The Profile of Polyphenolic Compounds, Contents of Total Phenolics and Flavonoids, and Antioxidant and Antimicrobial Properties of Bee Products
Abstract
:1. Introduction
2. Results and Discussion
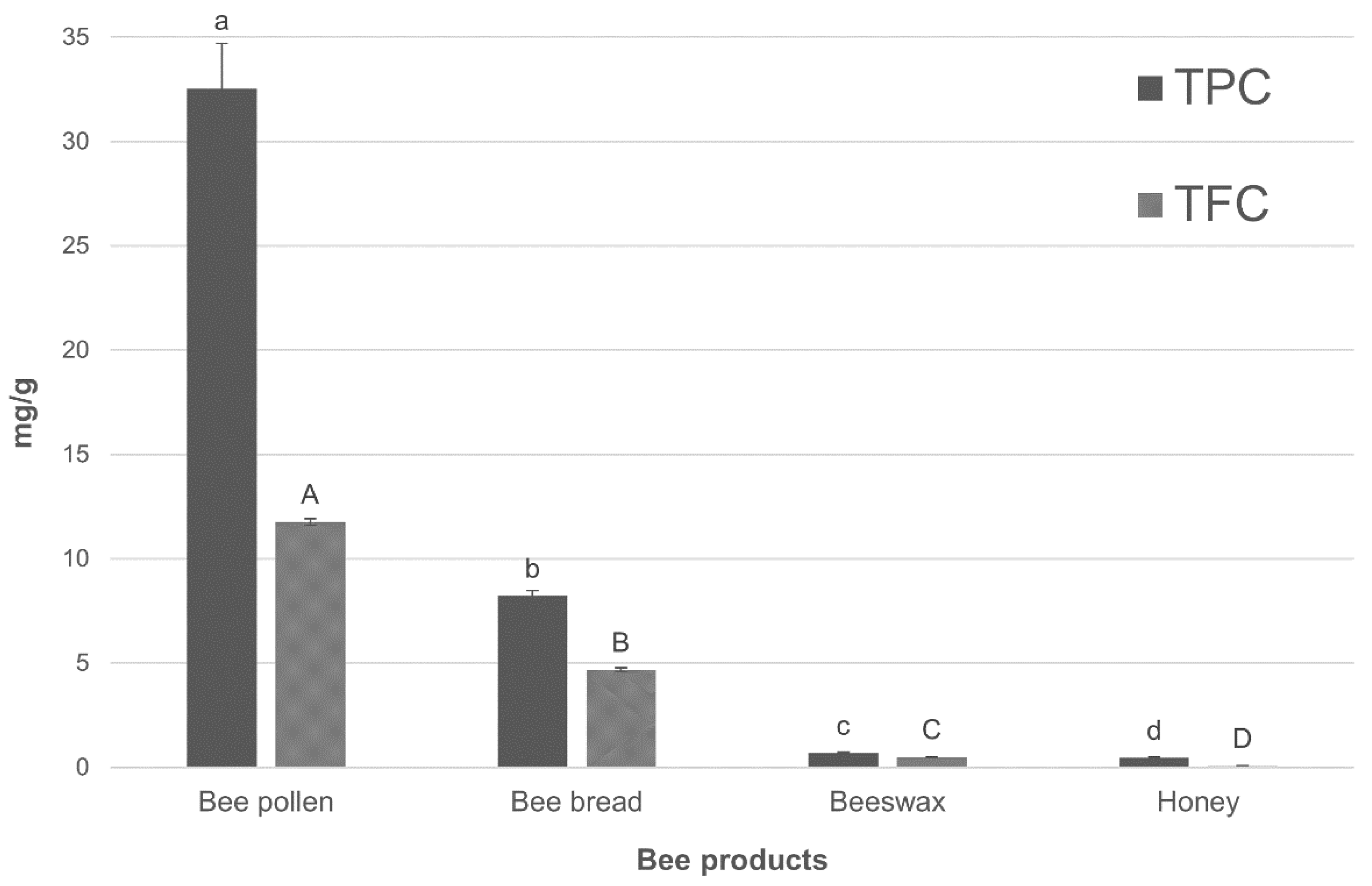
2.1. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.2. Polyphenolic Profile in the Bee Products
2.3. Antioxidant Activity and Reducing Potential of Bee Products
2.4. Antimicrobial Activity
2.5. Principal Component Analysis (PCA)
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Research Material
3.3. Extraction of Polyphenols
3.4. Identification and Quantification of Polyphenols
3.5. Determination of Total Phenolics and Flavonoids Content (TPC, TFC)
3.6. Determination of Antioxidant Activity (DPPH, ABTS, and PCL) and Reducing Potential (FRAP)
3.7. AntimicrobialActivity
3.7.1. Determination of Antimicrobial Activity by the Well Method
3.7.2. Determination of Minimum Inhibitory Concentration (MIC)
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kieliszek, M.; Piwowarek, K.; Kot, A.M.; Błażejak, S.; Chlebowska-Śmigiel, A.; Wolska, I. Pollen and bee bread as a new health-oriented products: A review. Trends Food Sci. Technol. 2018, 71, 170–180. [Google Scholar] [CrossRef]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic properties of bioactive compounds from different honeybee products. Front. Pharmacol. 2017, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Denisow, B.; Denisow-Pietrzyk, M. Biological and therapeutic properties of bee pollen: A review. J. Sci. Food Agric. 2016, 96, 4303–4309. [Google Scholar] [CrossRef]
- Baltršaitytė, V.; Rimantas Veskutonis, P.; Čeksterytė, V. Radical scavenging activity of different floral origin honey and beebread phenolic extracts. Food Chem. 2007, 101, 502–514. [Google Scholar] [CrossRef]
- Socha, R.; Juszczak, L.; Pietrzyk, S.; Gałkowska, D.; Fortuna, T.; Witczak, T. Phenolic profile and antioxidant properties of Polish honeys. Int. J. Food Sci. Technol. 2011, 46, 528–534. [Google Scholar] [CrossRef]
- Mosić, M.; Trifković, J.; Vovk, I.; Gašić, U.; Tešić, Ž.; Šikoparija, B.; Milojković-Opsenica, D. Phenolic composition influences the health-promoting potential of bee-pollen. Biomolecules 2019, 26, 783. [Google Scholar] [CrossRef] [Green Version]
- Negri, G.; Teixeira, E.W.; Alves, M.L.; Moreti, A.C.; Otsuk, I.P.; Borguini, R.G.; Salatino, A. Hydroxycinnamic acid amide derivatives, phenolic compounds and antioxidant activities of extracts of pollen samples from Southeast Brazil. J. Agric. Food Chem. 2011, 59, 5516–5522. [Google Scholar] [CrossRef]
- Martinello, M.; Mutinelli, F. Antioxidant activity in bee products: A review. Antioxidants 2021, 10, 71. [Google Scholar] [CrossRef]
- Saral, Ö.; Kiliçarslan, M.; Şahin, H.; Yildiz, O.; Dinçer, B. Evaluation of antioxidant activity of bee products of different bee races in Turkey. Turk. J. Vet. Anim. Sci. 2019, 43, 441–447. [Google Scholar] [CrossRef]
- Sawicki, T.; Bączek, N.; Starowicz, M. Characterisation of the total phenolic, vitamins C and E content and antioxidant properties of the beebread and honey from the same batch. Czech. J. Food Sci. 2020, 38, 158–163. [Google Scholar] [CrossRef]
- Wesołowska, M.; Dżugan, M. The use of PHOTOCHEM device in evaluation of antioxidant activity of Polish honey. Food Anal. Methods 2017, 10, 1568–1574. [Google Scholar] [CrossRef]
- Felicioli, A.; Cilia, G.; Mancini, S.; Turchi, B.; Galaverna, G.; Cirlini, M.; Cerri, D.; Frantini, F. In vitro antibacterial activity and volatile characterization of organic Apis mellifera ligustica (Spinola, 1906) beeswax ethanol extracts. Food Biosci. 2019, 29, 102–109. [Google Scholar] [CrossRef]
- Kacániová, M.; Vuković, N.; Chlebo, R.; Haščik, P.; Cubon, J.; Dżugan, M.; Pasternakiewicz, A. The antimicrobial activity of honey, bee pollen loads and beeswax from Slovakia. Arch. Biol. Sci. 2012, 64, 927–934. [Google Scholar] [CrossRef]
- Mayda, N.; Özkök, A.; Ecem Bayram, N.; Gerçek, Y.C.; Sorkun, K. Bee bread and bee pollen of different plant sources: Determination of phenolic content, antioxidant activity, fatty acid and element profiles. J. Food Meas. Charact. 2020, 14, 1795–1809. [Google Scholar] [CrossRef]
- Gośliński, M.; Nowak, D.; Kłębukowska, L. Antioxidant properties and antimicrobial activity of manuka honey versus Polish honeys. J. Food Sci. Technol. 2020, 57, 1269–1277. [Google Scholar] [CrossRef]
- Kafantaris, I.; Amoutzias, G.D.; Mossialos, D. Foodomics in bee product research: A systematic literature review. Eur. Food Res. Technol. 2021, 247, 309–331. [Google Scholar] [CrossRef]
- Fratini, F.; Cilia, G.; Turchi, B.; Felicioli, A. Beeswax: A minireview of its antimicrobial activity and its application in medicine. Asian Pac. J. Trop. Med. 2016, 9, 839–843. [Google Scholar] [CrossRef]
- Gabriele, M.; Parri, E.; Felicioli, A.; Sagona, S.; Pozzo, L.; Biondi, C.; Domenici, V. Phytochemical composition and antioxidant activity of Tuscan bee pollen of different botanic origins. Ital. J. Food Sci. 2015, 27, 248–259. [Google Scholar]
- Rocchetti, G.; Castiglioni, S.; Maldarizzi, G.; Carloni, P.; Lucini, L. UHPLC-ESI-QTOF-MS phenolic profiling and antioxidant capacity of bee pollen from different botanical origin. Int. J. Food Sci. Technol. 2018, 54, 335–346. [Google Scholar] [CrossRef]
- Rzepecka-Stojko, A.; Stojko, J.; Kurek-Górecka, A.; Górecki, M.; Sobczak, A.; Stojko, R.; Buszman, E. Polyphenol content and antioxidant activity of bee pollen extracts from Poland. J. Apic. Res. 2015, 54, 482–490. [Google Scholar] [CrossRef]
- Čeksteryté, V.; Kurtinaitienė, B.; Venskutonis, P.R.; Pukalskas, A.; Kazernavičiūtė, R.; Balžekas, J. Evaluation of antioxidant activity and flavonoid composition in differently preserved bee products. Czech. J. Food Sci. 2016, 34, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Socha, R.; Habryka, C.; Juszczak, L. Effect of propolis as additive on content of selected phenolic compounds and antioxidant activity of honey. Food Sci. Technol. Qual. 2016, 23, 127–139. [Google Scholar]
- Navarro-Hortal, M.D.; Orantes-Bermejo, F.J.; Sánchez-González, C.; Varela-López, A.; Giampieri, F.; Torres Fernández-Piñar, C.; Serra-Bonvehí, J.; Forbes-Hernández, T.Y.; Reboredo-Rodríguez, P.; Llopis, J.; et al. Industrial-scale decontamination procedure effects on the content of acaricides, heavy metals and antioxidant capacity of beeswax. Molecules 2019, 24, 1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giampieri, F.; Quiles, J.; Orantes-Bermejo, F.J.; Gasparrini, M.; Forbes-Hernández, T.; Sánchez-González, C.; Llopis, J.; Rivas-García, L.; Afrin, S.; Varela-López, A.; et al. Are by-products from beeswax recycling process a new promising source of bioactive compounds with biomedical properties? Food Chem. Toxicol. 2018, 112, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, C.M.; Serratob, J.C.; Quicazana, M.C. Chemical, nutritional and bioactive characterization of Colombian bee-bread. Chem. Eng. Trans. 2015, 43, 175–180. [Google Scholar]
- Habryka, C.; Socha, R.; Juszczak, L. Effect of bee pollen addition on the polyphenolic content, antioxidant activity, and quality parameters of honey. Antioxidants 2021, 10, 810. [Google Scholar] [CrossRef]
- Yucel, B.; Topal, E.; Kosoglu, M. Bee products as functional food. In Superfood and Functional Food—An Overview of Their Processing and Utilization; Waisundara, V., Shiomi, N., Eds.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Bakour, M.; Fernandes, A.; Barros, L.; Sokovic, M.; Ferreira, I.C.F.R.; Iyoussi, B. Bee bread as a functional product: Chemical composition and bioactive properties. LWT-Food Sci. Technol. 2019, 109, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Isidorov, V.A.; Isidorova, A.G.; Sczczepaniak, L.; Czyżewska, U. Gas chromatographic-mass spectrometric investigation of the chemical composition of beebread. Food Chem. 2009, 115, 1056–1063. [Google Scholar] [CrossRef]
- Sieniewska, E.; Baj, T.; Los, R.; Skalicka-Wozniak, K.; Malm, A.; Glowniak, K. Phenolic acids content, antioxidant and antimicrobial activity of Ligusticum mutellina L. Nat. Prod. Res. 2013, 27, 1108–1110. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, M.; Ma, G.; Fang, Y.; Yang, W.; Ma, N.; Fang, D.; Hu, Q.; Pei, F. The antioxidant and antimicrobial activities of different phenolic acids grafted onto chitose. Carbohydr. Polym. 2019, 225, 115238. [Google Scholar] [CrossRef]
- Bayram, N.E.; Gercek, Y.C.; Celik, S.; Mayda, N.; Kostic, A.Z.; Dramicanin, A.M.; Ozkok, A. Phenolic and free aino acid profiles of bee bread and bee pollen with the same botanical origin–similarities and differences. Arab. J. Chem. 2021, 14, 103004. [Google Scholar] [CrossRef]
- Ivanišová, E.; Kačániová, M.; Frančáková, H.; Petrová, J.; Hutková, J.; Brovarskyi, V.; Velychko, S.; Adamczuk, L.; Schubertová, Z.; Musilova, J. Bee bread-perspective source of bioactive compounds for future. Potravinarstvo 2015, 9, 592–598. [Google Scholar] [CrossRef] [Green Version]
- Dżugan, M.; Grabek-Lejko, D.; Sidor, E.; Tomczyk, M. The impact of ultrasound decrystallization on enzymatic, antioxidant and antibacterial properties of honey. Innov. Food Sci. Emerg. Technol. 2021, 71, 102709. [Google Scholar] [CrossRef]
- Dżugan, M.; Grabek-Lejko, D.; Swacha, S.; Tomczyk, M.; Bednarska, S.; Kapusta, I. Physicochemical quality parameters, antibacterial properties and cellular antioxidant activity of Polish buckwheat honey. Food Biosci. 2020, 34, 100538. [Google Scholar] [CrossRef]
- Tomczyk, M.; Miłek, M.; Sidor, E.; Kapusta, I.; Litwińczuk, W.; Puchalski, C.; Dżugan, M. The effect of adding the leaves and fruits of Morus alba to rape honey on its antioxidant properties, polyphenolic profile, and amylase activity. Molecules 2020, 25, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, F.; Chen, W.F.; Zhou, B. Antioxidant synergism of green tea polyphenols with alpha-tocopherol and L-ascorbic acid in SDS micelles. Biochimie 2008, 90, 1499–1505. [Google Scholar] [CrossRef]
- Herranz-López, M.; Fernández-Arroyo, S.; Pérez-Sanchez, A.; Barrajón-Catalán, E.; Beltrán-Debón, R.; Menéndez, J.A.; Alonso-Villaverde, C.; Segura-Carretero, A.; Joven, J.; Micol, V. Synergism of plant-derived polyphenols in adipogenesis: Perspectives and implications. Phytomedicine 2012, 19, 253–261. [Google Scholar] [CrossRef]
- Tang, Y.; Lana, X.; Lianga, C.; Zhonga, Z.; Xiea, R.; Zhoua, Y.; Miaoa, X.; Wangc, H.; Wangd, W. Honey loaded alginate/PVA nanofibrous membrane as potential bioactive wound dressing. Carbohydr. Polym. 2019, 219, 113–120. [Google Scholar] [CrossRef]
- Wilczyńska, A. Phenolic content and antioxidant activity of different types of Polish honey—A short report. Pol. J. Food Nutr. Sci. 2010, 60, 309–313. [Google Scholar]
- Horszwald, A.; Andlauer, W. Characterisation of bioactive compounds in berry juices by traditional photometric and modern microplate methods. J. Berry Res. 2011, 1, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1996, 28, 25–30. [Google Scholar] [CrossRef]

| No. | Compound | Rt [min] | MS [m/z] | MS/MS [m/z] | λmax [nm] | Sample |
|---|---|---|---|---|---|---|
| 1 | gallic acid | 1.12 | 169 | 125 | 271 | H, BB, BP |
| 2 | ellagic acid | 1.16 | 301 | 283/200/175 | 267 | H, BB, BP |
| 3 | neochlorogenic acid | 1.71 | 353 | 191/179 | 262 | H, BB, BP |
| 4 | chlorogenic acid | 2.03 | 353 | 191/179 | 281 | H, BB, BP |
| 5 | protocatechuic acid | 2.23 | 153 | 109 | 291 | H, BB, BP |
| 6 | sakuranetin dimer | 3.19 | 551 | 285/179/164 | 307 | H |
| 7 | caffeic acid | 3.96 | 179 | 135 | 300 | H |
| 8 | rutin | 4.44 | 609 | 301 | 286/338 | BB, BP |
| 9 | sinapic acid | 4.73 | 223 | 175/164 | 295 | H, BB, BP |
| 10 | isorhamnetine 3-O-rutinoside | 4.76 | 623 | 315/314 | 266, 309 | BB, BP |
| 11 | 3,4-di-O-caffeoylquinic acid | 5.09 | 515 | 179/191 | - | W |
| 12 | quercetin 3-O-glucuronide | 5.35 | 477 | 301 | 269, 324 | BB, BP |
| 13 | orientin | 5.39 | 447 | 357/339 | 265, 316 | BB |
| 14 | vitexin | 6.12 | 431 | 341/311 | 264, 315 | BB |
| 15 | quercetin | 6.45 | 301 | 179/151 | 256/355 | H |
| 16 | epicatechin | 7.44 | 289 | 245/203 | 319 | BB, BP |
| 17 | kaempferol | 7.53 | 285 | 257/201/185 | 286 | W |
| 18 | protocatechuic acid- O-hexoside | 8.15 | 315 | 153 | 287 | BB, BP, W |
| 19 | pinobanksin | 8.24 | 271 | 185/151 | 290 | BB, BP, W |
| 20 | apigenin | 8.65 | 269 | 179/225 | 338/ 346 | W |
| No. | Compound | Bee Product | |||
|---|---|---|---|---|---|
| Bee Pollen | Beebread | Honey | Beeswax | ||
| 1 | gallic acid | 21.3 | 32.6 | 69.2 | ND |
| 2 | ellagic acid | 2.2 | 2.6 | 6.6 | ND |
| 3 | neochlorogenic acid | 0.9 | 0.7 | 1.5 | ND |
| 4 | chlorogenic acid | 0.7 | 0.8 | 13.2 | ND |
| 5 | protocatechuic acid | 0.1 | 0.8 | 0.2 | ND |
| 6 | sakuranetin dimer | ND | ND | 4.6 | ND |
| 7 | caffeic acid | ND | ND | 0.8 | ND |
| 8 | rutin | 10.0 | 5.1 | ND | ND |
| 9 | sinapic acid | 42.8 | 27.3 | 1.8 | ND |
| 10 | isorhamnetine 3-O-rutinoside | 5.8 | 2.5 | ND | ND |
| 11 | 3.4-di-O-caffeoylquinic acid | ND | ND | ND | 1.9 |
| 12 | quercetin 3-O-glucuronide | 7.9 | 0.3 | ND | ND |
| 13 | orientin | ND | 7.1 | ND | ND |
| 14 | vitexin | ND | 15.2 | ND | ND |
| 15 | quercetin | ND | ND | 2.2 | ND |
| 16 | epicatechin | 7.2 | 2.6 | ND | ND |
| 17 | kaempferol | ND | ND | ND | 6.0 |
| 18 | protocatechuic acid-O-hexoside | 0.2 | 1.6 | ND | 3.4 |
| 19 | pinobanksin | 0.7 | 0.8 | ND | 85.7 |
| 20 | apigenin | ND | ND | ND | 3.0 |
| Total [µg/g] | 79.39 ± 0.31 a | 56.27 ± 0.89 b | 1.61 ± 0.02 c | 0.39 ± 0.00 d | |
| Parameter/Sample | PCL [µmol Trolox/g] | DPPH [µmol Trolox/g] | ABTS [mmol Trolox/g] | FRAP [µmol Trolox/g] | |
|---|---|---|---|---|---|
| ACL | ACW | ||||
| Bee pollen | 410.13 ± 19.56 b | 129.29 ± 2.75 b | 16.97 ± 1.19 a | 32.56 ± 0.30 a | 76.94 ± 4.48 a |
| Bee bread | 1017.83 ± 56.03 a | 162.16 ± 2.83 a | 10.26 ± 2.27 b | 31.60 ± 0.16 b | 31.23 ± 1.96 b |
| Beeswax | 210.59 ± 0.41 c | 4.72 ± 0.04 c | 0.53 ± 0.02 c | 5.96 ± 0.05 d | 8.14 ± 0.58 c |
| Honey | 1.53 ± 0.02 d | 4.72 ± 0.17 c | 0.18 ± 0.04 d | 15.68 ± 0.60 c | 35.36 ± 1.03 b |
| Test Strain | Sample Concentration [%] | Diameters of the Growth Inhibition Zones [mm] | |||
|---|---|---|---|---|---|
| Honey | Bee Pollen | Bee Bread | Beeswax | ||
| Gram-positive strains | |||||
| Staphylococcus aureus G3 | 90 | 12.0 ± 0.0 c | 16.0 ± 1.0 b | 22.0 ± 2.0 a | 0 |
| 75 | 0 | 14.0 ± 0.0 | 18.0 ± 2.0 | 0 | |
| 50 | 0 | 12.0 ± 0.0 | 16.0 ±1.0 | 0 | |
| 45 | 0 | 12.0 ± 0.0 | 14.0 ±1.0 | 0 | |
| 35 | 0 | 0 | 12.0 ± 0.0 | 0 | |
| Staphylococcus aureus 629G | 90 | 16.0 ± 1.0 | 16.0 ± 1.0 | 20.0 ± 2.0 | 0 |
| 75 | 14.0 ± 1.0 | 14.0 ± 1.0 | 18.0 ± 2.0 | 0 | |
| 50 | 12.0 ± 0.0 b | 12.0 ± 0.0 b | 18.0 ± 1.0 a | 0 | |
| 45 | 0 | 0 | 16.0 ± 1.0 | 0 | |
| 35 | 0 | 0 | 12.0 ± 0.0 | 0 | |
| Staphylococcus aureus ATCC29213 | 90 | 16.0 ± 1.0 | 18.0 ± 2.0 | 18.0 ± 1.0 | 0 |
| 75 | 14.0 ± 1.0 | 14.0 ± 1.0 | 16.0 ± 1.0 | 0 | |
| 50 | 12.0 ± 0.0 b | 14.0 ± 1.0 a | 14.0 ± 1.0 a | 0 | |
| 45 | 0 | 12.0 ± 0.0 | 12.0 ± 0.0 | 0 | |
| 35 | 0 | 0 | 12.0 ± 0.0 | 0 | |
| Enterococcus faecalis 24 | 90 | 0 | 14.0 ± 1.0 | 18.0 ± 1.0 | 0 |
| 75 | 0 | 12.0 ± 0.0 | 16.0 ± 1.0 | 0 | |
| 50 | 0 | 0 | 14.0 ± 1.0 | 0 | |
| 45 | 0 | 0 | 12.0 ± 0.0 | 0 | |
| 35 | 0 | 0 | 0 | 0 | |
| Enterococcus faecalis ss1-1 | 90 | 0 | 14.0 ± 1.0 | 16.0 ± 1.0 | 0 |
| 75 | 0 | 12.0 ± 0.0 | 16.0 ± 1.0 | 0 | |
| 50 | 0 | 0 | 14.0 ± 1.0 | 0 | |
| 45 | 0 | 0 | 12.0 ± 0.0 | 0 | |
| 35 | 0 | 0 | 0 | 0 | |
| Listeria monocytogenes 67 | 90 | 14.0 ± 1.0 b | 12.0 ± 0.0 c | 20.0 ± 2.0 a | 0 |
| 75 | 0 | 0 | 16.0 ± 1.0 | 0 | |
| 50 | 0 | 0 | 14.0 ± 1.0 | 0 | |
| 45 | 0 | 0 | 12.0 ± 0.0 | 0 | |
| 35 | 0 | 0 | 12.0 ± 0.0 | 0 | |
| Listeria monocytogenes 74 | 90 | 0 | 12.0 ± 0.0 | 18.0 ± 2.0 | 0 |
| 75 | 0 | 12.0 ± 0.0 | 16.0 ± 1.0 | 0 | |
| 50 | 0 | 0 | 14.0 ± 1.0 | 0 | |
| 45 | 0 | 0 | 12.0 ± 0.0 | 0 | |
| 35 | 0 | 0 | 12.0 ± 0.0 | 0 | |
| Listeria monocytogenes ATCC1912 | 90 | 12.0 ± 0.0 b | 12.0 ± 0.0 b | 16.0 ± 1.0 a | 0 |
| 75 | 0 | 0 | 14.0 ± 1.0 | 0 | |
| 50 | 0 | 0 | 12.0 ± 0.0 | 0 | |
| 45 | 0 | 0 | 12.0 ± 0.0 | 0 | |
| 35 | 0 | 0 | 12.0 ± 0.0 | 0 | |
| Gram-negative strains | |||||
| Escherichia coli 14169 | 90 | 12.0 ± 0.0 c | 18.0 ± 2.0 b | 24.0 ± 2.0 a | 0 |
| 75 | 0 | 16.0 ± 2.0 | 20.0 ± 2.0 | 0 | |
| 50 | 0 | 14.0 ± 1.0 | 20.0 ± 2.0 | 0 | |
| 45 | 0 | 14.0 ± 1.0 | 18.0 ± 1.0 | 0 | |
| 35 | 0 | 12.0 ± 0.0 | 14.0 ±1.0 | 0 | |
| Escherichia coli 25922 | 90 | 12.0 ± 0.0 b | 20.0 ± 2.0 a | 20.0 ± 2.0 a | 0 |
| 75 | 0 | 18.0 ± 2.0 | 20.0 ± 2.0 | 0 | |
| 50 | 0 | 14.0 ± 1.0 | 18.0 ± 1.0 | 0 | |
| 45 | 0 | 14.0 ± 1.0 | 16.0 ± 1.0 | 0 | |
| 35 | 0 | 12.0 ± 0.0 | 14.0 ± 1.0 | 0 | |
| Escherichia coli ATCC8793 | 90 | 0 | 16.0 ± 2.0 | 20.0 ± 2.0 | 0 |
| 75 | 0 | 16.0 ±1.0 | 18.0 ± 2.0 | 0 | |
| 50 | 0 | 14.0 ± 1.0 | 16.0 ± 1.0 | 0 | |
| 45 | 0 | 12.0 ± 0.0 | 14.0 ± 1.0 | 0 | |
| 35 | 0 | 12.0 ± 0.0 | 14.0 ± 1.0 | 0 | |
| Salmonella Typhimurium | 90 | 0 | 16.0 ± 1.0 | 18.0 ± 1.0 | 0 |
| 75 | 0 | 14.0 ± 1.0 | 18.0 ± 1.0 | 0 | |
| 50 | 0 | 12.0 ± 0.0 | 16.0 ± 1.0 | 0 | |
| 45 | 0 | 12.0 ± 0.0 | 14.0 ± 1.0 | 0 | |
| 35 | 0 | 12.0 ± 0.0 | 12.0 ± 0.0 | 0 | |
| Salmonella Typhimurium 235 | 90 | 0 | 14.0 ± 1.0 | 16.0 ± 1.0 | 0 |
| 75 | 0 | 12.0 ± 0.0 | 16.0 ± 1.0 | 0 | |
| 50 | 0 | 12.0 ± 0.0 | 14.0 ± 1.0 | 0 | |
| 45 | 0 | 0 | 12.0 ± 0.0 | 0 | |
| 35 | 0 | 0 | 12.0 ± 0.0 | 0 | |
| Salmonella Typhimurium 63 | 90 | 0 | 14.0 ± 1.0 | 18.0 ± 2.0 | 0 |
| 75 | 0 | 12.0 ± 0.0 | 18.0 ± 1.0 | 0 | |
| 50 | 0 | 0 | 16.0 ± 1.0 | 0 | |
| 45 | 0 | 0 | 14.0 ± 0.0 | 0 | |
| 35 | 0 | 0 | 12.0 ± 0.0 | 0 | |
| No. | Test Strain | Sample | |
|---|---|---|---|
| Bee Pollen | Bee Bread | ||
| S1 | Staphylococcus aureus G3 | 50.0 | 50.0 |
| S2 | Staphylococcus aureus 629G | 50.0 | 25.0 |
| S3 | Staphylococcus aureus ATCC29213 | 50.0 | 25.0 |
| S4 | Enterococcus faecalis 24 | 50.0 | 50.0 |
| S5 | Enterococcus faecalis ss1-1 | 50.0 | 50.0 |
| S6 | Listeria monocytogenes 67 | 50.0 | 50.0 |
| S7 | Listeria monocytogenes 74 | 50.0 | 25.0 |
| S8 | Listeria monocytogenes ATCC1912 | 50.0 | 25.0 |
| S9 | Escherichia coli 14169 | 25.0 | 15.0 |
| S10 | Escherichia coli 25922 | 25.0 | 15.0 |
| S11 | Escherichia coli ATCC8793 | 25.0 | 20.0 |
| S12 | Salmonella Typhimurium | 50.0 | 25.0 |
| S13 | Salmonella Typhimurium 235 | 50.0 | 25.0 |
| S14 | Salmonella Typhimurium 63 | 50.0 | 25.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawicki, T.; Starowicz, M.; Kłębukowska, L.; Hanus, P. The Profile of Polyphenolic Compounds, Contents of Total Phenolics and Flavonoids, and Antioxidant and Antimicrobial Properties of Bee Products. Molecules 2022, 27, 1301. https://doi.org/10.3390/molecules27041301
Sawicki T, Starowicz M, Kłębukowska L, Hanus P. The Profile of Polyphenolic Compounds, Contents of Total Phenolics and Flavonoids, and Antioxidant and Antimicrobial Properties of Bee Products. Molecules. 2022; 27(4):1301. https://doi.org/10.3390/molecules27041301
Chicago/Turabian StyleSawicki, Tomasz, Małgorzata Starowicz, Lucyna Kłębukowska, and Paweł Hanus. 2022. "The Profile of Polyphenolic Compounds, Contents of Total Phenolics and Flavonoids, and Antioxidant and Antimicrobial Properties of Bee Products" Molecules 27, no. 4: 1301. https://doi.org/10.3390/molecules27041301






