemulsions during dynamic in vitro digestion. J. Funct. Foods 2018, 48, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.-H.; Chiang, B.-H. Modification of curcumin-loaded liposome with edible compounds to enhance ability of crossing blood brain barrier. Colloids Surf. A Physicochem. Eng. Asp. 2020, 599, 124862. [Google Scholar] [CrossRef]
- Cuomo, F.; Cofelice, M.; Venditti, F.; Ceglie, A.; Miguel, M.; Lindman, B.; Lopez, F. In-vitro digestion of curcumin loaded chitosan-coated liposomes. Colloids Surf. B Biointerfaces 2018, 168, 29–34. [Google Scholar] [CrossRef]
- De Leo, V.; Di Gioia, S.; Milano, F.; Fini, P.; Comparelli, R.; Mancini, E.; Agostiano, A.; Conese, M.; Catucci, L. Eudragit S100 entrapped liposome for curcumin delivery: Anti-oxidative effect in Caco-2 cells. Coatings 2020, 10, 114. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Deng, L.; Cai, Z.; Zhang, S.; Wang, K.; Li, L.; Ding, S.; Zhou, C. Liposomes coated with thiolated chitosan as drug carriers of curcumin. Mater. Sci. Eng. C 2017, 80, 156–164. [Google Scholar] [CrossRef]
- Tai, K.; Rappolt, M.; Mao, L.; Gao, Y.; Li, X.; Yuan, F. The stabilization and release performances of curcumin-loaded liposomes coated by high and low molecular weight chitosan. Food Hydrocoll. 2020, 99, 105355. [Google Scholar] [CrossRef]
- Wei, X.-Q.; Ba, K. Construction a Long-Circulating Delivery System of Liposomal Curcumin by Coating Albumin. ACS Omega 2020, 5, 16502–16509. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Mou, B.; Song, S.; Tan, C.-P.; Lai, O.-M.; Shen, C.; Cheong, L.-Z. Curcumin-loaded liposomes prepared from bovine milk and krill phospholipids: Effects of chemical composition on storage stability, in-vitro digestibility and anti-hyperglycemic properties. Food Res. Int. 2020, 136, 109301. [Google Scholar] [CrossRef] [PubMed]
- Ban, C.; Jo, M.; Park, Y.H.; Kim, J.H.; Han, J.Y.; Lee, K.W.; Kweon, D.-H.; Choi, Y.J. Enhancing the oral bioavailability of curcumin using solid lipid nanoparticles. Food Chem. 2020, 302, 125328. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-P.; Li, B.-S.; Tang, C.-H. Nanocomplexation of soy protein isolate with curcumin: Influence of ultrasonic treatment. Food Res. Int. 2015, 2015, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; He, J.; Cao, L.; Lin, H.; Zhang, W.; Zhong, Q. Improved Physicochemical Properties of Curcumin-Loaded Solid Lipid Nanoparticles Stabilized by Sodium Caseinate-Lactose Maillard Conjugate. J. Agric. Food Chem. 2020, 68, 7072–7081. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, G.; Baby, T.; Chen, D.; Weitz, D.A.; Zhao, C.X. Stable polymer nanoparticles with exceptionally high drug loading by sequential nanoprecipitation. Angew. Chem. Int. Ed. 2020, 59, 4720–4728. [Google Scholar] [CrossRef]
- Park, H.R.; Rho, S.-J.; Kim, Y.-R. Solubility, stability, and bioaccessibility improvement of curcumin encapsulated using 4-α-glucanotransferase-modified rice starch with reversible pH-induced aggregation property. Food Hydrocoll. 2019, 95, 19–32. [Google Scholar] [CrossRef]
- Peng, S.; Zhou, L.; Cai, Q.; Zou, L.; Liu, C.; Liu, W.; McClements, D.J. Utilization of biopolymers to stabilize curcumin nanoparticles prepared by the pH-shift method: Caseinate, whey protein, soy protein and gum Arabic. Food Hydrocoll. 2020, 107, 105963. [Google Scholar] [CrossRef]
- Sun, C.; Xu, C.; Mao, L.; Wang, D.; Yang, J.; Gao, Y. Preparation, characterization and stability of curcumin-loaded zein-shellac composite colloidal particles. Food Chem. 2017, 228, 656–667. [Google Scholar] [CrossRef]
- Wei, Y.; Cai, Z.; Wu, M.; Guo, Y.; Wang, P.; Li, R.; Ma, A.; Zhang, H. Core-shell pea protein-carboxymethylated corn fiber gum composite nanoparticles as delivery vehicles for curcumin. Carbohydr. Polym. 2020, 240, 116273. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, H.; Zhu, J.; Liu, C.; Sun, X.; Wang, D.; Xu, Y. Fabrication and characterization of zein nanoparticles by dextran sulfate coating as vehicles for delivery of curcumin. Int. J. Biol. Macromol. 2020, 151, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Dai, L.; Zhang, L.; Gao, Y. Entrapment of curcumin in whey protein isolate and zein composite nanoparticles using pH-driven method. Food Hydrocoll. 2020, 106, 105839. [Google Scholar] [CrossRef]

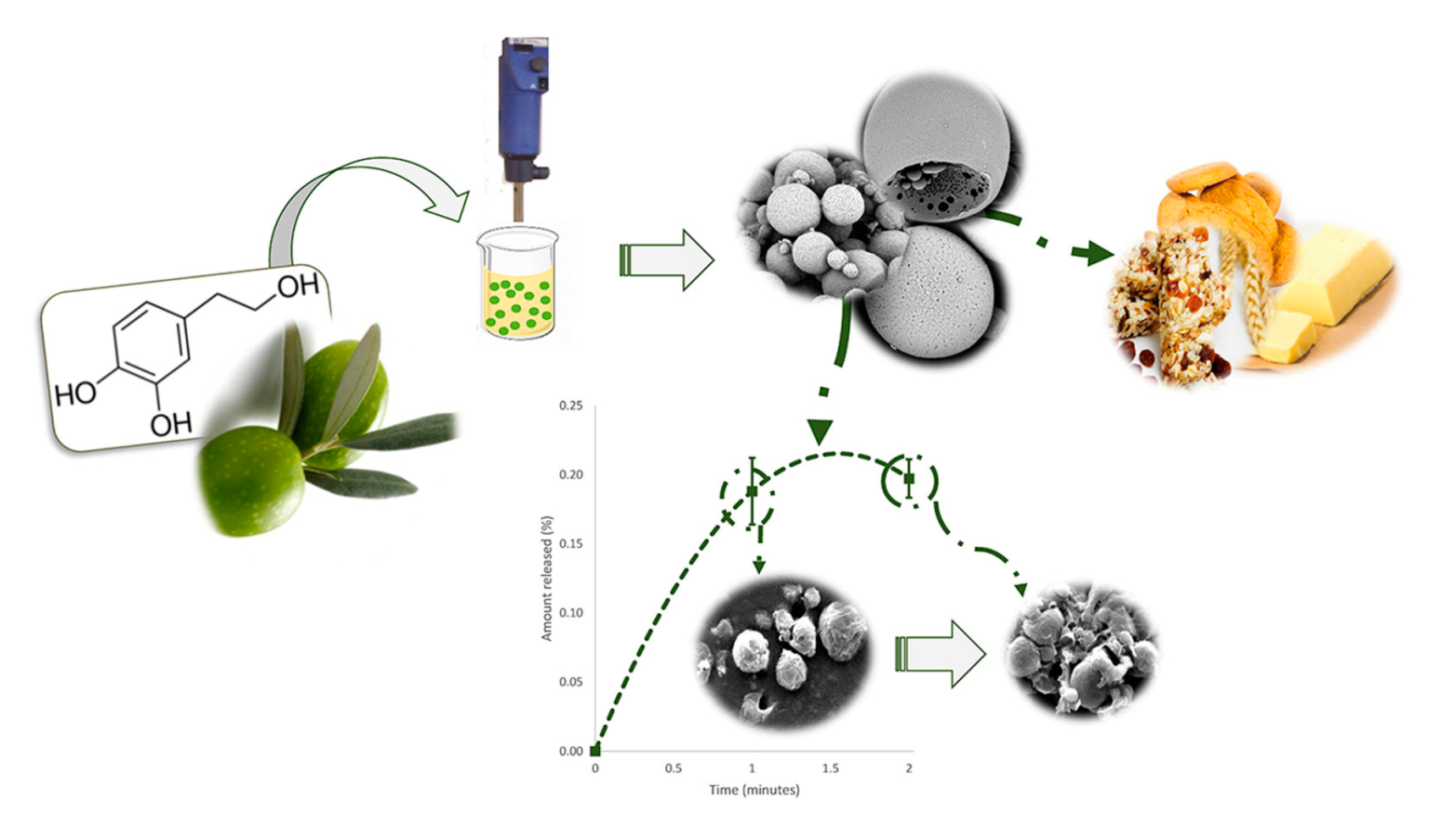

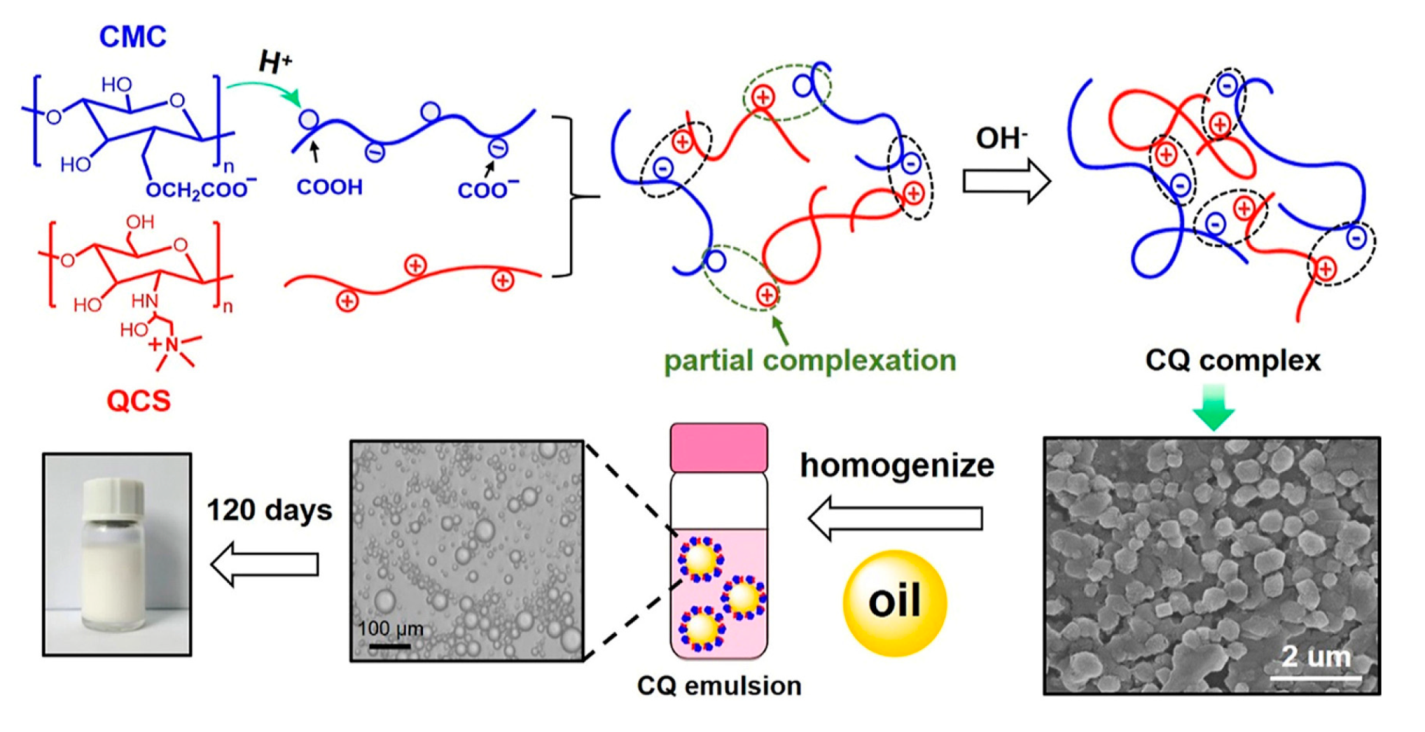
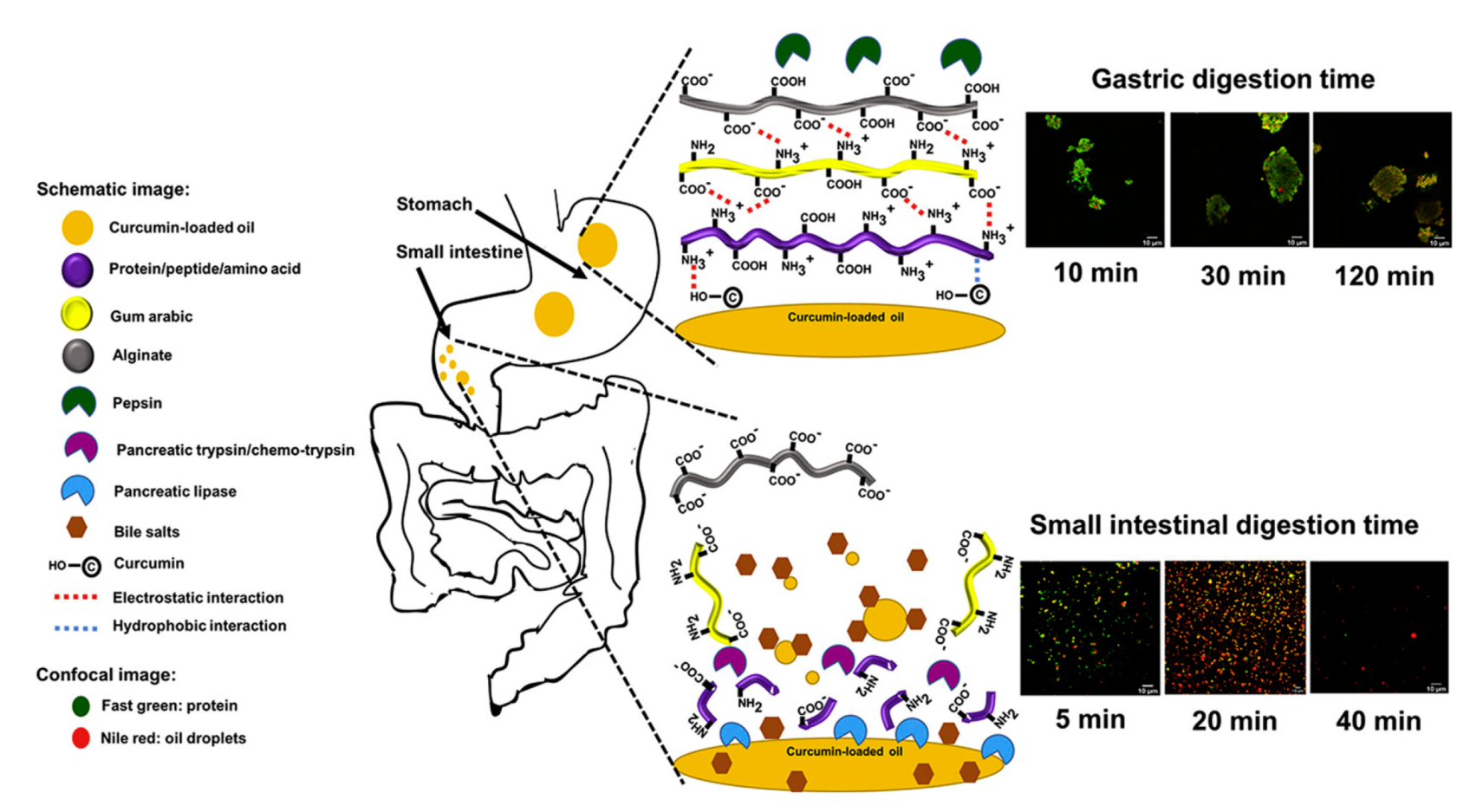
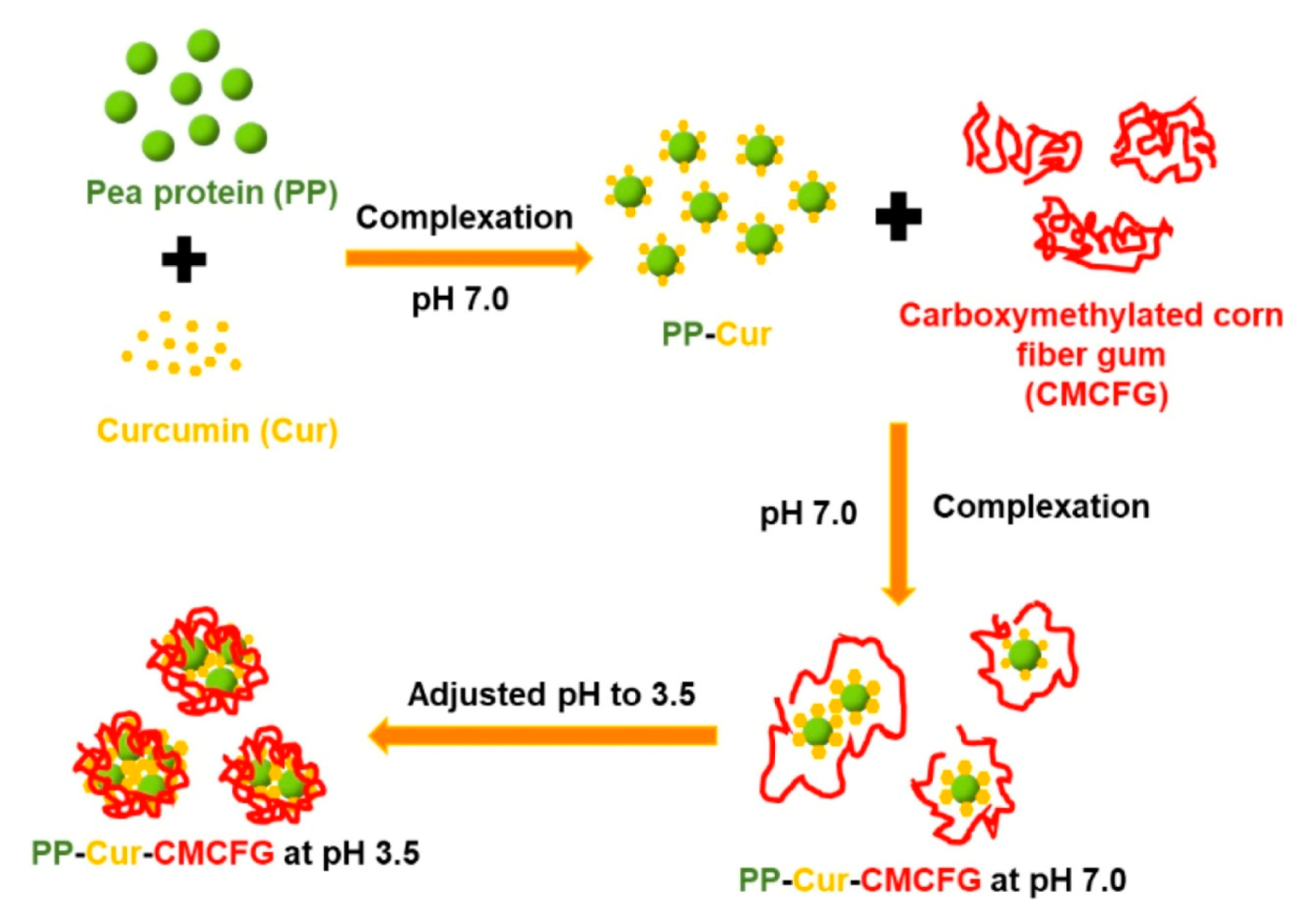
| Delivery System | Applications | References |
|---|---|---|
| Macroemulsions | Antioxidant activity, releasing efficacy, shelf life, solubility and gastrointestinal stability. | [91,92,93,94,95] |
| Multiple emulsions | Antioxidant activity, loading losses | [93,94] |
| Gelled emulsions | Antioxidant activity, HYT losses and HYT release | [71,96,97,98] |
| Liposomes | Stability, antioxidant activity | [72,99,100,101,102,103,104] |
| Solid particles | Loading capacity, in vitro antioxidant activity | [105,106,107,108,109,110] |
| Delivery System | Applications | References |
|---|---|---|
| Macroemulsions | Encapsulation efficiency, stability, and bioaccessibility | [113,114,115,116,117,118,119,120,121] |
| Nanoemulsions | Dispersibility, digestion, release kinetic, stability, antioxidant capacity, encapsulation efficiency | [43,122,123,124,125,126,127,128,129] |
| Pickering emulsions | Stability, release kinetic, encapsulation efficiency, temperature response, digestibility, bioaccessibility, cytotoxicity, anticancer/antifungal/antimicrobial activities | [41,127,130,131,132,133,134,135] |
| Multilayer emulsions | Stability, bioaccessibility, antioxidant activity, digestibility, permeability, and bioeffectives | [136,137,138,139,140] |
| Liposomes | Solubility, stability, anticancer activity, adsorption, release kinetic | [42,141,142,143,144,145,146,147] |
| Solid particles | Encapsulation efficiency, bioaccessibility, stability, antioxidant activity, biocompatibility, and in-vitro gastrointestinal release kinetic | [45,148,149,150,151,152,153,154,155,156,157] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuomo, F.; Iacovino, S.; Sacco, P.; De Leonardis, A.; Ceglie, A.; Lopez, F. Progress in Colloid Delivery Systems for Protection and Delivery of Phenolic Bioactive Compounds: Two Study Cases—Hydroxytyrosol and Curcumin. Molecules 2022, 27, 921. https://doi.org/10.3390/molecules27030921
Cuomo F, Iacovino S, Sacco P, De Leonardis A, Ceglie A, Lopez F. Progress in Colloid Delivery Systems for Protection and Delivery of Phenolic Bioactive Compounds: Two Study Cases—Hydroxytyrosol and Curcumin. Molecules. 2022; 27(3):921. https://doi.org/10.3390/molecules27030921
Chicago/Turabian StyleCuomo, Francesca, Silvio Iacovino, Pasquale Sacco, Antonella De Leonardis, Andrea Ceglie, and Francesco Lopez. 2022. "Progress in Colloid Delivery Systems for Protection and Delivery of Phenolic Bioactive Compounds: Two Study Cases—Hydroxytyrosol and Curcumin" Molecules 27, no. 3: 921. https://doi.org/10.3390/molecules27030921
APA StyleCuomo, F., Iacovino, S., Sacco, P., De Leonardis, A., Ceglie, A., & Lopez, F. (2022). Progress in Colloid Delivery Systems for Protection and Delivery of Phenolic Bioactive Compounds: Two Study Cases—Hydroxytyrosol and Curcumin. Molecules, 27(3), 921. https://doi.org/10.3390/molecules27030921









