New Insights on Rotenone Resistance of Complex I Induced by the m.11778G>A/MT-ND4 Mutation Associated with Leber’s Hereditary Optic Neuropathy
Abstract
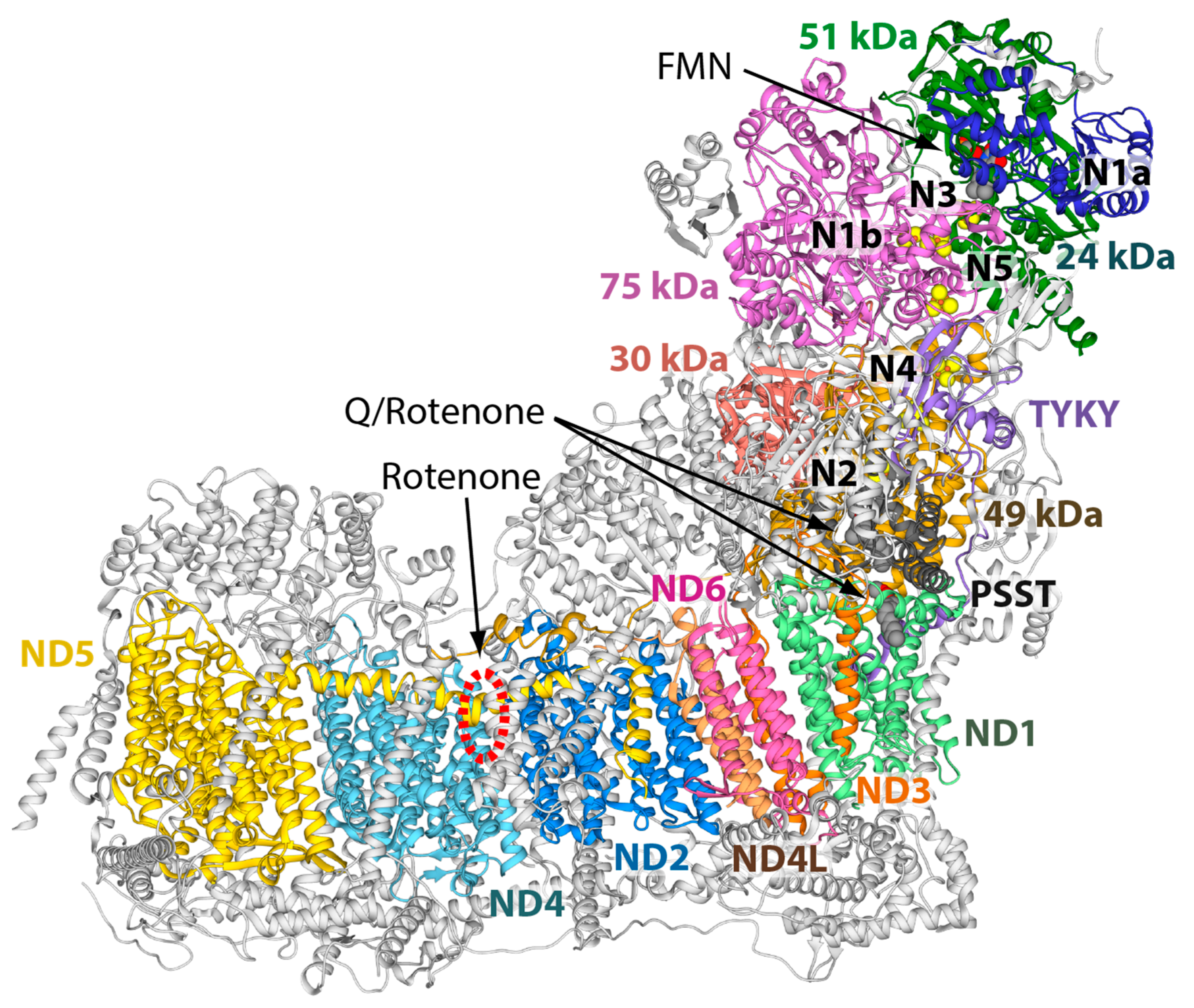
1. Introduction
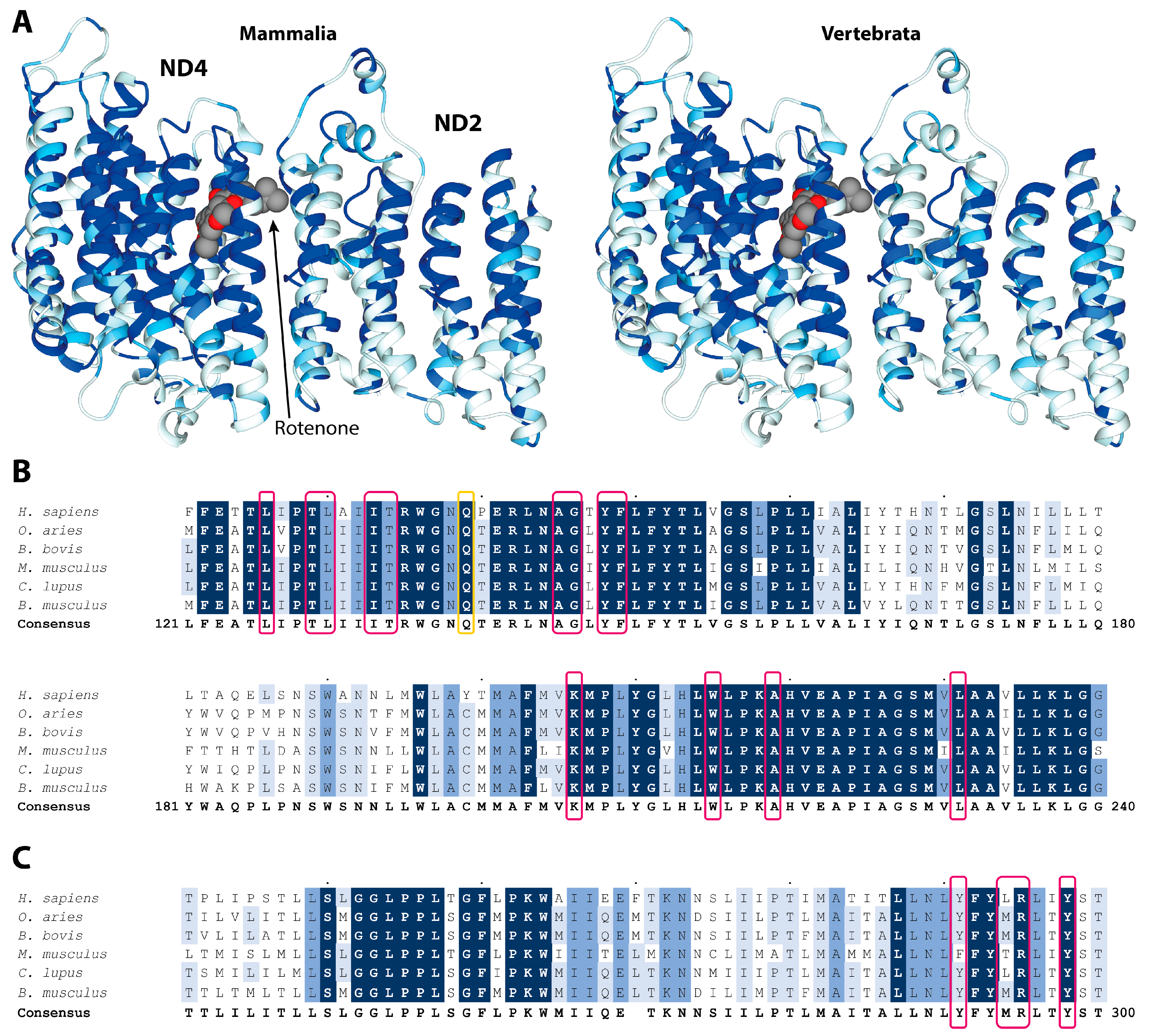
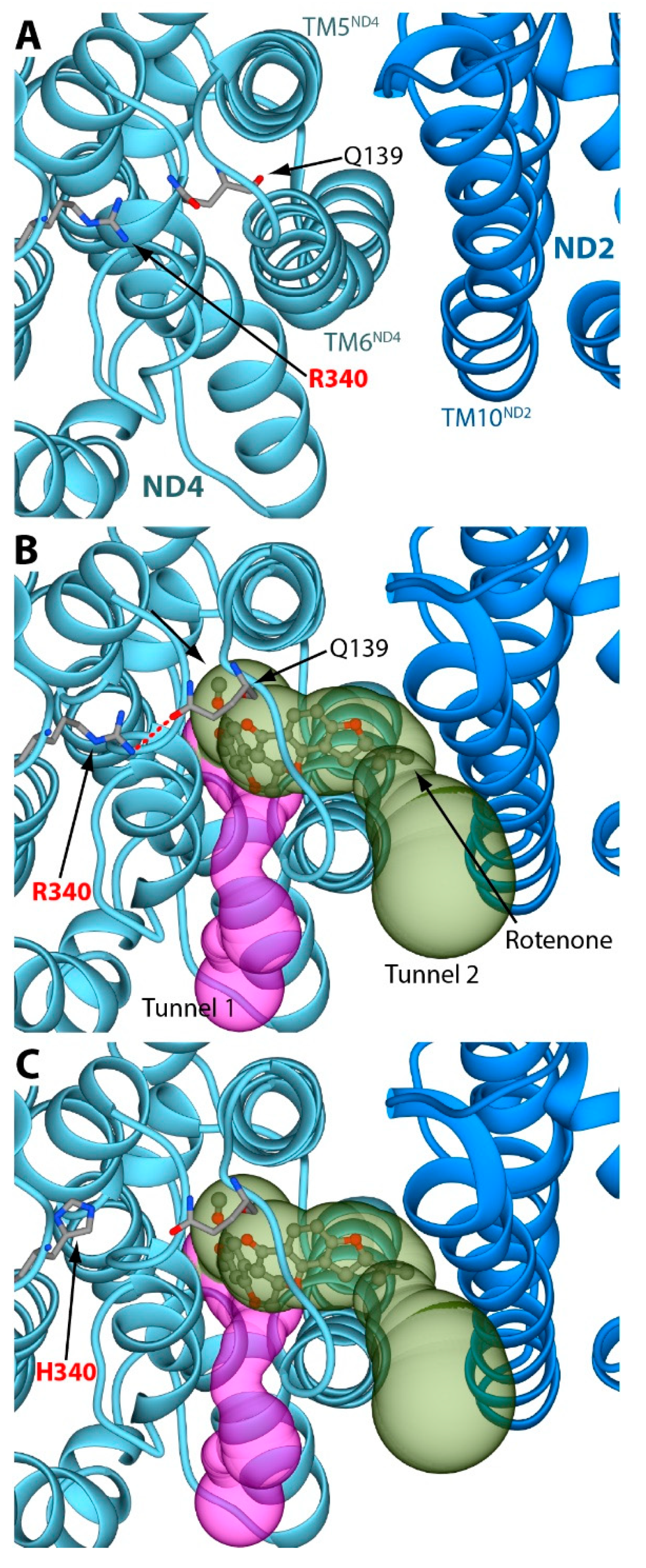
2. Results and Discussion
3. Materials and Methods
3.1. Sequence Alignment and Conservation Determination
3.2. Structural Analyses of the Rotenone Binding Site in the ND4 Subunit
3.3. Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Galemou Yoga, E.; Angerer, H.; Parey, K.; Zickermann, V. Respiratory complex I—Mechanistic insights and advances in structure determination. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148153. [Google Scholar] [CrossRef] [PubMed]
- Parey, K.; Wirth, C.; Vonck, J.; Zickermann, V. Respiratory complex I—Structure, mechanism and evolution. Curr. Opin. Struct. Biol. 2020, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Brandt, U. Energy converting NADH: Quinone oxidoreductase (complex I). Annu. Rev. Biochem. 2006, 75, 69–92. [Google Scholar] [CrossRef]
- Stroud, D.A.; Surgenor, E.E.; Formosa, L.E.; Reljic, B.; Frazier, A.E.; Dibley, M.G.; Osellame, L.D.; Stait, T.; Beilharz, T.H.; Thorburn, D.R.; et al. Accessory subunits are integral for assembly and function of human mitochondrial complex I. Nature 2016, 538, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Vinothkumar, K.R.; Hirst, J. Structure of mammalian respiratory complex I. Nature 2016, 536, 354–358. [Google Scholar] [CrossRef]
- Hunte, C.; Zickermann, V.; Brandt, U. Functional modules and structural basis of conformational coupling in mitochondrial complex I. Science 2010, 329, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Degli Esposti, M. Inhibitors of NADH-ubiquinone reductase: An overview. Biochim. Biophys. Acta 1998, 1364, 222–235. [Google Scholar] [CrossRef]
- Murai, M.; Miyoshi, H. Current topics on inhibitors of respiratory complex I. Biochim. Biophys. Acta (BBA) Bioenerg. 2016, 1857, 884–891. [Google Scholar] [CrossRef]
- Degli Esposti, M. Genome Analysis of Structure–Function Relationships in Respiratory Complex I, an Ancient Bioenergetic Enzyme. Genome Biol. Evol. 2016, 8, 126–147. [Google Scholar] [CrossRef] [PubMed]
- Bridges, H.R.; Fedor, J.G.; Blaza, J.N.; Di Luca, A.; Jussupow, A.; Jarman, O.D.; Wright, J.J.; Agip, A.-N.A.; Gamiz-Hernandez, A.P.; Roessler, M.M.; et al. Structure of inhibitor-bound mammalian complex I. Nat. Commun. 2020, 11, 5261. [Google Scholar] [CrossRef]
- Kampjut, D.; Sazanov, L.A. The coupling mechanism of mammalian respiratory complex I. Science 2020, 370, eabc4209. [Google Scholar] [CrossRef]
- Baradaran, R.; Berrisford, J.M.; Minhas, G.S.; Sazanov, L.A. Crystal structure of the entire respiratory complex I. Nature 2013, 494, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Zickermann, V.; Wirth, C.; Nasiri, H.; Siegmund, K.; Schwalbe, H.; Hunte, C.; Brandt, U. Structural biology. Mechanistic insight from the crystal structure of mitochondrial complex I. Science 2015, 347, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Grba, D.N.; Blaza, J.N.; Bridges, H.R.; Agip, A.-N.A.; Yin, Z.; Murai, M.; Miyoshi, H.; Hirst, J. Cryo-electron microscopy reveals how acetogenins inhibit mitochondrial respiratory complex I. J. Biolog. Chem. 2022, 2022, 101602. [Google Scholar] [CrossRef] [PubMed]
- Esposti, M.D.; Ghelli, A. Ubiquinone and inhibitor sites in complex I: One, two or three? Biochem. Soc. Transact. 1999, 27, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Roessler, M.M.; King, M.S.; Robinson, A.J.; Armstrong, F.A.; Harmer, J.; Hirst, J. Direct assignment of EPR spectra to structurally defined iron-sulfur clusters in complex I by double electron–electron resonance. Proc. Natl. Acad. Sci. USA 2010, 107, 1930–1935. [Google Scholar] [CrossRef] [PubMed]
- Degli Esposti, M.; Carelli, V.; Ghelli, A.; Ratta, M.; Crimi, M.; Sangiorgi, S.; Montagna, P.; Lenaz, G.; Lugaresi, E.; Cortelli, P. Functional alterations of the mitochondrially encoded ND4 subunit associated with Leber’s hereditary optic neuropathy. FEBS Lett. 1994, 352, 375–379. [Google Scholar] [CrossRef]
- Carelli, V.; Ghelli, A.; Ratta, M.; Bacchilega, E.; Sangiorgi, S.; Mancini, R.; Leuzzi, V.; Cortelli, P.; Montagna, P.; Lugaresi, E.; et al. Leber’s hereditary optic neuropathy: Biochemical effect of 11778/ND4 and 3460/ND1 mutations and correlation with the mitochondrial genotype. Neurology 1997, 48, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Grivennikova, V.G.; Maklashina, E.O.; Gavrikova, E.V.; Vinogradov, A.D. Interaction of the mitochondrial NADH-ubiquinone reductase with rotenone as related to the enzyme active/inactive transition. Biochim. Biophys. Acta 1997, 1319, 223–232. [Google Scholar] [CrossRef]
- Majander, A.; Huoponen, K.; Savontaus, M.L.; Nikoskelainen, E.; Wikström, M. Electron transfer properties of NADH:ubiquinone reductase in the ND1/3460 and the ND4/11778 mutations of the Leber hereditary optic neuroretinopathy (LHON). FEBS Lett. 1991, 292, 289–292. [Google Scholar] [CrossRef]
- Brown, M.D.; Trounce, I.A.; Jun, A.S.; Allen, J.C.; Wallace, D.C. Functional Analysis of Lymphoblast and Cybrid Mitochondria Containing the 3460, 11778, or 14484 Leber’s Hereditary Optic Neuropathy Mitochondrial DNA Mutation. J. Biolog. Chem. 2000, 275, 39831–39836. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.R.; Cooper, J.M.; Govan, G.G.; Harding, A.E.; Schapira, A.H. Platelet mitochondrial function in Leber’s hereditary optic neuropathy. J. Neurol. Sci. 1994, 122, 80–83. [Google Scholar] [CrossRef]
- Brown, M.D.; Allen, J.C.; Van Stavern, G.P.; Newman, N.J.; Wallace, D.C. Clinical, genetic, and biochemical characterization of a Leber hereditary optic neuropathy family containing both the 11778 and 14484 primary mutations. Am. J. Med. Genet. 2001, 104, 331–338. [Google Scholar] [CrossRef]
- Vergani, L.; Martinuzzi, A.; Carelli, V.; Cortelli, P.; Montagna, P.; Schievano, G.; Carrozzo, R.; Angelini, C.; Lugaresi, E. MtDNA mutations associated with Leber’s hereditary optic neuropathy: Studies on cytoplasmic hybrid (cybrid) cells. Biochem. Biophys. Res. Commun. 1995, 210, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Hofhaus, G.; Johns, D.R.; Hurko, O.; Attardi, G.; Chomyn, A. Respiration and growth defects in transmitochondrial cell lines carrying the 11778 mutation associated with Leber’s hereditary optic neuropathy. J. Biol. Chem. 1996, 271, 13155–13161. [Google Scholar] [CrossRef] [PubMed]
- Carelli, V.; Ghelli, A.; Bucchi, L.; Montagna, P.; De Negri, A.; Leuzzi, V.; Carducci, C.; Lenaz, G.; Lugaresi, E.; Degli Esposti, M. Biochemical features of mtDNA 14484 (ND6/M64V) point mutation associated with Leber’s hereditary optic neuropathy. Ann. Neurol. 1999, 45, 320–328. [Google Scholar] [CrossRef]
- Howell, N.; Bindoff, L.A.; McCullough, D.A.; Kubacka, I.; Poulton, J.; Mackey, D.; Taylor, L.; Turnbull, D.M. Leber hereditary optic neuropathy: Identification of the same mitochondrial ND1 mutation in six pedigrees. Am. J. Hum. Genet. 1991, 49, 939–950. [Google Scholar] [PubMed]
- Cock, H.R.; Cooper, J.M.; Schapira, A.H. Functional consequences of the 3460-bp mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. J. Neurol. Sci. 1999, 165, 10–17. [Google Scholar] [CrossRef]
- Ghelli, A.; Zanna, C.; Porcelli, A.M.; Schapira, A.H.V.; Martinuzzi, A.; Carelli, V.; Rugolo, M. Leber’s hereditary optic neuropathy (LHON) pathogenic mutations induce mitochondrial-dependent apoptotic death in transmitochondrial cells incubated with galactose medium. J. Biol. Chem. 2003, 278, 4145–4150. [Google Scholar] [CrossRef]
- Carelli, V.; Rugolo, M.; Sgarbi, G.; Ghelli, A.; Zanna, C.; Baracca, A.; Lenaz, G.; Napoli, E.; Martinuzzi, A.; Solaini, G. Bioenergetics shapes cellular death pathways in Leber’s hereditary optic neuropathy: A model of mitochondrial neurodegeneration. Biochim. Biophys. Acta 2004, 1658, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Lodi, R.; Taylor, D.J.; Tabrizi, S.J.; Kumar, S.; Sweeney, M.; Wood, N.W.; Styles, P.; Radda, G.K.; Schapira, A.H. In vivo skeletal muscle mitochondrial function in Leber’s hereditary optic neuropathy assessed by 31P magnetic resonance spectroscopy. Ann. Neurol. 1997, 42, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Lodi, R.; Carelli, V.; Cortelli, P.; Iotti, S.; Valentino, M.L.; Barboni, P.; Pallotti, F.; Montagna, P.; Barbiroli, B. Phosphorus MR spectroscopy shows a tissue specific in vivo distribution of biochemical expression of the G3460A mutation in Leber’s hereditary optic neuropathy. J. Neurol. Neurosurg. Psychiatry 2002, 72, 805–807. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baracca, A.; Solaini, G.; Sgarbi, G.; Lenaz, G.; Baruzzi, A.; Schapira, A.H.V.; Martinuzzi, A.; Carelli, V. Severe impairment of complex I-driven adenosine triphosphate synthesis in leber hereditary optic neuropathy cybrids. Arch. Neurol. 2005, 62, 730–736. [Google Scholar] [CrossRef]
- Brown, M.D. The enigmatic relationship between mitochondrial dysfunction and Leber’s hereditary optic neuropathy. J. Neurol. Sci. 1999, 165, 1–5. [Google Scholar] [CrossRef]
- Moster, M.L.; Sergott, R.C.; Newman, N.J.; Yu-Wai-Man, P.; Carelli, V.; Bryan, M.S.; Smits, G.; Biousse, V.; Vignal-Clermont, C.; Klopstock, T.; et al. For the LHON study group, Cross-Sectional Analysis of Baseline Visual Parameters in Subjects Recruited Into the RESCUE and REVERSE ND4-LHON Gene Therapy Studies. J. Neuro-Ophthalmol. 2021, 41, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Carelli, V.; Achilli, A.; Valentino, M.L.; Rengo, C.; Semino, O.; Pala, M.; Olivieri, A.; Mattiazzi, M.; Pallotti, F.; Carrara, F.; et al. Haplogroup Effects and Recombination of Mitochondrial DNA: Novel Clues from the Analysis of Leber Hereditary Optic Neuropathy Pedigrees. Am. J. Hum. Genet. 2006, 78, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Hudson, G.; Carelli, V.; Spruijt, L.; Gerards, M.; Mowbray, C.; Achilli, A.; Pyle, A.; Elson, J.; Howell, N.; La Morgia, C.; et al. Clinical Expression of Leber Hereditary Optic Neuropathy Is Affected by the Mitochondrial DNA–Haplogroup Background. Am. J. Hum. Genet. 2007, 81, 228–233. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2--a multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Pravda, L.; Sehnal, D.; Toušek, D.; Navrátilová, V.; Bazgier, V.; Berka, K.; Svobodová Vařeková, R.; Koča, J.; Otyepka, M. MOLEonline: A web-based tool for analyzing channels, tunnels and pores (2018 update). Nucleic Acids Res. 2018, 46, W368–W373. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera?A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Mills, J.E.J.; Dean, P.M. Three-dimensional hydrogen-bond geometry and probability information from a crystal survey. J. Comput.-Aided Mol. Des. 1996, 10, 607–622. [Google Scholar] [CrossRef] [PubMed]
- Shapovalov, M.V.; Dunbrack, R.L. A Smoothed Backbone-Dependent Rotamer Library for Proteins Derived from Adaptive Kernel Density Estimates and Regressions. Structure 2011, 19, 844–858. [Google Scholar] [CrossRef] [PubMed]
| ND1 | ND2 | ND3 | ND4 | ND4L | ND5 | ND6 | ||
|---|---|---|---|---|---|---|---|---|
| Eukaryota | Aligned sequences | 159 | 166 | 158 | 105 | 331 | 109 | 129 |
| Average conservation (%) | 70 | 60 | 61 | 59 | 69 | 55 | 44 | |
| Conserved residues (%) | 56 | 34 | 42 | 35 | 57 | 26 | 11 | |
| Invariant residues (%) | 2 | 1 | 2 | 3 | 1 | 4 | 0 | |
| Vertebrata | Aligned sequences | 109 | 127 | 101 | 62 | 279 | 59 | 84 |
| Average conservation (%) | 82 | 70 | 75 | 77 | 77 | 73 | 58 | |
| Conserved residues (%) | 77 | 51 | 62 | 63 | 64 | 56 | 29 | |
| Invariant residues (%) | 26 | 10 | 20 | 20 | 5 | 17 | 6 | |
| Mammalia | Aligned sequences | 79 | 95 | 74 | 44 | 256 | 39 | 43 |
| Average conservation (%) | 85 | 77 | 80 | 83 | 80 | 79 | 71 | |
| Conserved residues (%) | 79 | 62 | 70 | 77 | 69 | 68 | 55 | |
| Invariant residues (%) | 39 | 20 | 31 | 38 | 17 | 32 | 23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musiani, F.; Rigobello, L.; Iommarini, L.; Carelli, V.; Degli Esposti, M.; Ghelli, A.M. New Insights on Rotenone Resistance of Complex I Induced by the m.11778G>A/MT-ND4 Mutation Associated with Leber’s Hereditary Optic Neuropathy. Molecules 2022, 27, 1341. https://doi.org/10.3390/molecules27041341
Musiani F, Rigobello L, Iommarini L, Carelli V, Degli Esposti M, Ghelli AM. New Insights on Rotenone Resistance of Complex I Induced by the m.11778G>A/MT-ND4 Mutation Associated with Leber’s Hereditary Optic Neuropathy. Molecules. 2022; 27(4):1341. https://doi.org/10.3390/molecules27041341
Chicago/Turabian StyleMusiani, Francesco, Laura Rigobello, Luisa Iommarini, Valerio Carelli, Mauro Degli Esposti, and Anna Maria Ghelli. 2022. "New Insights on Rotenone Resistance of Complex I Induced by the m.11778G>A/MT-ND4 Mutation Associated with Leber’s Hereditary Optic Neuropathy" Molecules 27, no. 4: 1341. https://doi.org/10.3390/molecules27041341
APA StyleMusiani, F., Rigobello, L., Iommarini, L., Carelli, V., Degli Esposti, M., & Ghelli, A. M. (2022). New Insights on Rotenone Resistance of Complex I Induced by the m.11778G>A/MT-ND4 Mutation Associated with Leber’s Hereditary Optic Neuropathy. Molecules, 27(4), 1341. https://doi.org/10.3390/molecules27041341









