A Review on the Main Phytoconstituents, Traditional Uses, Inventions, and Patent Literature of Gum Arabic Emphasizing Acacia seyal
Abstract
1. Introduction
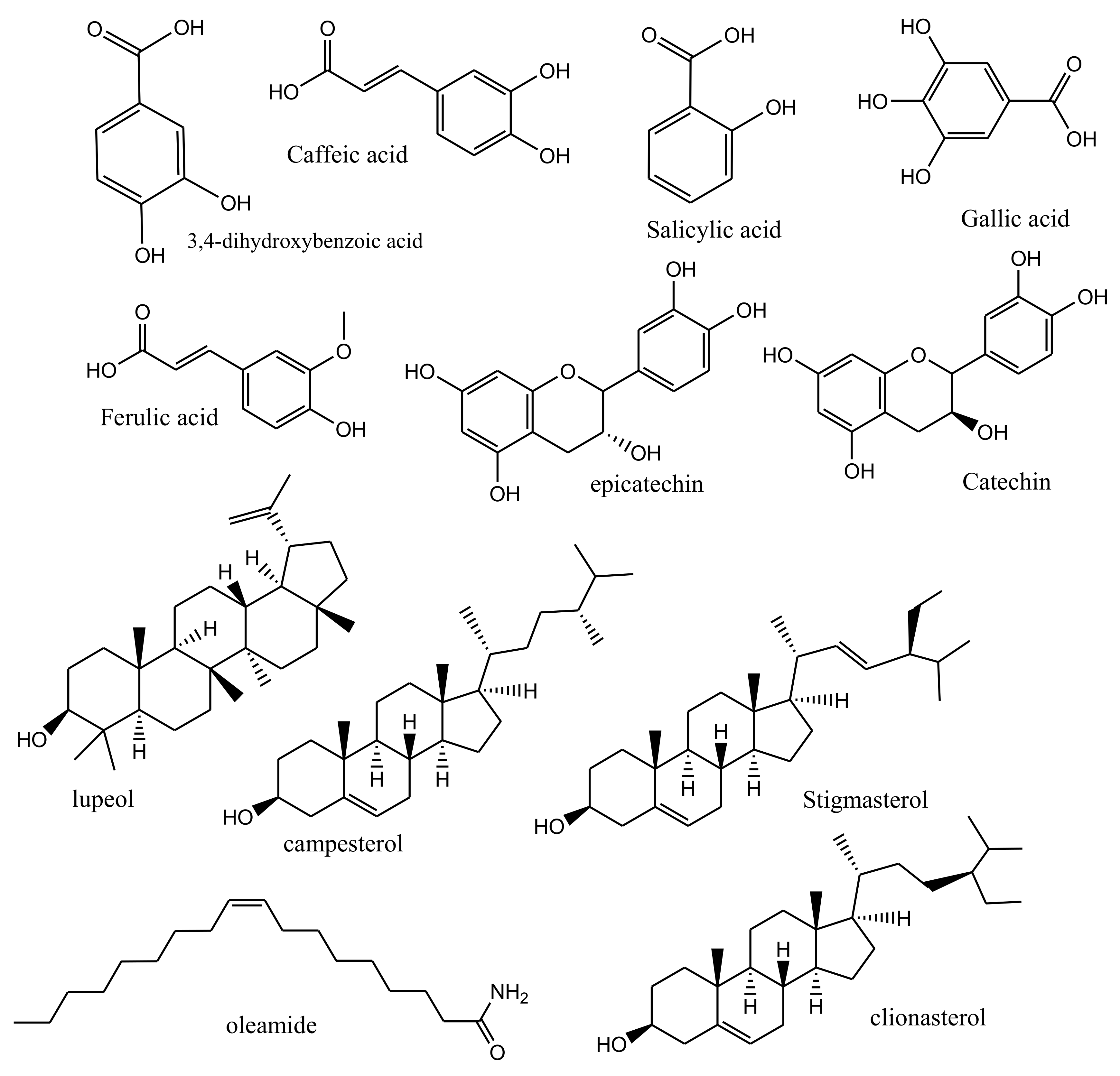
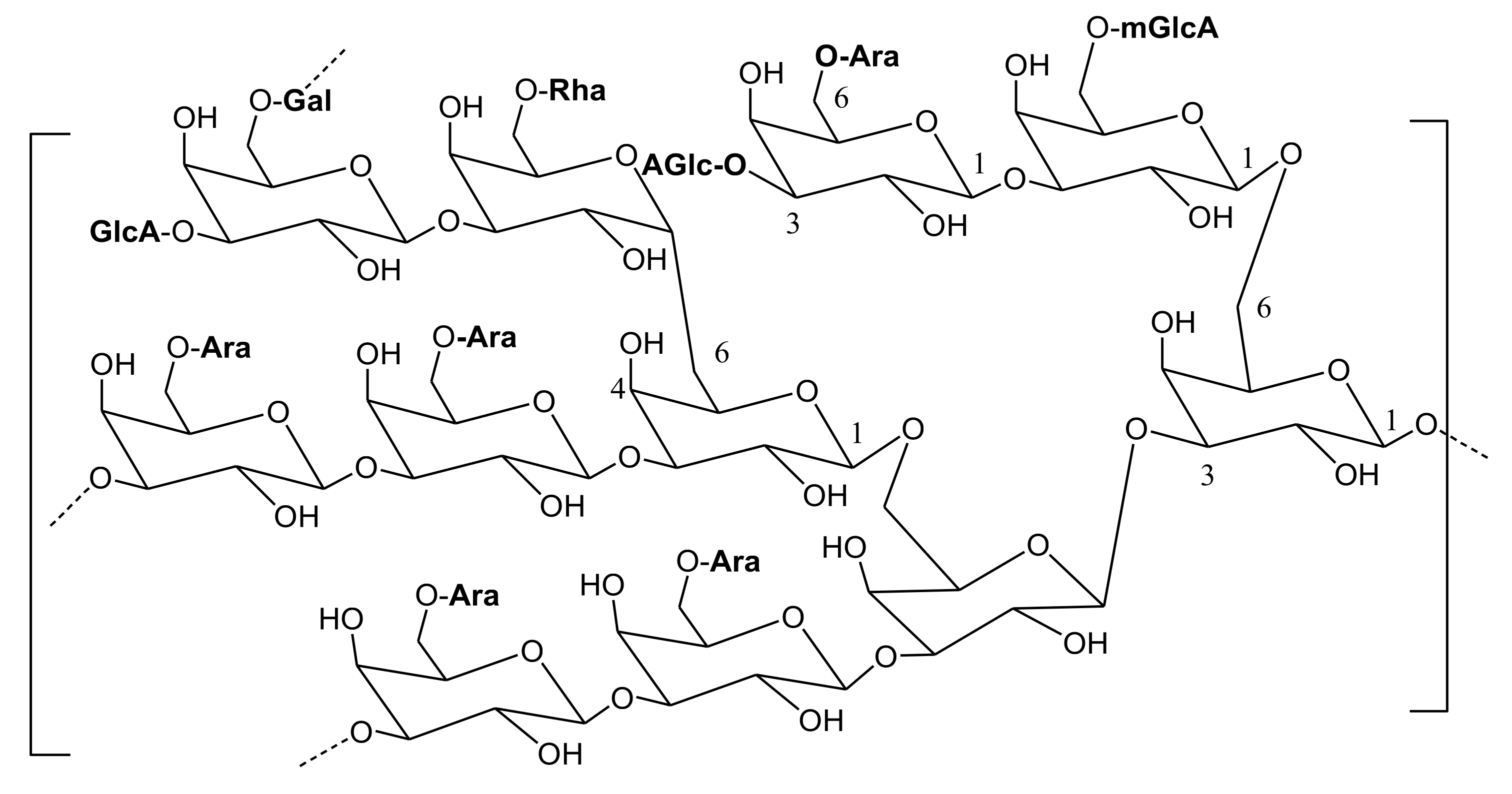
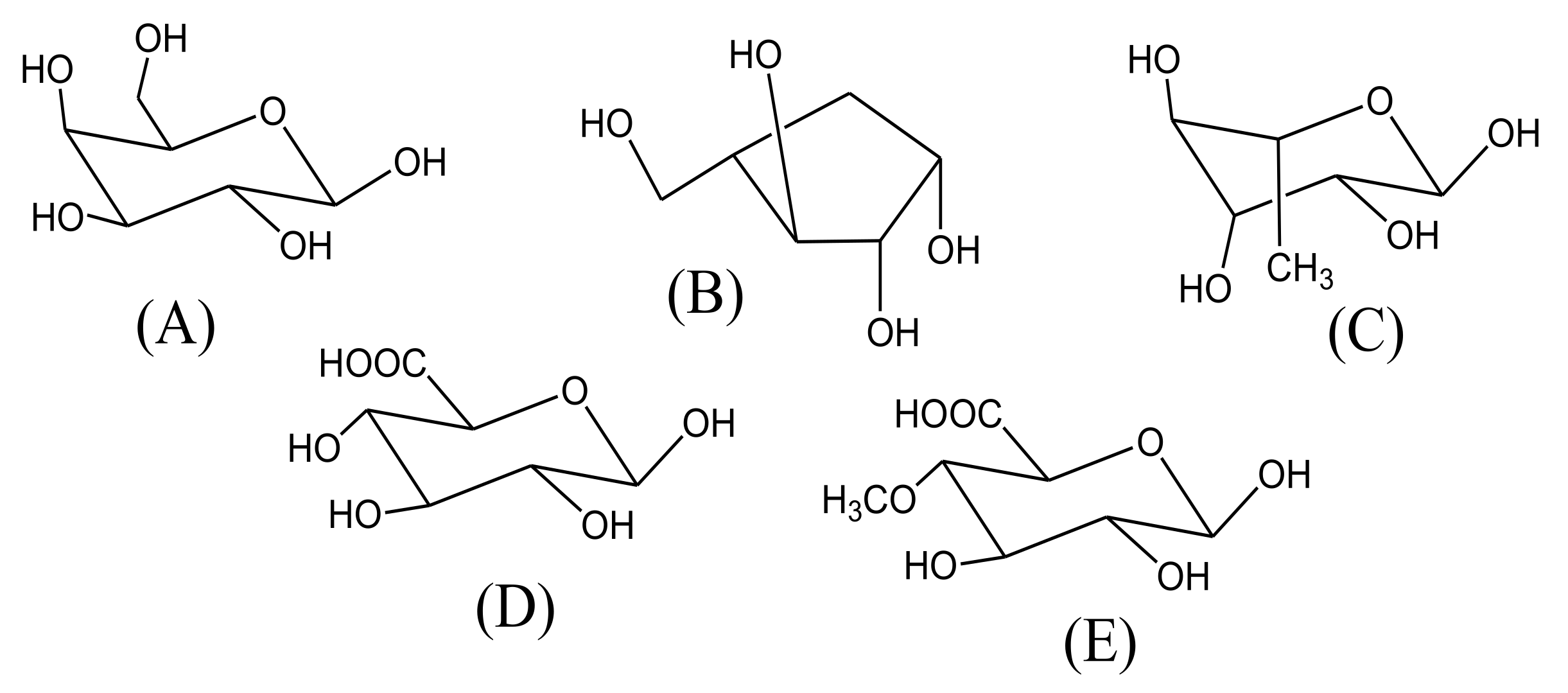
2. Main Phytoconstituents
3. Traditional Uses
4. Medicinal Uses
5. Pharmacological Relevance and Industrial Applications
6. Nutritive Value
7. Patent Literature
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Miller, P. The Gardeners Dictionary, 4th ed.; John and James Rivington: London, UK, 1754; Volume 1, p. 33. [Google Scholar]
- Pedley, L. A synopsis of Racosperma C. Mart. (Leguminosae: Mimosoideae). Austrobaileya 2003, 6, 445–496. [Google Scholar]
- Maslin, B.R.; Miller, J.; Seigler, D.S. Overview of the generic status of Acacia (Leguminosae: Mimosoideae). Aust. Syst. Bot. 2003, 16, 1–18. [Google Scholar] [CrossRef]
- Brown, G.K.; Murphy, D.J.; Miller, J.T.; Ladiges, P.Y. Acacia s.s. and its relationship among tropical legumes, tribe Ingeae (Leguminosae: Mimosoideae). Syst. Bot. 2008, 33, 739–751. [Google Scholar] [CrossRef]
- Bouchenak-Khelladi, Y.; Maurin, O.; Hurter, J.; van der Bank, M. The evolutionary history and biogeography of Mimosoideae (Leguminosae): An emphasis on African Acacias. Mol. Phylogen. Evol. 2010, 57, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Thiele, K.R.; Funk, V.A.; Iwatsuki, K.; Morat, P.; Peng, C.-I.; Raven, P.H.; Sarukhán, J.; Seberg, O. The controversy over the retypification of Acacia Mill. with an Australian type: A pragmatic view. Taxon 2011, 60, 194–198. [Google Scholar] [CrossRef]
- Moore, A.; Cotterill, F.P.D. The Acacia retypification debate: Perspectives of African amateur botanists. Taxon 2011, 60, 858–859. [Google Scholar] [CrossRef]
- Hussein, S.A. Utilization of tannins extract of Acacia seyal bark (Taleh) in tannage of leather. J. Chem. Eng. Process Technol. 2017, 8, 334. [Google Scholar] [CrossRef]
- Swarna, V.K.; Venba, R.; Madhan, B.; Chandrababu, N.K.; Sadulla, S. Cleaner tanning practices for tannery pollution abatement: Role of enzymes in eco-friendly vegetable tanning. J. Clean. Prod. 2009, 17, 507–515. [Google Scholar]
- Azzaoui, K.; Hammouti, B.; Lamhamdi, A.; Mejdoubi, E.; Berrabah, M. The gum Arabic in the southern region of Morocco. Morocco J. Chem. 2015, 3, 99–107. [Google Scholar]
- Mariod, A.A. 6—Chemical properties of gum Arabic. In Gum Arabic: Structure, Properties, Application, and Economics; Mariod, A.A., Ed.; Elsevier Science: London, UK, 2018; pp. 67–73. [Google Scholar]
- Abdel-Farid, I.B.; Sheded, M.G.; Mohamed, E.A. Metabolomic profiling and antioxidant activity of some Acacia species. Saudi J. Biol. Sci. 2014, 21, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, M.; Brima, E.I. Phytochemicals, trace element contents, and antioxidant activities of bark of Taleh (Acacia seyal) and desert rose (Adenium obesum). Biol. Trace Elem. Res. 2021, 199, 3135–3146. [Google Scholar] [CrossRef] [PubMed]
- Abdllha, H.B.; Mohamed, A.I.; Almoniem, K.A.; Adam, N.; Alhaadi, W.; Elshikh, A.; Ali, A.; Makuar, I.; Elnazeer, A.; Elrofaei, N.; et al. Evolution of antimicrobial, antioxidant potentials and phytochemical studies of three solvent extracts of five species from Acacia used in Sudanese ethnomedicine. Adv. Microbiol. 2016, 6, 691–698. [Google Scholar] [CrossRef]
- Garba, U.; Sadiq, F.S.D.; Abdullahi, Y. Anticonvulsant Screening of Ethanol and N-Hexane extracts of Acacia seyal Del. (Stem Bark) in rats. Niger. J. Pharm. Biomed. Res. 2018, 3, 17–21. [Google Scholar]
- Mekbib, S.B.; Regnier, T.J.; Sivakumar, D.; Korsten, L. Evaluation of Ethiopian plant extracts, Acacia seyal and Withania somnifera, to control green mould and ensure quality maintenance of citrus (Citrus sinensis L.). Fruits 2009, 64, 285–294. [Google Scholar] [CrossRef]
- Mekbib, S.B. In vitro antimicrobial assay of selected medicinal plants against medically important plant and foodborne pathogens. J. Med. Plants Stud. 2016, 4, 163–169. [Google Scholar]
- Magnini, R.D.; Hilou, A.; Millogo-Koné, H.; Compaore, S.; Pagès, J.-M.; Davin-Regli, A. A review on ethnobotanical uses, biological activities, and phytochemical aspects of Acacia senegal (L.) Willd. and Acacia seyal Delile. (Fabaceae). Int. J. Plant Sci. Hor. 2020, 2, 32–55. [Google Scholar] [CrossRef]
- Elmi, A.; Spina, R.; Risler, A.; Philippot, S.; Mérito, A.; Duval, R.E.; Abdoul-Latif, F.M.; Laurain-Mattar, D. Evaluation of antioxidant and antibacterial activities, cytotoxicity of Acacia seyal Del bark extracts and isolated compounds. Molecules 2020, 25, E2392. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.-P.; Wang, C.; Cui, S.W.; Wang, Q.; Xie, M.-Y.; Phillips, G.O. A further amendment to the classical core structure of gum Arabic (Acacia senegal). Food Hydrocoll. 2013, 31, 42–48. [Google Scholar] [CrossRef]
- Eltayeb, I.M.; Elhassan, I.A.; Elrasoul, J.H.; Eldin, E.S. A comparative study of chemical composition of Acacia seyal stem, stem wood and stem bark dry distillates used by Sudanese women as cosmetic and medicine. Int. J. Pharm. Pharm. Sci. 2017, 9, 218–224. [Google Scholar] [CrossRef][Green Version]
- Awad, S.S.; Rabah, A.A.; Ali, H.I.; Mahmoud, T.E. 1—Acacia seyal gums in Sudan: Ecology and economic contribution. In Gum Arabic: Structure, Properties, Application and Economics; Mariod, A.A., Ed.; Elsevier Science: London, UK, 2018; pp. 3–11. [Google Scholar]
- Sanchez, C.; Nigen, M.; Mejia-Tamayo, V.; Doco, T.; Williams, P.; Amine, C.; Renard, D. Acacia gum: History of the future. Food Hydrocoll. 2018, 78, 140–160. [Google Scholar] [CrossRef]
- Karamalla, K.A. Gum Arabic: Production, Chemistry and Application; Manager Research and Development Department; Gandil Agricultural Company Ltd.: Khartoum, Sudan, 1999. [Google Scholar]
- Li, J.; Deng, Q.; Yu, X.; Wang, W. Structural studies of a new fraction obtained by gradient ethanol precipitation from Acacia seyal gum. Food Hydrocoll. 2020, 107, 105932. [Google Scholar] [CrossRef]
- Idris, O.H.M.; Haddad, G.M. Gum Arabic’s (gum Acacia’s) journey from tree to end user. In Gum Arabic, special ed.; Kennedy, J.F., Phillips, G.O., Williams, P.A., Eds.; RSC Publishing: Cambridge, UK, 2012; pp. 3–19. [Google Scholar]
- Gashua, I.B. An Investigation of the Molecular Structure, Composition and Biophysical Properties of Gum Arabic. Ph.D. Thesis, University of Wolverhampton, Wolverhampton, UK, 2016. [Google Scholar]
- Menzies, A.R.; Osman, M.E.; Malik, A.A.; Baldwin, T.C. A comparison of the physicochemical and immunological properties of the plant gum exudates of Acacia senegal (gum Arabic) and Acacia seyal (gum tahla). Food Addit. Cont. 1996, 13, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Idris, O.H.M. What is gum Arabic? An overview. Int. J. Sudan Res. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Al-Assaf, S.; Phillips, G.O.; Williams, P.A. Studies on Acacia exudate gums. Part I: The molecular weight of Acacia senegal gum exudate. Food Hydrocoll. 2005, 19, 647–660. [Google Scholar] [CrossRef]
- Siddig, N.E.; Osman, M.E.; Al-Assaf, S.; Phillips, G.O.; Williams, P.A. Studies on Acacia exudate gums, part IV. Distribution of molecular components in Acacia seyal in relation to Acacia senegal. Food Hydrocoll. 2005, 19, 679–686. [Google Scholar] [CrossRef]
- Elnour, A.A.M.; Mirghani, M.E.S.; Kabbashi, N.A.; Md Alam, Z.; Musa, K.H. Study of antioxidant and anti-inflammatory crude methanol extract and fractions of Acacia seyal gum. Am. J. Pharmacol. Pharmacother. 2018, 5, 3. [Google Scholar] [CrossRef]
- Awad, S.S.; Rabah, A.A.; Ali, H.I.; Mahmoud, T. Acacia Seyal gums in Sudan: A review. In Proceedings of the 7th Annual Conference for Postgraduate Studies and Scientific Research Basic Sciences and Engineering Studies-University of Khartoum, Khartoum, Sudan, 20–23 February 2016; Volume 6, pp. 94–98. [Google Scholar]
- Abdalla, M.S.A.; Babiker, I.A.; Idris, A.M.; Elkalifa, K.F. Potential nutrient composition of Acacia seyal fruits as fodder for livestock in the dry lands in Sudan. Dev. Anal. Chem. 2014, 1, 25–30. [Google Scholar]
- Talaat, G.E.-M.; Abdel-Magid, D. The Potential of Acacia seyal as a Resourceful Tree for Gum Arabic in Sudan: Khartoum—2014 December. Available online: https://www.researchgate.net/publication/309731254 (accessed on 15 November 2021).
- Mariod, A.A. 12—Enhancement of color stability in foods by Gum Arabic. In Gum Arabic: Structure, Properties, Application and Economics; Mariod, A.A., Ed.; Elsevier Science: London, UK, 2018; pp. 143–150. [Google Scholar]
- Bi, B.; Yang, H.; Fang, Y.; Nishinari, K.; Phillips, G.O. Characterization and emulsifying properties of β-lactoglobulin-gum Acacia seyal conjugates prepared via the Maillard reaction. Food Chem. 2017, 214, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Eldeen, I.M.S.; Van Staden, J. In vitro pharmacological investigation of extracts from some trees used in Sudanese traditional medicine. South Afr. J. Bot. 2007, 73, 435–440. [Google Scholar] [CrossRef]
- Abdoul-Latif, F.M.; Osman, D.A.; Fourreh, A.E.; Abdallah, A.H.; Merito, A.; Hassan, S.; Asfaw, Z.; Kelbessa, E. Candidate medicinal plant species of djiboutian pharmacopeia for testing pharmacological activities on common microbial diseases. Int. J. Pharm. Pharm. Sci. 2016, 8, 78–84. [Google Scholar] [CrossRef]
- Muthaura, C.N.; Keriko, J.M.; Mutai, C.; Yenesew, A.; Gathirwa, J.W.; Irungu, B.N.; Nyangacha, R.; Mungai, G.M.; Derese, S. Antiplasmodial potential of traditional antimalarial phytotherapy remedies used by the Kwale community of the Kenyan Coast. J. Ethnopharmacol. 2015, 21, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Eldeen, I.M.S.; Van Staden, J. Cyclooxygenase inhibition and antimycobacterial effects of extracts from Sudanese medicinal plants. South Afr. J. Bot. 2008, 74, 225–229. [Google Scholar] [CrossRef][Green Version]
- Ismail, M.A.; Koko, W.S.; Osman, E.E.; Dahab, M.M.; Garbi, M.I.; Alsadeg, A.M.; Kabbashi, A.M. Molluscicidal activity of Acacia seyal (Dell) bark methanolic extract against Biomphalaria pfeifferi snails. Int. Biol. Biomed. J. 2016, 2, 73–79. [Google Scholar]
- Saeed, M.E.; Abdelgadir, H.; Sugimoto, Y.; Khalid, H.E.; Efferth, T. Cytotoxicity of 35 medicinal plants from Sudan towards sensitive and multidrug-resistant cancer cells. J. Ethnopharmacol. 2015, 174, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Zingue, S.; Njuh, A.N.; Tueche, A.B.; Tamsa, J.; Tchoupang, E.N.; Kakene, S.D.; Sipping, M.; Njamen, D. In vitro cytotoxicity and in vivo antimammary tumor effects of the hydroethanolic extract of Acacia seyal (Mimosaceae) stem bark. BioMed. Res. Int. 2018, 2018, 2024602. [Google Scholar] [CrossRef]
- Lindsay, R.; Hepper, F. Medicinal Plants of Marakwet, Kenya; Royal Botanic Gardens Kew: Richmond, UK, 1978. [Google Scholar]
- Nguta, J.M.; Mbaria, J.M.; Gakuya, D.W.; Gathumbi, P.K.; Kiama, S.G. Traditional antimalarial phytotherapy remedies used by the South coast community, Kenya. J. Ethnopharmacol. 2010, 131, 256–267. [Google Scholar] [CrossRef]
- Wambugu, S.N.; Mathiu, P.M.; Gakuya, D.W.; Kanui, T.I.; Kabasa, J.D.; Kiama, S.G. Medicinal plants used in the management of chronic joint pains in Machakos and Makueni counties, Kenya. J. Ethnopharmacol. 2011, 137, 945–955. [Google Scholar] [CrossRef]
- Doka, I.; Yagi, S. Ethnobotanical survey of medicinal plants in West Kordofan (Western Sudan). Ethnobot. Leafl. 2009, 13, 1409–1416. [Google Scholar]
- El-Ghazali, G.B.; El Tohami, M.S.; El Egams, A.B.; Abdalla, S.; Mohammed, M. Medicinal Plants of the Sudan: Part 4. Medicinal Plants of Northern Kordofan; Medicinal and Aromatic Plants Research Institute, National Center for Research: Khartoum, Sudan, 1997. [Google Scholar]
- Teklehaymanot, T. An ethnobotanical survey of medicinal and edible plants of Yalo Woreda in Afar regional state, Ethiopia. J. Ethnobiol. Ethnomed. 2017, 13, 40. [Google Scholar] [CrossRef]
- Lulekal, E.; Kelbessa, E.; Bekele, T.; Yineger, H. An ethnobotanical study of medicinal plants in Mana Angetu district, southeastern Ethiopia. J. Ethnobiol. Ethnomed. 2008, 4, 10. [Google Scholar] [CrossRef]
- Gradé, J.T.; Tabuti, J.R.; Van Damme, P. Ethnoveterinary knowledge in pastoral Karamoja, Uganda. J. Ethnopharmacol. 2009, 122, 273–293. [Google Scholar] [CrossRef] [PubMed]
- Hassan-Abdallah, A.; Merito, A.; Hassan, S.; Aboubaker, D.; Djama, M.; Asfaw, Z.; Kelbessa, E. Medicinal plants and their uses by the people in the Region of Randa, Djibouti. J. Ethnopharmacol. 2013, 148, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Hammiche, V.; Maiza, K. Traditional medicine in Central Sahara: Pharmacopoeia of Tassili N’ajjer. J. Ethnopharmacol. 2006, 105, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Boulos, L. Medicinal Plants of North Africa; Medicinal Plants of the World; Reference Publications, Inc.: Algonac, MI, USA, 1983; Volume 1, p. 286. [Google Scholar]
- Abdulrahman, A.I.; AL-Yahya, M.A. Antiulcer activity of gum Arabic and its interaction with antiulcer effect of ranitidine in rats. Biomed. Res. 2016, 4, 1102–1106. [Google Scholar]
- Ryan-Harshman, M.; Aldoori, W. How diet and lifestyle affect duodenal ulcers. Review of the evidence. Can. Fam. Physician 2004, 50, 727–732. [Google Scholar] [PubMed]
- Samy, W.M.; Ghoneim, A.I.; Elgindy, N.A. Novel microstructured sildenafil dosage forms as wound healing promoters. Expert Opin. Drug Deliv. 2014, 11, 1525–1536. [Google Scholar] [CrossRef]
- Ahmed, A.A. 16—Health benefits of Gum Arabic and medical use. In Gum Arabic: Structure, Properties, Application and Economics; Mariod, A.A., Ed.; Elsevier Science: London, UK, 2018; pp. 184–210. [Google Scholar]
- Singh, J.; Pal, R.; Hooda, M.S.; Bias, C.S. Hepatoprotective activity of Acacia senegal Pod against carbon tetrachloride-induced hepatotoxicity in rats. Int. J. Pharm. Sci. Rev. Res. 2014, 1, 165–168. [Google Scholar]
- Ali, B.H.; Al-Husseni, I.; Beegam, S.; Al-Shukaili, A.; Nemmar, A.; Schierling, S.; Queisser, N.; Schupp, N. Effect of gum Arabic on oxidative stress and inflammation in adenine–induced chronic renal failure in rats. PLoS ONE 2013, 8, e55242. [Google Scholar] [CrossRef]
- Gado, A.M.; Aldahmash, B.A. Antioxidant effect of Arabic gum against mercuric chloride-induced nephrotoxicity. Drug Des. Dev. Ther. 2013, 7, 1245–1252. [Google Scholar] [CrossRef]
- Nasir, O.; Babiker, S.; Salim, A.M. Protective effect of gum Arabic supplementation for type 2 diabetes mellitus and its complications. Int. J. Multidiscip. Curr. Res. 2016, 4, 288–294. [Google Scholar]
- Musa, H.H.; Ahmed, A.A.; Fedail, J.S.; Musa, T.H.; Sifaldin, A.Z. Gum Arabic attenuates the development of nephropathy in type 1 diabetes rat. In Gums and Stabilisers for the Food Industry 18: Hydrocolloid Functionality for Affordable and Sustainable Global Food Solutions, Proceedings of the 18th Gums and Stabilisers for the Food Industry Conference, Wrexham, UK, 23–26 June 2015; Williams, P.A., Phillips, G.O., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2016; pp. 245–255. [Google Scholar]
- Glover, D.A.; Matsumoto, N.; Riley, S.G.; Wolever, T.; Ushida, K.; Al-Assaf, S.; Phillips, G.O.; Phillips, A.O. Acacia (SEN) supergumTM (gum Arabic): In vivo and in vitro evaluation of potential health benefits in renal disease. In Gum Arabic; Kennedy, J.F., Phillips, G.O., Williams, P.A., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2011. [Google Scholar] [CrossRef]
- Abd-Allah, A.R.; Al-Majed, A.A.; Mostafa, A.M.; Al-Shabanah, O.A.; Din, A.G.; Nagi, M.N. Protective effect of Arabic gum against cardiotoxicity induced by doxorubicin in mice: A possible mechanism of protection. J. Biochem. Mol. Toxicol. 2002, 16, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Jarrar, A.H.; Stojanovska, L.; Apostolopoulos, V.; Feehan, J.; Bataineh, M.F.; Ismail, L.C.; Al Dhaheri, A.S. The effect of gum Arabic (Acacia senegal) on cardiovascular risk factors and gastrointestinal symptoms in adults at risk of metabolic syndrome: A randomized clinical trial. Nutrients 2021, 13, E194. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Ziada, A.; Blunden, G. Biological effects of gum Arabic: A review of some recent research. Food Chem. Toxicol. 2009, 47, 1–8. [Google Scholar] [CrossRef]
- Kong, H.; Yang, J.; Zhang, Y.; Fang, Y.; Nishinari, K.; Phillips, G.O. Synthesis and antioxidant properties of gum Arabic-stabilized selenium nanoparticles. Int. J. Biol. Macromol. 2014, 65, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Kamal, E.; Kaddam, L.A.; Dahawi, M.; Osman, M.; Salih, M.A.; Alagib, A.; Saeed, A. Gum Arabic fibers decreased inflammatory markers and disease severity score among rheumatoid arthritis patients, phase II trial. Int. J. Rheumatol. 2018, 2018, 4197537. [Google Scholar] [CrossRef]
- Osman, M.E.; Abo Zeid, I.M.; Adam, F.A. Gum Arabic: A reducing agent od uric acid and supportive treatment of gout. In Gum Arabic; Kennedy, J.F., Phillips, G.O., Williams, P.A., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2011. [Google Scholar] [CrossRef]
- Ushida, K. Gum Arabic and its anti-obese effect. In Gum Arabic; Kennedy, J.F., Phillips, G.O., Williams, P.A., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2012; pp. 285–290. [Google Scholar]
- Schneeman, B.O. Dietary fiber: Comments on interpreting recent research. J. Am. Diet. Assoc. 1987, 87, 1163. [Google Scholar] [CrossRef]
- Babiker, R.; Merghani, T.H.; Elmusharaf, K.; Badi, R.M.; Lang, F.; Saeed, A.M. Effects of gum Arabic ingestion on body mass index and body fat percentage in healthy adult females: Two-arm randomized, placebo controlled, double-blind trial. Nutr. J. 2012, 11, 111. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Musa, H.H.; Fedail, J.S.; Sifaldin, A.Z.; Musa, T.H. Gum Arabic suppressed diet-induced obesity by alteration the expression of mRNA levels of genes involved in lipid metabolism in mouse liver. Bioact. Carbohydr. Diet. Fibre 2016, 7, 15–20. [Google Scholar] [CrossRef]
- Pasman, W.J.; Saris, W.H.; Wauters, M.A.; Westerterp-Plantenga, M.S. Effect of one week of fibre supplementation on hunger and satiety ratings and energy intake. Appetite 1997, 29, 77–87. [Google Scholar] [CrossRef]
- Aleixandre, A.; Miguel, M. Dietary fiber in the prevention and treatment of metabolic syndrome: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 905–912. [Google Scholar] [CrossRef]
- Keenan, M.J.; Zhou, J.; McCutcheon, K.L.; Raggio, A.M.; Bateman, H.G.; Todd, E.; Jones, C.K.; Tulley, R.T.; Melton, S.; Martin, R.J.; et al. Effects of resistant starch, a nondigestible fermentable fiber, on reducing body fat. Obesity 2006, 14, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Aloqbi, A.A. Gum Arabic as a natural product with antimicrobial and anticancer activities. Arch. Pharm. Pract. 2020, 11, 107–112. [Google Scholar]
- Mohamed, R.E.; Gadour, M.O.; Adam, I. The lowering effect of gum Arabic on hyperlipidemia in Sudanese patients. Front. Physiol. 2015, 6, 160. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Musa, H.H.; Fedail, J.S.; Sifaldin, A.Z.; Musa, T.H. Gum Arabic decreased visceral adipose tissue associated with downregulation of 11β-hydroxysteroid dehydrogenase type I in liver and muscle of mice. Bioact. Carbohydr. Dietary Fibre 2015, 6, 31–36. [Google Scholar] [CrossRef]
- Parnell, J.A.; Reimer, R.A. Effect of prebiotic fibre supplementation on hepatic gene expression and serum lipids: A dose-response study in JCR:LA-cp rats. Br. J. Nutr. 2010, 103, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef] [PubMed]
- Chandalia, M.; Garg, A.; Lutjohann, D.; von Bergmann, K.; Grundy, S.M.; Brinkley, L.J. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2000, 342, 1392–1398. [Google Scholar] [CrossRef]
- Weickert, M.O. What dietary modification best improves insulin sensitivity and why? Clin. Endocrinol. 2012, 77, 508–512. [Google Scholar] [CrossRef]
- Kim, E.K.; Oh, T.J.; Kim, L.-K.; Cho, Y.M. Improving effect of the acute administration of dietary fiber-enriched cereals on blood glucose levels and gut hormone secretion. J. Korean Med. Sci. 2016, 31, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, D.; Quiñones, M.; Moulay, L.; Muguerza, B.; Miguel, M.; Aleixandre, A. Soluble fiber-enriched diets improve inflammation and oxidative stress biomarkers in Zucker fatty rats. Pharmacol. Res. 2011, 64, 31–35. [Google Scholar] [CrossRef]
- Xuan, N.T.; Shumilina, E.; Nasir, O.; Bobbala, D.; Gotz, F.; Lang, F. Stimulation of mouse dendritic cells by gum Arabic. Cellular physiology and biochemistry. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2010, 25, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F. Traditional medicines in Africa: An appraisal of ten potent African medicinal plants. Evid. Based Complement. Altern. Med. 2013, 2013, 617459. [Google Scholar] [CrossRef] [PubMed]
- Kaddam, L.; FdleAlmula, I.; Eisawi, O.A.; Abdelrazig, H.A.; Elnimeiri, M.; Lang, F.; Saeed, A.M. Gum Arabic as fetal hemoglobin inducing agent in sickle cell anemia; in vivo study. BMC Hematol. 2015, 15, E19. [Google Scholar] [CrossRef] [PubMed]
- Ballal, A.; Bobbala, D.; Qadri, S.M.; Foller, M.; Kempe, D.; Nasir, O.; Saeed, A.; Lang, F. Anti-malarial effect of gum Arabic. Malar. J. 2011, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Gamal-Eldeen, A.M.; Moustafa, D.; El-Daly, S.M.; Abo-Zeid, M.A.; Saleh, S.; Khoobchandani, M.; Katti, K.; Shukla, R.; Katti, K.V. Gum Arabic encapsulated gold nanoparticles for a non-invasive photothermal ablation of lung tumor in mice. Biomed. Pharmacother. 2017, 89, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Nasir, O.; Wang, K.; Föller, M.; Bhandaru, M.; Sandulache, D.; Artunc, F.; Ackermann, T.F.; Ebrahim, A.; Palmada, M.; Klingel, K.; et al. Downregulation of angiogenin transcript levels and inhibition of colonic carcinoma by Gum Arabic (Acacia senegal). Nutr. Cancer 2010, 62, 802–810. [Google Scholar] [CrossRef]
- Smolinske, S.C. Handbook of Food, Drug, and Cosmetic Excipients; CRC Press: Boca Raton, FL, USA, 1992; p. 7. [Google Scholar]
- Ali, I.A.K.E. Use of acacia gum in the treatment of skin lesions of two children with Kwashiorkor. In Gum Arabic; Mariod, A.A., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2018; pp. 221–228. [Google Scholar]
- Obaid, S.S. The Medical Uses of gum acacia-gum Arabic (GA) in human. Acad. J. Res. Sci. Pub. 2020, 1, 5–7. [Google Scholar]
- Turvill, J.L.; Wapnir, R.A.; Wingertzahn, M.A.; Teichberg, S.; Farthing, M.J. Cholera toxin-induced secretion in rats is reduced by a soluble fiber, gum Arabic. Dig. Dis. Sci. 2000, 45, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Calame, W.; Weseler, A.R.; Viebke, C.; Flynn, C.; Siemensma, A.D. Gum Arabic establishes prebiotic functionality in healthy human volunteers in a dose-dependent manner. Br. J. Nutr. 2008, 100, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Rawi, M.H.; Abdullah, A.; Ismail, A.; Sarbini, S.R. Manipulation of gut microbiota using acacia gum polysaccharide. ACS Omega 2021, 6, 17782–17797. [Google Scholar] [CrossRef] [PubMed]
- Singhal, R.; Agarwal, V.; Rastogi, P.; Khanna, R.; Tripathi, S. Efficacy of Acacia arabica gum as an adjunct to scaling and root planing in the treatment of chronic periodontitis: A randomized controlled clinical trial. Saudi Dental J. 2018, 30, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Roy, S.; Parida, P.K.; Dutta, A.; Bardhan, S.; Das, S.; Jana, K.; Karmakar, P. Folic acid conjugated curcumin loaded biopolymeric gum acacia microsphere for triple negative breast cancer therapy in vitro and in vivo model. Mater. Sci. Eng. C. 2019, 95, 204–216. [Google Scholar] [CrossRef]
- Padil, V.V.; Wacławek, S.; Černík, M.; Varma, R.S. Tree gum-based renewable materials: Sustainable applications in nanotechnology, biomedical and environmental fields. Biotechnol. Adv. 2018, 36, 1984–2016. [Google Scholar] [CrossRef] [PubMed]
- Idris, O.H.M.; Haddad, G.M. Gum Arabic (gum acacia’s) journey from tree to end user. In Gum Arabic; Kennedy, J.F., Phillips, G.O., Williams, P.A., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2011. [Google Scholar] [CrossRef]
- Glicksman, M. Food Hydrocolloids; Glicksman, M., Ed.; CRC Press: Boca Raton, FL, USA, 1983; Volume 2, p. 7. [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C. Re-evaluation of acacia gum (E 414) as a food additive. EFSA J. 2017, 15, 04741. [Google Scholar] [PubMed]
- Younes, M.; Aquilina, G.; Castle, L.; Engel, K.H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gürtler, R.; Husøy, T.; Mennes, W.; et al. Opinion on the re-evaluation of Acacia gum (E 414) as a food additive in foods for infants below 16 weeks of age and the follow-up of its re-evaluation as a food additive for uses in foods for all population groups. EFSA J. 2019, 17, 05922. [Google Scholar] [PubMed]
- Page, T.L.; Apfel, J. Medical Compound. U.S. Patent 481815, 30 August 1892. [Google Scholar]
- Cheetham, P.S.J.; Quail, M.A. Process for Preparing L-Rhamnose. U.S. Patent 5077206, 31 December 1991. [Google Scholar]
- Tanner, G.J.; Joseph, R.G.; Larkin, P.J. Manipulation of Proanthocyanidin Biosynthesis. U.S. Patent WO9807836A1, 26 February 1998. [Google Scholar]
- Kieliszewski, M.J. Synthetic Genes for Plant Gums and other Hydroxyproline-Rich Glycoproteins. U.S. Patent 6570062, 27 May 2003. [Google Scholar]
- Phillips, G.O.; Du, P.T.A.; Al-Assaf, S.; Williams, P.A. Biopolymers Obtained by Solid State Irradiation in an Unsaturated Gaseous Atmosphere. U.S. Patent 6610810, 26 August 2003. [Google Scholar]
- Phillips, G.O.; Du, P.T.A.; Al-Assaf, S.; Williams, P.A. Biopolymers Obtained by Solid State Irradiation in an Unsaturated Gaseous Atmosphere. U.S. Patent 6841644, 11 January 2005. [Google Scholar]
- Al-Assaf, S.; Phillips, G.O.; Sasaki, Y.; Katayama, T. Modified Acacia and Use Thereof. U.S. Patent WO2004089992A1, 21 October 2004. [Google Scholar]
- Heikkilae, H.; Koivikko, H.; Nurmi, J.; Mattila, J.; Saari, P.; Nurmi, N.; Sarmala, P.; Lindroos, M.; Lewandowski, J. Separation. Process. Patent No. WO2005042788A1, 12 May 2005. [Google Scholar]
- Pinhasi, A.; Gomberg, M. A Solid Composition for Intra-Oral Delivery of Insulin. U.S. Patent WO2006103657A2, 5 October 2006. [Google Scholar]
- Prakash, I.; Dubois, G.E.; Jella, P.; King, G.A.; San, M.R.; Specic, K.H.; Weerasinghe, D.K.; White, N. Natural High-Potency Sweetener Compositions with Improved Temporal Profile and/or Flavor Profile, Methods for Their Formulation, and Uses. U.S. Patent 9011956, 21 April 2015. [Google Scholar]
- Prakash, I.; Dubois, G.E.; Jella, P.; King, G.A.; San, M.R.; Specic, K.H.; Weerasinghe, D.K.; White, N.R. Synthetic Sweetener Compositions with Improved Temporal Profile and/or Flavor Profile, Methods for Their Formulation, and Uses. U.S. Patent 2007275147, 29 November 2007. [Google Scholar]
- Lang, F.; Nasir, O. Angioinhibin. U.S. Patent WO2008074437A2, 26 June 2008. [Google Scholar]
- Nakahama, H. Tannin-Free Talha Gum and Method of Removing Tannin from Talha Gum. U.S. Patent JP5139719B2, 6 February 2013. [Google Scholar]
- Lang, F.; Nasir, O. Composition for the Prophylaxis and Treatment of Osteoporosis. U.S. Patent WO2009021661A1, 19 February 2009. [Google Scholar]
- Zheng, H. Method for Uniformly Colorizing Fish Flesh. U.S. Patent No. CN102845737A, 2 January 2013. [Google Scholar]
- Morales, A.; Abbassmovahedi, H.; Figueroa, M.E.; Morales, J. Oral Devices Having Natural Gum Based Materials Therein. U.S. Patent 20130177867, 11 July 2013. [Google Scholar]
- Li, H. Blood-Cooling and Liquid-Engendering Bread by Utilizing Water Chestnuts and Chayote and Preparation Method Thereof. U.S. Patent CN105341064A, 24 February 2016. [Google Scholar]
- Liu, H.; Wang, Q.; Hu, H.; Chen, X.; Xu, F.; Shi, A.; Liu, L. Peanut Protein Solid Beverage Containing Yeast Mannan and Preparation Method Thereof. U.S. Patent CN105341611B, 29 June 2018. [Google Scholar]
- Bernett, N. Protein Based Frozen Dessert Using Alternative Sugars and Methods of Making the Same. U.S. Patent 2017071229, 16 March 2017. [Google Scholar]
- Haseleu, A.; Barkalow, D.G.; Orr, U.; Soto, M. Hard and Crunchy Confectionary Coating. U.S. Patent 20130101706, 25 April 2013. [Google Scholar]
- Hitzfeld, A.; Leuenberger, B.H.; Vidoni, O. Compositions of Fat-Soluble Active Ingredients Containing Gum Ghatti. U.S. Patent US8680161B2, 25 March 2014. [Google Scholar]
- Al-Assaf, S.; Lukanowski, J.; Tretzel, J. Gum Arabic from Acacia seyal. U.S. Patent EP3328901B1, 11 September 2019. [Google Scholar]
- Chen, X. Functional Surfactant and Preparation Method Thereof. U.S. Patent No. CN106377450A, 8 February 2017. [Google Scholar]
- Edwards, S. Adherent Dental Synbiotic Lozenge for ORal and General Health. U.S. Patent 20170232048, 17 August 2017. [Google Scholar]
- Savova, E.; Dullemond, W. Calorie Reduced Sugar Substitute Compositions. U.S. Patent 2020187535, 18 June 2020. [Google Scholar]
- Hara, M.; Tsuchida, M. Vegetable Proteoglycan and Use Therefor. U.S. Patent JP2019172718A, 10 October 2019. [Google Scholar]
- Sato, M. Method of Determining Contamination of Heterogeneous Gum, Apparatus for Determining Contamination of Heterogeneous gum and Computer Program for Determining Contamination of Heterogeneous Gum. U.S. Patent JP2019191085A, 31 October 2019. [Google Scholar]
- Fioretti, B.; Leonardi, L. Use of a Berberis and Resveratrol Mixture to Control Dyslipidemia. U.S. Patent WO2020128802A1, 25 June 2020. [Google Scholar]
- Hara, M.; Tsuchida, M. Plant-Derived Proteoglycan and Application Thereof. U.S. Patent 2021059344, 1 April 2021. [Google Scholar]
- Paredes, S.; Amann, A. Water-Soluble Microencapsulated Cannabinoid Extract Powder and Method of Making the Same. U.S. Patent 20210267907, 2 September 2021. [Google Scholar]
| Fraction | AY60 (Fraction) | AY 80 (Fraction) | AYS (Fraction) | AY (Entire Substance) |
|---|---|---|---|---|
| Weight percentage (%) | 44 | 39 | 2.4 | 100 |
| Average molecular weight | 924,900 Da | ND | ND | ND |
| % moisture content | 12.67 ± 0.04 | 13.59 ± 0.21 | ND | 14.41 ± 0.11 |
| % Ash content | 4.44 ± 0.01 | 4.51 ± 0.02 | ND | 3.50 ± 0.02 |
| % total protein content | 0.14 ± 0.01 | 0.13 ± 0.06 | 0.45 ± 0.02 | 0.32 ± 0.02 |
| % neutral sugar content | 61.24 ± 3.44 | 63.82 ± 2.76 | 67.82± 1.62 | 60.90 ±2.13 |
| % uronic acid content | 15.26 ± 0.25 | 16.17 ± 0.19 | 1.83 ± 0.07 | 17.43 ± 0.62 |
| the total molar percentage (mol%) of rhamnose | 2.13 | 2.24 | 2.28 | 3.09 |
| mol% of arabinose | 43.54 | 44.80 | 40.13 | 47.29 |
| mol% of galactose | 39.38 | 37.22 | 49.61 | 33.00 |
| mol% of galacturonic acid | 14.95 | 15.74 | 1.54 | 16.62 |
| Country | Use | Part | Ref. |
|---|---|---|---|
| Kenya | Pneumonia | Bark, stem, trunk, twig | [45] |
| Kenya | Malaria | Roots | [46] |
| Kenya | Joint pain | Bark, stems, leaves | [47] |
| Sudan | Bleeding, leprosy | Bark, leaves | [48] |
| Sudan | Arthritis, rheumatisms, rheumatoid fever | Wood | [49] |
| Ethiopia | Intestinal parasites | Roots, leaves | [50] |
| Ethiopia | Chest pain | Roots | [51] |
| Uganda | Diarrhea, Viral skin necrosis nodules | Roots, bark, leaves | [52] |
| Djibouti | Dysentery | Bark, roots | [53] |
| Algeria, Egypt, Morocco | Infected wounds, fever, dysmenorrhea, eye infections, stomach ulcers, rheumatisms | Seed | [54] |
| Algeria, Egypt, Morocco | Rheumatisms, respiratory tract infection, gastric ulcer | Gum | [55] |
| Pharmacological Activity | Possible Mechanism of Action | Refs. |
|---|---|---|
| Antiulcerative effect | It provides an antisecretory and cytoprotective effect on GIT. | [56,57] |
| Wound healing effect | Inhibits periodontic bacterial growth and early deposition of plaque. | [58] |
| Protective effect on the reproductive system | GA protects the ovary from oxidative stress damage in mice fed with a high-fat diet and increases sperm and semen qualities in the diabetic rat. | [59] |
| Hepatoprotective effect | GA decreases serum bilirubin level and other liver function markers (ALT, AST) and decreases symptoms of liver damage by restoring the architecture of liver tissue. | [60] |
| Activity against adenine-induced renal failure | GA mitigates the adenine-induced inflammation and generation of free radicals, resulting in reduced concentrations of plasma urea and creatinine. | [61] |
| Activity against Hg-induced nephrotoxicity | It prevented Hg-induced degenerative changes of kidney tissues. | [62] |
| Activity on renal function | It has a significant reduction in blood urea and creatinine concentrations in diabetic nephropathy patients. | [63,64] |
| Improvement of chronic renal failure | GA can activate colonic bacteria to produce ureases that hydrolyze urea to NH3 and CO2, NH3 excreted in feces through incorporation into bacterial protein. GA increases serum level of butyrate, which prevents the generation of pro-fibrotic cytokine TGF-B1 that contributes to renal fibroblast. | [65] |
| Activity against doxorubicin induced-cardiotoxicity | It has significant reduction effects on serum creatine kinase and cardiac lipid peroxides. | [66] |
| Health benefits on the cardiovascular system | GA showed a significant decrease in systolic and diastolic blood pressure. It has a hypocholesterolemic effect, decreasing low-density lipoproteins (LDL) and very-low-density lipoproteins (VLDL). | [67] |
| Antioxidant activity | GA increases the activity of superoxide dismutase, catalase, and glutathione peroxidase in the liver of diabetic rats by either directly scavenging free radicals or reactive oxygen metabolites or via increasing the synthesis of antioxidant biomolecules. | [59,61,68,69] |
| Anti-inflammatory effects | GA fibers decreased inflammatory markers and disease severity scores among rheumatoid arthritis patients. | [70] |
| Supportive treatment of gout | GA reduces in a dose-dependent manner the serum levels of uric acid, urea, creatinine, and erythrocyte sedimentation rate level while increasing the hemoglobin and packed cell volume. | [71] |
| Effects on fat metabolism and obesity | GA lowers sugar and fat absorption and lowers the caloric density of the diet. It improves the fat utilization in adipose tissues, alternating the expression of mRNA levels of genes involved in lipid metabolism. It has a downregulation effect on 11β-hydroxysteroid dehydrogenase type 1 and increases the viscosity of gastrointestinal contents, thus delaying the evacuation of GIT and contributing to a feeling of satiety. GA influences the gut hormones and enzymes that regulate food intake, satiety, and pancreatic functions. It has metabolic energy dilution, bulking, and satiety effects and aids fermentation to produce short-chain fatty acids and increase GLP-1 and PYY. GA diminishes intestinal SGLT1 expression and activity and glucose-actuated overweight. | [72,73,74,75,76,77,78,79] |
| Antihypercholesterolimic effect | GA decreases plasma triglyceride, total cholesterol, low-density lipoprotein (LDL), and very-low-density lipoprotein. GA disrupts the enterohepatic circulation of bile acids, leading to increased bile acid excretion. | [75,80,81,82] |
| Antidiabetic effect | The gel-forming and viscosity of GA inhibit intestinal absorption of macronutrients, enhancement of insulin sensitivity, and modification of certain gut hormones secretion affects a variety of metabolic and inflammatory biomarkers. | [83,84,85,86,87] |
| Immunomodulatory effects | GA increased the percentage of CD11c+CD40+, CD11c+MHCII+, CD11c+CD86+, and CD54− expressing DCs; in addition, it stimulated the production of IL-6, IL-10, IL12p70, and TNF-α in a p38- and/or extracellular signal-regulated kinases (ERK)-dependent manner. | [59,88] |
| Antibacterial activity | Due to poly-phenolic (tannins) and saponin contents, GA has antibacterial activities against pathogenic bacteria. GA can also stimulate the growth of probiotic bacteria that protect the body against pathogenic bacteria. | [79,89] |
| Anti-sickle-cell anemia | GA increases fetal hemoglobin (HbF) level, mean corpuscular volume, and hematocrit level. | [90] |
| Antimalaria effect | GA metabolites (short-chain fatty acids) increase the level of HbF, which is known to hamper the intra-erythrocytic growth of Plasmodium parasites. | [59,91] |
| Anticarcinogenic effect | GA modifies cancer-related genes’ mRNA expression. Antioxidant amino acids contents of GA have radical scavenging activities. GA is involved as a nanomaterial for the preparation of anticancer nano-pharmaceuticals, e.g., gold nanoparticles and selenium nanoparticles. GA decreased the colonic mRNA levels of the angiogenetic factors and diminished ss-catenin expression. | [59,69,79,92,93] |
| Dermatological activity | It is used as an antiallergic, smoothing, protective, binding, and/or stabilizing agent in cosmetic preparations. It has an anti-inflammatory effect against Kwashiorkor skin lesions and decreases skin inflammation (redness). | [94,95,96] |
| Water and electrolyte up-taking | GA increases water and electrolyte movement from the intestinal lumen to the bloodstream. | [97] |
| Gut probiotic effect | GA increases the growth of colonic beneficial strains of Lactobacillus and Bifidobacterium. GA selectively nourishes gut microbiota and aid to produces short-chain fatty acids, especially butyrate, and inhibits pathogenic organisms, e.g., the Clostridium histolyticum group, that are commonly associated with gut dysbiosis. | [98,99] |
| Dentistry applications | It upgrades dental re-mineralization and has some antimicrobial effects. It showed antiplaque on the gums and teeth and anti-gingivitis actions. | [68,100] |
| Industrial Relevance | Its Role | Refs. |
|---|---|---|
| Adjustment of medication delivery | GA microspheres facilitate absorption and expand the bioavailability of drugs. | [101] |
| Nanotechnology | GA is a renewable, biocompatible, biodegradable, and non-harmful nanomaterial. GA has the optimum capacity to experience simple synthetic alterations with higher economic values. | [102] |
| Additive in Food and pharmaceutical industry | GA has many applications as an emulsifier, stabilizer, thickener, processing aid, firming agent, texturizer, adhesive, plasticizer, and formulation aid. GA protects against unstable oils and flavors from the development of rancidity and off-tastes. | [67,103,104,105,106] |
| Confectionery industry | GA prevents sugar crystallization, modifies texture, emulsifies, acts as a binder, and keeps fatty components evenly distributed. | [103] |
| Baking products | GA has comparatively low water absorption and favorable adhesive properties. It imparts stability in bun glaze with free-flowing and adhesive characteristics. | [103] |
| high-quality emulsifying conjugate | A. seyal gum was incorporated with β-lactoglobulin through Maillard reaction to obtain emulsifying conjugate with high-quality properties. | [37] |
| S. No. | Patent/Patent Application Number (Applicant; Publication Date; Priority Country) | International Patent Classification | Status on 15 November 2021 (Family Members) | Summary |
|---|---|---|---|---|
| 1 | US481815A (Thomas Page; 30 August 1892; USA) | A61K36/48 (EP, US) | Expired patent (None) | It claims a medical composition comprising an aqueous solution (prepared in boiling water) of Acacia constricta or its equivalent such as A. seyal (two parts) to treat/cure kidney and bladder affections [107]. |
| 2 | US5077206A (Unilever Patent Holdings; 31 December 1991; United Kingdom) | C07H3/08 C12P19/02 C12P19/14 (IPC1-7): C07G17/00 C07H15/00; C12N9/24; C12P19/14; | Expired patent (AT92109T CA1333780C DE3882655T2 EP0317033B1 ES2058241T3 JPH02502248A MX170209B PT89040B WO8904870A1) | It claims an enzymatic process for preparing L-rhamnose from plant material such as A. Seyal [108]. |
| 3 | WO9807836A1 (Commonwealth Scientific and Industrial Research; 26 February 1998; Australia) | C07K16/40, C12N15/29, C12N15/82, C12N9/04, (IPC1-7): A01H1/00, C12N15/29, C12N15/53, C12N15/61, C12N9/02, C12N9/90 | Lapsed (AR009294A1, CA2264201A1, NZ334224A) | It claims isolated nucleic acid molecules that encode leucoanthocyanidin reductases of plants such as A. seyal [109]. |
| 4 | US6570062B1 (Ohio University; 27 May 2003; USA) | C07K14/415, C12N15/29, C12N15/82, (IPC1-7): C12N15/29, C12N15/82, C12P19/04, C12P21/02 | Expired patent (WO9903978A1) | It claims an isolated plant gum polynucleotide or synthetic genes that help to improve gum Arabic production in plants (A. senegal and A. seyal) [110]. |
| 5 | US6610810B2 (Phillips Hydrocolloids Research Limited; 26 August 2003; USA) | A61L27/00, C08B11/12, C08B11/20, C08B37/00, C08B37/06, C08F2/46, C08G63/00, C08H1/06, C08H6/00, C08H7/00, C08J3/28 (IPC1-7): C08F2/46, C08G63/00, C08H5/02 | Expired patents (CA2440863A1 EP1565483A2 JP2004536624A RU2280038C2 WO02072862A2 ZA200307398B) | US6610810B2 [111] and US6841644B2 [112] are members of the same patent family and claim new biopolymers of A. seyal with improved physicochemical properties. |
| 6 | US6841644B2 (Phillips Hydrocolloids Research Limited; 11 January 2005; USA) | |||
| 7 | WO2004089992A1 (Phillips Hydrocolloids Research Limited; 21 October 2004; Japan) | A23L1/308, A23L29/20, A61K31/736, A61P1/14, A61P3/06, A61P3/10, A61P35/00, C08B37/00 (IPC1-7): A23L1/308, A61K31/736, A61P1/14, A61P3/06, A61P3/10, A61P35/00, C08B37/00 | Lapsed (CA2521692A1, CN100447160C, EP1612225A1, JPWO2004089992A1, US2006240166A1) | It claims a water-soluble modified gum Arabic that has a total dietary fiber content of 90% or more, which was prepared by heating gum Arabic (A. seyal and A. senegal) [113]. |
| 8 | WO2005042788A1 (Danisco Sweeteners Oy; 12 May 2005; USA) | C07H1/08, C13K13/00, (IPC1-7): C13K13/00 | Lapsed (EP1678330A1, NO20062457L, US2005096464A1, US2007112187A1) | It claims a process of recovering arabinose from vegetable fiber (exudate gum such as gum Arabic, gum ghatti, and gum tragacanth) [114]. |
| 9 | WO2006103657A2 (Dexcel Pharma Technologies; 5 October 2006; Israel) | A61K9/006 (EP), A61K9/127 (EP), A61K9/7007 (EP) | No national phase entry (None) | It claims a solid composition for intra-oral/buccal delivery of insulin encompassing insulin, a hydrophilic polymer (such as gum Talha or A. seyal) matrix, and a phospholipid (such as lecithin or phosphotidylcholin), providing insulin bioavailability of 5–20% [115]. |
| 10 | US9011956B2 (Prakash Indra; 21 April 2015; USA) | A23L27/00, A23L27/30, A23L1/305, A23L2/52, A23L2/60, A61K31/575 | Patented case (AR056220A1, AU2006318781B2, BRPI0618945A2, CA2630049C, CA2969364C, CN103393062B, DK2526783T3, EP2526778B1, EP2526783B1, EP3199033B1, ES2611887T3, JP6113974B2, JP6609587B2, KR101374346B1, KR101385710B1, MX2008006583A, MY149619A, TW200738168A, UY29928A1, WO2007061795A1, ZA200804458B) | It claims a sweetener composition comprising rebaudioside A (purity > 97%), erythritol, and a sweet taste improving polymer (such as gum A. senegal and gum A. seyal) or combinations thereof [116]. |
| 11 | US2007275147A1 (The Coca-Cola Company; 29 November 2007; USA) | A23L27/00, A23L27/30 | Abandoned in 2011 (AR056180A1, AU2006335251A1, BRPI0619068A2, CA2629556A1, EP1965667A2, JP2009517037A, KR20080071606A, MX2008006587A, TW200738169A, WO2007081442A2) | It claims a synthetic sweetener composition with an improved taste profile comprising a sweet taste improving polymer (A. senegal and gum A. seyal) [117]. |
| 12 | WO2008074437A2 (Eberhard-Karls-Universitaet Tuebingen Universitaetsklinikum; 26 June 2008; Germany) | A61K35/00, A61K36/48 | Lapsed (DE102006061517A1, EP2109453A2) | It claims the use of gum Arabic (A. senegal and A. seyal) as an active ingredient of an angioinhibin (angiogenesis inhibitors) [118]. |
| 13 | JP5139719B2 (Kamisu Kagaku; 6 February 2013; Japan) | C02F1/58, C08B37/00 | Patented case (None) | It claims a tannin-free talha gum (A. seyal) having acceptable quality for use in the food industry [119]. |
| 14 | WO2009021661A1 (Eberhard-Karls-Universitaet Tuebingen Universitaetsklinikum; 19 February 2009; Germany) | A61K36/48, A61P19/10 | Lapsed (DE102007039310A1) | It claims the use of gum Arabic (A. senegal and A. seyal) for the prophylaxis and treatment of osteoporosis [120]. |
| 15 | CN102845737A (Tianjin Tiankangyuan Biological Technology; 2 January 2013; China) | A23L1/275, A23L17/00 | Withdrawn (None) | It claims a method for uniformly coloring fish meat (salmon meat) utilizing a 10% aqueous solution of A. senegal or A. seyal [121]. |
| 16 | US20130177867A1 (Morales Anthony; 11 July 2013; USA) | A61C19/06, A61C5/14, A61J17/00 | Abandoned in 2014 (None) | It claims an oral device to dispense substances in an oral cavity comprising a natural gum (gum Arabic such as A. senegal and A. seyal) or a combination of natural gum with a medicament [122]. |
| 17 | CN105341064A (Li Hua; 24 February 2016; China) | A21D13/00, A21D2/36 | Withdrawn (None) | It claims a nutritional chayote bread containing gum Arabic (A. senegal and A. seyal) [123]. |
| 18 | CN105341611B (Chinese Academy of Agricultural Sciences; 29 June 2018; China) | A23L2/39, A23L2/62 | Patented case (None) | It claims a stable and nutritional peanut protein solid beverage comprising gum Arabic (A. senegal and A. seyal) [124]. |
| 19 | US2017071229A1 (Coolwhey Inc.; 16 March 2017; USA) | A23G9/32, A23G9/38 | Abandoned in 2018 (CA2942266C) | It claims a protein-fortified frozen dessert formulation utilizing gum Arabic (A. senegal and A. seyal) as a stabilizing agent [125]. |
| 20 | US20130101706A1 (Haseleu Andrea; 25 April 2013; USA) | A23G3/38, A23G3/54 | Abandoned in 2017 (AU2010336955B2 CA2785060C CN102695425A EP2519113A1 EP2519113B1 PL2519113T3 RU2517862C2 WO2011082050A1) | It claims a confectionary product (chewing gums and candies) comprising a film-forming agent (gum tahla) [126]. |
| 21 | US8680161B2 (Hitzfeld Andrea; 25 March 2014; Europe) | A23L33/155, A61K31/07 | Patented case (CN102056497B, EP2280611B1, ES2436167T3, JP2011521658A, PL2280611T3, WO2009147158A2) | It claims a composition of dried particles of gum ghatti, gum Acacia (A. senegal and A. seyal), and at least one fat-soluble active ingredient (carotenoid) useful for the enrichment, fortification, and/or coloration of food, beverages, animal feed, cosmetics, or pharmaceutical compositions [127]. |
| 22 | EP3328901B1 (Döhler GMBH; 11 September 2019; Europe) | A23L29/25, C08B37/00, C08L5/00, A23L2/52 | Patented case (BR112018001226A2, ES2758363T3, HRP20192112T1, HUE046765T2, JP2018523494A, MX2018001147A, PL3328901T3, PT3328901T, RS59685B1, RU2725959C2, SI3328901T1, US2018215841A1, US2021070892A1, WO2017017248A1) | It claims an improved gum Arabic (A. seyal) having a tannin content > 700 ppm (w/w) with superior emulsification performance [128]. |
| 23 | CN106377450A (Chen Xiong; 8 February 2017; China) | A23L29/10, A23L33/10, A61K36/14, A61K47/36, A61K8/73, A61K8/9761, A61P11/10, A61P11/14, A61P9/12, A61Q5/02, A61Q5/12 | Withdrawn (None) | It claims a functional surfactant/emulgent based on A. seyal gum [129]. |
| 24 | US20170232048A1 (Renuzoral; 17 August 2017; USA) | A61K31/715, A61K31/723, A61K31/733, A61K35/744, A61K35/747, A61K36/48, A61K47/02, A61K47/10, A61K47/12, A61K47/26, A61K47/36, A61K47/38, A61K47/46, A61K8/02, A61K8/24, A61K8/34, A61K8/36, A61K8/60, A61K8/73, A61K8/97, A61K8/99, A61K9/00, A61Q11/00 | Abandoned in 2019 (None) | It claims a dental synbiotic lozenge encompassing adhesive prebiotics (inulin, A. seyal gum, Konjac mannan, Xanthan gum) and one or more species of probiotic organisms [130]. |
| 25 | US2020187535A1 (FTC International Consulting Ltd.; 18 June 2020; Canada) | A23L27/00, A23L27/30, A23L33/21 | Under examination (CA3063233A1 MX2019013378A WO2018205039A1) | It claims a sugar substitute composition comprising a digestion resistant soluble fiber (A. senegal and A. seyal) between 99.00% and 99.99% by weight and a stevia leaf extract (0.01% and 1.00% by weight) [131]. |
| 26 | JP2019172718A (NOF Corporation; 10 October 2019; Japan) | A61K36/48, A61K38/02, A61K8/73, A61K8/9789, A61P17/16, A61P43/00, A61Q19/00, C08B37/00 | Under examination (None) | It claims a plant proteoglycan (molecular weight of 900,000-3,500,000; total aldehyde content = 2.0 μmol equivalent/g or less) obtained from A. senegal or A. seyal [132]. |
| 27 | JP2019191085A (Sanei Gen FFI Inc.; 31 October 2019; Japan) | G01N21/359 | Under examination (None) | It claims a method for determining the mixing/contamination of different types of gum into gum Arabic (A. senegal and A. seyal) or gadhi gum by measuring diffuse reflection using a near-infrared spectrophotometer [133]. |
| 28 | WO2020128802A1 (S&R Farmaceutici; 25 June 2020; Italy) | A61K31/05, A61K31/4375, A61P3/06 | Entered into national phase (CA3122918A1 CN113242733A EP3897596A1 IT201800011155A1) | It claims a food supplement comprising a mixture of berberine, resveratrol, and one nutrient with properties of regulating the lipid profile (A. senegal and A. seyal) for use in the treatment and/or control of dyslipidemia [134]. |
| 29 | WO2021059344A1 (NOF Corporation; 1 April 2021; Japan) | A61K8/9789, A61Q19/00, C08B37/00, A23L33/105, A61K38/02 | No national phase entry (None) | WO2021059344A1 [135] claims the same invention as described in JP2019172718A [132]. |
| 30 | US20210267907A1 (Prinova Flavors; 2 September 2021; USA) | A61K31/05, A61K9/50 | Under examination (CN113304703A, EP3875076A1) | It claims a water-dispersible microencapsulated composition containing 10–20% of cannabinoid and at least one gum Acacia (A. senegal and A. seyal) for use as an ingredient in food and cosmetics [136]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashour, M.A.; Fatima, W.; Imran, M.; Ghoneim, M.M.; Alshehri, S.; Shakeel, F. A Review on the Main Phytoconstituents, Traditional Uses, Inventions, and Patent Literature of Gum Arabic Emphasizing Acacia seyal. Molecules 2022, 27, 1171. https://doi.org/10.3390/molecules27041171
Ashour MA, Fatima W, Imran M, Ghoneim MM, Alshehri S, Shakeel F. A Review on the Main Phytoconstituents, Traditional Uses, Inventions, and Patent Literature of Gum Arabic Emphasizing Acacia seyal. Molecules. 2022; 27(4):1171. https://doi.org/10.3390/molecules27041171
Chicago/Turabian StyleAshour, Mohamed A., Waseem Fatima, Mohd. Imran, Mohammed M. Ghoneim, Sultan Alshehri, and Faiyaz Shakeel. 2022. "A Review on the Main Phytoconstituents, Traditional Uses, Inventions, and Patent Literature of Gum Arabic Emphasizing Acacia seyal" Molecules 27, no. 4: 1171. https://doi.org/10.3390/molecules27041171
APA StyleAshour, M. A., Fatima, W., Imran, M., Ghoneim, M. M., Alshehri, S., & Shakeel, F. (2022). A Review on the Main Phytoconstituents, Traditional Uses, Inventions, and Patent Literature of Gum Arabic Emphasizing Acacia seyal. Molecules, 27(4), 1171. https://doi.org/10.3390/molecules27041171








