Preliminary Study of Resistance Mechanism of Botrytis cinerea to SYAUP-CN-26
Abstract
:1. Introduction
2. Results
2.1. Generation of B. cinerea Mutant Resistance to SYAUP-CN-26
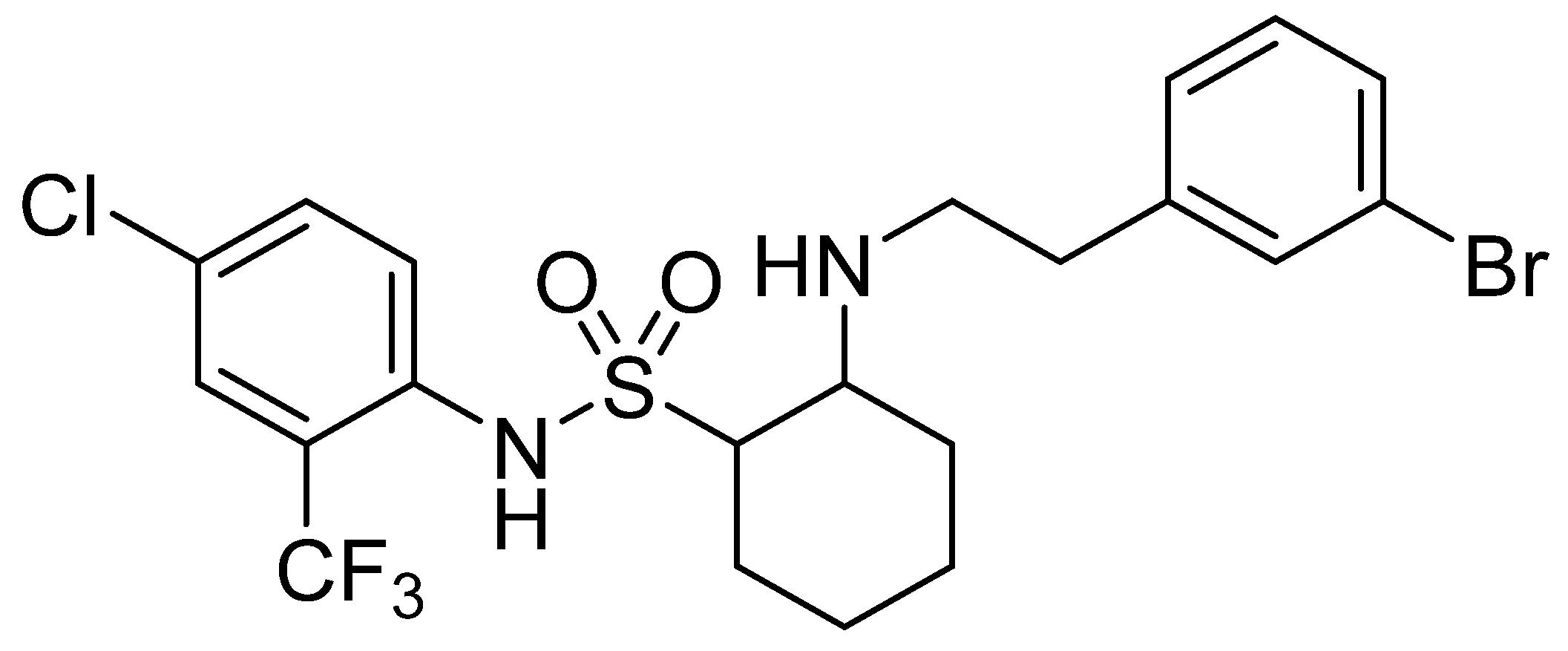
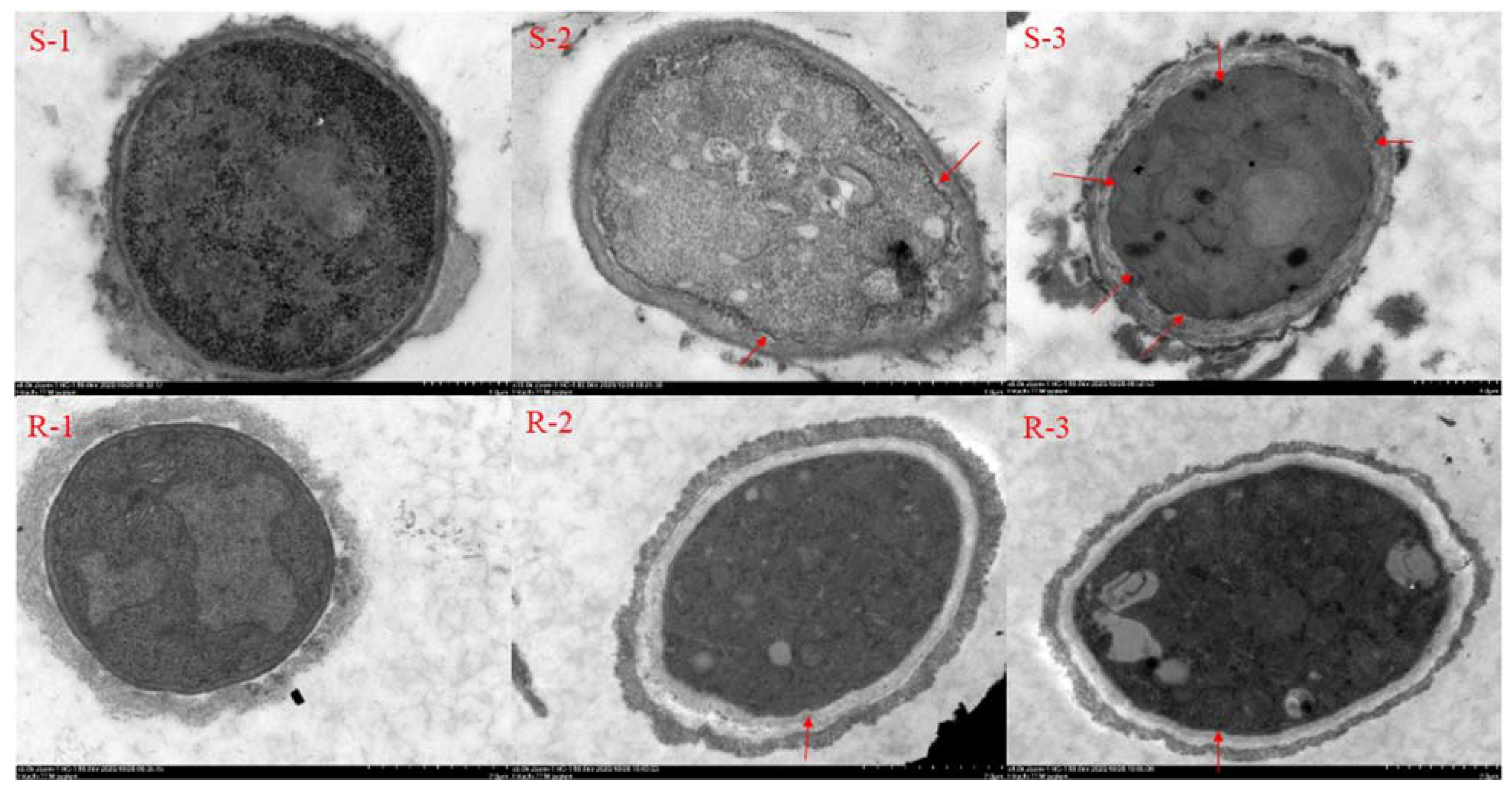
2.2. Effect of SYAUP-CN-26 on Cell Membrane
2.3. Measurement of Relative Electric Conductivity
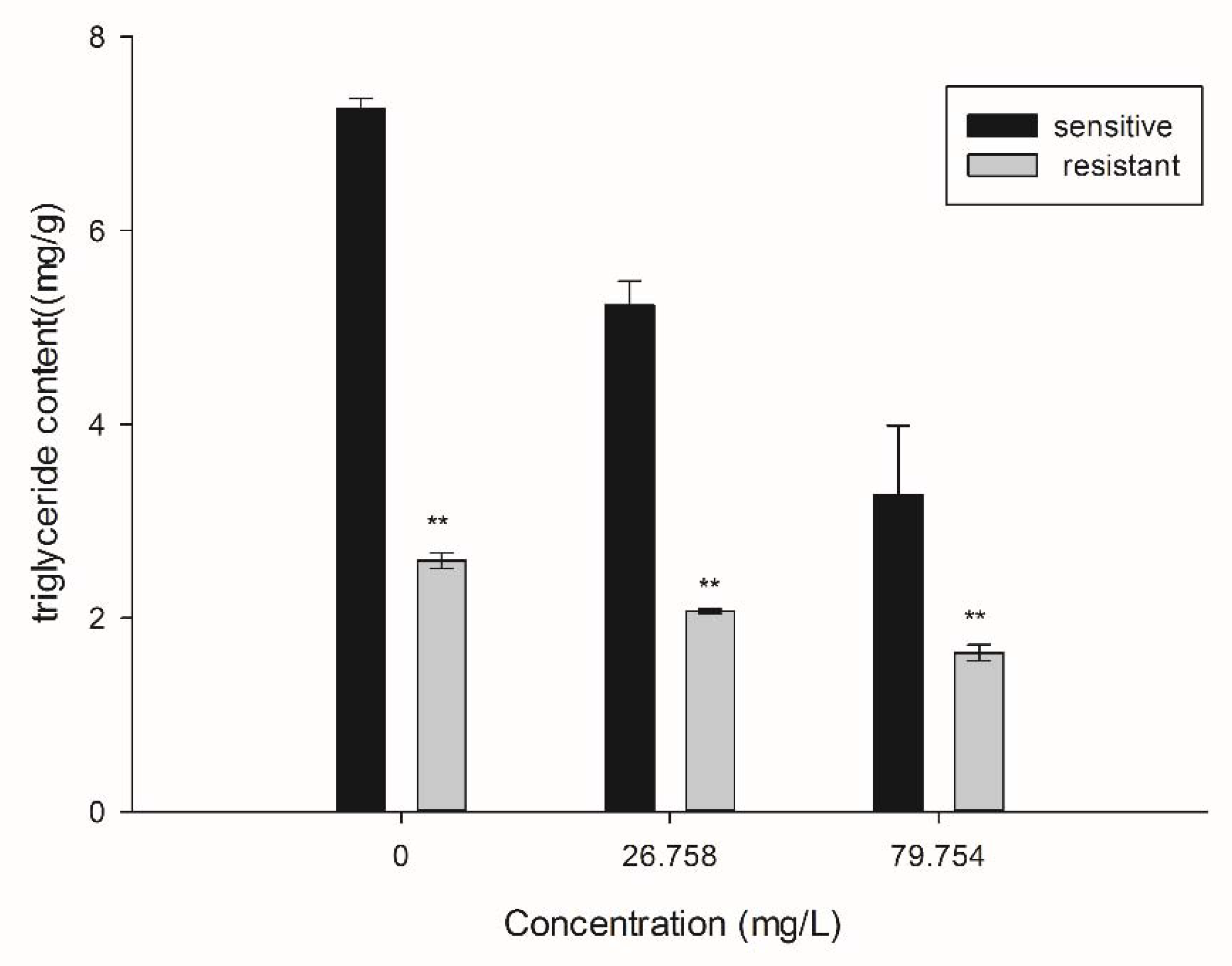
2.4. Effect of SYAUP-CN-26 on the Triglyceride Synthesis (TG) of B. cinerea
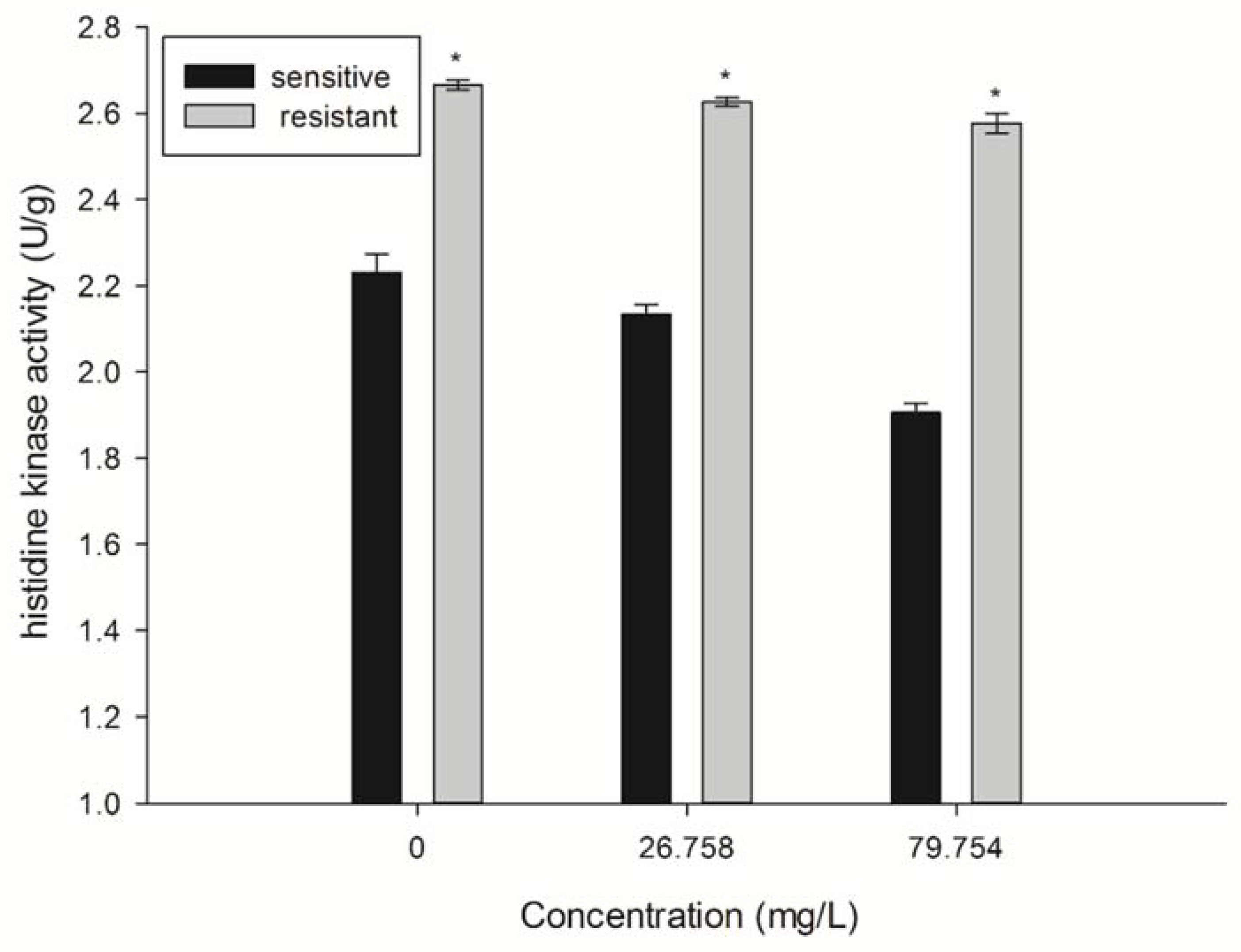
2.5. Detection of Histidine Kinase (HK) Activity
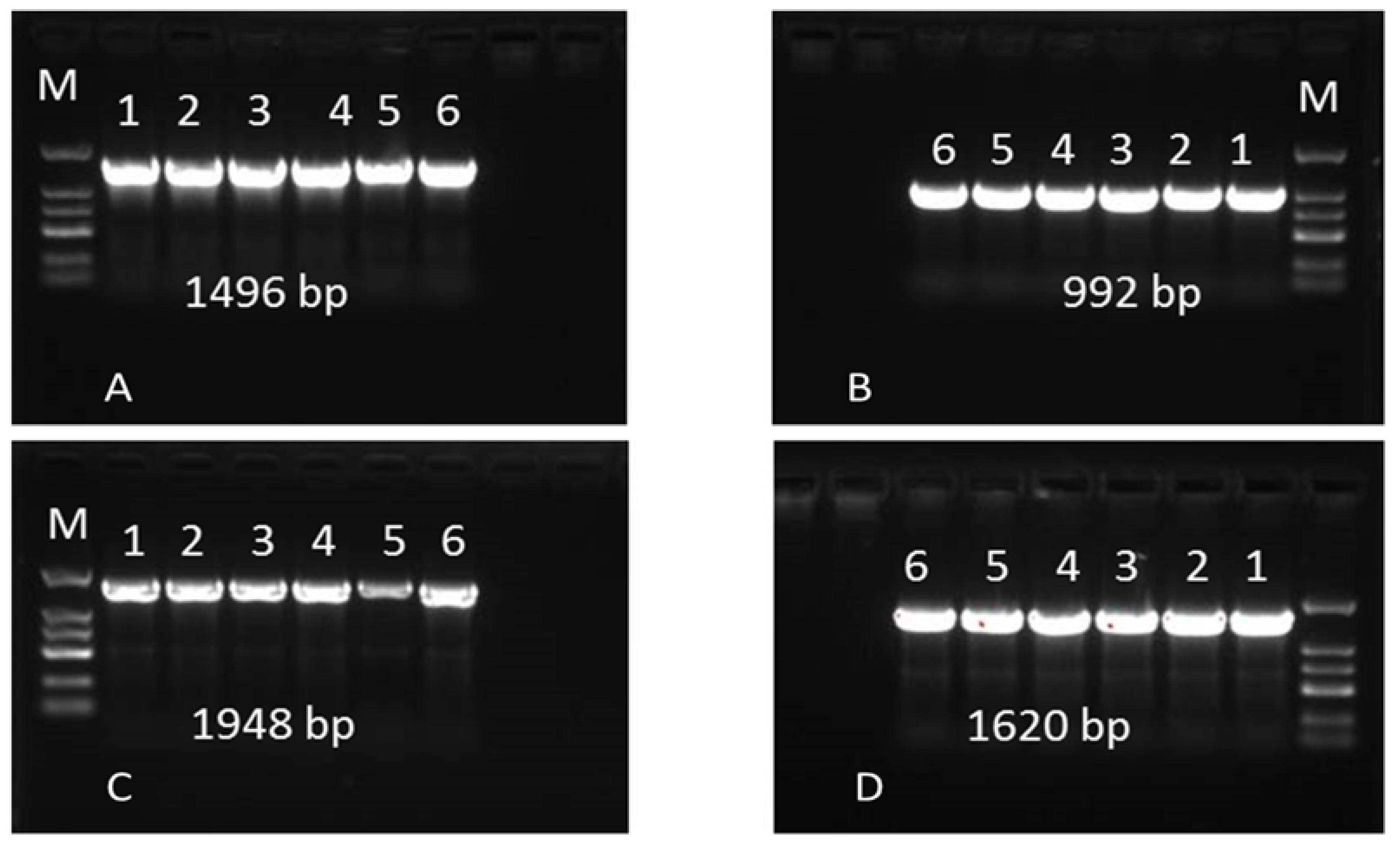
2.6. Sequence Variation in the Bos1 Gene
3. Discussion
4. Materials and Methods
4.1. Fungal Isolates, Chemicals, and Culture Media
4.2. Generation of Resistant Mutants of B. cinerea to SYAUP-CN-26
4.3. Level and Stability of Resistance of Mutants to SYAUP-CN-26
4.4. Effect of SYAUP-CN-26 on Cell Membrane Structure
4.5. Measurement of Relative Electric Conductivity
4.6. Effect of SYAUP-CN-26 on Triglyceride Synthesis (TG)
4.7. Effect of SYAUP-CN-26 on Histidine Kinase Activity
4.8. Sequences of the Bos1 Gene in Sensitive and Resistant Strains
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benito-Pescador, D.; Santander, D.; Arranz, M.; Díaz-Mínguez, J.M.; Eslava, A.P.; van Kan, J.A.; Benito, E. PBcmimp1, a Botrytis cinerea gene transiently expressed in planta, encodes a mitochondrial protein. Front. Microbiol. 2016, 7, 213. [Google Scholar] [CrossRef]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; Van Kan, J.A. Botrytis cinereal L.: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef]
- Dik, A.J.; Elad, Y. Comparison of antagonists of Botrytis cinerea in greenhouse-grown cucumber and tomato under different climatic conditions. Eur. J. Plant Pathol. 1999, 105, 123–137. [Google Scholar] [CrossRef]
- Baptista, F.J.; Bailey, B.J.; Meneses, J.F. Effect of nocturnal ventilation on the occurrence of Botrytis cinerea in mediterranean unheated tomato greenhouses. Crop Prot. 2012, 32, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Widiastuti, A.; Yoshino, M.; Saito, H.; Maejima, K.; Zhou, S.Y.; Odani, H.; Hasegawa, M.; Nitta, Y.; Sato, T. Induction of disease resistance against Botrytis cinerea by heat shock treatment in melon (Cucumis melo L.). Physiol. Mol. Plant Pathol. 2011, 75, 157–162. [Google Scholar] [CrossRef]
- Sun, H.Y.; Wang, H.C.; Chen, Y.; Li, H.X.; Chen, C.J.; Zhou, M.G. Multiple resistance of Botrytis cinerea from vegetable crops to carbendazim, diethofencarb, procymidone, and pyrimethanil in China. Plant Dis. 2010, 94, 551–556. [Google Scholar] [CrossRef] [Green Version]
- Ren, W.C.; Shao, W.Y.; Han, X.; Zhou, M.G.; Chen, C.J. Molecular and biochemical characterization of laboratory and field mutants of Botrytis cinerea resistant to fludioxonil. Plant Dis. 2016, 100, 1414–1423. [Google Scholar] [CrossRef] [Green Version]
- Yin, D.; Chen, X.; Hamada, M.S.; Yu, M.; Yin, Y.; Ma, Z. Multiple resistances to QoIs and other classes of fungicides in Botrytis cinerea populations from strawberry in Zhejiang Province, China. Eur. J. Plant Pathol. 2015, 141, 169–177. [Google Scholar] [CrossRef]
- Dolores, F.O.; Alejandro, P.G.; Manuel, C.; Eduardo DL, P.; Antonio, D.V.; Antonio, T.J. Resistance to the SDHI Fungicides boscalid, fluopyram, fluxapyroxad, and penthiopyrad in Botrytis cinerea from commercial strawberry fields in Spain. Plant Dis. 2017, 101, 1306–1313. [Google Scholar]
- Liu, S.M.; Che, Z.P.; Chen, G.Q. Multiple-fungicide resistance to carbendazim, diethofencarb, procymidone, and pyrimethanil in field isolates of Botrytis cinerea from tomato in Henan Province, China. Crop Prot. 2016, 84, 56–61. [Google Scholar] [CrossRef]
- Habib, W.; Saab, C.; Malek, R.; Kattoura, L.; Rotolo, C.; Gerges, E.; Baroudy, F.; Pollastro, S.; Faretra, F.; De Miccolis Angelini, R.M. Resistance profiles of Botrytis cinerea populations to several fungicide classes on greenhouse tomato and strawberry in Lebanon. Plant Pathol. 2020, 69, 1453–1468. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, W.M.; Liu, K.; Yang, S.; Fan, H.T.; Bhadury, P.; Hu, D.Y.; Zhang, Y.P. Synthesis and antiviral activity of 5-(4-chlorophenyl)-1,3,4-thiadiazole sulfonamides. Molecules 2010, 15, 9046–9056. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.L.; Rui, P.; Liu, C.X.; Du, Y.; Qin, P.W.; Qi, Z.Q.; Ji, M.S.; Li, X.H.; Cui, Z.N. Design, synthesis and fungicidal activity of 2-substituted phenyl-2-oxo-, 2-hydroxy-and 2-acyloxyethylsulfonamides. Molecules 2017, 22, 738. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.; Kochi, S.I.; Kunita, H.; Ito, S.I.; Kameya-Iwaki, M. Biological mode of action of the fungicide, flusulfamide, against plasmodiophora brassicae (clubroot). Eur. J. Plant Pathol. 1999, 105, 577–584. [Google Scholar] [CrossRef]
- Yang, J.C.; Guan, A.Y.; Yang, F.; Liu, C.L. Progress of fungicides in China and Abroad. Mod. Agrochem. 2015, 14, 1–9. [Google Scholar]
- Mitani, S.; Araki, S.; Yamaguchi, T.; Takii, Y.; Ohshima, T.; Matsuo, N. Antifungal activity of the novel fungicide cyazofamid against phytophthora infestans and other plant pathogenic fungi in vitro. Pestic. Biochem. Physiol. 2001, 70, 92–99. [Google Scholar] [CrossRef]
- Ezabadi, I.R.; Camoutsis, C.; Zoumpoulakis, P.; Geronikaki, A.; Soković, M.; Glamoćilija, J.; Ćirić, A. Sulfonamide-1,2,4-triazole derivatives as antifungal and antibacterial agents: Synthesis, biological evaluation, lipophilicity, and conformational studies. Bioorganic Med. Chem. 2008, 16, 1150–1161. [Google Scholar] [CrossRef]
- Wang, X.P.; Wang, D.Q. Synthesis and bactericidal activity of 2-oxocyclododecyl sulfonamide. Chem. J. Chin. Univ. 1997, 18, 889–893. [Google Scholar]
- Yang, H.Y.; Yan, X.J.; Yuan, H.Z.; Liang, X.M.; Zhang, J.J.; Wang, D.Q. Synthesis and fungicidal activity of 2-oxocyclohe xylsulfonamide s containing fluorine. Chin. J. Pestic. Sci. 2010, 12, 449–452. [Google Scholar]
- Qi, Z.Q.; Sun, Q.B.; Li, X.H.; Gu, Z.M.; Li, X.W.; Ji, M.S. Inhibitory effect of N-(2,4,5-trichlorophenyl)-2-oxocyclohexylsulfonamide against Botrytis cinerea. Chin. J. Pestic. Sci. 2014, 16, 523–528. [Google Scholar]
- Xue, C.S.; He, R.H.; Li, X.H.; Xiao, S.Q.; Niu, M.Y.; Feng, H. Inhibition effect of cycloakylsulfonamide on Phytophthora capsici. Plant Prot. 2016, 42, 214–218. [Google Scholar]
- Li, X.H.; Ji, M.S.; Qi, Z.Q.; Li, X.W.; Shen, Y.X.; Gu, Z.M.; Zhang, Y.; Wei, S.H.; Wang, Y.Z.; Wang, D.Q. Synthesis of 2-amino-6-oxocyclohexenylsulfonamides and their activity against Botrytis cinerea. Pest Manag. Sci. 2011, 67, 986–992. [Google Scholar] [CrossRef]
- Liu, C.X.; Yan, X.J.; Wang, M.L.; Qin, P.W.; Qi, Z.Q.; Ji, M.S. Design, synthesis and fungicidal activity of novel 2-substituted aminocycloalkyl sulfonamides. Bioorg. Med. Chem. Lett. 2017, 27, 271–276. [Google Scholar] [CrossRef]
- Cai, N.; Liu, C.X.; Feng, Z.H.; Li, X.H.; Qi, Z.Q.; Ji, M.S.; Qin, P.W.; Ahmed, W.; Cui, Z.N. Design, synthesis, and SAR of novel 2-glycinamide cyclohexyl sulfonamide derivatives against Botrytis cinerea. Molecules 2018, 23, 740. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.N.; Wang, K.; Feng, T.Y.; Zhang, H.Z.; Li, X.H.; Qi, Z.Q. The Effect of (1S, 2R-((3-bromophenethyl)amino)-N-(4-chloro-2-trifluoromethylphenyl) cyclohexane-1-sulfonamide) on Botrytis cinerea through the membrane damage mechanism. Molecules 2019, 25, 94. [Google Scholar] [CrossRef] [Green Version]
- Feng, T.Y.; Li, J.L.; Sun, M.F.; Peng, J.N.; Li, X.H.; Qi, Z.Q. SYAUP–CN–26 applies its antifungal activity against Botrytis cinerea by disrupting mitochondrial structure and function. Biochimie 2020, 176, 162–168. [Google Scholar] [CrossRef]
- Cui, W.; Beever, E.R.; Parkes, L.S.; Weeds, L.P.; Templeton, D.M. An osmosensing histidine kinase mediates dicarboximide fungicide resistance in Botryotinia fuckeliana (Botrytis cinerea). Fungal Genet. Biol. 2002, 36, 187–198. [Google Scholar] [CrossRef]
- Zhu, M.L.; Yan, J.P.; Sun, Q.L.; Mo, M.H.; Zhang, K.Q. Molecular mechanisms of the resistance to dicarboximides fungicides (DCFs) in Phytopathogenic Fungi. Biotechnology 2005, 15, 95–97. [Google Scholar]
- Orth, A.B.; Rzhetskaya, M.; Pell, E.J.; Tien, M. A serine (threonine) protein kinase confers fungicide resistance in the phytopathogenic fungus Ustilago maydis. Appl. Environ. Microbiol. 1995, 61, 2341–2345. [Google Scholar] [CrossRef] [Green Version]
- Elad, Y.; Yunis, H.; Katan, T. Multiple fungicide resistance to benzimidazoles, dicarboximides and diethofencarb in field isolates of Botrytis cinerea in Israel. Plant Pathol. 2010, 41, 41–46. [Google Scholar] [CrossRef]
- Myresiotis, C.K.; Karaoglanidis, G.S.; Tzavella-Klonari, K. Resistance of Botrytis cinerea isolates from vegetable crops to anilinopyrimidine, phenylpyrrole, hydroxyanilide, benzimidazole, and dicarboximide fungicides. Plant Dis. 2007, 91, 407–413. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.X.; Zhang, R.S.; Han, X.; Wang, J.X.; Zhou, M.G.; Chen, C.J. Resistance risk assessment for fludioxonil in Stemphylium solani. Ann. Appl. Biol. 2015, 167, 277–284. [Google Scholar] [CrossRef]
- Pang, Z.L.; Shao, J.P.; Chen, L.; Lu, X.H.; Hu, J.; Qin, Z.H.; Liu, X.L. Resistance to the novel fungicide pyrimorph in Phytophthora capsici: Risk assessment and detection of point mutations in CesA3 that confer resistance. PLoS ONE 2013, 8, e56513. [Google Scholar]
- Zhang, X.; Wu, D.; Duan, Y.B.; Ge, C.; Wang, J.X.; Zhou, M.G.; Chen, C.J. Biological characteristics and resistance analysis of the novel fungicide SYP-1620 against Botrytis cinerea. Pestic. Biochem. Physiol. 2014, 114, 72–78. [Google Scholar] [CrossRef]
- Rubinstein Shmuel, M.; Kolodkin-Gal, I.; McLoon, A.; Liraz, C.; Roberto, K.; Richard, L.; Weitz David, A. Osmotic pressure can regulate matrix gene expression in Bacillus subtilis. Mol. Microbiol. 2012, 86, 426–436. [Google Scholar] [CrossRef] [Green Version]
- Choi, G.J.; Lee, H.J.; Cho, K.Y. Involvement of catalase and superoxide dismutase in resistance of Botrytis cinerea to dicarboximide fungicide vinclozolin. Pestic. Biochem. Physiol. 1997, 59, 1–10. [Google Scholar] [CrossRef]
- Ma, Z.H.; Yan, L.Y.; Luo, Y.; Michailides, T.J. Sequence variation in the two-component histidine kinase gene of Botrytis cinerea associated with resistance to dicarboximide fungicides. Pestic. Biochem. Physiol. 2007, 88, 300–306. [Google Scholar] [CrossRef]
- Ryan Kaler Kylie, M.; Nix Jay, C.; Schubot Florian, D. RetS inhibits Pseudomonas aeruginosa biofilm formation by disrupting the canonical histidine kinase dimerization interface of GacS. J. Biol. Chem. 2021, 297, 101193. [Google Scholar] [CrossRef]
- Leroux, P.; Fritz, R.; Debieu, D.; Albertini, C.; Lanen, C.; Bach, J.; Gredt, M.; Chapeland, F. Mechanisms of resistance to fungicides in field strains of Botrytis cinerea. Pest Manag. Sci. 2002, 58, 876–888. [Google Scholar] [CrossRef]
- Duan, Y.B.; Ge, C.Y.; Liu, S.M.; Wang, J.X.; Zhou, M.G. A two-component histidine kinase Shk1 controls stress response, sclerotial formation and fungicide resistance in Sclerotinia sclerotiorum. Mol. Plant Pathol. 2013, 14, 708–718. [Google Scholar] [CrossRef]
- Ochiai, N.; Fujimura, M.; Motoyama, T.; Ichiishi, A.; Usami, R.; Horikoshi, K.; Yamaguchi, I. Characterization of mutations in the two-component histidine kinase gene that confer fludioxonil resistance and osmotic sensitivity in the os-1 mutants of Neurospora crassa. Pest Manag. Sci. 2001, 57, 437–442. [Google Scholar] [CrossRef]
- Oshima, M.; Banno, S.; Okada, K.; Takeuchi, T.; Kimura, M.; Ichiishi, A.; Yamaguchi, I.; Fujimura, M. Survey of mutations of a histidine kinase gene BcOS1 in dicarboximide-resistant field isolates of Botrytis cinerea. Gen. Plant Pathol. 2006, 72, 65–73. [Google Scholar] [CrossRef]
- Dry Ian, B.; Yuan Khor, H.; Hutton Don, G. Dicarboximide resistance in field isolates of Alternaria alternata, is mediated by a mutation in a two-component histidine kinase gene. Fungal Genet. Biol. 2004, 41, 102–108. [Google Scholar] [CrossRef]
- Oshima, M.; Fujimura, M.; Banno, S.; Hashimoto, C.; Motoyama, T.; Ichiishi, A.; Yamaguchi, I. A point mutation in the two-component histidine kinase bcos-1 gene confers dicarboximide resistance in field isolates of Botrytis cinerea. Phytopathology 2002, 92, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Abedin, M.J.; Wang, D.; McDonnell, M.A.; Lehmann UKelekar, A. Autophagy delays apoptotic death in breast cancer cells following DNA damage. Cell Death Differ. 2007, 14, 500–510. [Google Scholar] [CrossRef]
- Arany, Z.; Neinast, M. Branched Chain Amino Acids in Metabolic Disease. Curr. Diabetes Rep. 2018, 18, 76–78. [Google Scholar] [CrossRef]
- Yin, T.; Xu, B.L.; Liang, Q.L.; Gu, L.J.; Li, R.F. UV mutagenesis and screening for fungicide resistant strains of Trichoderma aureoviride T2. Acta Prataculturae Sin. 2013, 22, 117–122. [Google Scholar]
- Qi, Y.; Li, H.; Su, Y.; Zhen, W. Sensitivity to trifluzamide and main biological characteristics of resistant mutants of Rhizoctonia cerealis. Chin. J. Pestic. Sci. 2014, 16, 271–280. [Google Scholar]


| EC50 (µg/mL) | RF | FSC | |||
|---|---|---|---|---|---|
| 1st | 10th | 1st | 10th | ||
| 5055 | 1.6047 | 1.6561 | -- | -- | |
| 5055-R4 | 111.0821 | 115.7630 | 69.2230 | 69.9010 | 1.0098 |
| AF396827.2 | 329 | ******* | 348 | ****** | 368 | 369 | 370 | 371 | 372 | 373 |
| CAA | ******* | CCG | ****** | GTC | CAG | CGC | ATG | TGG | AAC | |
| Q | ******* | P | ****** | D | Q | R | M | W | N | |
| 5505 | CAA | ****** | CCG | ****** | GTC | CAG | CGC | ATG | TGG | AAC |
| Q | ****** | P | ****** | D | Q | R | M | W | N | |
| 5505-R1 | CAA | ****** | CCG | ****** | GTC | CAG | CGC | ATG | TGG | AAC |
| Q | ****** | P | ****** | D | Q | R | M | W | N | |
| 5505-R2 | CAA | ****** | CTG | ****** | GTC | CAG | CGC | ATG | TGG | AAC |
| Q | ****** | L | ****** | D | Q | R | M | W | N | |
| 5505-R3 | CAA | ****** | CTG | ****** | GTC | CAG | CGC | ATG | TGG | AAC |
| Q | ****** | L | ****** | D | Q | R | M | W | N | |
| 5505-R4 | CAA | ****** | CTG | ****** | GTC | CAG | CGC | ATG | TGG | AAC |
| Q | ****** | L | ****** | D | Q | R | M | W | N | |
| 5505-R5 | CAA | ****** | CTG | ****** | GTC | CAG | CGC | ATG | TGG | AAC |
| Q | ****** | L | ****** | D | Q | R | M | W | N |
| Primers | Sequences | Annealing Temperature | Gene Sizes |
|---|---|---|---|
| F1: | AACGCGTAGCAGCTCTCGAA | 62 | 1496 |
| R1 | ATGGTAAGATGGGTGGCCAA | ||
| F2 | GCGGGTGAAATACTCATACT | 60 | 992 |
| R2 | ACGCTATCAAGTTCACAGAG | ||
| F3 | AACCCAAAGTCCCAATCCCAA | 58 | 1948 |
| R3 | TTCTCGGTGGACAAGCCAA | ||
| F4 | TTACCGCGTAGATAGCTCAGTT | 60 | 1620 |
| R4 | GCCGAAGTTCGATGATAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Zhang, H.; Zhu, W.; Peng, J.; Li, X.; Wang, Y.; Qi, Z. Preliminary Study of Resistance Mechanism of Botrytis cinerea to SYAUP-CN-26. Molecules 2022, 27, 936. https://doi.org/10.3390/molecules27030936
Wang K, Zhang H, Zhu W, Peng J, Li X, Wang Y, Qi Z. Preliminary Study of Resistance Mechanism of Botrytis cinerea to SYAUP-CN-26. Molecules. 2022; 27(3):936. https://doi.org/10.3390/molecules27030936
Chicago/Turabian StyleWang, Kai, Huazhong Zhang, Wanying Zhu, Jingnan Peng, Xinghai Li, Yingzi Wang, and Zhiqiu Qi. 2022. "Preliminary Study of Resistance Mechanism of Botrytis cinerea to SYAUP-CN-26" Molecules 27, no. 3: 936. https://doi.org/10.3390/molecules27030936
APA StyleWang, K., Zhang, H., Zhu, W., Peng, J., Li, X., Wang, Y., & Qi, Z. (2022). Preliminary Study of Resistance Mechanism of Botrytis cinerea to SYAUP-CN-26. Molecules, 27(3), 936. https://doi.org/10.3390/molecules27030936






