Abstract
Betulinic acid (BA) and its derivatives exhibit a variety of biological activities, especially their anti-HIV-1 activity, but generally have only modest inhibitory potency against influenza virus. The entry of influenza virus into host cells can be competitively inhibited by multivalent derivatives targeting hemagglutinin. In this study, a series of hexa-, hepta- and octavalent BA derivatives based on α-, β- and γ-cyclodextrin scaffolds, respectively, with varying lengths of flexible oligo(ethylene glycol) linkers was designed and synthesized using a microwave-assisted copper-catalyzed 1,3-dipolar cycloaddition reaction. The generated BA-cyclodextrin conjugates were tested for their in vitro activity against influenza A/WSN/33 (H1N1) virus and cytotoxicity. Among the tested compounds, 58, 80 and 82 showed slight cytotoxicity to Madin-Darby canine kidney cells with viabilities ranging from 64 to 68% at a high concentration of 100 μM. Four conjugates 51 and 69–71 showed significant inhibitory effects on influenza infection with half maximal inhibitory concentration values of 5.20, 9.82, 7.48 and 7.59 μM, respectively. The structure-activity relationships of multivalent BA-cyclodextrin conjugates were discussed, highlighting that multivalent BA derivatives may be potential antiviral agents against influenza infection.
1. Introduction
Influenza viruses are widespread human respiratory pathogens that can cause serious infections with significant morbidity and mortality [1]. Recently, coinfection of influenza virus with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been reported [2], highlighting that the prevention and treatment of influenza will be more important than ever. Due to the lack of activity against influenza B and the widespread resistance of M2 ion channel inhibitors among circulating influenza strains, the antiviral drugs currently recommended for the treatment of influenza are limited to neuraminidase (NA) [3] and polymerase acidic protein (PA) inhibitors [4]. Although variants resistant to NA and PA inhibitors are much less than M2 inhibitors, the high variability of influenza viruses, such as seasonal H1N1 viruses carrying the H275Y, H275Y and I38T mutations [5] and the H7N9 virus carrying the R294K mutation [6], enables the rapid evolution of antiviral resistance to drugs, underscoring the urgent need for the development of new anti-influenza drugs.
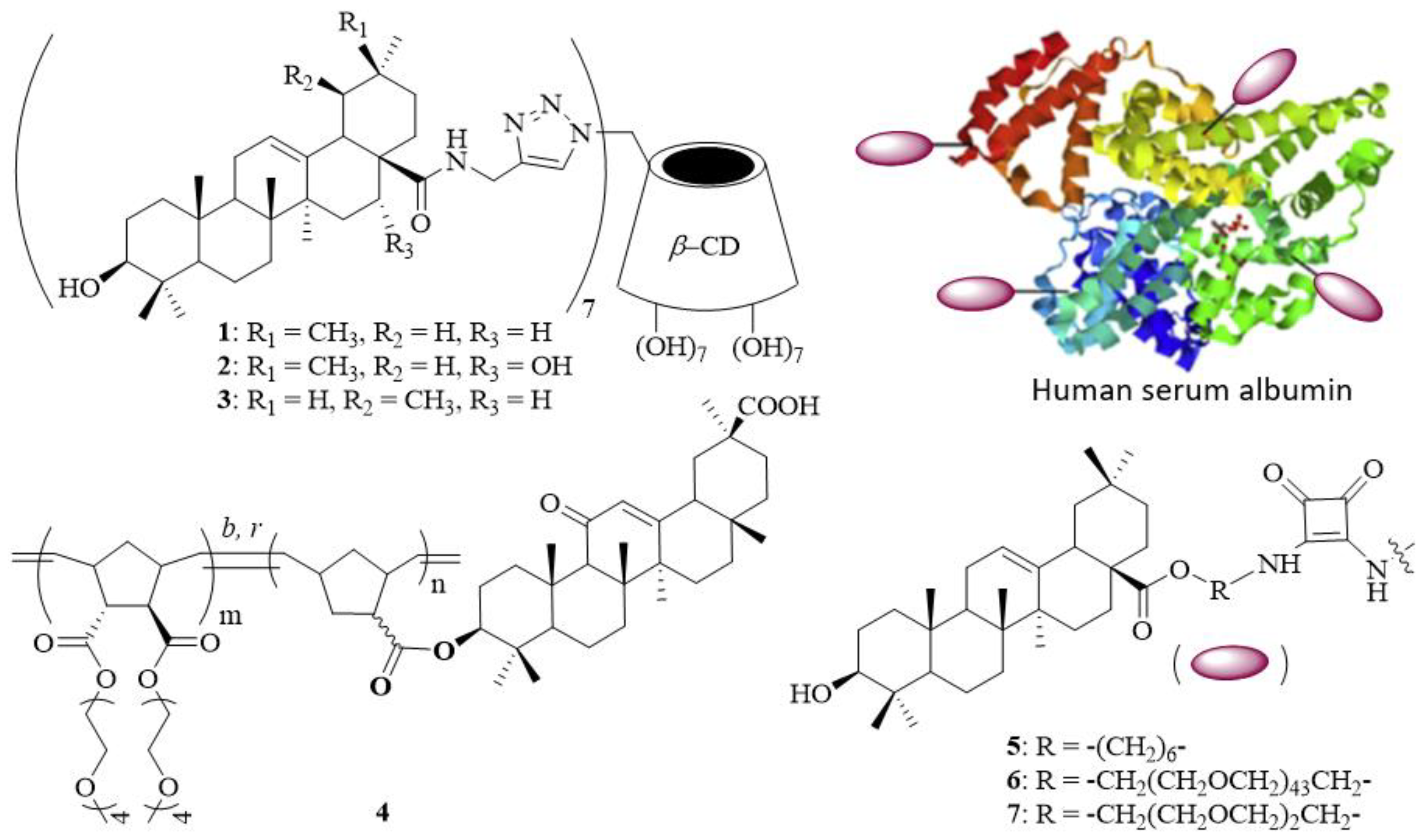
The entry of influenza virus into host cells is a six-step dynamic process [7], which represents an attractive target for antiviral therapy. Influenza viruses attach to host cells by binding the globular head of hemagglutinin (HA), a homotrimeric type I membrane glycoprotein expressed on the virion surface, to sialylated host cells. The interaction between HA and sialic acid is usually weak with an association constant of 103 M−1 [8]. However, the interactions between multiple HA trimers on the viral surface (~600–1200 molecules per virus particle) and sialic acid-terminated glycoproteins and glycolipids on the cell surface (~50–200 residues per 100 nm2) substantially increase through multivalent effects [9]. To this end, linear polymers [10], dendritic polymers [11], and nanoparticles [12] have been used as different display systems for highly potent influenza virus inhibitors. We have previously reported the synthesis of multivalent pentacyclic triterpene conjugates [13] and found that three heptavalent pentacyclic triterpene derivatives 1–3 display broad-spectrum anti-influenza virus activity with half maximal inhibitory concentration (IC50) values in the 1.60–18.74 μM range (Figure 1). Two years later, Li et al. demonstrated the synthesis of a series of random glycyrrhetinic acid/oligo(ethylene glycol) (OEG)-appended norbomene copolymers 4 as potential nanocarriers for drug delivery [14]. In another study, Yang et al. reported the synthesis of PEGylated oleanolic acid-functionalized human serum albumin conjugates 5–7 and their potential use as anti-infective agents [15]. These results rationalize the construction of multivalent pentacyclic triterpenes as potential inhibitors to block the replication of influenza viruses.
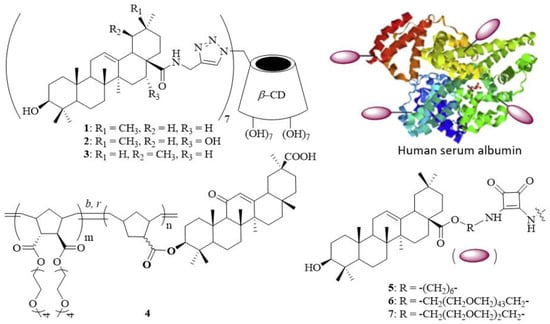
Figure 1.
Structures of multivalent pentacyclic triterpene conjugates (1–7).
Betulinic acid (BA) is a naturally occurring pentacyclic triterpenoid found in several species of plants, notably Betula pubescens, commonly known as white birch. Owing to its unusual multiple biological effects, BA has garnered attention from researchers in the scientific community and pharmaceutical industry in recent years [16]. The remarkable anti-HIV-1 potency of BA derivatives, such as bevirimat and BMS-955176, is one of their most important properties [17,18]. The interesting anti-HIV-1 properties of BA derivatives led to the examination of their anti-influenza activity. Hong et al. [19]. reported that BA shows weak anti-influenza activity against A/PR/8/34 virus (10 μM: ~30%). Antiviral-guided isolation of the leafstalk extract of Schefflera heptaphylla led to the identification of two 3-epi-betulinic acid derivatives with anti-influenza A (H1N1) virus activity [20]. Betulinic aldehyde isolated from Alnus japonica, which is used in folk remedies for influenza, exhibits certain anti-influenza effects against avian influenza KBNP-0028 (H9N2) virus with an EC50 value of 28.4 μM [21]. Simple modifications of BA at position C-3 or C-28 provide compounds with significant activities against influenza A virus [22,23]. However, the very poor water solubility of these compounds hampers their further development in vivo and inspires more research on better hydrophilic derivatives with potential pharmaceutical applications.
Based on these literature results of the antiviral activities of lupane-type triterpenoids and our interest in the development of natural products as potential anti-influenza agents [13,24,25], it was valuable to design and synthesize a variety of multivalent triterpenoid conjugates to disclose the relationship between their structure and activity. In our recent study, we found that one multivalent BA-α-cyclodextrin (CD) conjugate, CYY1-11, showed good anti-influenza activity (IC50 = 5.20 μM) against A/WSN/33 virus [25]. In the present work, we further describe the synthesis of a range of well-defined hexa-, hepta- and octavalent BA derivatives based on α-, β- and γ-CD scaffolds, respectively, with varying OEG chains (0, 1, 2, 4, 6 and 8 OEG units) as linkers via click chemistry. A total of 36 BA-CD conjugates, including compound CYY1-11 (named 51 in this manuscript), were examined to determine their anti-influenza activity against A/WSN/33 virus, and four conjugates, 51 and 69–71, showed significant antiviral activities. The structure-activity relationships (SAR) of multivalent BA-CD conjugates were discussed to explore potential therapeutic agents for influenza infection.
2. Results and Discussion
2.1. Chemistry
The multivalent presentation of bioactive molecules to polymers, such as poly(ethylene glycol) (PEG), has aroused extensive interest and been widely applied in many different fields [26,27], especially in drug delivery systems [28]. Difficulties in loading a quantitative amount of drugs at a specific position of polymeric carriers, such as polymethyl methacrylate, make drug delivery systems hard to work with. To pursue our research interests in natural products with significant anti-influenza activity, we planned to synthesize a series of multivalent BA derivatives based on CD scaffolds linked by a variable length OEG chain via click chemistry. Three natural CD scaffolds and six OEG linkers were selected because of their beneficial effects on the grafted ligands, such as water solubility and good biocompatibility and immune compatibility [29].
2.2. Synthesis of BA-Based Alkynes 33–38
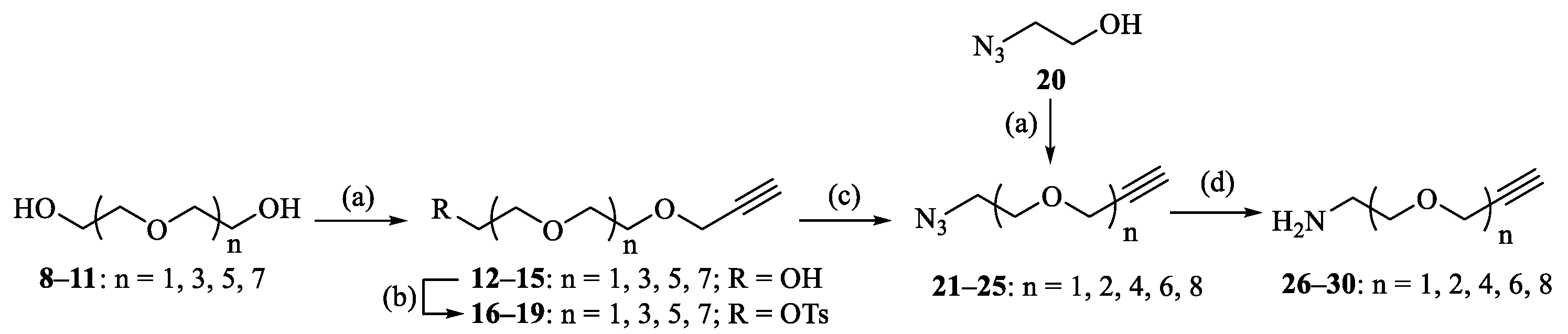
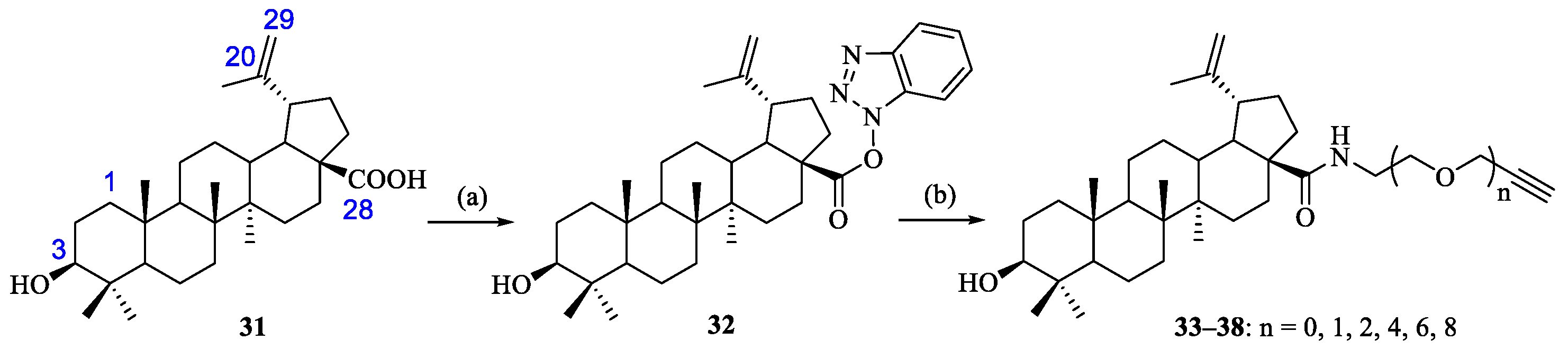
As described above, PEGs and OEGs are ubiquitously used in the pharmaceutical industry and biomedical research for the modification of proteins, peptides or nonpeptides. Therefore, OEGs were selected as the linkers between the BA pharmacophore and CD scaffold. Designed bifunctional amino alkyne linkers of different lengths (1, 2, 4, 6 and 8 OEG units) 26–30 were prepared from commercially available 2-azidoethanol 20, di(ethylene glycol) 8, tetra(ethylene glycol) 9, hexa(ethylene glycol) 10 or octa(ethylene glycol) 11 in 27–64% yields over four steps according to conventional methods (Scheme 1) [30,31]. Compound 31 could be accessed by synthesis from the cheaper precursor betulin, as reported previously [25,32]. Subsequent activation of the carboxylic acid by using TBTU/DIPEA in THF gave compound 32 in 83% yield, which was then subjected to aminolysis with commercially available propargylamine or bifunctional aminoalkyne linkers 26–30 to afford BA derivatives 33–38 bearing a terminal alkynyl group at the C-28 position (Scheme 2), and their structures were characterized with 1H and 13C NMR (Supplementary Materials). 1H NMR spectra of 33–38 showed one proton of the terminal alkynyl group at δ 2.41~2.43 ppm, while 13C NMR spectra displayed two carbons of the terminal alkynyl group at δ 71.06 and 80.14 ppm for 33 [33] and δ 74.49~74.65 and 79.44~79.58 ppm for 34–38.
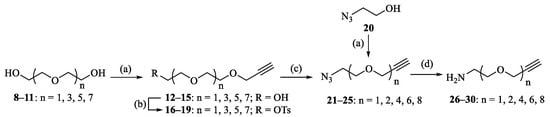
Scheme 1.
Reagents and conditions: (a) propargyl bromide, NaH, THF, 48–82%; (b) TsCl, triethylamine, DCM, 85–91%; (c) NaN3, DMF, 70 °C, 89–93%; and (d) Ph3P, H2O, THF, 73–90%.
Scheme 2.
Reagents and conditions: (a) TBTU, THF, DIPEA, 83%; and (b) propargylamine or 26–30, DMF, K2CO3, 34–70%.
2.3. Synthesis of Multivalent BA-CD Conjugates 69–86
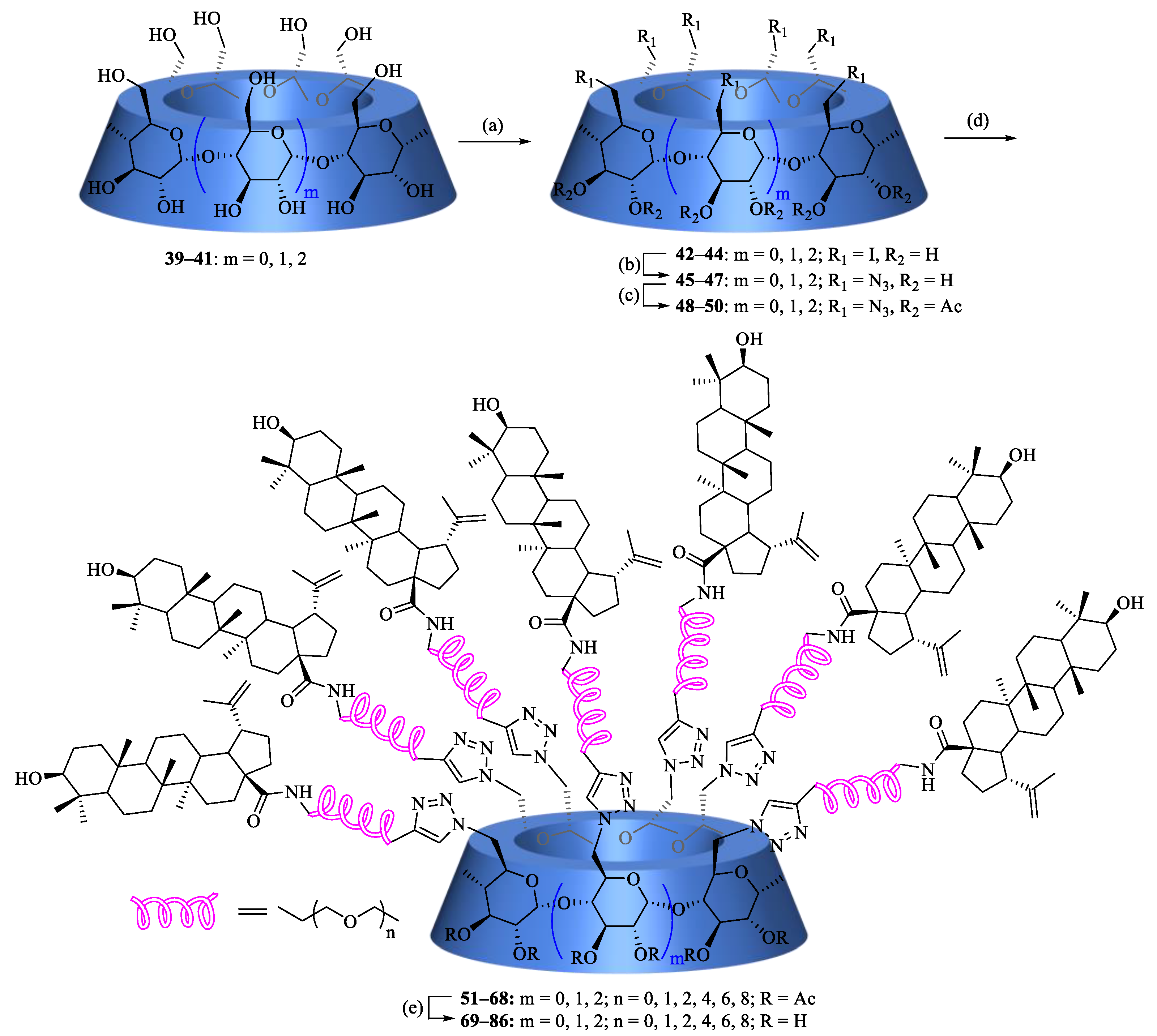
Multiazide-substituted CD scaffolds 48–50 were synthesized in 58–71% yields over three steps according to the methods described elsewhere (Scheme 3) [34,35]. The copper-catalyzed 1,3-dipolar cycloaddition between each terminal alkyne-modified BA, 33–38, and each multiazide-appended CD scaffold, 48–50, was performed at 100 °C in the presence of sodium ascorbate and a copper sulfate catalytic system in THF/H2O (1:1, v/v) under microwave irradiation to yield a series of acetyl-protected BA-CD conjugates, 51–68, in 42–55% yields, followed by a deacetylation reaction under Zemblén transesterification conditions to afford the desired homomultivalent conjugates 69–86 in good to excellent yields.
Scheme 3.
Reagents and conditions: (a) Ph3P, I2, DMF, 65–75%; (b) NaN3, DMF, 80 °C, ~100%; (c) Ac2O, pyridine, DMAP, 89–95%; (d) CuSO4·5H2O, sodium ascorbate, THF/H2O (1:1, v/v), microwave, 100 °C, 42–83%; and (e) CH3ONa/CH3OH, 82–94%.
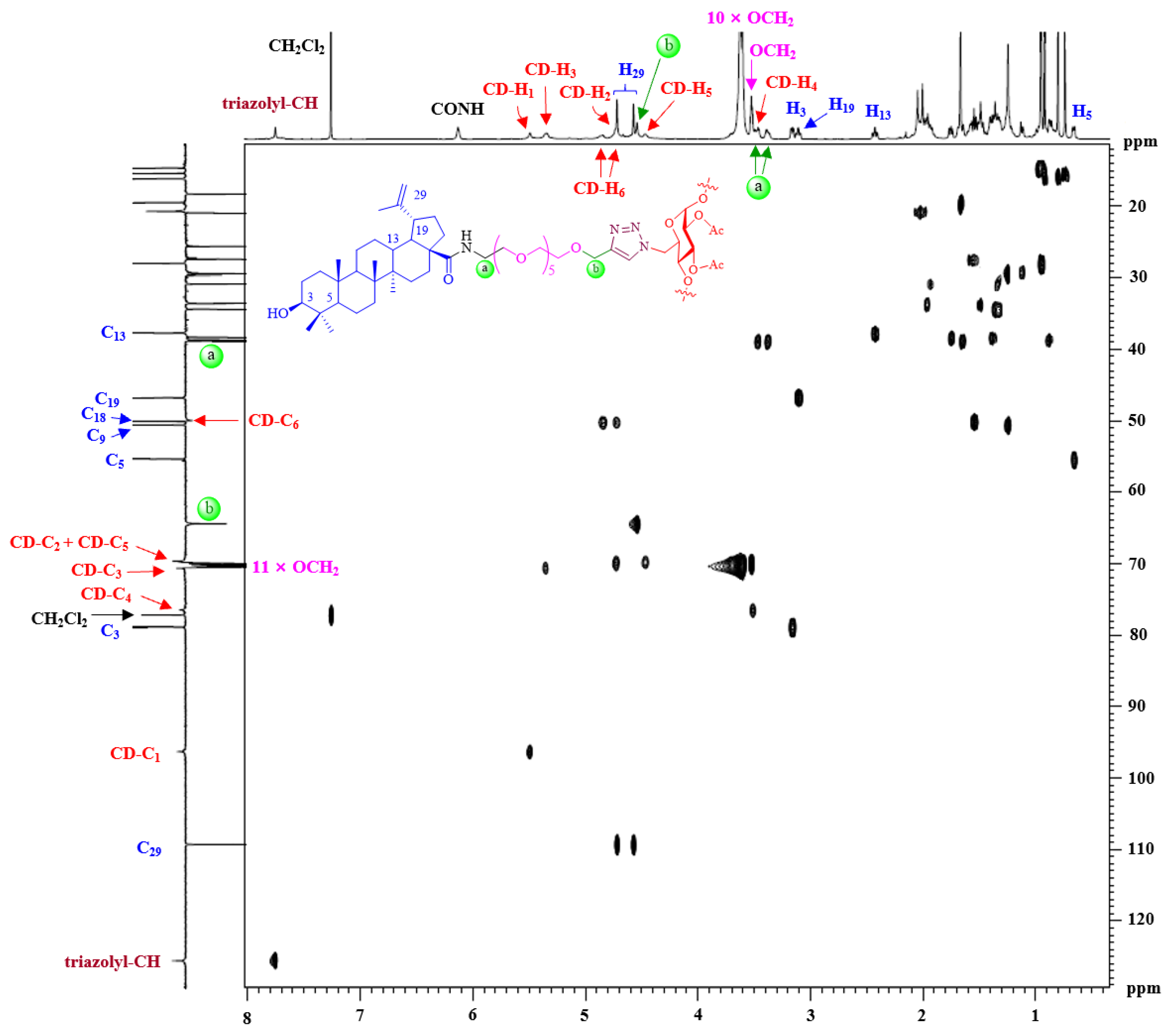
The structures of synthesized multivalent BA-CD conjugates 51–86 were characterized by NMR spectroscopy and MALDI-TOF mass spectrometry (Supplementary Materials). Except for the signals of the linker, the 1H and 13C NMR spectra of 51–68 are similar to each other; therefore, only the assignment of conjugate 64 is discussed in detail as an example. The 2D 1H-13C HSQC spectrum of conjugate 64 is shown in Figure 2, and inspection of it led to the assignment of most of the peaks. In the low-field region, the signal at δH 7.75 ppm (Supplementary Materials), according to the 1H-13C correlation spectrum, was assigned to triazolyl-CH. The proton of CONH at δH 6.13 ppm was easily identified, as there was no correlation in the 2D NMR spectrum. As a C7-symmetric macromolecular triazole adduct, conjugate 64 showed only one set of characteristic anomeric resonances [δH 5.49 ppm (β-CD-H1)]. Likewise, the other protons H2-6 of the β-CD scaffold were also assigned based on the HSQC spectrum. Two sets of peaks were clearly observed at 3.62–3.60 and 3.53–3.46 ppm (overlap with β-CD-H4 and NHCH2), which were assigned to the eleven OCH2 protons of the OEG group. Based on the literature data [36], major 1H NMR chemical shifts of the BA residue were attributed. For example, the occurrence of a vinyl residue was shown to be identified by very distinct signals at δH 4.72 and 4.57 ppm. Additionally, six methyl singlets were displayed at δH 1.67, 0.95, 0.94, 0.92, 0.80 and 0.74 ppm in the high-field region. The 13C NMR spectrum showed the expected number of signals (Supplementary Materials), which were assigned with the assistance of the HSQC spectrum. In the MALDI-TOF mass spectra (Supplementary Materials), a conjugate 64 molecular ion peak was observed at m/z 7228.56 (Calcd for C385H616N28NaO98+, 7228.25, Δ = 42.8 ppm), further confirming its identity as a fully substituted heptavalent conjugate.
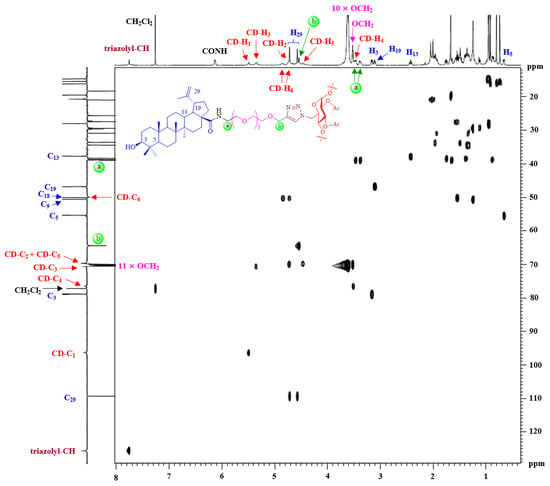
Figure 2.
The 1H-13C HSQC spectrum (600 MHz, 25 °C, CDCl3) of conjugate 64 demonstrates that it was a fully substituted heptavalent conjugate.
The 1H and 13C NMR signals of conjugates 69–86 were assigned based on precursors 51–65. As expected, in the majority of cases, the de-O-acetylation of the CD scaffold caused an upfield shift in CD-H2 and CD-H3 peaks of ~1.3 and ~1.5 ppm, respectively, but a downfield shift in both CD-C2 and CD-C3 peaks of ~2.7–3.3 ppm. For example, for conjugate 70, β-CD-H2 and β-CD-H3 were observed as a triplet and broad doublet at δ 3.45 and 3.86 ppm, respectively, which is upfield compared to the corresponding signals in conjugate 52 (δ 4.77 and 5.32 ppm, respectively).
2.4. Cytotoxicity of Multivalent BA-CD Conjugates to MDCK Cells
Cell viability assays are widely used to assess potential compound-induced toxicity. Measurement of intracellular ATP levels using ATP/luminescence readouts, such as the CellTiter-Glo reagent, is one of the most conventional and commonly used methods. Here, we evaluated the cytotoxicity of BA-CD conjugates 51–86 in MDCK cells before determining the anti-influenza virus activity. Culture medium containing 1% DMSO was used as a vehicle control. No significant effects on cell viability were observed with most of the multivalent BA-CD conjugates at a concentration of 100 µM, except for three conjugates 58, 80 and 82, which were slightly cytotoxic, with a MDCK cell viability of less than 70% (68%, 64% and 67%, respectively) (Figure S1). However, parental compound 31 possessed strong cytotoxicity towards host MDCK cells with a viability of 8.5% at the same concentration, which may due to its better cell permeability [37], encompassing the role of membrane damage in BA induced apoptosis.
2.5. Anti-influenza A/WSN/33 Virus Activity of Multivalent BA-CD Conjugates
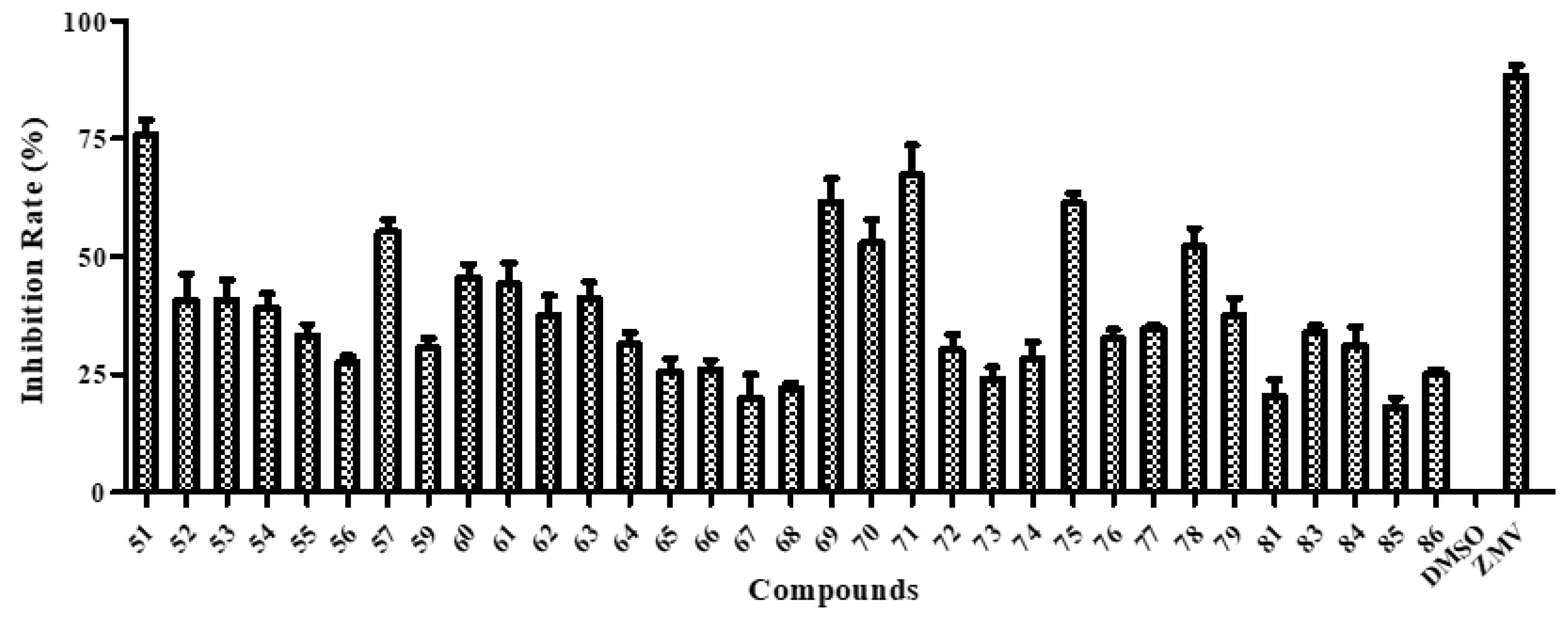
Next, we employed the cytopathic effect (CPE) reduction assay to investigate the anti-influenza activity of the multivalent BA derivatives. Except for three conjugates 58, 80 and 82 with weak cytotoxicity, the other 33 conjugates were evaluated. SAR analysis suggested that the α-CD scaffold-based conjugates exhibited higher antiviral activity against A/WSN/33 virus than the other two CD scaffold-based conjugates (e.g., 51 vs. 52 and 53, 57 vs. 59, and 75 vs. 76 and 77) (Figure 3). One of the most likely reasons was that steric hindrance caused by the multiple crowded BAs inhibited the interaction between the ligand and the target protein. An exception was conjugate 81, for which approximately 1.5-fold decreases in activity was observed compared with that of conjugate 83. In general, the linker between BA and the CD scaffold had no obvious effect on antiviral activity. Seven conjugates 51, 57, 69–71, 75 and 78 exhibited an inhibition rate against influenza virus A/WSN/33 (H1N1) of over 50% at a concentration of 20 μM. Further viral yield reduction studies with A/WSN/33 virus showed that they displayed dose-dependent inhibition of influenza virus replication (Table 1). Among them, conjugates 57, 75 and 78 only showed weak anti-influenza activity with IC50 over 10 μM, therefore the 50% cytotoxic concentration, CC50, values in MDCK cells were not further determined. The other four conjugates 51 and 69–71 showed potent antiviral activities with IC50 values falling within the low micromolar range (IC50: 5.20–9.82 μM). More specifically, hexavalent conjugate 51 (IC50 of 5.20 µM) showed the highest activity and was at least 20-42 times more active than its parent compound BA (50 µg/mL: 27.6% [38], EC50 > 219.0 µM [39]). In addition, the CC50 was not determined for 51 because the dose-response curve was not achieved at the highest concentration tested (200 μM), displaying over 38.4-fold selectivity. Detailed studies on the biological activities of 51 have been described by Chen et al. [25]. The CC50 values of conjugates 69–71 in MDCK cell were also not determined because they had a value over 100 µM, in other words they were also not cytotoxic. These results indicated that the grafting of multiple BAs onto the primary face of CD scaffolds was an effective strategy for enhancing the anti-influenza activity of BAs.
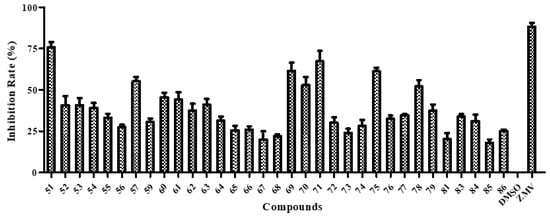
Figure 3.
CPE-based screening of BA-CD conjugates 51–57, 59–79, 81 and 83–86 at a single concentration (20 µM). DMSO (1%) and zanamivir (ZMV, 5 μM) were used as negative and positive controls, respectively. Error bars indicate standard deviations of measurements of triplicate samples.

Table 1.
In vitro cytotoxicity and anti-influenza activity of BA and its CD conjugates.
During the last two decades, BA and its derivatives have attracted special interest due to their remarkable anti-HIV-1 activity with three derivatives (bevirimat [40], BMS-955176 [41] and GSK-2838232 [42]) entering clinical trials. In recent years, an increasing number of studies with regard to their potential applications against other viruses have been performed [43,44,45]. As a class of anti-HIV-1 agents with new mechanisms of action (entry [17] and maturation [41]), however, the anti-influenza mechanism of action of BA and its derivatives has not yet been clearly elucidated. The primary study of the antiviral mechanism of 51 based on surface plasmon resonance assay indicated that multivalent BA derivatives can bind specifically with influenza HA protein with KD value of 1.50 μM [25], thus blocking influenza virus entry into host cells. Compared to 51, some conjugates, such as 81 and 83, showed relative weak binding affinity to influenza HA protein with KD values over 10 μM (Figure S2), which agreed well with their anti-influenza activities. Further efforts to uncover the function of the HA protein binding domain of multivalent BA-CD conjugates and to investigate how the structural features contribute to the binding domain will provide important insights into the multivalent binding mechanism and help to guide the design of more effective multivalent pentacyclic triterpene derivatives targeting this important protein.
3. Materials and Methods
3.1. Materials
α-, β- and γ-CD were purchased from Kaiguo Science & Technology Co., Ltd. (Beijing, China). BA was purchased from Bide Pharmatech. Ltd (Beijing, China). All the other chemical reagents and solvents were commercially available and used as received. The MDCK cell line was obtained from Crown Bioscience Inc. (San Diego, CA, USA), which were grown in Dulbecco’s Modified Eagle Medium (DMEM; Gibco BRL Life Technologies Inc., Grand Island, NY, USA) and supplemented with 10% fetal bovine serum (FBS; PAA Laboratories, Pasching, Austria) at 37 °C in a humidified atmosphere of 5% CO2. The CellTiter-Glo luminescent cell viability assay kit was purchased from Promega Corp. (Madison, WI, USA).
The NMR spectra were obtained on Bruker 400 and 600 MHz spectrometers (Bruker Daltonics., Billerica, MA, USA). The value of chemical shifts (δ) are given in ppm and coupling constants (J) in hertz (Hz). High-resolution electrospray mass spectra (HRMS) and MALDI-TOF-MS were recorded by a Bruker APEX IV FT_MS (7.0 T) mass spectrometer (Bruker Daltonics Inc., Billerica, MA, USA) and an AB Sciex TOF/TOF™ 72115 mass spectrometer (AB Sciex, Redwood City, CA, USA), respectively. The reaction progress and chromatography fractions were monitored by analytical thin-layer chromatography (TLC) on 0.25 mm thickness E. Merck pre-coated plates of silica gel 60 F254. The spots were visualized by immersion of the TLC plate in an appropriate solution followed by heating with a hot gun. The following staining solutions were applied: ninhydrin staining solution [ninhydrin (10.0 g) and ethanol (300 mL)], cerium molybdate staining solution [Ce(NH4)2(NO3)6 (0.5 g, 0.9 mmol), (NH4)6Mo7O24·4H2O (24.0 g, 19.4 mmol), concentrated aqueous H2SO4 (30 mL) and H2O (470 mL)]. Column chromatography was performed on silica gel (200–300 mesh). Linkers 26–30 [30,31], BA derivatives 32–33 [33] and hexavalent BA-α-CD conjugate 51 [25] were synthesized by literature methods, and the data were consistent with those published.
3.2. General Procedure A for the Synthesis of Terminal Propargylated OEG-Tethered BA Derivatives (34–38)
Na2CO3 (0.68 mmol, 2.0 equiv.) was added to a solution of 1-benzotriazolyl 3β-hydroxy-lup-20(29)-en-28-oate (32) (0.34 mmol, 1.0 equiv.) and terminal propargylated OEG-tethered amine (26–30) (0.41 mmol, 1.2 equiv.) in DMF (4 mL), and the mixture was stirred at room temperature for 24 h. After completion of the reaction, as indicated by TLC, the solvent was evaporated under reduced pressure, and the obtained residue was extracted with CH2Cl2 (10 mL × 3), dried with anhydrous Na2SO4, filtered, and evaporated. The pure product was obtained by column chromatography performed on silica gel.
3.2.1. Synthesis of N-(2-(2-Propyn-1-yloxy)ethyl)-3β-hydroxy-lup-20(29)-en-28-amide (34)
Prepared from 32 and 2-(propyn-1-yloxy)-ethanamine (26) according to general procedure A, the residue was purified by flash chromatography (eluent: CH2Cl2:(CH3)2CO = 40:1) to afford 34 as a white product with a yield of 93%. Rf = 0.36 (CH2Cl2:(CH3)2CO = 20:1); 1H NMR (400 MHz, CDCl3): δ 5.96 (t, 1H, J = 5.6 Hz), 4.73 (d, 1H, J = 1.7 Hz), 4.58 (s, 1H), 4.15 (d, 2H, J = 2.4 Hz), 3.62–3.38 (m, 4H), 3.17 (dd, 1H, J = 10.5, 5.6 Hz), 3.11 (dt, 1H, J = 11.5, 4.6 Hz), 2.46–2.39 (m, 2H), 2.00–0.85 (m, other aliphatic ring protons), 1.68, 0.96, 0.95, 0.93, 0.81, 0.75 (s, each 3H, 6 × CH3), 0.67 (d, 1H, J = 9.0 Hz); 13C NMR (100 MHz, CDCl3): δ 176.19, 150.95, 109.32, 79.45, 78.96, 74.64, 69.07, 58.25, 55.71, 55.37, 50.61, 50.07, 46.82, 42.48, 40.74, 38.84, 38.79, 38.71, 38.34, 37.78, 37.19, 34.39, 33.68, 30.88, 29.42, 27.97, 27.41, 25.61, 20.91, 19.46, 18.29, 16.12, 15.34, 14.64; ESI-HRMS (m/z) Calcd for C35H56NO3 [M + H]+: 538.4255. Found 538.4247.
3.2.2. Synthesis of N-(2-(2-(2-Propyn-1-yloxy)ethoxy)ethyl)-3β-hydroxy-lup-20(29)-en-28-amide (35)
Prepared from 32 and 2-(2-(2-propyn-1-yloxy)ethoxy)-ethanamine (27) according to general procedure A, the residue was purified by flash chromatography (eluent: PE:EtOAc = 2:1) to afford 35 as a white product with a yield of 58%. Rf = 0.16 (PE:EtOAc = 2:1); 1H NMR (400 MHz, CDCl3): δ 6.05 (t, 1H, J = 5.6 Hz), 4.72 (s, 1H), 4.58 (s, 1H), 4.19 (d, 2H, J = 2.4 Hz), 3.69–3.62 (m, 4H), 3.56–3.36 (m, 4H), 3.17 (dd, 1H, J = 11.4, 5.0 Hz), 3.11 (dt, 1H, J = 12.7, 5.8 Hz), 2.45–2.38 (m, 2H), 1.98–0.85 (m, other aliphatic ring protons), 1.67, 0.96, 0.95, 0.92, 0.80, 0.74 (s, each 3H, 6 × CH3), 0.66 (d, 1H, J = 9.0 Hz); 13C NMR (100 MHz, CDCl3): δ 176.18, 150.98, 109.28, 79.44, 78.94, 74.65, 70.08, 69.98, 68.96, 58.37, 55.69, 55.37, 50.60, 50.08, 46.81, 42.46, 40.73, 38.84, 38.78, 38.70, 38.33, 37.76, 37.19, 34.39, 33.65, 30.89, 29.40, 27.97, 27.41, 25.61, 20.91, 19.46, 18.29, 16.14, 16.11, 15.34, 14.63; ESI-HRMS (m/z) Calcd for C37H60NO4 [M + H]+: 582.4517. Found 582.4510.
3.2.3. Synthesis of N-(3,6,9,12-Tetraoxapentadec-14-yn-1-yl)-3β-hydroxy-lup-20(29)-en-28-amide (36)
Prepared from 32 and 3,6,9,12-tetraoxapentadec-14-yn-1-amine (28) according to general procedure A, the residue was purified by flash chromatography (eluent: PE:EtOAc = 1:3) to afford 36 as a white product with a yield of 69%. Rf = 0.30 (PE:EtOAc = 1:3); 1H NMR (400 MHz, CDCl3): δ 6.06 (s, 1H), 4.72 (s, 1H), 4.57 (s, 1H), 4.19 (s, 2H), 3.68–3.40 (m, 16H), 3.13 (m, 2H), 2.42 (t, 2H, J = 13.0 Hz), 1.94 (m, 2H), 1.77–0.95 (m, other aliphatic ring protons), 1.67 (s, 3H), 0.95 (s, 6H, 2 × CH3 ), 0.92 (s, 3H), 0.86 (d, 1H, J = 12.6 Hz), 0.86, 0.74 (s, each 3H, 2 × CH3 ), 0.66 (d, 1H, J = 7.7 Hz); 13C NMR (100 MHz, CDCl3): δ 176.15, 150.97, 109.26, 79.58, 78.91, 74.51, 70.59, 70.51, 70.40, 70.20, 70.05, 69.07, 58.37, 55.65, 55.36, 50.60, 50.09, 46.78, 42.44, 40.72, 38.82, 38.69, 38.31, 37.72, 37.17, 34.39, 33.62, 30.88, 29.39, 27.96, 27.40, 25.60, 20.90, 19.46, 18.28, 16.14, 16.10, 15.33, 14.62; ESI-HRMS (m/z) Calcd for C41H71N2O6 [M + NH4]+: 687.5307. Found 687.5324.
3.2.4. Synthesis of N-(3,6,9,12,15,18-Hexaoxaheneicos-20-yn-1-yl)-3β-hydroxy-lup-20(29)-en-28-amide (37)
Prepared from 32 and 3,6,9,12,15,18-hexaoxaheneicos-20-yn-1-amine (29) according to general procedure A, the residue was purified by flash chromatography (eluent: PE:EtOAc = 1:3) to afford 37 as a colourless oil with a yield of 61%. Rf = 0.17 (PE:EtOAc = 1:3); 1H NMR (400 MHz, CDCl3): δ 6.12 (t, 1H, J = 5.5 Hz), 4.71 (d,1H, J = 1.9 Hz), 4.56 (s, 1H), 4.18 (d, 2H, J = 2.4 Hz), 3.69–3.57 (m, 20H), 3.51 (t, 2H, J = 4.8 Hz), 3.48–3.42 (m, 1H), 3.40–3.33 (m, 1H), 3.15 (dd, 1H, J = 11.2, 5.1 Hz), 3.10 (dt, 1H, J = 11.6, 4.7 Hz), 2.45–2.38 (m, 2H), 1.97–1.87 (m, 2H), 1.77–0.79 (m, other aliphatic ring protons), 1.66 (s, 3H, CH3), 0.94 (s, 6H, 2 × CH3), 0.91, 0.79, 0.73 (each 3H, 3 × CH3), 0.65 (d, 1H, J = 8.9 Hz); 13C NMR (100 MHz, CDCl3): δ 176.14, 150.95, 109.22, 79.59, 78.85, 74.49, 70.51, 70.43, 70.34, 70.14, 70.05, 69.03, 58.33, 55.60, 55.32, 50.56, 50.06, 46.73, 42.39, 40.68, 38.79, 38.66, 38.28, 37.67, 37.14, 34.36, 33.55, 30.85, 29.35, 27.93, 27.36, 25.57, 20.87, 19.43, 18.25, 16.11, 16.07, 15.31, 14.59; ESI-HRMS (m/z) Calcd for C45H79N2O8 [M + NH4]+: 775.5831. Found 775.5829.
3.2.5. Synthesis of N-(3,6,9,12,15,18,21,24-Octaoxaheneicos-26-Yn-1-Yl)-3β-Hydroxy-lup-20(29)-en-28-amide (38)
Prepared from 32 and 3,6,9,12,15,18,21,24-octaoxaheptacos-26-yn-1-amine (30) according to general procedure A, the residue was purified by flash chromatography (eluent: PE:EtOAc = 1:3) to afford 38 as a colourless oil with a yield of 64%. Rf = 0.16 (PE:EtOAc = 1:3); 1H NMR (400 MHz, CDCl3): δ 6.08 (s, 1H), 4.69 (s, 1H), 4.54 (s, 1H), 4.17 (d, 2H, J = 1.5 Hz), 3.66–3.58 (m, 28H), 3.50–3.33 (m, 4H), 3.14 (dd, 1H, J = 10.7, 4.8 Hz), 3.12–3.06 (m, 1H), 2.42–2.32 (m, 2H), 1.94–1.86 (m, 2H), 1.75–1.70 (m, 1H), 1.64–0.82 (m, other aliphatic ring protons), 1.64 (s, 3H, 1 × CH3), 0.92 (s, 6H, 2 × CH3), 0.89, 0.77, 0.71 (s, each 3H, 3 × CH3), 0.63 (d, 1H, J = 8.7 Hz); 13C NMR (100 MHz, CDCl3): 176.07, 150.90, 109.20, 79.52, 78.75, 74.51, 70.44, 70.37, 70.28, 70.08, 69.96, 68.96, 58.28, 55.53, 55.23, 50.46, 49.95, 46.67, 42.32, 40.59, 38.73, 38.58, 38.23, 37.60, 37.05, 34.26, 33.50, 30.76, 29.28, 27.89, 27.28, 25.47, 20.79, 19.37, 18.18, 16.04, 15.29, 14.54; ESI-HRMS (m/z) Calcd for C49H87N2O10 [M + NH4+]+: 863.6361. Found 863.6345.
3.3. General procedure B for the Synthesis of Multivalent BA-CD Conjugates (52–68)
CuSO4·5H2O (0.10 mmol, 1.0 equiv.) and sodium L-ascorbate (1.1 equiv. per mol azide) were added to a solution of multiazide-substituted α-, β- and γ-CD (48–50) (0.10 mmol, 1.0 equiv.) and terminal propargylated OEG-tethered BA derivatives (33–38) (1.1 equiv. per mol azide) in THF-H2O (10 mL, v/v 1:1). The reaction vessel was placed in a vigorously stirred CEM Discover SP microwave reactor (100 °C, 50 W) and heated for 1 h. After the reaction mixture was extracted with CH2Cl2 (10 mL × 3), it was washed with water and brine and dried with anhydrous Na2SO4. The solvent was evaporated under reduced pressure, and the crude product was purified by column chromatography.
3.3.1. Synthesis of Heptakis 6-Deoxy-6-[4-N-(3β-Hydroxy-lup-20(29)-en-28-oyl)-aminomethyl-1H-1,2,3-triazol-1-yl]-2,3-di-O-acetyl-β-CD (52)
Prepared from 33 and 49 according to general procedure B, the residue was purified by flash chromatography (eluent: CH2Cl2:CH3OH = 12:1) to afford 52 as a white foam with a yield of 28%. Rf = 0.40 (CH2Cl2:CH3OH = 8:1); 1H NMR (400 MHz, CDCl3): δ 7.66 (s, 7H), 6.75 (s, 7H), 5.44 (s, 7H), 5.37–5.27 (m, 7H), 4.94 (d, 7H, J = 14.1 Hz), 4.81–4.54 (m, 35H), 4.74 (s, 7H), 4.19–4.01 (m, 7H), 3.53 (t, 7H, J = 8.0 Hz), 3.21–3.05 (m, 14H), 2.48 (m, 7H), 2.07 (s, 21H), 2.02 (s, 21H), 1.91–0.71 (m, other aliphatic ring protons), 1.65, 0.95, 0.95, 0.92, 0.81, 0.76 (s, each 21H, 42 × CH3), 0.67 (d, 7H, J = 8.4 Hz); 13C NMR (150 MHz, CDCl3): δ 176.47, 170.33, 169.37, 150.75, 145.45, 124.48, 109.53, 96.57, 78.91, 70.42, 69.96, 69.65, 55.57, 55.33, 50.61, 50.07, 46.60, 42.45, 40.73, 38.83, 38.70, 38.18, 37.59, 37.18, 34.82, 34.43, 33.52, 30.79, 27.96, 27.38, 27.18, 25.58, 20.91, 20.72, 20.65, 19.37, 18.30, 16.14, 15.37, 14.63; MALDI-TOF MS (m/z) Calcd for C301H448N28NaO56 [M + Na]+: 5374.30. Found 5374.64.
3.3.2. Synthesis of Octakis 6-Deoxy-6-[4-N-(3β-Hydroxy-lup-20(29)-en-28-oyl)-aminomethyl-1H-1,2,3-triazol-1-yl]-2,3-di-O-acetyl-γ-CD (53)
Prepared from 33 and 50 according to general procedure B, the residue was purified by flash chromatography (eluent: CH2Cl2:CH3OH = 10:1) to afford 53 as a white foam with a yield of 31%. Rf = 0.45 (CH2Cl2:CH3OH = 8:1); 1H NMR (400 MHz, CDCl3): δ 7.64 (s, 8H), 6.69 (s, 8H), 5.45 (s, 8H), 5.33 (t, 8H, J = 8.7 Hz), 4.85 (d, 8H, J = 13.6 Hz), 4.87–4.56 (m, 48H), 4.42 (s, 8H, J = 7.2 Hz), 4.08 (d, 8H, J = 14.9 Hz), 3.51 (t, 8H, J = 8.6 Hz), 3.17 (dd, 8H, J = 10.4, 3.2 Hz), 3.09 (dt, 8H, J = 11.0, 4.2 Hz), 2.47 (t, 8H, J = 10.0 Hz), 2.06 (s, 24H), 2.05 (m, 8H), 2.03 (s, 24H), 1.87–0.89 (m, other aliphatic ring protons), 1.64 (s, 24H, 8 × CH3), 0.95 (s, 48H, 16 × CH3), 0.92, 0.80, 0.74 (s, each 24H, 24 × CH3), 0.67 (d, 8H, J = 9.7 Hz); 13C NMR (150 MHz, CDCl3): δ 176.48, 170.29, 169.44, 150.76, 145.39, 124.56, 109.56, 96.22, 78.92, 76.04, 70.27, 69.95, 55.61, 55.34, 50.61, 50.09, 49.91, 46.65, 42.46, 40.75, 38.84, 38.71, 38.21, 37.63, 37.19, 34.85, 34.44, 33.51, 30.83, 29.52, 27.39, 25.58, 20.93, 20.78, 20.64, 19.36, 18.31, 16.16, 15.39, 14.64; MALDI-TOF MS (m/z) Calcd for C344H512N32NaO64 [M + Na]+: 6143.03. Found 6143.16.
3.3.3. Synthesis of Hexakis 6-Deoxy-6-[4-N-[(2-(2-Propyn-1-yloxy)ethyl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-2,3-di-O-acetyl-α-CD (54)
Prepared from 34 and 48 according to general procedure B, the residue was purified by flash chromatography (eluent: CH2Cl2:CH3OH = 20:1) to afford 54 as a white foam with a yield of 61%. Rf = 0.38 (CH2Cl2:CH3OH = 10:1); 1H NMR (600 MHz, CDCl3): δ 7.64 (s, 6H), 6.50 (s, 6H), 5.51 (s, 1H), 5.40 (s, 6H), 4.71–4.52 (m, 48H), 3.56–3.38 (m, 30H), 3.17 (m, 6H), 3.10 (m, 6H), 2.49 (m, 6H), 2.04–2.00 (m, 42H), 1.89–0.86 (m, other aliphatic ring protons), 1.66 (s, 18H, 6 × CH3), 0.95 (s, 36H, 12 × CH3), 0.93, 0.80, 0.74 (s, each 18H, 18 × CH3), 0.66 (d, 6H, J = 8.6 Hz); 13C NMR (150 MHz, CDCl3): δ 176.55, 170.32, 169.07, 150.93, 144.56, 125.55, 109.36, 96.40, 78.90, 70.63, 70.02, 69.69, 64.17, 55.58, 55.36, 50.61, 50.15, 46.67, 42.41, 40.73, 39.05, 38.83, 38.69, 38.29, 37.57, 37.18, 34.41, 33.56, 30.88, 29.41, 27.99, 27.39, 25.59, 20.94, 20.69, 19.47, 18.31, 16.19, 16.14, 15.37, 14.63; MALDI-TOF MS (m/z) Calcd for C270H408N24NaO54 [M + Na]+: 4877.34. Found 4877.08.
3.3.4. Synthesis of Heptakis 6-Deoxy-6-[4-N-[(2-(2-Propyn-1-yloxy)ethyl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-2,3-di-O-acetyl-β-CD (55)
Prepared from 34 and 49 according to general procedure B, the residue was purified by flash chromatography (eluent: CH2Cl2:CH3OH = 15:1) to afford 55 as a white foam with a yield of 20%. Rf = 0.29 (CH2Cl2:CH3OH = 10:1); 1H NMR (600 MHz, CDCl3): δ 7.71 (s, 7H), 6.46 (s, 7H), 5.46 (s, 7H), 5.34 (m, 7H), 4.89–4.44 (m, 56H), 3.54–3.36 (m, 35H), 3.16 (dd, 7H, J = 11.3, 4.4 Hz), 3.10 (dt, 7H, J = 10.9, 4.2 Hz), 2.49 (m, 7H), 2.06–2.00 (m, 49H), 1.90–0.86 (m, other aliphatic ring protons), 1.66 (s, 21H, 7 × CH3), 0.94 (s, 42H, 14 × CH3), 0.92, 0.80, 0.74 (s, each 21H, 21 × CH3), 0.66 (d, 7H, J = 8.9 Hz); 13C NMR (150 MHz, CDCl3): δ 176.52, 170.33, 169.30, 150.92, 144.80, 125.45, 109.38, 96.45, 78.89, 70.34, 69.83, 69.67, 64.15, 55.60, 55.35, 50.60, 50.13, 46.68, 42.41, 40.73, 39.00, 38.83, 38.69, 38.32, 37.57, 37.18, 34.41, 33.56, 30.86, 29.42, 27.99, 27.39, 25.58, 22.65, 20.93, 20.72, 20.67, 19.45, 18.31, 16.17, 16.14, 15.37, 14.63; MALDI-TOF MS (m/z) Calcd for C315H476N28NaO63 [M + Na]+: 5686.40. Found 5686.94.
3.3.5. Synthesis of Octakis 6-Deoxy-6-[4-N-[(2-(2-Propyn-1-yloxy)ethyl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-Yl]-2,3-di-O-acetyl-γ-CD (56)
Prepared from 34 and 50 according to general procedure B, the residue was purified by flash chromatography (eluent: CH2Cl2:CH3OH = 15:1) to afford 56 as a white foam with a yield of 34%. Rf = 0.15 (CH2Cl2:CH3OH = 10:1); 1H NMR (600 MHz, CDCl3): δ 7.75 (s, 8H), 6.48 (s, 8H), 5.48 (s, 8H), 5.35 (m, 8H), 4.83–4.40 (m, 64H), 3.56–3.39 (m, 40H), 3.17 (d, 8H, J = 7.0 Hz), 3.10 (dt, 8H, J = 10.9, 4.3 Hz), 2.49 (t, 8H, J = 9.7 Hz), 2.07–2.02 (m, 56H), 1.92–0.86 (m, other aliphatic ring protons), 1.66 (s, 24H, 8 × CH3), 0.95 (s, 48H, 16 × CH3), 0.93, 0.80, 0.74 (s, each 24H, 24 × CH3), 0.66 (d, 8H, J = 8.9 Hz); 13C NMR (150 MHz, CDCl3): δ 176.54, 170.26, 169.40, 150.93, 144.85, 125.54, 109.38, 96.18, 78.90, 69.97, 69.65, 64.24, 55.61, 55.36, 50.61, 50.13, 49.91, 46.68, 42.42, 40.74, 38.93, 38.84, 38.70, 38.34, 37.58, 37.19, 34.41, 33.57, 30.88, 29.42, 28.00, 27.40, 25.60, 20.95, 20.75, 20.65, 19.46, 18.32, 16.18, 16.15, 15.39, 14.64; MALDI-TOF MS (m/z) Calcd for C360H545N32O72 [M + H]+: 6473.47. Found 6473.59.
3.3.6. Synthesis of Hexakis 6-Deoxy-6-[4-N-[(2-(2-(2-Propyn-1-yloxy)ethoxy)ethyl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-2,3-di-O-acetyl-α-CD (57)
Prepared from 35 and 48 according to general procedure B, the residue was purified by flash chromatography (eluent: CH2Cl2:CH3OH = 20:1) to afford 57 as a white foam with a yield of 74%. Rf = 0.40 (CH2Cl2:CH3OH = 10:1); 1H NMR (600 MHz, CDCl3): δ 7.73 (s, 6H), 6.24 (t, 6H, J = 5.5 Hz), 5.49 (t, 6H, J = 9.5 Hz), 5.41 (d, 6H, J = 3.1 Hz), 4.71–4.50 (m, 48H), 3.64–3.34 (m, 54H), 3.16 (d, 6H, J = 11.0 Hz), 3.10 (dt, 6H, J = 11.0, 4.3 Hz), 2.47–2.43 (m, 6H), 2.03–1.99 (m, 42H), 1.93–1.88 (m, 6H), 1.78–0.86 (m, other aliphatic ring protons), 1.66 (s, 18H, 6 × CH3), 0.95 (s, 36H, 12 × CH3), 0.92, 0.80, 0.74 (s, each 18H, 18 × CH3), 0.66 (d, 6H, J = 9.4 Hz); 13C NMR (150 MHz, CDCl3): δ 176.30, 170.30, 169.07, 150.96, 144.70, 125.63, 109.33, 96.74, 78.89, 70.83, 70.06, 70.01, 69.94, 64.40, 55.62, 55.34, 50.58, 50.09, 46.73, 42.43, 40.72, 38.83, 38.75, 38.68, 38.33, 37.65, 37.17, 34.40, 33.57, 30.87, 29.40, 27.98, 27.39, 25.59, 20.91, 20.71, 19.45, 18.30, 16.16, 16.13, 15.37, 14.63; MALDI-TOF MS (m/z) Calcd for C282H432N24NaO60 [M + Na]+: 5141.66. Found 5141.23.
3.3.7. Synthesis of Heptakis 6-Deoxy-6-[4-N-[(2-(2-(2-Propyn-1-yloxy)ethoxy)ethyl)-3β-hydroxy-lup-20(29)-en-28-Oyl]-aminomethyl-1H-1,2,3-triazol-1-Yl]-2,3-di-O-acetyl-β-CD (58)
Prepared from 35 and 49 according to general procedure B, the residue was purified by flash chromatography (eluent: CH2Cl2:CH3OH = 15:1) to afford 58 as a white foam with a yield of 60%. Rf = 0.39 (CH2Cl2:CH3OH = 10:1); 1H NMR (600 MHz, CDCl3): δ 7.76 (s, 7H), 6.23 (t, 7H, J = 5.5 Hz), 5.49 (s, 7H), 5.35 (t, 7H, J = 8.6 Hz), 4.87–4.45 (m, 56H), 3.64–3.34 (m, 63H), 3.16 (dd, 7H, J = 11.5, 4.5 Hz), 3.10 (dt, 7H, J = 11.1, 4.4 Hz), 2.44 (dt, 7H, J = 12.7, 3.3 Hz), 2.05–1.88 (m, 56H), 1.77–0.84 (m, other aliphatic ring protons), 1.66, 0.95, 0.94, 0.92, 0.80, 0.73 (s, each 21H, 42 × CH3), 0.66 (d, 7H, J = 9.4 Hz); 13C NMR (150 MHz, CDCl3): δ 176.30, 170.35, 169.32, 150.93, 144.86, 125.53, 109.35, 96.33, 78.88, 76.53, 70.54, 70.06, 70.04, 69.90, 69.71, 64.39, 55.63, 55.34, 50.58, 50.09, 46.74, 42.43, 40.72, 38.83, 38.79, 38.69, 38.34, 37.66, 37.18, 34.40, 33.59, 30.88, 29.40, 27.99, 27.39, 25.59, 20.91, 20.72, 20.67, 19.46, 18.30, 16.16, 16.13, 15.38, 14.64; MALDI-TOF MS (m/z) Calcd for C329H504N28NaO70 [M + Na]+: 5994.77. Found 5994.38.
3.3.8. Synthesis of Octakis 6-Deoxy-6-[4-N-[(2-(2-(2-Propyn-1-yloxy)ethoxy)ethyl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-2,3-di-O-acetyl-γ-CD (59)
Prepared from 35 and 50 according to general procedure B, the residue was purified by flash chromatography (eluent: CH2Cl2:CH3OH = 15:1) to afford 59 as a white foam with a yield of 68%. Rf = 0.33 (CH2Cl2:CH3OH = 10:1); 1H NMR (600 MHz, CDCl3): δ 7.77 (s, 8H), 6.24 (t, 8H, J = 5.5 Hz), 5.52 (s, 8H), 5.35 (t, 8H, J = 8.7 Hz), 4.79–4.44 (m, 64H), 3.65–3.34 (m, 72H), 3.16 (d, 8H, J = 10.9 Hz), 3.10 (dt, 8H, J = 11.0, 4.4 Hz), 2.44 (dt, 8H, J = 12.6, 3.2 Hz), 2.05–1.97 (m, 56H), 1.95–0.86 (m, other aliphatic ring protons), 1.66, 0.95, 0.94, 0.91, 0.79, 0.73 (s, each 24H, 48 × CH3), 0.66 (d, 8H, J = 9.4 Hz); 13C NMR (150 MHz, CDCl3): δ 176.29, 170.26, 169.42, 150.92, 144.85, 125.58, 109.33, 95.98, 78.85, 75.71, 70.25, 70.06, 70.03, 69.88, 69.82, 64.40, 55.62, 55.33, 50.57, 50.08, 46.73, 42.42, 40.71, 38.82, 38.78, 38.68, 38.33, 37.65, 37.16, 34.39, 33.57, 30.87, 29.39, 27.98, 27.38, 25.58, 20.90, 20.76, 20.63, 19.45, 18.29, 16.15, 16.12, 15.38, 14.63; MALDI-TOF MS (m/z) Calcd for C376H577N32O80 [M + H]+: 6825.90. Found 6824.69.
3.3.9. Synthesis of Hexakis 6-Deoxy-6-[4-N-[(3,6,9,12-Tetraoxapentadec-14-yn-1-yl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-2,3-di-O-acetyl-α-CD (60)
Prepared from 36 and 48 according to general procedure B, the residue was purified by flash chromatography (eluent: CH2Cl2:CH3OH = 7:1) to afford 60 as a white foam with a yield of 39%. Rf = 0.31 (CH2Cl2:CH3OH = 10:1); 1H NMR (600 MHz, CDCl3): δ 7.68 (s, 6H), 6.11 (t, 6H, J = 5.3 Hz), 5.46 (t, 6H, J = 9.1 Hz), 5.40 (d, 6H, J = 2.8 Hz), 4.64 (m, 48H), 3.66–3.50 (m, 84H), 3.45 (m, 6H), 3.37 (m, 6H), 3.16 (dd, 6H, J = 11.5, 4.6 Hz), 3.10 (dt, 6H, J = 11.1, 4.4 Hz), 2.42 (m, 6H), 2.07–1.90 (m, 48H), 1.75 (m, 6H), 1.68–0.97 (m, other aliphatic ring protons), 1.66, 0.95, 0.94, 0.91, 0.80, 0.73 (s, each 18H, 36 × CH3), 0.87 (m, 6H), 0.66 (d, 6H, J = 9.2 Hz); 13C NMR (150 MHz, CDCl3): δ 176.17, 170.25, 168.99, 150.93, 144.64, 125.60, 109.29, 96.69, 78.85, 70.94, 70.47, 70.43, 70.14, 70.02, 69.94, 69.79, 64.39, 55.63, 55.34, 50.58, 50.51, 50.08, 46.77, 42.43, 40.71, 38.82, 38.68, 38.30, 37.70, 37.16, 34.38, 33.60, 30.87, 29.64, 29.38, 29.26, 27.98, 27.38, 27.15, 25.58, 20.89, 20.71, 20.67, 19.45, 18.28, 16.14, 16.11, 15.36, 14.63; MALDI-TOF MS (m/z) Calcd for C306H481N24O72 [M + H]+: 5648.31. Found 5648.34.
3.3.10. Synthesis of Heptakis 6-Deoxy-6-[4-N-[(3,6,9,12-Tetraoxapentadec-14-yn-1-yl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-2,3-di-O-acetyl-β-CD (61)
Prepared from 36 and 49 according to general procedure B, the residue was purified by flash chromatography (eluent: CH2Cl2:CH3OH = 10:1) to afford 61 as a white foam with a yield of 31%. Rf = 0.28 (CH2Cl2:CH3OH = 10:1); 1H NMR (600 MHz, CDCl3): δ 7.75 (s, 7H), 6.12 (s, 7H), 5.49 (s, 7H), 5.35 (m, 7H), 4.87–4.47 (m, 56H), 3.63 (m, 84H), 3.52 (m, 28H), 3.37 (m, 7H), 3.16 (dd, 7H, J = 11.5, 4.6 Hz), 3.10 (dt, 7H, J = 11.2, 4.4 Hz), 2.42 (dt, 7H, J = 12.8, 3.2 Hz), 1.99 (m, 56H), 1.75 (m, 7H), 1.66–1.11 (m, other aliphatic ring protons), 1.66, 0.95, 0.94, 0.92, 0.80, 0.73 (s, each 21H, 42 × CH3), 0.87 (m, 7H), 0.66 (d, 7H, J = 9.3 Hz); 13C NMR (150 MHz, CDCl3): δ 176.19, 170.33, 169.33, 150.93, 144.86, 125.53, 109.31, 96.29, 78.86, 76.47, 70.48, 70.44, 70.39, 70.15, 70.04, 69.92, 69.65, 64.41, 55.64, 55.35, 50.59, 50.09, 49.96, 46.78, 42.44, 40.72, 38.83, 38.69, 38.31, 37.72, 37.17, 34.39, 33.60, 29.65, 29.39, 29.27, 27.98, 27.39, 27.16, 25.59, 25.50, 20.90, 20.71, 20.67, 19.46, 18.29, 16.15, 16.11, 15.37, 14.64; MALDI-TOF MS (m/z) Calcd for C357H560N28NaO84 [M + Na]+: 6611.51. Found 6611.99.
3.3.11. Synthesis of Octakis 6-Deoxy-6-[4-N-[(3,6,9,12-Tetraoxapentadec-14-yn-1-yl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-2,3-di-O-acetyl-γ-CD (62)
Prepared from 36 and 50 according to general procedure B, the residue was purified by flash chromatography (eluent: CH2Cl2:CH3OH = 7:1) to afford 62 as a white foam with a yield of 56%. Rf = 0.38 (CH2Cl2:CH3OH = 10:1); 1H NMR (600 MHz, CDCl3): δ 7.76 (s, 8H), 6.12 (t, 8H, J = 5.2 Hz), 5.53 (s, 8H), 5.35 (t, 8H, J = 8.6 Hz), 4.76 (m, 32H), 4.57 (s, 24H), 4.44 (s, 8H), 3.67 (s, 16H), 3.61 (m, 80H), 3.52 (m, 24H), 3.46 (m, 8H), 3.37 (m, 8H), 3.16 (dd, 8H, J = 11.4, 4.6 Hz), 3.10 (dt, 8H, J = 11.1, 4.3 Hz), 2.42 (dt, 8H, J = 12.7, 3.1 Hz), 1.97 (m, 64H), 1.75 (m, 8H), 1.68–1.11 (m, other aliphatic ring protons), 1.66, 0.95, 0.94, 0.92, 0.80, 0.73 (s, each 24H, 48 × CH3), 0.87 (m, 8H), 0.66 (d, 8H, J = 9.2 Hz); 13C NMR (150 MHz, CDCl3): δ 176.18, 170.25, 169.46, 150.93, 144.88, 125.57, 109.31, 95.94, 78.85, 70.49, 70.45, 70.41, 70.15, 70.04, 69.91, 69.80, 64.43, 55.64, 55.35, 50.58, 50.09, 49.87, 46.78, 42.44, 40.72, 38.83, 38.69, 38.31, 37.72, 37.17, 34.39, 33.60, 29.65, 29.39, 29.27, 27.98, 27.39, 25.59, 20.90, 20.77, 20.64, 19.46, 18.29, 16.15, 16.12, 15.38, 14.64; MALDI-TOF MS (m/z) Calcd for C408H641N32O96 [M + H]+: 7530.74. Found 7529.87.
3.3.12. Synthesis of Hexakis 6-Deoxy-6-[4-N-[(3,6,9,12,15,18-Hexaoxaheneicos-20-yn-1-yl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yL]-2,3-dI-O-acetyl-α-CD (63)
Prepared from 37 and 48 according to general procedure B, the residue was purified by flash chromatography (eluent: CH2Cl2:CH3OH = 10:1) to afford 63 as a white foam with a yield of 90%. Rf = 0.42 (CH2Cl2:CH3OH = 10:1); 1H NMR (600 MHz, CDCl3): δ 7.68 (s, 6H), 6.12 (t, 6H, J = 5.0 Hz), 5.45 (t, 6H, J = 8.2 Hz), 5.39 (s, 6H), 4.70–4.55 (m, 48H), 3.64–3.58 (m, 120H), 3.51–3.44 (m, 24H), 3.38–3.34 (m, 6H), 3.14 (dd, 6H, J = 11.4, 4.6 Hz), 3.09 (dt, 6H, J = 11.1, 4.3 Hz), 2.41 (dt, 6H, J = 12.7, 3.3 Hz), 2.03–1.88 (m, 60H), 1.75 –0.84 (m, other aliphatic ring protons), 1.65, 0.93, 0.92, 0.90, 0.78, 0.72 (s, each 18H, 36 × CH3), 0.64 (d, 6H, J = 9.2 Hz); 13C NMR (150 MHz, CDCl3): δ 176.14, 170.22, 169.06, 150.91, 144.62, 125.58, 109.23, 96.48, 78.79, 77.00, 70.41, 70.40, 70.34, 70.10, 70.03, 69.86, 64.33, 55.59, 55.31, 50.54, 50.42, 50.04, 46.72, 42.38, 40.67, 38.78, 38.65, 38.26, 37.66, 37.12, 34.34, 33.53, 30.83, 29.34, 27.94, 27.34, 25.55, 20.85, 20.67, 20.63, 19.41, 18.24, 16.10, 16.06, 15.33, 14.59; MALDI-TOF MS (m/z) Calcd for C330H528N24NaO84 [M + Na]+: 6198.93. Found 6198.17.
3.3.13. Synthesis of Heptakis 6-Deoxy-6-[4-N-[(3,6,9,12,15,18-Hexaoxaheneicos-20-yn-1-yl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-2,3-di-O-acetyl-β-CD (64)
Prepared from 37 and 49 according to general procedure B, the residue was purified by flash chromatography (eluent: CH2Cl2:CH3OH = 23:2) to afford 64 as a white foam with a yield of 19%. Rf = 0.25 (CH2Cl2:CH3OH = 10:1); 1H NMR (600 MHz, CDCl3): δ 7.75 (s, 7H), 6.13 (s, 7H), 5.49 (s, 7H), 5.35 (s, 7H), 4.84 (m, 7H), 4.72–4.66 (m, 21H), 4.57–4.47 (m, 28H), 3.62–3.60 (m, 140H), 3.53–3.46 (m, 28H), 3.39–3.36 (m, 7H), 3.16 (dd, 7H, J = 11.3, 4.4 Hz), 3.11 (dt, 7H, J = 11.1, 4.1 Hz), 2.43 (t, 7H, J = 10.7 Hz), 2.05–1.92 (m, 70H), 1.77–0.86 (m, other aliphatic ring protons), 1.67, 0.95, 0.94, 0.92, 0.80, 0.74 (s, each 21H, 42 × CH3), 0.66 (d, 7H, J = 9.4 Hz); 13C NMR (150 MHz, CDCl3): δ 176.20, 170.34, 169.37, 150.97, 144.87, 125.58, 109.30, 96.30, 78.88, 77.21, 70.65, 70.50, 70.47, 70.37, 70.17, 70.09, 69.91, 69.77, 69.67, 64.41, 55.66, 55.36, 50.60, 50.10, 49.98, 46.79, 42.45, 40.73, 38.84, 38.71, 38.32, 37.73, 37.18, 34.40, 33.60, 31.89, 30.89, 29.74, 29.66, 29.62, 29.58, 29.52, 29.48, 29.44, 29.40, 29.32, 29.28, 29.22, 27.99, 27.40, 25.61, 20.92, 20.73, 20.68, 19.47, 18.30, 16.16, 16.12, 15.38, 14.65; MALDI-TOF MS (m/z) Calcd for C385H616N28NaO98 [M + Na]+: 7228.25. Found 7228.56.
3.3.14. Synthesis of Octakis 6-Deoxy-6-[4-N-[(3,6,9,12,15,18-Hexaoxaheneicos-20-yn-1-yl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-2,3-di-O-acetyl-γ-CD (65)
Prepared from 37 and 50 according to general procedure B, the residue was purified by flash chromatography (eluent: CH2Cl2:CH3OH = 10:1) to afford 65 as a white foam with a yield of 83%. Rf = 0.34 (CH2Cl2:CH3OH = 10:1); 1H NMR (600 MHz, CDCl3): δ 7.77 (s, 8H), 6.12 (s, 8H), 5.52 (s, 8H), 5.34 (t, 8H, J = 7.9 Hz), 4.80–4.44 (m, 64H), 3.65–3.60 (m, 160H), 3.53–3.45 (m, 32H), 3.39–3.36 (m, 8H), 3.16 (d, 8H, J = 10.9 Hz), 3.10 (dt, 8H, J = 11.2, 4.4 Hz), 2.42 (dt, 8H, J = 12.8, 3.4 Hz), 2.05–1.89 (m, 80H), 1.77–0.85 (m, other aliphatic ring protons), 1.66, 0.95, 0.94, 0.92, 0.80, 0.73 (s, each 24H, 48 × CH3), 0.66 (d, 8H, J = 9.3 Hz); 13C NMR (150 MHz, CDCl3): δ 176.19, 170.26, 169.45, 150.96, 144.84, 125.63, 109.28, 95.94, 78.85, 77.00, 75.60, 70.48, 70.45, 70.36, 70.15, 70.08, 69.88, 64.39, 55.64, 55.35, 50.59, 50.08, 46.77, 42.43, 40.72, 38.83, 38.69, 38.31, 37.72, 37.17, 34.39, 33.59, 30.88, 29.39, 27.98, 27.39, 25.59, 20.90, 20.76, 20.64, 19.46, 18.28, 16.15, 16.11, 15.37, 14.63; MALDI-TOF MS (m/z) Calcd for C440H704N32NaO112 [M + Na]+: 8257.57. Found 8257.04.
3.3.15. Synthesis of Hexakis 6-Deoxy-6-[4-N-[(3,6,9,12,15,18,21,24-Octaoxaheptacos-26-yn-1-yl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-2,3-di-O-acetyl-α-CD (66)
Prepared from 38 and 48 according to general procedure B, the residue was purified by flash chromatography (eluent: CH2Cl2:CH3OH = 10:1) to afford 66 as a white foam with a yield of 90%. Rf = 0.29 (CH2Cl2:CH3OH = 10:1); 1H NMR (600 MHz, CDCl3): δ 7.69 (s, 1H), 6.09 (s, 1H), 5.47 (t, 1H, J = 8.6 Hz), 5.41 (s, 1H), 4.72–4.57 (m, 8H), 3.66–3.60 (m, 28H), 3.54–3.45 (m, 4H), 3.41–3.36 (m, 1H), 3.18–3.15 (m, 1H), 3.11 (dt, 1H, J = 11.2, 4.4 Hz), 2.43 (dt, 1H, J = 12.8, 3.5 Hz), 2.04–1.94 (m, 8H), 1.77–0.86 (m, other aliphatic ring protons), 1.67, 0.96, 0.95, 0.92, 0.80, 0.74 (s, each 3H, 6 × CH3), 0.66 (d, 1H, J = 9.2 Hz); 13C NMR (150 MHz, CDCl3): 176.17, 170.27, 169.07, 150.98, 144.69, 125.63, 109.29, 96.60, 78.89, 77.00, 70.53, 70.51, 70.48, 70.40, 70.18, 70.08, 69.94, 64.39, 55.66, 55.36, 50.60, 50.10, 46.79, 42.45, 40.73, 38.84, 38.70, 38.32, 37.73, 37.18, 34.40, 33.62, 30.89, 29.40, 27.98, 27.41, 25.61, 20.91, 20.73, 20.69, 19.47, 18.29, 16.16, 16.12, 15.36, 14.64; MALDI-TOF MS (m/z) Calcd for C354H576N24NaO96 [M + Na]+: 6727.56. Found 6727.62.
3.3.16. Synthesis of Heptakis 6-Deoxy-6-[4-N-[(3,6,9,12,15,18,21,24-Octaoxaheptacos-26-yn-1-yl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-2,3-di-O-acetyl-β-CD (67)
Prepared from 38 and 49 according to general procedure B, the residue was purified by flash chromatography (eluent: CH2Cl2:CH3OH = 10:1) to afford 67 as a white foam with a yield of 64%. Rf = 0.34 (CH2Cl2:CH3OH = 10:1); 1H NMR (600 MHz, CDCl3): δ 7.76 (s, 1H), 6.12 (s,1 H), 5.50 (s,1 H), 5.35 (s, 1H), 4.85–4.48 (m, 8H), 3.64–3.36 (m, 33H), 3.17 (d, 1H, J = 10.3 Hz), 3.11 (dt, 1H, J = 11.2, 4.5 Hz), 2.43 (dt, 1H, J = 12.8, 3.5 Hz), 2.05–1.91 (m, 8H), 1.82–0.85 (m, other aliphatic ring protons), 1.67, 0.96, 0.95, 0.92, 0.81, 0.74 (s, each 3H, 6 × CH3), 0.67 (d, 1H, J = 9.2 Hz); 13C NMR (150 MHz, CDCl3): 176.20, 170.34, 169.41, 150.99, 144.83, 125.60, 109.29, 96.30, 78.90, 77.00, 70.54, 70.51, 70.49, 70.37, 70.19, 70.10, 69.94, 64.42, 55.67, 55.37, 50.61, 50.11, 46.80, 42.46, 40.74, 38.85, 38.71, 38.33, 37.74, 37.19, 34.41, 33.62, 30.90, 29.41, 28.00, 27.42, 25.62, 20.92, 19.48, 18.31, 16.17, 16.13, 15.38, 14.65. MALDI-TOF MS (m/z) Calcd for C413H672N28NaO112 [M + Na]+: 7844.99. Found 7845.54.
3.3.17. Synthesis of Octakis 6-Deoxy-6-[4-N-[(3,6,9,12,15,18,21,24-Octaoxaheptacos-26-yn-1-yl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-2,3-di-O-acetyl-γ-CD (68)
Prepared from 38 and 50 according to general procedure B, the residue was purified by flash chromatography (eluent: CH2Cl2:CH3OH = 10:1) to afford 68 as a white foam with a yield of 73%. Rf = 0.34 (CH2Cl2:CH3OH = 10:1); 1H NMR (600 MHz, CDCl3): δ 7.76 (s, 1H), 6.10 (t, 1H, J = 8.6 Hz), 5.52 (s, 1H), 5.34 (t, 1H, J = 8.3 Hz), 4.80–4.44 (m, 8H), 3.66–3.60 (m, 28H), 3.53–3.45 (m, 4H), 3.40–3.35 (m, 1H), 3.16 (dd, 1H, J = 11.0, 3.8 Hz), 3.11 (dt, 1H, J = 11.2, 4.4 Hz), 2.44 (dt, 1H, J = 11.6, 3.5 Hz), 2.05–1.90 (m, 8H), 1.77–0.85 (m, other aliphatic ring protons), 1.67, 0.95, 0.94, 0.92, 0.80, 0.74 (s, each 3H, 6 × CH3), 0.66 (d, 1H, J = 9.1 Hz); 13C NMR (150 MHz, CDCl3): 176.16, 170.24, 169.46, 150.96, 144.87, 125.59, 109.28, 95.93, 78.87, 77.00, 75.60, 70.52, 70.50, 70.48, 70.37, 70.17, 70.06, 69.90, 69.77, 64.40, 55.64, 55.36, 50.59, 50.09, 46.78, 42.44, 40.72, 38.83, 38.70, 38.31, 37.72, 37.17, 34.39, 33.60, 30.88, 29.39, 27.98, 27.40, 25.60, 20.90, 20.77, 20.65, 19.47, 18.29, 16.15, 16.11, 15.36, 14.63; MALDI-TOF MS (m/z) Calcd for C472H768N32NaO128 [M + Na]+: 8962.42. Found 8961.29.
3.4. General Procedure C for the Synthesis of Multivalent BA-CD Conjugates (69–86)
The per-O-acetylated multivalent BA-CD conjugates (51–68) were dissolved in CH3OH (~5 mL per 100 mg of conjugate). CH3ONa (0.1 eq per mol of acetate, 30% in CH3OH) was added, and the solution was stirred at room temperature for 6 h. The solution was neutralized with Amberlite IR-120 H+ resin and filtered, the solvent was evaporated under reduced pressure, and the crude product was purified by short RP column chromatography (eluted by CH3OH) to afford the desired products.
3.4.1. Synthesis of Hexakis 6-Deoxy-6-[4-N-(3β-Hydroxy-lup-20(29)-en-28-oyl)-aminomethyl-1H-1,2,3-triazol-1-yl]-α-CD (69)
Prepared from 51 according to general procedure C, the residue was purified by RP flash chromatography (eluent: methanol) to afford 69 as a white foam with a yield of 89%; 1H NMR (600 MHz, CDCl3/CD3OD = 1:1 v/v): δ 7.64 (s, 6H), 5.08 (d, 6H, J = 2.6 Hz), 4.65(s, 6H), 4.57 (s, 6H, overlap with H2O), 4.54(s, 6H), 4.38(d, 6H, J = 15.3 Hz), 4.33(d, 6H, J = 9.9 Hz), 4.23(m, 6H), 4.10(d, 6H, J = 15.3 Hz), 3.97(t, 6H, J = 9.1 Hz), 3.42(dd, 6H, J = 10.0, 2.5 Hz), 3.23(t, 6H, J = 8.8 Hz), 3.11(dd, 6H, J = 10.3, 5.6 Hz), 3.02(m, 6H), 2.49(t, 6H, J = 10.0 Hz), 2.09(d, 6H, J = 12.3 Hz), 2.05–0.88(m, other aliphatic ring protons), 1.63, 0.94, 0.92, 0.90, 0.81, 0.72 (s, each 18H, 36 × CH3), 0.66(d, 6H, J = 9.1 Hz); 13C NMR (150 MHz, CDCl3/CD3OD = 1:1 v/v): δ 177.97, 151.35, 145.87, 125.39, 109.94, 102.34, 83.42, 79.12, 73.67, 72.39, 70.82, 56.23, 56.06, 51.26, 50.98, 50.68, 47.24, 42.95, 41.33, 39.42, 39.36, 38.77, 38.16, 37.72, 35.06, 34.99, 33.65, 31.33, 29.9928.30, 27.39, 26.19, 21.54, 19.60, 18.91, 16.59, 16.53, 15.84; MALDI-TOF MS (m/z) Calcd for C234H360N24NaO36 [M + Na]+: 4105.70. Found 4105.12.
3.4.2. Synthesis of Heptakis 6-Deoxy-6-[4-N-(3β-Hydroxy-lup-20(29)-en-28-oyl)-aminomethyl-1H-1,2,3-triazol-1-yl]-β-CD (70)
Prepared from 52 according to general procedure C, the residue was purified by RP flash chromatography (eluent: methanol) to afford 70 as a white foam with a yield of 85%; 1H NMR (400 MHz, CDCl3/CD3OD = 1:1 v/v): δ 7.66 (s, 7H), 5.09 (d, 7H, J = 2.6 Hz), 4.54 (s, 14H), 4.43–4.33 (m, 14H), 4.16–4.12 (m, 14H), 3.86 (t, 7H, J = 9.0 Hz), 3.47–3.43 (m, 7H), 3.25 (t, 7H, J = 9.1 Hz), 3.14–3.10 (m, 7H), 3.04 (s, 7H), 2.52 (t, 7H, J = 10.0 Hz), 2.14–2.11 (m, 7H), 1.76–0.86 (m, other aliphatic ring protons), 1.64, 0.95 (s, each 21H, 14 × CH3), 0.93 (s, 42H, 14 × CH3), 0.82, 0.73 (s, each 21H, 14 × CH3), 0.67 (d, 7H, J = 7.4 Hz); 13C NMR (100 MHz, CDCl3/CD3OD = 1:1 v/v): δ 178.29, 151.53, 146.31, 125.47, 110.10, 102.89, 83.62, 79.23, 73.57, 73.03, 71.13, 56.39, 56.27, 51.47, 51.13, 50.86, 47.43, 43.12, 41.52, 39.53, 38.96, 38.33, 37.89, 35.18, 33.81, 31.53, 30.30, 30.19, 30.03, 29.91, 28.44, 27.55, 26.39, 21.74, 19.66, 19.12, 16.75, 16.00, 15.11, 14.38; MALDI-TOF MS (m/z) Calcd for C234H360N24NaO36 [M + Na]+: 4105.70. Found 4105.12.
3.4.3. Synthesis of Octakis 6-Deoxy-6-[4-N-(3β-Hydroxy-lup-20(29)-en-28-oyl)-aminomethyl-1H-1,2,3-triazol-1-yl]-γ-CD (71)
Prepared from 53 according to general procedure C, the residue was purified by RP flash chromatography (eluent: methanol) to afford 71 as a white foam with a yield of 85%; 1H NMR (600 MHz, CDCl3/CD3OD = 1:1 v/v): δ 7.64 (s, 8H), 5.11 (s, 8H), 4.65 (s, 8H), 4.53 (s, 8H, overlap with H2O), 4.41–4.14 (m, 24H), 3.85 (s, 8H), 3.46 (s, 8H), 3.24 (s, 8H), 3.11 (s, 8H), 3.03 (s, 8H), 2.51 (s, 8H), 2.11 (s, 8H), 1.76–0.94 (m, other aliphatic ring protons), 1.63, 0.94 (s, each 24H, 16 × CH3), 0.92 (s, 48H, 16 × CH3), 0.81, 0.72 (s, each 24H, 16 × CH3), 0.66 (s, 8H); 13C NMR (150 MHz, CDCl3/CD3OD = 1:1 v/v): δ 177.89, 151.36, 145.97, 125.18, 109.90, 102.51, 82.92, 79.09, 73.08, 70.71, 63.77, 56.19, 56.02, 51.23, 50.66, 47.18, 42.93, 41.30, 39.39, 39.32, 38.74, 38.10, 37.69, 35.12, 34.97, 33.64, 31.32, 30.10, 29.96, 28.29, 27.36, 26.17, 21.52, 19.60, 18.88, 16.58, 16.53, 15.82, 14.99; MALDI-TOF MS (m/z) Calcd for C312H480N32NaO48 [M + Na]+: 5466.60. Found 5467.72.
3.4.4. Synthesis of Hexakis 6-Deoxy-6-[4-N-[(2-(2-Propyn-1-yloxy)ethyl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-α-CD (72)
Prepared from 54 according to general procedure C, the residue was purified by RP flash chromatography (eluent: methanol) to afford 72 as a white foam with a yield of 88%; 1H NMR (600 MHz, CDCl3/CD3OD = 1:1 v/v): δ 7.86 (s, 6H), 5.10 (s, 6H), 4.68–4.45 (m, 36H, overlap with H2O), 4.23 (s, 6H), 3.99 (m, 6H), 3.50–3.41 (m, 18H), 3.24 (m, 6H), 3.12 (dd, 6H, J = 10.9, 5.2 Hz), 3.05 (m, 6H), 2.49 (t, 6H, J = 11.1 Hz), 2.09 (d, 6H, J = 12.5 Hz), 1.86–1.77 (m, 12H), 1.65–0.85 (m, other aliphatic ring protons), 1.65, 0.95 (s, each 18H, 12 × CH3), 0.92 (s, 36H, 12 × CH3), 0.81, 0.72 (s, each 18H, 12 × CH3), 0.66 (d, 6H, J = 9.9 Hz); 13C NMR (150 MHz, CDCl3/CD3OD = 1:1 v/v): δ 178.33, 151.56, 145.31, 126.41, 109.92, 102.68, 83.53, 79.18, 73.79, 72.57, 70.96, 70.11, 64.44, 58.01, 56.42, 56.22, 51.40, 50.83, 49.85, 47.48, 43.06, 41.46, 39.56, 39.48, 38.97, 38.35, 37.84, 35.13, 33.80, 31.50, 30.10, 28.41, 27.51, 26.34, 21.67, 19.70, 19.02, 16.64, 15.94, 15.11; MALDI-TOF MS (m/z) Calcd for C246H384N24NaO42 [M + Na]+: 4369.85. Found 4370.96.
3.4.5. Synthesis of Heptakis 6-Deoxy-6-[4-N-[(2-(2-Propyn-1-yloxy)ethyl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-β-CD (73)
Prepared from 55 according to general procedure C, the residue was purified by RP flash chromatography (eluent: methanol) to afford 73 as a white foam with a yield of 85%; 1H NMR (600 MHz, CDCl3/CD3OD = 1:1 v/v): δ 7.86 (s, 7H), 5.12 (d, 7H, J = 3.0 Hz), 4.68 (s, 7H), 4.58–4.44 (m, 35H), 4.17 (m, 7H), 3.88 (t, 7H, J = 9.2 Hz), 3.54–3.49 (m, 14H), 3.44–3.42 (m, 7H), 3.23 (t, 7H, J = 9.1 Hz), 3.12 (dd, 7H, J = 11.0, 5.2 Hz), 3.05 (dt, 7H, J = 11.2, 4.4 Hz), 2.50 (t, 7H, J = 12.7 Hz), 2.09 (d, 7H, J = 12.4 Hz), 1.85–1.77 (m, 14H), 1.65–0.85 (m, other aliphatic ring protons), 1.65, 0.95 (s, each 21H, 14 × CH3), 0.93 (s, 42H, 14 × CH3), 0.82, 0.73 (s, each 21H, 14 × CH3), 0.67 (d, 7H, J = 9.6 Hz); 13C NMR (150 MHz, CDCl3/CD3OD = 1:1 v/v): δ 178.39, 151.61, 145.35, 126.41, 109.96, 102.96, 83.50, 79.22, 73.56, 73.03, 70.96, 70.12, 64.51, 58.03, 56.47, 56.29, 51.47, 50.90, 49.86, 47.53, 43.11, 41.52, 39.62, 39.53, 39.03, 38.39, 37.89, 35.20, 33.84, 31.56, 30.16, 28.46, 27.56, 26.40, 21.72, 19.73, 19.08, 16.69, 15.98, 15.14; MALDI-TOF MS (m/z) Calcd for C287H448N28NaO49 [M + Na]+: 5097.88. Found 5097.29.
3.4.6. Synthesis of Octakis 6-Deoxy-6-[4-N-[(2-(2-Propyn-1-yloxy)ethyl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-γ-CD (74)
Prepared from 56 according to general procedure C, the residue was purified by RP flash chromatography (eluent: methanol) to afford 74 as a white foam with a yield of 86%; 1H NMR (600 MHz, CDCl3/CD3OD = 1:1 v/v): δ 7.84 (s, 8H), 5.16 (d, 8H, J = 2.8 Hz), 4.68–4.49 (m, 48H), 4.16 (m, 8H), 3.88 (t, 8H, J = 9.2 Hz), 3.53–3.41 (m, 24H), 3.20 (t, 8H, J = 9.2 Hz), 3.12–3.09 (m, 8H), 3.04 (dt, 8H, J = 11.1, 4.5 Hz), 2.46 (t, 8H, J = 9.9 Hz), 2.05 (d, 8H, J = 12.5 Hz), 1.85–1.75 (m, 16H), 1.63–0.84 (m, other aliphatic ring protons), 1.63, 0.93, 0.91, 0.90, 0.79, 0.71 (s, each 24H, 48 × CH3), 0.64 (d, 8H, J = 9.6 Hz); 13C NMR (150 MHz, CDCl3/CD3OD = 1:1 v/v): δ 177.90, 151.36, 145.09, 126.08, 109.76, 102.39, 82.44, 79.03, 73.13, 72.96, 70.47, 69.95, 64.37, 57.90, 56.17, 55.94, 51.13, 50.58, 49.86, 47.21, 42.85, 41.21, 39.30, 39.26, 38.77, 38.10, 37.61, 34.90, 33.67, 31.28, 29.87, 28.26, 27.30, 26.10, 21.42, 19.63, 18.78, 16.49, 16.45, 15.76, 14.98; MALDI-TOF MS (m/z) Calcd for C328H512N32NaO56 [M + Na]+: 5822.86. Found 5822.75.
3.4.7. Synthesis of Hexakis 6-Deoxy-6-[4-N-[(2-(2-(2-Propyn-1-yloxy)ethoxy)ethyl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-α-CD (75)
Prepared from 57 according to general procedure C, the residue was purified by RP flash chromatography (eluent: methanol) to afford 75 as a white foam with a yield of 91%; 1H NMR (600 MHz, CDCl3/CD3OD = 1:1 v/v): δ 7.92 (s, 6H), 5.12 (d, 6H, J = 2.7 Hz), 4.68–4.47 (m, 36H, overlap with H2O), 4.25 (m, 6H), 3.99 (t, 6H, J = 9.1 Hz), 3.62–3.56 (m, 24H), 3.50–3.45 (m, 12H), 3.42–3.40 (m, 6H), 3.24 (t, 6H, J = 8.9 Hz), 3.12 (dd, 6H, J = 11.0, 5.2 Hz), 3.06 (dt, 6H, J = 10.9, 4.3 Hz), 2.48 (dt, 6H, J = 12.5, 3.2 Hz), 2.08 (d, 6H, J = 13.0 Hz), 1.88–1.77 (m, 12H), 1.66–0.85 (m, other aliphatic ring protons), 1.66, 0.96, 0.93, 0.92, 0.81, 0.72 (s, each 18H, 36 × CH3), 0.67 (d, 6H, J = 10.0 Hz); 13C NMR (150 MHz, CDCl3/CD3OD = 1:1 v/v): δ 178.29, 151.58, 145.07, 126.76, 109.89, 102.56, 83.42, 79.19, 73.86, 72.58, 70.98, 70.75, 70.50, 64.66, 56.44, 56.23, 54.14, 51.41, 50.84, 47.52, 43.08, 41.46, 39.56, 39.49, 38.96, 38.39, 37.84, 35.14, 33.84, 31.51, 30.11, 28.42, 27.52, 26.36, 21.65, 19.72, 19.02, 18.16, 16.63, 15.93, 15.12; MALDI-TOF MS (m/z) Calcd for C258H408N24NaO48 [M + Na]+: 4637.21. Found 4636.52.
3.4.8. Synthesis of Heptakis 6-Deoxy-6-[4-N-[(2-(2-(2-Propyn-1-yloxy)ethoxy)ethyl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-β-CD (76)
Prepared from 58 according to general procedure C, the residue was purified by RP flash chromatography (eluent: methanol) to afford 76 as a white foam with a yield of 82%; 1H NMR (600 MHz, CDCl3/CD3OD = 1:1 v/v): δ 7.93 (s, 7H), 5.13 (d, 7H, J = 2.8 Hz), 4.70–4.44 (m, 42H, overlap with H2O), 4.18 (m, 7H), 3.87 (t, 7H, J = 9.1 Hz), 3.63–3.56 (m, 28H), 3.50–3.42 (m, 21H), 3.23 (t, 7H, J = 10.4 Hz), 3.12 (dd, 7H, J = 11.0, 5.2 Hz), 3.06 (dt, 7H, J = 10.9, 4.1 Hz), 2.49 (t, 7H, J = 11.6 Hz), 2.09 (d, 7H, J = 12.8 Hz), 1.88–1.78 (m, 14H), 1.66–0.82 (m, other aliphatic ring protons), 1.66, 0.96, 0.93, 0.92, 0.82, 0.73 (s, each 21H, 42 × CH3), 0.67 (d, 7H, J = 10.1 Hz); 13C NMR (150 MHz, CDCl3/CD3OD = 1:1 v/v): δ 178.33, 151.61, 145.06, 126.80, 109.92, 102.87, 83.39, 79.21, 73.62, 73.05, 70.97, 70.79, 70.52, 64.70, 58.02, 56.46, 56.27, 51.45, 50.88, 49.86, 47.54, 43.11, 41.50, 39.60, 39.52, 39.48, 39.00, 38.41, 37.88, 35.19, 33.86, 31.55, 30.14, 28.45, 27.55, 26.40, 21.69, 19.74, 19.05, 18.18, 16.66, 15.96, 15.14; MALDI-TOF MS (m/z) Calcd for C301H476N28NaO56 [M + Na]+: 5402.52. Found 5402.60.
3.4.9. Synthesis of Octakis 6-Deoxy-6-[4-N-[(2-(2-(2-Propyn-1-yloxy)ethoxy)ethyl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-γ-CD (77)
Prepared from 59 according to general procedure C, the residue was purified by RP flash chromatography (eluent: methanol) to afford 77 as a white foam with a yield of 83%; 1H NMR (600 MHz, CDCl3/CD3OD = 1:1 v/v): δ 7.93 (s, 8H), 5.18 (d, 8H, J = 2.8 Hz), 4.68–4.45 (m, 48H, overlap with H2O), 4.19 (m, 8H), 3.89 (t, 8H, J = 9.2 Hz), 3.63–3.58 (m, 32H), 3.52–3.43 (m, 24H), 3.25–3.22 (m, 8H), 3.12 (dd, 8H, J = 10.9, 5.2 Hz), 3.06 (dt, 8H, J = 10.9, 4.3 Hz), 2.49 (dt, 8H, J = 12.6, 3.0 Hz), 2.09 (d, 8H, J = 12.8 Hz), 1.90–1.77 (m, 16H), 1.65–0.86 (m, other aliphatic ring protons), 1.65, 0.96 (s, each 24H, 16 × CH3), 0.93 (s, 48H, 16 × CH3), 0.82, 0.73 (s, each 24H, 16 × CH3), 0.67 (d, 8H, J = 10.0 Hz); 13C NMR (150 MHz, CDCl3/CD3OD = 1:1 v/v): δ 178.28, 151.58, 145.04, 126.77, 109.91, 102.66, 82.87, 79.19, 73.45, 73.28, 70.76, 70.50, 64.72, 58.01, 56.44, 56.25, 51.42, 50.85, 49.86, 47.51, 43.09, 41.47, 39.58, 39.50, 39.47, 38.98, 38.39, 37.86, 35.17, 33.85, 31.52, 30.12, 28.44, 27.52, 26.37, 21.68, 19.74, 19.03, 18.17, 16.65, 15.95, 15.14; MALDI-TOF MS (m/z) Calcd for C344H544N32NaO64 [M + Na]+: 6175.29. Found 6175.66.
3.4.10. Synthesis of Hexakis 6-Deoxy-6-[4-N-[(3,6,9,12-Tetraoxapentadec-14-yn-1-yl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-α-CD (78)
Prepared from 60 according to general procedure C, the residue was purified by RP flash chromatography (eluent: methanol) to afford 78 as a white foam with a yield of 92%; 1H NMR (600 MHz, CDCl3/CD3OD = 1:1 v/v): δ 7.94 (s, 6H), 5.14 (s, 6H), 4.71 (s, 6H), 4.58–4.51 (m, 18H), 4.27 (s, 6H), 4.01 (t, 6H, J = 9.0 Hz), 3.63–3.52 (m, 90H), 3.44–3.25 (m, 30H), 3.14 (dd, 6H, J = 10.9, 5.3 Hz), 3.08 (dt, 6H, J = 11.0, 4.3 Hz), 2.49 (t, 6H, J = 11.8 Hz), 2.09 (d, 6H, J = 13.0 Hz), 1.90 (m, 6H), 1.81 (m, 6H), 1.68–0.88 (m, other aliphatic ring protons), 1.68, 0.98 (s, each 18H, 12 × CH3), 0.95 (s, 36H, 12 × CH3), 0.84, 0.75 (s, each 18H, 12 × CH3), 0.69 (d, 6H, J = 10.2 Hz); 13C NMR (150 MHz, CDCl3/CD3OD = 1:1 v/v): δ 178.22, 151.55, 145.01, 126.74, 109.85, 102.57, 83.42, 79.16, 73.81, 72.54, 71.04, 71.01, 70.71, 70.51, 70.48, 64.56, 56.42, 56.19, 51.37, 51.12, 50.80, 47.51, 43.05, 41.43, 39.53, 39.52, 39.46, 38.90, 38.40, 37.81, 35.10, 33.80, 31.47, 30.20, 30.06, 29.83, 28.39, 27.70, 27.50, 26.33, 23.22, 21.62, 19.70, 18.97, 16.60, 15.90, 15.10, 14.33; MALDI-TOF MS (m/z) Calcd for C282H456N24NaO60 [M + Na]+: 5165.85. Found 5165.20.
3.4.11. Synthesis of Heptakis 6-Deoxy-6-[4-N-[(3,6,9,12-Tetraoxapentadec-14-yn-1-yl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-β-CD (79)
Prepared from 61 according to general procedure C, the residue was purified by RP flash chromatography (eluent: methanol) to afford 79 as a white foam with a yield of 88%; 1H NMR (600 MHz, CDCl3/CD3OD = 1:1 v/v): δ 7.96 (s, 7H), 5.15 (s, 7H), 4.72 (s, 7H), 4.59–4.53 (m, 21H), 4.18 (s, 7H), 3.89 (m, 7H), 3.64–3.34 (m, 133H), 3.26 (t, 7H, J = 9.0 Hz), 3.15 (dd, 7H, J = 10.8, 5.4 Hz), 3.09 (dt, 7H, J = 11.0, 4.1 Hz), 2.49 (t, 7H, J = 11.6 Hz), 2.09 (d, 7H, J = 13.1 Hz), 1.91 (m, 7H), 1.81 (m, 7H), 1.69–0.89 (m, other aliphatic ring protons), 1.69, 0.99 (each 21H, 14 × CH3), 0.96 (s, 42H, 14 × CH3), 0.84, 0.76 (s, each 21H, 14 × CH3), 0.70 (d, 7H, J = 10.3 Hz); 13C NMR (150 MHz, CDCl3/CD3OD = 1:1 v/v): δ 178.17, 151.52, 144.87, 126.81, 109.85, 102.72, 83.35, 79.15, 73.48, 72.87, 71.01, 70.99, 70.68, 70.56, 70.46, 64.47, 56.39, 56.16, 51.33, 51.13, 50.77, 47.49, 43.03, 41.40, 39.50, 39.48, 39.43, 38.89, 38.38, 37.79, 35.08, 33.79, 31.45, 30.17, 30.04, 29.81, 28.38, 27.68, 27.47, 26.30, 23.19, 21.59, 19.69, 18.95, 16.59, 15.89, 15.09, 14.31; MALDI-TOF MS (m/z) Calcd for C329H533N28O70 [M + H]+: 6001.01. Found 6001.28.
3.4.12. Synthesis of Octakis 6-Deoxy-6-[4-N-[(3,6,9,12-Tetraoxapentadec-14-yn-1-yl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-γ-CD (80)
Prepared from 62 according to general procedure C, the residue was purified by RP flash chromatography (eluent: methanol) to afford 80 as a white foam with a yield of 94%; 1H NMR (600 MHz, CDCl3/CD3OD = 1:1 v/v): δ 7.94 (s, 8H), 5.21 (s, 8H), 4.72 (s, 8H), 4.21 (s, 8H), 3.92 (s, 8H), 3.64–3.33 (m, 144H), 3.26 (s, 8H), 3.15 (dd, 8H, J = 10.4, 5.8 Hz), 3.09 (dt, 8H, J = 11.0, 4.3 Hz), 2.48 (m, 8H), 2.08 (d, 8H, J = 13.0 Hz), 1.91 (m, 8H), 1.81 (m, 8H), 1.69–0.89 (m, other aliphatic ring protons), 1.69, 0.99, 0.95, 0.94, 0.84, 0.76 (each 24H, 48 × CH3), 0.69 (d, 8H, J = 10.0 Hz); 13C NMR (150 MHz, CDCl3/CD3OD = 1:1 v/v): δ 177.96, 151.41, 109.76, 102.39, 82.57, 79.06, 73.22, 73.04, 70.91, 70.89, 70.57, 70.40, 64.52, 56.27, 56.02, 51.20, 50.64, 47.35, 42.93, 41.28, 39.38, 39.33, 38.78, 38.26, 37.68, 34.96, 33.71, 31.34, 30.07, 29.92, 29.71, 28.31, 27.59, 27.38, 26.18, 23.10, 21.48, 19.66, 18.84, 16.51, 16.50, 15.81, 15.03, 14.29; MALDI-TOF MS (m/z) Calcd for C376H608N32NaO80 [M + Na]+: 6880.13. Found 6880.93.
3.4.13. Synthesis of Hexakis 6-Deoxy-6-[4-N-[(3,6,9,12,15,18-Hexaoxaheneicos-20-yn-1-yl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-α-CD (81)
Prepared from 63 according to general procedure C, the residue was purified by RP flash chromatography (eluent: methanol) to afford 81 as a white foam with a yield of 90%; 1H NMR (600 MHz, CDCl3/CD3OD = 1:1 v/v): δ 7.92 (s, 6H), 5.13 (s, 6H), 4.60–4.45 (m, 30H), 4.26 (s, 6H), 3.98 (t, 6H, J = 8.9 Hz), 3.63–3.38 (m, 144H), 3.29–3.22 (m, 6H), 3.12 (dd, 6H, J = 10.3, 5.8 Hz), 3.06 (dt, 6H, J = 13.5, 6.7 Hz), 2.46 (dt, 6H, J = 12.6, 3.2 Hz), 2.08–2.05 (m, 6H), 1.93–1.76 (m, 12H), 1.66–0.86 (m, other aliphatic ring protons), 1.66, 0.97 (s, each 18H, 12 × CH3), 0.93 (s, 36H, 12 × CH3), 0.82, 0.73 (s, each 18H, 12 × CH3), 0.67 (d, 6H, J = 9.2 Hz); 13C NMR (150 MHz, CDCl3/CD3OD = 1:1 v/v): δ 178.32, 151.61, 145.12, 126.78, 109.85, 102.58, 83.52, 79.21, 73.89, 72.59, 71.12, 71.09, 71.04, 70.76, 70.53, 64.63, 56.48, 56.26, 51.44, 51.12, 50.87, 49.85, 47.57, 43.10, 41.49, 39.59, 39.51, 38.94, 38.46, 37.87, 35.15, 33.82, 31.52, 30.11, 28.42, 27.55, 26.39, 21.67, 19.72, 19.02, 18.18, 16.62, 15.93, 15.11; MALDI-TOF MS (m/z) Calcd for C306H504N24NaO72 [M + Na]+: 5694.48. Found 5694.02.
3.4.14. Synthesis of Heptakis 6-Deoxy-6-[4-N-[(3,6,9,12,15,18-Hexaoxaheneicos-20-yn-1-yl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-β-CD (82)
Prepared from 64 according to general procedure C, the residue was purified by RP flash chromatography (eluent: methanol) to afford 82 as a white foam with a yield of 85%; 1H NMR (600 MHz, CDCl3/CD3OD = 1:1 v/v): δ 7.94 (s, 7H), 5.12 (s, 7H), 4.69 (s, 7H), 4.56–4.39 (m, 35H), 4.15 (s, 7H), 3.86 (t, 7H, J = 9.0 Hz), 3.63–3.39 (m, 168H), 3.29–3.25 (m, 7H), 3.12 (dd, 7H, J = 10.3, 5.8 Hz), 3.06 (dt, 7H, J = 13.4, 6.6 Hz), 2.47 (t, 7H, J = 11.4 Hz), 2.08–2.05 (m, 7H), 1.90–1.76 (m, 14H), 1.66–0.86 (m, other aliphatic ring protons), 1.66, 0.97 (s, each 21H, 14 × CH3), 0.93 (s, 42H, 14 × CH3), 0.82, 0.73 (s, each 21H, 14 × CH3), 0.67 (d, 7H, J = 9.3 Hz); 13C NMR (150 MHz, CDCl3/CD3OD = 1:1 v/v): δ 178.33, 151.61, 144.97, 126.92, 109.87, 102.82, 83.53, 79.21, 73.60, 72.98, 71.12, 71.10, 71.06, 70.77, 70.63, 70.53, 64.53, 56.48, 56.27, 51.45, 51.20, 50.87, 49.85, 47.58, 43.11, 41.50, 39.59, 39.51, 38.95, 38.46, 37.87, 35.16, 33.83, 31.53, 30.12, 28.43, 27.56, 26.40, 21.68, 19.72, 19.03, 18.18, 16.63, 15.94, 15.12; MALDI-TOF MS (m/z) Calcd for C357H588N28NaO84 [M + Na]+: 6639.73. Found 6639.63.
3.4.15. Synthesis of Octakis 6-Deoxy-6-[4-N-[(3,6,9,12,15,18-Hexaoxaheneicos-20-yn-1-yl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-γ-CD (83)
Prepared from 65 according to general procedure C, the residue was purified by RP flash chromatography (eluent: methanol) to afford 83 as a white foam with a yield of 89%; 1H NMR (400 MHz, CDCl3/CD3OD = 1:1 v/v): δ 7.93 (s, 8H), 5.18 (s, 8H), 4.56–4.48 (m, 40H), 4.17 (s, 8H), 3.88 (t, 8H, J = 9.0 Hz), 3.63–3.38 (m, 192H), 3.29–3.25 (m, 8H), 3.12 (dd, 8H, J = 10.3, 5.9 Hz), 3.06 (dt, 8H, J = 13.4, 6.4 Hz), 2.47 (t, 8H, J = 11.4 Hz), 2.08–2.05 (m, 8H), 1.90–0.86 (m, other aliphatic ring protons), 1.66, 0.97 (s, each 24H, 16 × CH3), 0.93 (s, 48H, 16 × CH3), 0.82, 0.73 (s, each 24H, 16 × CH3), 0.67 (d, 8H, J = 9.3 Hz); 13C NMR (150 MHz, CDCl3/CD3OD = 1:1 v/v): δ 178.33, 151.62, 145.08, 126.85, 109.87, 102.69, 83.05, 79.22, 73.47, 73.29, 71.13, 71.11, 71.07, 70.88, 70.77, 70.57, 70.53, 64.66, 56.49, 56.28, 51.46, 50.88, 49.86, 47.58, 43.11, 41.50, 39.60, 39.53, 38.95, 38.47, 37.88, 35.17, 33.83, 31.54, 30.13, 28.45, 27.57, 26.41, 21.69, 19.73, 19.04, 18.18, 16.64, 15.95, 15.13; MALDI-TOF MS (m/z) Calcd for C408H672N32NaO96 [M + Na]+: 7584.98. Found 7584.17.
3.4.16. Synthesis of Hexakis 6-Deoxy-6-[4-N-[(3,6,9,12,15,18,21,24-Octaoxaheptacos-26-yn-1-yl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-α-CD (84)
Prepared from 66 according to general procedure C, the residue was purified by RP flash chromatography (eluent: methanol) to afford 84 as a white foam with a yield of 90%; 1H NMR (600 MHz, CDCl3/CD3OD = 1:1 v/v): δ 7.92 (s, 1H), 5.11 (s, 1H), 4.69 (m, 1H), 4.56–4.48 (m, 4H), 4.38 (s, 1H), 4.22 (s, 1H), 3.98 (t, 1H, J = 8.9 Hz), 3.63–3.59 (m, 28H), 3.51 (t, 2H, J = 5.3 Hz), 3.45–3.39 (m, 2H), 3.25 (m, 1H), 3.12 (dd, 1H, J = 10.9, 5.4 Hz), 3.05 (dt, 1H, J = 11.0, 4.3 Hz), 2.45 (dt, 1H, J = 13.0, 3.1 Hz), 2.06 (m, 1H), 1.92–1.84 (m, 1H), 1.80–1.76 (m, 1H), 1.68–0.85 (m, other aliphatic ring protons), 1.66, 0.96, 0.93, 0.92, 0.81, 0.72 (s, each 3H, 6 × CH3), 0.66 (d, 1H, J = 10.0 Hz); 13C NMR (150 MHz, CDCl3/CD3OD = 1:1 v/v): 178.15, 151.52, 144.83, 126.82, 109.79, 102.51, 83.55, 79.14, 73.75, 72.44, 71.03, 71.02, 71.00, 70.97, 70.67, 70.54, 70.47, 64.43, 56.38, 56.14, 51.32, 50.75, 47.47, 43.01, 41.38, 39.49, 39.41, 38.85, 38.37, 37.77, 35.04, 33.76, 31.43, 30.01, 28.35, 27.46, 26.28, 21.57, 19.68, 18.92, 16.54, 15.85, 15.06; MALDI-TOF MS (m/z) Calcd for C330H552N24NaO84 [M + Na]+: 6223.12. Found 6223.38.
3.4.17. Synthesis of Heptakis 6-Deoxy-6-[4-N-[(3,6,9,12,15,18,21,24-Octaoxaheptacos-26-yn-1-yl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-β-CD (85)
Prepared from 67 according to general procedure C, the residue was purified by RP flash chromatography (eluent: methanol) to afford 85 as a white foam with a yield of 87%; 1H NMR (600 MHz, CDCl3/CD3OD = 1:1 v/v): δ 7.93 (s, 1H), 5.11 (s, 1H), 4.69 (m, 1H), 4.56–4.49 (m, 4H), 4.35 (m, 1H), 4.13 (s, 1H), 3.84 (t, 1H, J = 8.9 Hz), 3.63–3.59 (m, 28H), 3.51 (t, 2H, J = 5.2 Hz), 3.47–3.39 (m, 2H), 3.24 (m, 1H), 3.12 (dd, 1H, J = 10.9, 5.3 Hz), 3.05 (dt, 1H, J = 11.0, 4.4 Hz), 2.45 (dt, 1H, J = 12.8, 3.2 Hz), 2.06 (m, 1H), 1.92–1.84 (m, 1H), 1.80–1.76 (m, 1H), 1.66–0.85 (m, other aliphatic ring protons), 1.66, 0.96, 0.93, 0.92, 0.81, 0.72 (s, each 3H, 6 × CH3), 0.66 (d, 1H, J = 10.0 Hz); 13C NMR (150 MHz, CDCl3/CD3OD = 1:1 v/v): 178.14, 151.51, 144.79, 126.84, 109.79, 102.68, 83.40, 79.14, 73.45, 72.83, 71.01, 70.94, 70.66, 70.57, 70.47, 64.38, 56.38, 56.13, 51.31, 50.74, 47.47, 43.01, 41.38, 39.48, 39.41, 38.85, 38.37, 37.77, 35.04, 33.76, 31.42, 30.01, 28.35, 27.46, 26.28, 21.56, 19.68, 18.92, 16.54, 15.85, 15.06; MALDI-TOF MS (m/z) Calcd for C385H644N28NaO98 [M + Na]+: 7256.47. Found 7254.72.
3.4.18. Synthesis of Octakis 6-Deoxy-6-[4-N-[(3,6,9,12,15,18,21,24-Octaoxaheptacos-26-yn-1-yl)-3β-hydroxy-lup-20(29)-en-28-oyl]-aminomethyl-1H-1,2,3-triazol-1-yl]-γ-CD (86)
Prepared from 68 according to general procedure C, the residue was purified by RP flash chromatography (eluent: methanol) to afford 86 as a white foam with a yield of 92%; 1H NMR (600 MHz, CDCl3/CD3OD = 1:1 v/v): δ 7.90 (s, 1H), 5.16 (s, 1H), 4.68 (s, 1H), 4.55 (m, 5H), 4.16 (s, 1H), 3.87 (s, 1H), 3.63–3.59 (m, 28H), 3.51 (t, 2H, J = 5.2 Hz), 3.45–3.38 (m, 2H), 3.22 (s, 1H), 3.11 (dd, 1H, J = 10.2, 6.1 Hz), 3.05 (dt, 1H, J = 11.0, 4.3 Hz), 2.42 (dt, 1H, J = 12.9, 3.1 Hz), 2.03 (m, 1H), 1.91–1.84 (m, 1H), 1.78–1.75 (m, 1H), 1.65–0.85 (m, other aliphatic ring protons), 1.65, 0.95, 0.92, 0.91, 0.80 (s, each 3H, 6 × CH3), 0.65 (d, 1H, J = 10.0 Hz); 13C NMR (150 MHz, CDCl3/CD3OD = 1:1 v/v): 177.84, 151.36, 144.81, 126.57, 109.68, 102.30, 82.51, 79.03, 73.13, 72.93, 70.88, 70.84, 70.81, 70.49, 70.38, 70.33, 64.43, 56.21, 55.93, 51.11, 50.55, 47.29, 42.86, 41.20, 39.31, 39.29, 39.25, 38.70, 38.21, 37.60, 34.86, 33.65, 31.26, 29.83, 28.24, 27.32, 26.10, 21.39, 19.62, 18.75, 16.44, 16.42, 15.73, 14.97. MALDI-TOF MS (m/z) Calcd for C440H736N32NaO112 [M + Na]+: 8289.83. Found 8289.80.
3.5. Cytotoxicity Test
The cytotoxicity of the synthesized BA-CD conjugates was evaluated with the CellTiter-Glo luminescent cell viability assay kit. Briefly, 10,000 MDCK cells in DMEM supplemented with 1% FBS were grown in 96-well plates and incubated at 37 °C. After 24 h, the cells were treated or mock-treated with 100 µM test compounds and collected at 36 h. An equal volume of CellTiter-Glo reagent was added to the cells and mixed for 2 min on an orbital shaker. After stabilization at room temperature for 10 min, the luminescence intensity was measured by an Infinite M2000 PRO™ instrument (Tecan Group Ltd., Männedorf, Switzerland).
3.6. CPE Reduction Assay
MDCK cells were seeded at 1.0 × 104 cells per well in 96-well plates and cultured overnight. When the cells had grown to approximately 70-80% confluence, the media were removed, and the cells were infected with influenza A/WSN/33 virus at a multiplicity of infection (MOI) of 0.2 in DMEM (with 1% FBS and 2 µg/mL TPCK-treated trypsin) containing the corresponding concentration of test samples with two-fold serial dilution (0.78, 1.56, 3.13, 6.25, 12.50, 25.00, 50.00, and 100.00 µM) from the stock solutions (2.0 mM). All plates were incubated at 37 °C and 5% CO2 for 36 h, and cell viability was determined using CellTiter-Glo reagent, as described above.
3.7. Statistical Analysis
Data was analyzed using GraphPad Prism 8.3.0 (GraphPad Software Inc., San Diego, CA, USA). The half maximal inhibitory concentration (IC50) and cytotoxicity concentration for 50% cell death (CC50) was calculated using a non-linear regression dose response curve. Selectivity Index (SI) was calculated as the rate of CC50 to IC50.
4. Conclusions
In summary, an efficient and conventional method is presented here for the synthesis of multivalent BA derivatives by using α-, β- and γ-CD as scaffolds with different biocompatible OEG linker structures. Our approach involves the construction of two building blocks: terminal propargylated OEG-tethered BAs and multiazide substituted per-O-acetylated CDs in the first stage, followed by a regioselective 1,3-dipolar cycloaddition reaction and a subsequent de-O-acetylation reaction to provide the desired multivalent BA-CD conjugates. This general strategy is particularly suitable for the rapid assembly of structurally well-defined multivalent compound libraries based on CD scaffolds. Due to the numerous free hydroxyl groups at the CD scaffold and the OEG linker, the generated BA-CD derivatives are expected to display high solubility and good compatibility in biological environments. No obvious cytotoxicity to MDCK cells was observed for these conjugates at concentrations up to 100 μM. Further in vitro testing showed that four conjugates, 51 and 69–71, were potent against A/WSN/33 (H1N1) virus with IC50 values below 10 μM. The work presented herein demonstrated that multivalent BA derivatives have the potential to fight viral infection.
Supplementary Materials
The following are available online, Figure S1: Cytotoxicity of multivalent BA-CD conjugates to MDCK cells; Figure S2: Binding sensorgrams for conjugates 81 and 83 interaction with influenza HA protein; Selected NMR, ESI-HRMS or MALDI-TOF MS spectra.
Author Contributions
Y.C. and Q.G. synthesized and characterization of the conjugates; X.W. and S.L. carried out the in vitro anti-influenza activity and cytotoxicity experiments; E.V.T. and Y.Z. discussed the results and assisted in writing the manuscript; X.M. analyzed the NMR data; D.Z. and S.X. supervised the project and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the International Cooperation and Exchange Program of China (NSFC-RFBR, No. 82161148006), the National Natural Science Foundation of China (No. 21877007 and 82130100), Shenzhen Bay Laboratory Start-up Foundation (No. 21230071) and the foundation by RFBR and NSFC (No. 20-53-55001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data of this study is contained within the article or supplementary materials. The data presented in this study are available in this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 69–86 are available from the authors.
References
- Lagace-Wiens, P.R.S.; Rubinstein, E.; Gumel, A. Influenza epidemiology-past, present, and future. Crit. Care Med. 2010, 38, E1–E9. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado-Payan, E.; Montagud-Marrahi, E.; Torres-Elorza, M.; Bodro, M.; Blasco, M.; Poch, E.; Soriano, A.; Pineiro, G.J. SARS-CoV-2 and influenza virus co-infection. Lancet 2020, 395, E84. [Google Scholar] [CrossRef]
- Gubareva, L.; Mohan, T. Antivirals targeting the neuraminidase. Cold Spring Harb. Perspect. Med. 2022, 12, a038455. [Google Scholar] [CrossRef] [PubMed]
- Yang, T. Baloxavir Marboxil: The first cap-dependent endonuclease inhibitor for the treatment of influenza. Ann. Pharmacother. 2019, 53, 754–759. [Google Scholar] [CrossRef]
- Moscona, A. Global transmission of oseltamivir-resistant influenza. N. Engl. J. Med. 2009, 360, 953–956. [Google Scholar] [CrossRef] [Green Version]
- Kormuth, K.A.; Lakdawala, S.S. Emerging antiviral resistance. Nat. Microbiol. 2020, 5, 4–5. [Google Scholar] [CrossRef]
- Edinger, T.O.; Pohl, M.O.; Stertz, S. Entry of influenza A virus: Host factors and antiviral targets. J. Gen. Virol. 2014, 95, 263–277. [Google Scholar] [CrossRef]
- Cuellar-Camacho, J.L.; Bhatia, S.; Reiter-Scherer, V.; Lauster, D.; Liese, S.; Rabe, J.; Herrmann, A.; Haag, R. Quantification of multivalent interactions between sialic acid and influenza A virus spike proteins by single-molecule force spectroscopy. J. Am. Chem. Soc. 2020, 142, 12181–12191. [Google Scholar] [CrossRef]
- Mammen, M.; Choi, S.K.; Whitesides, G.M. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angew. Chem. Int. Ed. 1998, 37, 2755–2794. [Google Scholar] [CrossRef]
- Sigal, G.B.; Mammen, M.; Dahmann, G.; Whitesides, G.M. Polyacrylamides bearing pendant α-sialoside groups strongly inhibit agglutination of erythrocytes by influenza virus: The strong inhibition reflects enhanced binding through cooperative polyvalent interactions. J. Am. Chem. Soc. 1996, 118, 3789–3800. [Google Scholar] [CrossRef]
- Zanini, D.; Roy, R. Novel dendritic α-sialosides: Synthesis of glycodendrimers based on a 3,3’-iminobis(propylamine) core. J. Org. Chem. 1996, 61, 7348–7354. [Google Scholar] [CrossRef]
- Ogata, M.; Umemura, S.; Sugiyama, N.; Kuwano, N.; Koizumi, A.; Sawada, T.; Yanase, M.; Takaha, T.; Kadokawa, J.I.; Usui, T. Synthesis of multivalent sialyllactosamine-carrying glyco-nanoparticles with high affinity to the human influenza virus hemagglutinin. Carbohydr. Polym. 2016, 153, 96–104. [Google Scholar] [CrossRef]
- Xiao, S.; Si, L.; Tian, Z.; Jiao, P.; Fan, Z.; Meng, K.; Zhou, X.; Wang, H.; Xu, R.; Han, X.; et al. Pentacyclic triterpenes grafted on CD cores to interfere with influenza virus entry: A dramatic multivalent effect. Biomaterials 2016, 78, 74–85. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Wang, B.; Hao, J.; Hu, J.; Ju, Y. Natural triterpenoid- and oligo(ethylene glycol)-pendant-containing block and random copolymers: Aggregation and pH-controlled release. Chem. Asian J. 2018, 13, 2723–2729. [Google Scholar] [CrossRef]
- Yang, Y.; He, H.J.; Chang, H.; Yu, Y.; Yang, M.B.; He, Y.; Fan, Z.C.; Iyer, S.S.; Yu, P. Multivalent oleanolic acid human serum albumin conjugate as nonglycosylated neomucin for influenza virus capture and entry inhibition. Eur. J. Med. Chem. 2018, 143, 1723–1731. [Google Scholar] [CrossRef]
- Amiri, S.; Dastghaib, S.; Ahmadi, M.; Mehrbod, P.; Khadem, F.; Behrouj, H.; Aghanoori, M.R.; Machaj, F.; Ghamsari, M.; Rosik, J.; et al. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotechnol. Adv. 2020, 38, 107409. [Google Scholar] [CrossRef]
- Mayaux, J.F.; Bousseau, A.; Pauwels, R.; Huet, T.; Henin, Y.; Dereu, N.; Evers, M.; Soler, F.; Poujade, C.; De Clercq, E.; et al. Triterpene derivatives that block entry of human immunodeficiency virus type 1 into cells. Proc. Natl. Acad. Sci. USA 1994, 91, 3564–3568. [Google Scholar] [CrossRef] [Green Version]
- Kashiwada, Y.; Hashimoto, F.; Cosentino, L.M.; Chen, C.H.; Garrett, G.P.; Lee, K.H. Betulinic acid and dihydrobetulinic acid derivatives as potent anti-HIV agents. J. Med. Chem. 1996, 39, 1016–1017. [Google Scholar] [CrossRef]
- Hong, E.H.; Song, J.H.; Kang, K.B.; Sung, S.H.; Ko, H.J.; Yang, H. Anti-influenza activity of betulinic acid from Zizyphus jujuba on influenza A/PR/8 virus. Biomol. Ther. 2015, 23, 345–349. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Jiang, R.; Ooi, L.S.M.; But, P.P.H.; Ooi, V.E.C. Antiviral triterpenoids from the medicinal plant Schefflera heptaphylla. Phytother. Res. 2007, 21, 466–470. [Google Scholar] [CrossRef]
- Tung, N.H.; Kwon, H.J.; Kim, J.H.; Ra, J.C.; Kim, J.A.; Kim, Y.H. An anti-influenza component of the bark of Alnus japonica. Arch. Pharm. Res. 2010, 33, 363–367. [Google Scholar] [CrossRef]
- Baltina, L.A.; Flekhter, O.B.; Nigmatullina, L.R.; Boreko, E.I.; Pavlova, N.I.; Nikolaeva, S.N.; Savinova, O.V.; Tolstikov, G.A. Lupane triterpenes and derivatives with antiviral activity. Bioorg. Med. Chem. Lett. 2003, 13, 3549–3552. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Medvedeva, N.I.; Baikova, I.P.; Tolstikov, G.A.; Lopatina, T.V.; Yunusov, M.S.; Zaprutko, L. Synthesis of triterpenoid acylates: Effective reproduction inhibitors of influenza A (H1N1) and papilloma viruses. Russ. J. Bioorg. Chem. 2010, 36, 771–778. [Google Scholar] [CrossRef]
- Yu, M.; Si, L.; Wang, Y.; Wu, Y.; Yu, F.; Jiao, P.; Shi, Y.; Wang, H.; Xiao, S.; Fu, G.; et al. Discovery of pentacyclic triterpenoids as potential entry inhibitors of influenza viruses. J. Med. Chem. 2014, 57, 10058–10071. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Zhu, Y.; Si, L.; Zhang, B.; Zhang, Y.; Zhang, L.; Zhou, D.; Xiao, S. Synthesis of a hexavalent betulinic acid derivative as a hemagglutinin-targeted influenza virus entry inhibitor. Mol. Pharm. 2020, 17, 2546–2554. [Google Scholar] [CrossRef]
- Huang, X.; Leroux, J.C.; Castagner, B. Well-defined multivalent ligands for hepatocytes targeting via asialoglycoprotein receptor. Bioconjug. Chem. 2017, 28, 283–295. [Google Scholar] [CrossRef]
- Dubacheva, G.V.; Curk, T.; Auzely-Velty, R.; Frenkel, D.; Richter, R.P. Designing multivalent probes for tunable superselective targeting. Proc. Natl. Acad. Sci. USA 2015, 112, 5579–5584. [Google Scholar] [CrossRef] [Green Version]
- Ekladious, I.; Colson, Y.L.; Grinstaff, M.W. Polymer-drug conjugate therapeutics: Advances, insights and prospects. Nat. Rev. Drug Discov. 2019, 18, 273–294. [Google Scholar] [CrossRef]
- Veronese, F.M.; Pasut, G. PEGylation, successful approach to drug delivery. Drug Discov. Today. 2005, 10, 1451–1458. [Google Scholar] [CrossRef]
- Thompson, S.; Fleming, I.N.; O’Hagan, D. Enzymatic transhalogenation of dendritic RGD peptide constructs with the fluorinase. Org. Biomol. Chem. 2016, 14, 3120–3129. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.F.; Bi, Y.H.; Wu, Y.; Zhang, S.S.; Qi, J.X.; Li, Y.; Lu, X.C.; Zhang, Z.N.; Lv, X.; Yan, J.H.; et al. Structure-based tetravalent zanamivir with potent inhibitory activity against drug-resistant influenza viruses. J. Med. Chem. 2016, 59, 6303–6312. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.H.L.; Chen, Z.D.; Nguyen, T.; Pezzuto, J.M.; Qiu, S.X.; Lu, Z.Z. A concise semi-synthetic approach to betulinic acid from betulin. Synth. Commun. 1997, 27, 1607–1612. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, Q.; Si, L.; Shi, Y.; Wang, H.; Yu, F.; Zhang, Y.; Li, Y.; Zheng, Y.; Zhang, C.; et al. Synthesis and anti-HCV entry activity studies of β-cyclodextrin-pentacyclic triterpene conjugates. ChemMedChem 2014, 9, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- Gadelle, A.; Defaye, J. Selective halogenation at primary positions of cyclomaltooligosaccharides and a synthesis of per-3,6-anhydro cyclomaltooligosaccharides. Angew. Chem. Int. Ed. 1991, 30, 78–80. [Google Scholar] [CrossRef]
- Ashton, P.R.; Koniger, R.; Stoddart, J.F.; Alker, D.; Harding, V.D. Amino acid derivatives of β-cyclodextrin. J. Org. Chem. 1996, 61, 903–908. [Google Scholar] [CrossRef]
- Peng, C.; Bodenhausen, G.; Qiu, S.X.; Fong, H.H.S.; Farnsworth, N.R.; Yuan, S.G.; Zheng, C.Z. Computer-assisted structure elucidation: Application of CISOC-SES to the resonance assignment and structure generation of betulinic acid. Magn. Reson. Chem. 1998, 36, 267–278. [Google Scholar] [CrossRef]
- Tiwari, R.; Puthli, A.; Balakrishnan, S.; Sapra, B.K.; Mishra, K.P. Betulinic acid-induced cytotoxicity in human breast tumor cell lines MCF-7 and T47D and its modification by tocopherol. Cancer Investig. 2014, 32, 402–408. [Google Scholar] [CrossRef]
- Gong, K.K.; Li, P.L.; Qiao, D.; Zhang, X.W.; Chu, M.J.; Qin, G.F.; Tang, X.L.; Li, G.Q. Cytotoxic and antiviral triterpenoids from the mangrove plant Sonneratia paracaseolaris. Molecules 2017, 22, 1319. [Google Scholar] [CrossRef] [Green Version]
- Kazakova, O.B.; Smirnova, I.E.; Baltina, L.A.; Boreko, E.I.; Savinova, O.V.; Pokrovskii, A.G. Antiviral activity of acyl derivatives of betulin and betulinic and dihydroquinopimaric acids. Russ. J. Bioorg. Chem. 2018, 44, 740–744. [Google Scholar] [CrossRef]
- Smith, P.F.; Ogundele, A.; Forrest, A.; Wilton, J.; Salzwedel, K.; Doto, J.; Allaway, G.P.; Martin, D.E. Phase I and II study of the safety, virologic effect, and pharmacokinetics/pharmacodynamics of single-dose 3-O-(3′,3′-dimethylsuccinyl)betulinic acid (bevirimat) against human immunodeficiency virus infection. Antimicrob. Agents Chemother. 2007, 51, 3574–3581. [Google Scholar] [CrossRef] [Green Version]
- Nowicka-Sans, B.; Protack, T.; Lin, Z.Y.; Li, Z.F.; Zhang, S.R.; Sun, Y.N.; Samanta, H.; Terry, B.; Liu, Z.; Chen, Y.; et al. Identification and characterization of BMS-955176, a second-generation HIV-1 maturation inhibitor with improved potency, antiviral spectrum, and gag polymorphic coverage. Antimicrob. Agents Chemother. 2016, 60, 3956–3969. [Google Scholar] [CrossRef] [Green Version]
- DeJesus, E.; Harward, S.; Jewell, R.C.; Johnson, M.; Dumont, E.; Wilches, V.; Halliday, F.; Talarico, C.L.; Jeffrey, J.; Gan, J.; et al. A phase IIa study evaluating safety, pharmacokinetics, and antiviral activity of GSK2838232, a novel, second-generation maturation inhibitor, in participants with human immunodeficiency virus type 1 infection. Clin. Infect. Dis. 2020, 71, 1255–1262. [Google Scholar] [CrossRef]
- Alhadrami, H.A.; Sayed, A.M.; Sharif, A.M.; Azhar, E.I.; Rateb, M.E. Olive-derived triterpenes suppress SARS COV-2 main protease: A promising scaffold for future therapeutics. Molecules 2021, 26, 2654. [Google Scholar] [CrossRef]
- Loe, M.W.C.; Hao, E.; Chen, M.; Li, C.; Lee, R.C.H.; Zhu, I.X.Y.; Teo, Z.Y.; Chin, W.X.; Hou, X.; Deng, J.; et al. Betulinic acid exhibits antiviral effects against dengue virus infection. Antivir. Res. 2020, 184, 104954. [Google Scholar] [CrossRef]
- Cavalcante, B.R.R.; Aragão-França, L.S.; Sampaio, G.L.A.; Nonaka, C.K.V.; Oliveira, M.S.; Campos, G.S.; Sardi, S.I.; Dias, B.R.S.; Menezes, J.P.B.; Rocha, V.P.C.; et al. Betulinic acid exerts cytoprotective activity on Zika virus-infected neural progenitor cells. Front. Cell Infect. Microbiol. 2020, 10, 558324. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).