Biofortification—A Frontier Novel Approach to Enrich Micronutrients in Field Crops to Encounter the Nutritional Security
Abstract
1. Introduction
2. Global Status of Micronutrients in Soil
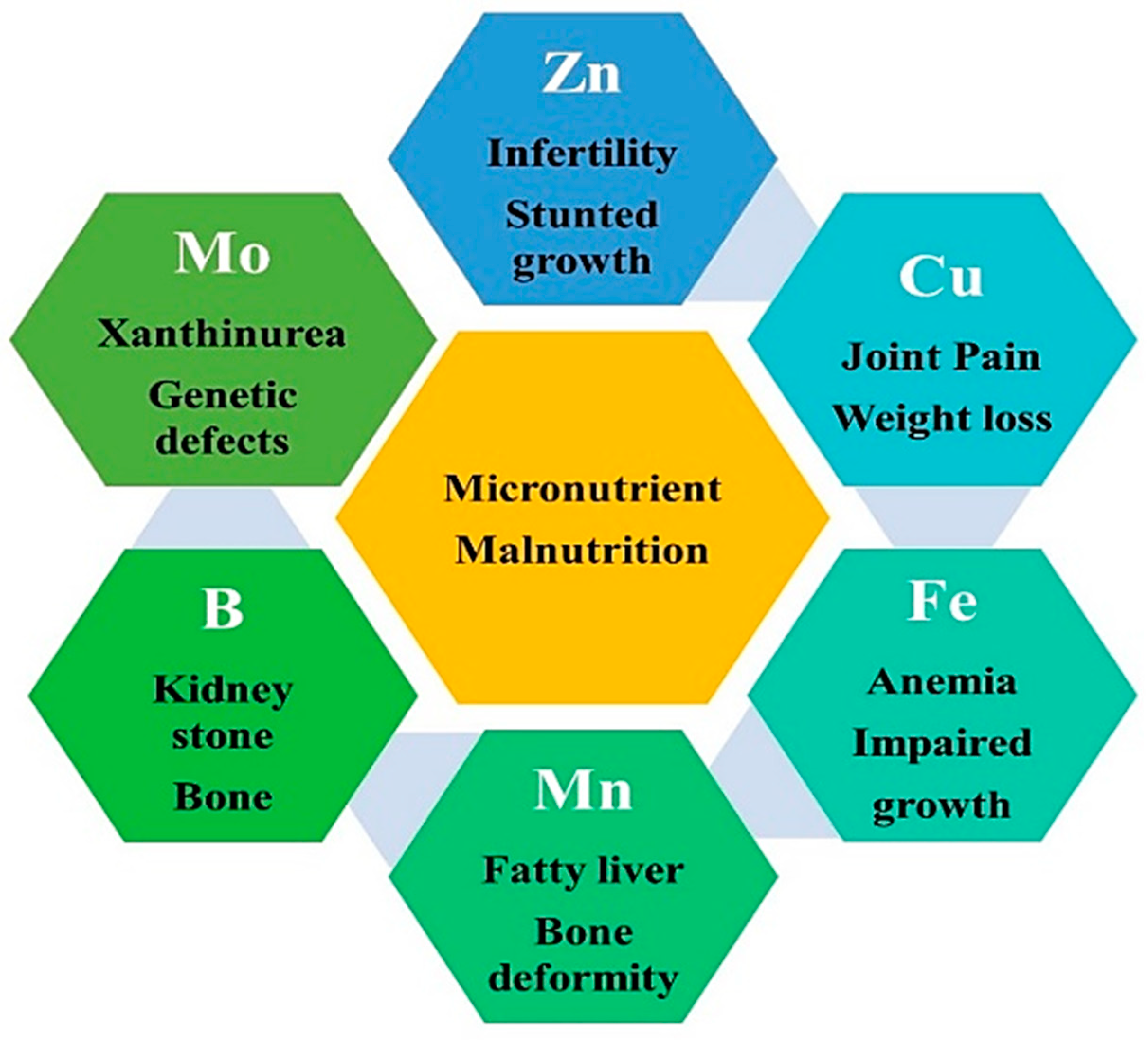
3. Global Status of Micronutrients Malnutrition
3.1. Malnutrition in Young Infants and Adult Humans
3.2. Malnutrition in Animals
4. Field Crops as a Rich Source of Minerals
4.1. Cereals Crops
4.2. Pulses Crops
4.3. Oilseed Crops
4.4. Fodder Crops
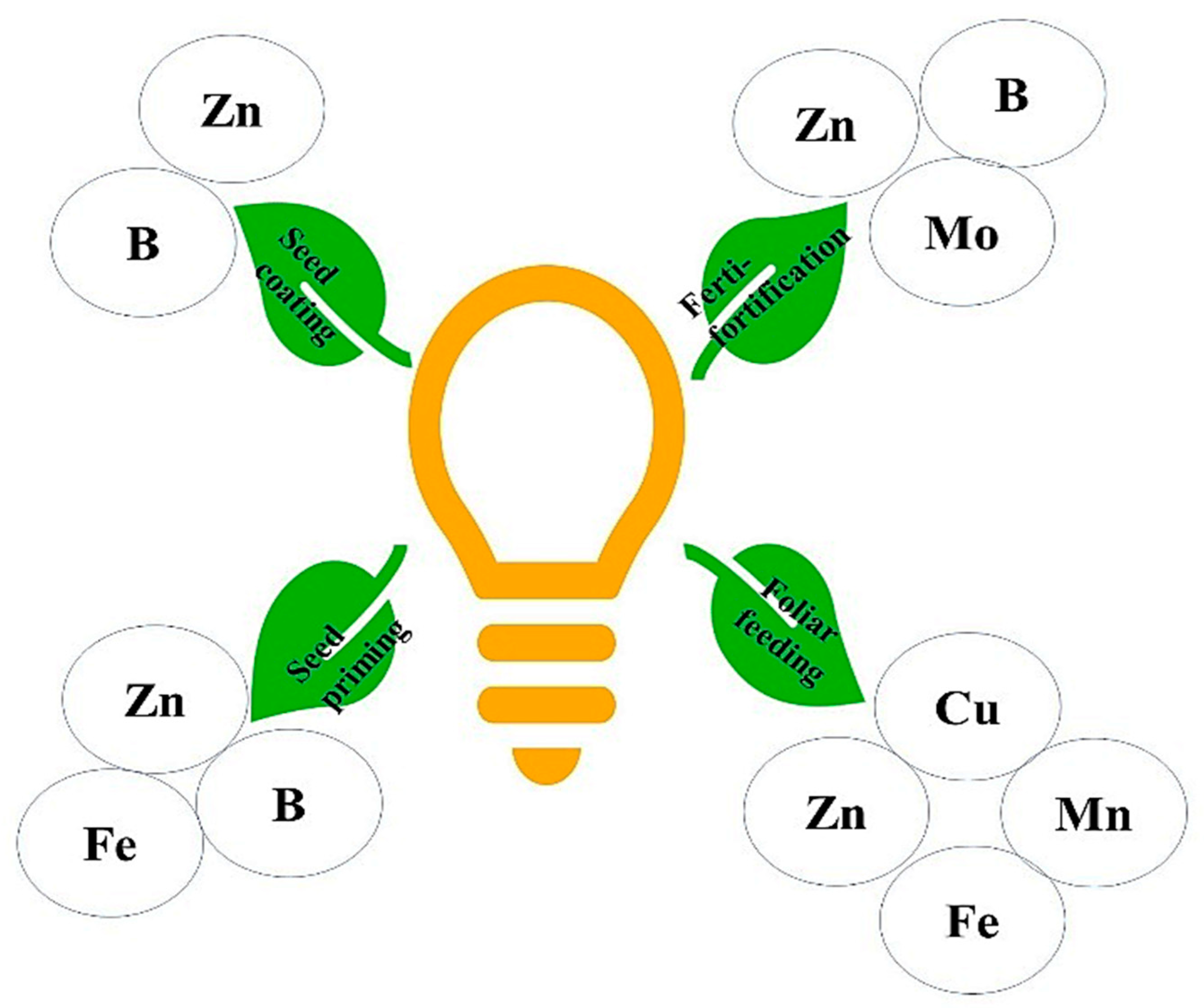
5. Biofortification through the Mode of Minerals Fertilization
5.1. Ferti-Fortification or Soil-Applied Fertilizers
5.2. Foliar Feeding
5.3. Seed Priming
5.4. Seed Coating
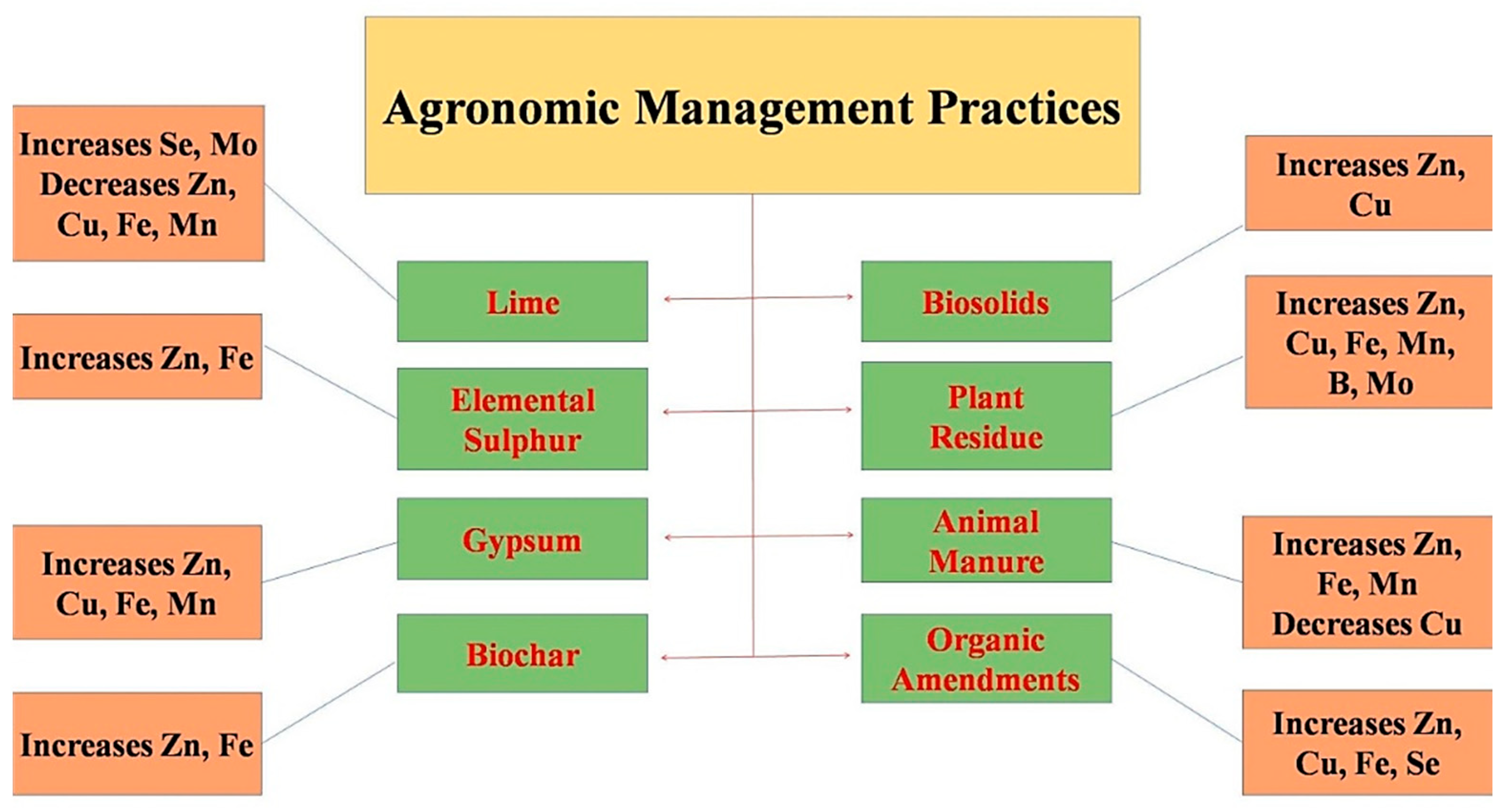
6. Biofortification through Agronomic Management Practices
6.1. Application of Lime
6.2. Application of Elemental Sulfur
6.3. Application of Gypsum (CaSO4·2H2O)
6.4. Application of Biochar
6.5. Application of Biosolids
6.6. Incorporation of Crop Residue
6.7. Application of Animal Manure
6.8. Addition of Compost
7. Selection of Suitable Crops and Cropping Systems
7.1. Suitable Crops
7.2. Suitable Cropping System
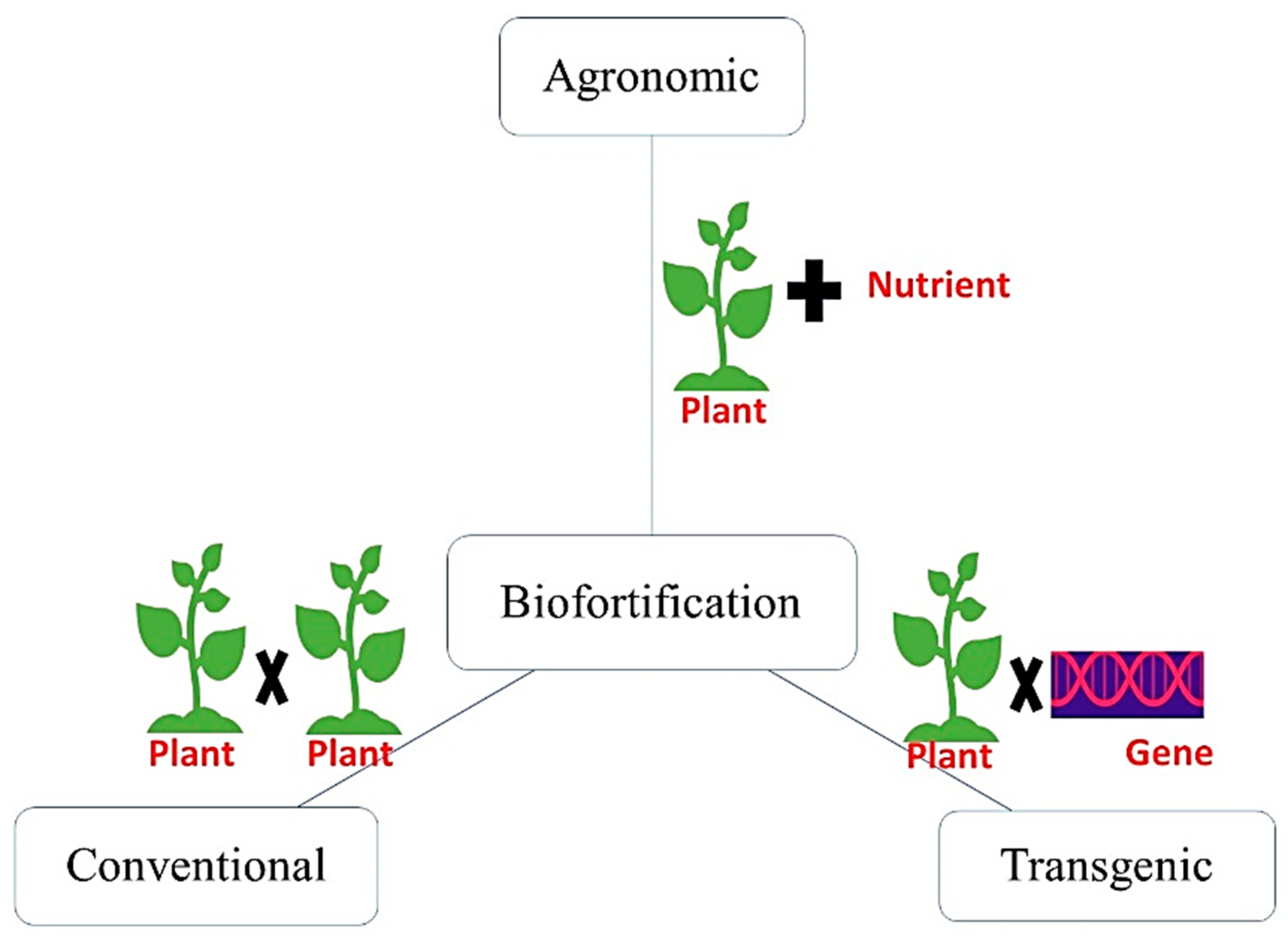
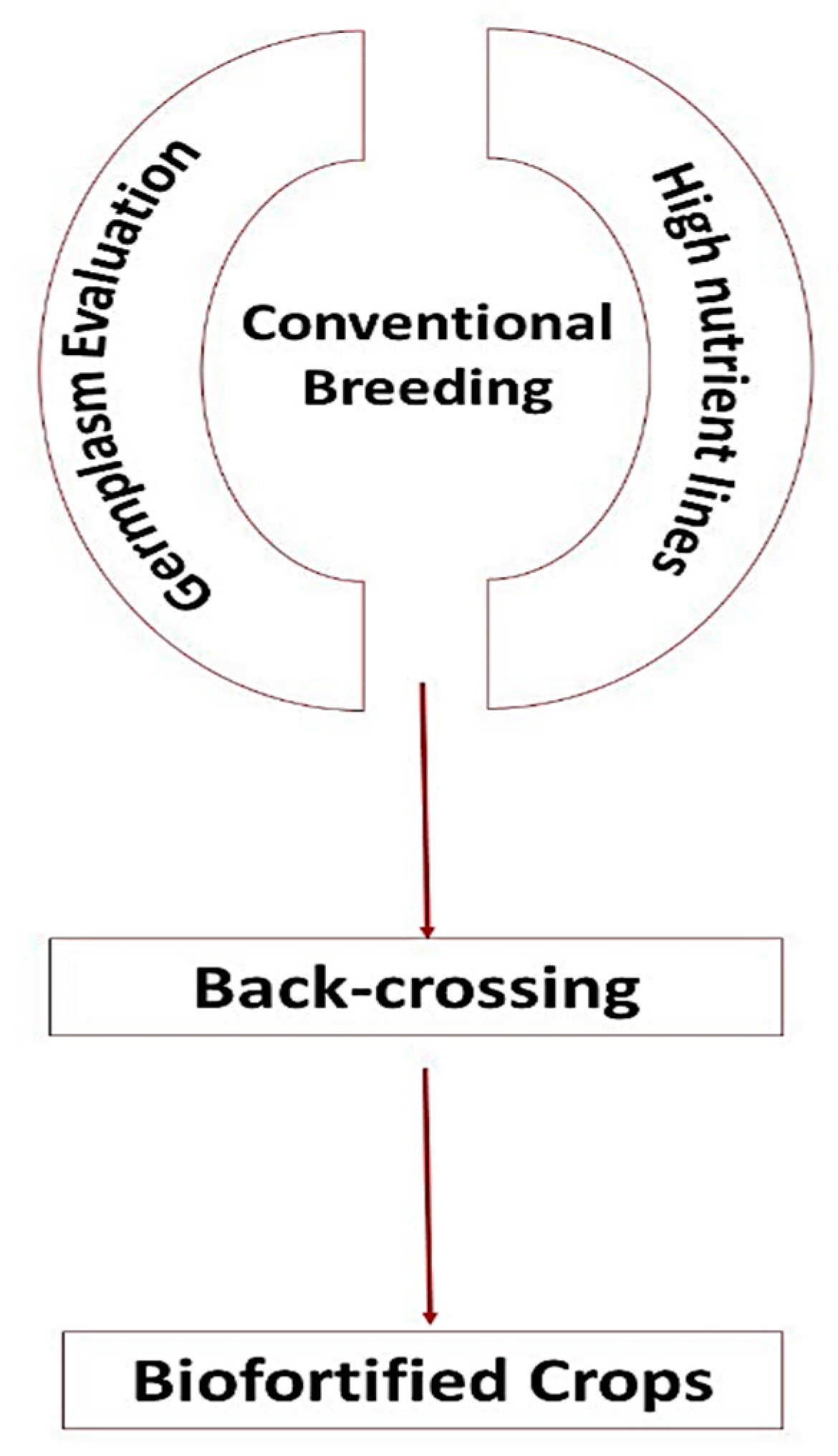
8. Biofortification through Breeding Approaches
8.1. Screening of Crop Cultivars
8.2. Conventional Breeding
8.3. Transgenic Technology/Biotechnological Approach
9. Micronutrients Sources for Mineral Enrichment
9.1. Mineral Solutions
| Source | Crop | Reference |
|---|---|---|
| ZnSO4 | Rice, Wheat, Maize | [6] |
| Zn-DTPA, Zn-HEDTA, Zn-EDTA | Maize | [102] |
| N + Zn + Fe | Wheat | [106] |
| Zn, Mn, Fe, Cu and B | Wheat | [107] |
| Zn, I, Se and Fe | Wheat | [108] |
| Na2SeO4 + ZnSO4 | Wheat | [109] |
| FeSO4·7H2O + ZnSO4·7H2O | Wheat | [110] |
| Cu + Zn + Mn + NPK | Winter-wheat | |
| ZnSO4 + Zn-HEDP | Wheat | [50] |
| EDDHA-Fe(III), EDTANa2Fe(II) | Rice | [111] |
9.2. Multi-Micronutrients Mixtures
9.3. Chelates and Chelating Agents
10. Biofortification through Nano-Technology
10.1. Micronutrients Nano Fertilizers
10.2. Macronutrients Nano-Fertilizers
11. Biofortification through Green Technology
11.1. Bacterial Biofortification
11.2. Endophytic Biofortification
12. Interactions between Foliar-Applied Minerals and Plant Nutrients
12.1. Micronutrients and Micronutrients
12.2. Micronutrients and Macronutrients
12.3. Micronutrient and Heavy Metals
13. Crop Yield Responses to Mineral Applications
13.1. Soil Application
13.2. Foliar Application
14. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, S.; Wang, P.; Yamaji, N.; Ma, J.F. Plant nutrition for human nutrition: Hints from rice research and future perspectives. Mol. Plant 2020, 13, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.; Jafari, A.; Stamoulis, K.G.; Callens, K. The first programme food and nutrition security, impact, resilience, sustainability and transformation: Review and future directions. Glob. Food Sec. 2020, 26, 100422. [Google Scholar] [CrossRef] [PubMed]
- Maertens, M.; Velde, K.V. Contract-farming in staple food chains: The case of rice in Benin. World Dev. 2017, 95, 73–87. [Google Scholar] [CrossRef]
- Klikocka, H.; Marks, M. Sulphur and nitrogen fertilization as a potential means of agronomic biofortification to improve the content and uptake of microelements in spring wheat grain DM. J. Chem. 2018, 2018, 9326820. [Google Scholar] [CrossRef]
- Steur, H.D.; Mehta, S.; Gellynck, X.; Finkelstein, J.L. GM biofortified crops: Potential effects on targeting the micronutrient intake gap in human populations. Curr. Opin. Biotechnol. 2017, 44, 181–188. [Google Scholar] [CrossRef]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2017, 69, 172–180. [Google Scholar] [CrossRef]
- Connorton, J.M.; Balk, J. Iron biofortification of staple crops: Lessons and challenges in plant genetics. Plant Cell Physiol. 2019, 60, 1447–1456. [Google Scholar] [CrossRef]
- Ligowe, I.S.; Bailey, E.H.; Young, S.D.; Ander, E.L.; Kabambe, V.; Chilimba, A.D.; Lark, R.M.; Nalivata, P.C. Agronomic iodine biofortification of leafy vegetables grown in Vertisols, Oxisols and Alfisols. Environ. Geochem. Health 2021, 43, 361–374. [Google Scholar] [CrossRef]
- Zheng, X.; Giuliano, G.; Al-Babili, S. Carotenoid biofortification in crop plants: Citius, altius, forties. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158664. [Google Scholar] [CrossRef]
- Saeid, A.; Patel, A.; Jastrzębska, M.; Korczyński, M. Food biofortification. J. Chem. 2019, 2019, 5718426. [Google Scholar] [CrossRef]
- Górniak, W.; Cholewińska, P.; Konkol, D. Feed additives produced on the basis of organic forms of micronutrients as a means of biofortification of food of animal origin. J. Chem. 2018, 2018, 8084127. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munné-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef] [PubMed]
- Torre-Roche, D.L.R.; Cantu, J.; Tamez, C.; Zuverza-Mena, N.; Hamdi, H.; Adisa, I.O.; Elmer, W.; Torresdey, J.G.; White, J.C. Seed biofortification by engineered nanomaterials: A pathway to alleviate malnutrition? J. Agric. Food Chem. 2020, 68, 12189–12202. [Google Scholar] [CrossRef]
- Kaur, T.; Rana, K.L.; Kour, D.; Sheikh, I.; Yadav, N.; Kumar, V.; Yadav, A.N.; Dhaliwal, H.S.; Saxena, A.K. Microbe-mediated biofortification for micronutrients: Present status and future challenges. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–17. [Google Scholar]
- Sillanpää, M. Micronutrient assessment at country level: An international study. In Soils Bulletin; No. 63; FAO: Rome, Italy, 1990; p. 208. [Google Scholar]
- Lešková, A.; Giehl, R.F.H.; Hartmann, A.; Fargašová, A.; von Wirén, N. Heavy metals induce iron deficiency responses at different hierarchic and regulatory levels. Plant Physiol. 2017, 174, 1648–1668. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.V. Evaluation of micronutrient status in different agroecological zones of India. Fertil. News 2001, 46, 25–42. [Google Scholar]
- Singh, A.P.; Singh, M.V.; Sakal, R.; Chaudhary, V.C. Boron nutrition of crops and soils of Bihar. Tech. Bull. 2006, 6, 1–80. [Google Scholar]
- Bhupalraj, G.; Patnaik, M.C.; Khadke, K.M. Molybdenum status in soils of Andhra Pradesh. AICRP Micro Second. Nutr. Soils Plants Pradesh 2002, 36, 1–87. [Google Scholar]
- Shaw, D.J. World Food Conference 1974. In World Food Security; Palgrave Macmillan: London, UK, 2007. [Google Scholar]
- Ohanenye, I.C.; Emenike, C.U.; Mensi, A.; Medina-Godoy, S.; Jin, J.; Ahmed, T.; Sun, X.; Udenigwe, C.C. Food fortification technologies: Influence on iron, zinc and vitamin A bioavailability and potential implications on micronutrient deficiency in sub-Saharan Africa. Sci. Afr. 2021, 11, e00667. [Google Scholar] [CrossRef]
- Stein, A.J.; Qaim, M. The human and economic cost of hidden hunger. Food Nutr. Bull. 2007, 28, 125–134. [Google Scholar] [CrossRef]
- India State-Level Disease Burden Initiative Collaborators. Nations within a nation: Variations in epidemiological transition across the states of India, 1990–2016 in the Global Burden of Disease Study. Lancet 2017, 390, 2437–2460. [Google Scholar] [CrossRef]
- West, K.P. Extent of vitamin A deficiency among preschool children and women of reproductive age. J. Nutr. 2002, 132, 2857S–2866S. [Google Scholar] [CrossRef] [PubMed]
- Cook, R. World Cattle Inventory. 2020. Available online: www.beef2live.com (accessed on 31 January 2020).
- Earagariyanna, M.Y.; Venkayala, J.; Kammardi, S.; Sriramaiah, M. Fodder resource management in India-A critical analysis. Int. J. Livest. Res. 2020, 7, 14–22. [Google Scholar] [CrossRef]
- McDowell, L.R. Minerals in Animal and Human Nutrition, 2nd ed.; Elsevier Science BV: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Suttle, N.F. Copper imbalances in ruminants and humans: Unexpected common ground. Adv. Nutr. 2012, 3, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E. Principles of human nutrition. Medicine 2019, 47, 140–144. [Google Scholar] [CrossRef]
- Rosa-Sibakov, N.; Poutanen, K.; Micard, V. How does wheat grain, bran and aleurone structure impact their nutritional and technological properties? Trends Food Sci. Technol. 2015, 41, 118–134. [Google Scholar] [CrossRef]
- Nuss, E.T.; Tanumihardjo, S.A. Maize: A paramount staple crop in the context of global nutrition. Food Soc. Food Saf. 2010, 9, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Callens, T.; del Castello, R.; Baratelli, M.; Xipsiti, M.; Navarro, D.K. The International Year of Pulses; Final Report; FAO: Rome, Italy, 2019; p. 40. [Google Scholar]
- Brueck, H.; Lammel, J. Impact of fertilizer N application on the grey water footprint of winter wheat in a NW-European temperate climate. Water 2016, 8, 356. [Google Scholar] [CrossRef]
- Rebello, C.J.; Greenway, F.L.; Finley, J.W. Whole grains and pulses: A comparison of the nutritional and health benefits. J Agric. Food Chem. 2014, 62, 7029–7049. [Google Scholar] [CrossRef]
- Venkidasamy, B.; Selvaraj, D.; Nile, A.S.; Ramalingam, S.; Kai, G.; Nile, S.H. Indian pulses: A review on nutritional, functional and biochemical properties with future perspectives. Trends Food Sci. Technol. 2019, 88, 228–242. [Google Scholar] [CrossRef]
- Zafar, S.; Li, Y.L.; Li, N.N.; Zhu, K.M.; Tan, X.L. Recent advances in enhancement of oil content in oilseed crops. J. Biotechnol. 2019, 301, 35–44. [Google Scholar] [CrossRef]
- Kowalska, G.; Kowalski, R.; Hawlena, J.; Rowiński, R. Seeds of oilseed rape as an alternative source of protein and mineral. J. Elementol. 2020, 25, 513–522. [Google Scholar] [CrossRef]
- Száková, J.; Praus, L.; Tremlová, J.; Kulhánek, M.; Tlustoš, P. Efficiency of foliar selenium application on oilseed rape (Brassica napus L.) as influenced by rainfall and soil characteristics. Arch. Agron. Soil Sci. 2017, 63, 1240–1254. [Google Scholar] [CrossRef]
- López-Alonso, M. Trace minerals and livestock: Not too much not too little. ISRN Vet. Sci. 2012, 2012, 704825. [Google Scholar] [CrossRef]
- Jadhav, P.V.; Magar, S.; Thakur, P.; Moharil, M.P.; Yadav, H.; Mandlik, R. Biofortified fodder crops: An approach to eradicate hidden hunger. In Advances in Agri-Food Biotechnology; Springer Nature: Singapore, 2020. [Google Scholar]
- Boller, B.; Greene, S.L. Genetic resources. In Fodder Crops and Amenity Grasses; Springer: New York, NY, USA, 2010; pp. 13–37. [Google Scholar]
- Humphreys, M.W.; O’Donovan, S.A.; Farrell, M.S.; Gay, A.P.; Kingston-Smith, A.H. The potential of novel Festulolium (2n = 4x = 28) hybrids as productive, nutrient-use-efficient fodder for ruminants. Food Energy Sec. 2014, 3, 98–110. [Google Scholar] [CrossRef]
- Cabral, G.B.; Carneiro, V.T.; Rossi, M.L.; da Silva, J.P.; Martinelli, A.P.; Dusi, D.M. Plant regeneration from embryogenic callus and cell suspensions of Brachiaria brizantha. In Vitro Cell. Dev. Biol. Plant 2015, 51, 369–377. [Google Scholar] [CrossRef]
- Badenhorst, P.; Panter, S.; Palanisamy, R.; Georges, S.; Smith, K.; Mouradov, A.; Mason, J.; Spangenberg, G. Molecular breeding of transgenic perennial ryegrass (Lolium perenne L.) with altered fructan biosynthesis through the expression of fructosyltransferases. Mol. Breed. 2018, 38, 21. [Google Scholar] [CrossRef]
- Gao, R.; Feyissa, B.A.; Croft, M.; Hannoufa, A. Gene editing by CRISPR/Cas9 in the obligatory outcrossing Medicago sativa. Planta 2018, 247, 1043–1050. [Google Scholar] [CrossRef]
- Kaur, J.; Bhatti, D.S.; Goyal, M. Influence of copper application on forage yield and quality of oats fodder in copper deficient soils. Indian J. Anim. Nutr. 2015, 32, 290–294. [Google Scholar]
- Sandhu, A.; Dhaliwal, S.S.; Shukla, A.K.; Sharma, V.; Singh, R. Fodder quality improvement and enrichment of oats with Cu through biofortification: A technique to reduce animal malnutrition. J. Plant Nutr. 2020, 43, 1378–1389. [Google Scholar] [CrossRef]
- Kumar, D.; Dhaliwal, S.S.; Naresh, R.K.; Salaria, A. Agronomic biofortification of paddy through nitrogen, zinc and iron fertilization: A review. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2942–2953. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Manchanda, J.S. Critical level of Boron in Typic Ustrochrepts for predicting response of mungbean (Phaseolus aureus L.) to boron application. Indian J. Ecol. 2009, 36, 22–27. [Google Scholar]
- Dhaliwal, S.S.; Sadana, U.S.; Khurana, M.P.; Sidhu, S.S. Enrichment of wheat grains with Zn through ferti-fortification. Indian J. Fertil. 2012, 8, 48–55. [Google Scholar]
- Ram, H.; Sohu, V.S.; Cakmak, I.; Singh, K.; Buttar, G.S.; Sodhi, G.P.S.; Gill, H.S.; Bhagat, I.; Singh, P.; Dhaliwal, S.S.; et al. Agronomic fortification of rice and wheat grains with zinc for nutritional security. Curr. Sci. 2015, 129, 1171–1176. [Google Scholar] [CrossRef]
- Manzeke, M.G.; Mtambanengwe, F.; Nezomba, H.; Watts, M.J.; Broadley, M.R.; Mapfumo, P. Zinc fertilization increases productivity and grain nutritional quality of cowpea (Vigna unguiculata [L.] Walp.) under integrated soil fertility management. Field Crop. Res. 2017, 213, 231–244. [Google Scholar] [CrossRef]
- Rasheed, N.; Maqsood, M.A.; Aziz, T.; Jabbar, A. Characterizing lentil germplasm for zinc biofortification and high grain output. J. Soil Sci. Plant Nutr. 2020, 20, 1336–1349. [Google Scholar] [CrossRef]
- Dai, H.; Wei, S.; Twardowska, I. Biofortification of soybean (Glycine max L.) with Se and Zn, and enhancing its physiological functions by spiking these elements to soil during flowering phase. Sci. Total Environ. 2020, 740, 139648. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Sadana, U.S.; Manchanda, J.S.; Dhadli, H.S. Biofortification of wheat grains with zinc (Zn) and iron (Fe) in typic Ustochrept soils of Punjab. Indian J. Fertil. 2009, 5, 13–16. [Google Scholar]
- Dhaliwal, S.S.; Sadana, U.S.; Khurana, M.P.S.; Dhadli, H.S.; Manchanda, J.S. Enrichment of paddy grains (Oryza sativa L.) with zinc and iron through ferti-fortification. Indian J. Fertil. 2010, 6, 28–35. [Google Scholar]
- Dhaliwal, S.S.; Sadana, U.S.; Manchanda, J.S. Relevance and essentiality of ferti-fortification of wheat grains with manganese and copper. Indian J. Fertil. 2011, 7, 48–55. [Google Scholar]
- Singh, P.; Dhaliwal, S.S.; Sadana, U.S. Iron enrichment of paddy grains through ferti-fortification. J. Res. (Punjab Agric. Univ.) 2013, 50, 32–38. [Google Scholar]
- Dhaliwal, S.S.; Sadana, U.S.; Manchanda, J.S.; Khurana, M.P.S.; Shukla, A.K. Differential response of maize cultivars to iron (Fe) applied through ferti-fortification. Indian J. Fertil. 2013, 9, 52–57. [Google Scholar]
- Ullah, M.; Farooq, A.; Rehman, M.S.; Arshad, H.; Shoukat, A.; Nadeem, A.; Nawaz, A.; Wakeel, A.; Nadeem, F. Manganese nutrition improves the productivity and grain biofortification of bread wheat in alkaline calcareous soil. Exp. Agric. 2018, 54, 744–754. [Google Scholar] [CrossRef]
- Kumar, D.; Dhaliwal, S.S.; Uppal, R.S.; Ram, H. Influence of nitrogen, zinc and iron fertilizer on growth parameters and yield of Parmal rice in transplanted condition. Indian J. Ecol. 2016, 43, 115–118. [Google Scholar]
- Singh, V.P.; Singh, G.; Dhaliwal, S.S. Agronomic biofortification of chickpea with zinc and iron through application of zinc and urea. Commun. Soil Sci. Plant Anal. 2019, 50, 1864–1877. [Google Scholar]
- Dhaliwal, S.S.; Sandhu, A.S.; Shukla, A.K.; Sharma, V.; Kumar, B.; Singh, R. Bio-fortification of oats fodder through zinc enrichment to reduce animal malnutrition. J. Agric. Sci. Technol. A 2020, 10, 98–108. [Google Scholar]
- Kumar, B.; Dhaliwal, S.S. Zinc biofortification of dualpurpose cowpea [Vigna unguiculata (L.) Walp] for enhancing the productivity and nutritional quality in a semi-arid regions of India. Arch. Agron. Soil Sci. 2021. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Tabassum, R.; Afzal, I. Enhancing the performance of direct seeded fine rice by seed priming. Plant Prod. Sci. 2006, 9, 446–456. [Google Scholar] [CrossRef]
- Farooq, M.; Cheema, Z.A.; Wahid, A. Seed priming with boron improves growth and yield of fine grain aromatic rice. Plant Growth Regul. 2012, 68, 189–201. [Google Scholar]
- Rehman, M.; Muhammad, F.; Muhammad, N.; Ahmad, N.; Babar, S. Seed priming of Zn with endophytic bacteria improves the productivity and grain biofortification of bread wheat. Eur. J. Agron. 2018, 94, 98–107. [Google Scholar] [CrossRef]
- Reis, C.A.; Pavia, S.; Lima-Brito, I.; Eduardo, J. Influence of seed priming with iron and/or zinc in the nucleolar activity and protein content of bread wheat. Protoplasma 2019, 256, 763–775. [Google Scholar]
- Rehman, M.; Faroo, Z.A. Zinc seed coating improves the growth, grain yield and grain biofortification of bread wheat. Acta Physiol. Plant. 2016, 38, 238. [Google Scholar] [CrossRef]
- Rehman, M.; Farooq, Z.A. Boron application through seed coating improves the water relations, panicle fertility, kernel yield, and biofortification of fine grain aromatic rice. Acta Physiol. Plant. 2013, 35, 411–418. [Google Scholar] [CrossRef]
- Selim, M.M. Introduction to the integrated nutrient management strategies and their contribution to yield and soil properties. Int. J. Agron. 2020, 2, 2821678. [Google Scholar] [CrossRef]
- Padbhushan, R.; Sharma, S.; Kumar, U.; Rana, D.S.; Kohli, A.; Kaviraj, M.; Parmar, B.; Kumar, R.; Annapurna, K.; Sinha, A.K.; et al. Meta-analysis approach to measure the effect of integrated nutrient management on crop performance, microbial activity, and carbon stocks in indian soils. Front. Environ. Sci. 2021, 9, 724702. [Google Scholar] [CrossRef]
- Barman, M.; Shukla, L.M.; Datta, S.; Rattan, R.K. Effect of applied lime and boron on the availability of nutrients in an acid soil. J. Plant Nutr. 2014, 37, 357–373. [Google Scholar] [CrossRef]
- Soltani, S.M.; Mohamed, M.H.; Abdol, W.S.; Sharifah, K.S.M.; Mohammad, A.H. Rice growth improvement and grains bio-fortification through lime and zinc application in zinc deficit tropical acid sulphate soils. Chem. Speciat. Bioavailab. 2016, 28, 152–162. [Google Scholar] [CrossRef][Green Version]
- Ramzani, P.M.A.; Khalid, M.; Naveed, M.; Ahmad, R.; Shahid, M. Iron biofortification of wheat grains through integrated use of organic and chemical fertilizers in pH affected calcareous soil. Plant Physiol. Biochem. 2016, 104, 284–293. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Uddin, S.; Rengasamy, P.; McDonald, G.K. Field applications of gypsum reduce pH and improve soil C in highly alkaline soils in southern Australia’s dryland cropping region. Soil Use Manag. 2022, 38, 466–477. [Google Scholar] [CrossRef]
- Nagabovanalli, B.P.; Prabhudev, D.; Shruthi, C.T.; Shrenivas, A. Performance of slag-based gypsum on maize yield and available soil nutrients over commercial gypsum under acidic and neutral soil. Commun. Soil Sci. Plant Anal. 2020, 51, 1780–1798. [Google Scholar]
- Farooq, M.; Ullah, A.; Usman, M.; Siddique, K.H.M. Application of zinc and biochar help to mitigate cadmium stress in bread wheat raised from seeds with high intrinsic zinc. Chemosphere 2020, 260, 127652. [Google Scholar] [CrossRef]
- Khum-in, V.; Suk-in, J.; In-ai, P.; Piaowan, K.; Phaimisap, Y.; Supanpaiboon, W.; Phenrat, T. Combining biochar and zerovalent iron (BZVI) as a paddy field soil amendment for heavy cadmium (Cd) contamination decreases Cd but increases zinc and iron concentrations in rice grains: A field-scale evaluation. Process Saf. Environ. Prot. 2020, 141, 222–233. [Google Scholar] [CrossRef]
- Kalembasa, D.; Malinowska, E. Bioaccumulation of zinc under the influence of sewage sludge and liming and its speciation in soil. Fresen Environ. Bull. 2013, 22, 3359–3369. [Google Scholar]
- Malinowska, E. The effects of soil liming and sewage sludge application on dynamics of copper fractions and total copper concentration. Environ. Monitor. Assess. 2016, 188, 597. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Srivastva, P.C. Effect of zinc sulphate application and the cyclic incorporation of cereal straw on yields, the tissue concentration and uptake of Zn by crops and availability of Zn in soil under rice–wheat rotation. Int. J. Recycl. Org. Waste Agric. 2014, 3, 53. [Google Scholar] [CrossRef]
- Khoshgoftarmanesh, A.H.; Norouzi, M.; Afyuni, M.; Schulin, R. Zinc biofortification of wheat through preceding crop residue incorporation into the soil. Eur. J. Agron. 2017, 89, 131–139. [Google Scholar] [CrossRef]
- Behera, S.B.; Shukla, A.K.; Brahma, S.; Dwivedi, R.; Lakaria, B.L. Lime and zinc application influence soil zinc availability, dry matter yield and zinc uptake by maize grown on Alfisols. SOIL Discuss. 2016. [Google Scholar] [CrossRef]
- Schweizer, S.A.; Seitz, B.; van der Heijden, M.G.A.; Schulin, R.; Tandy, S. Impact of organic and conventional farming systems on wheat grain uptake and soil bioavailability of zinc and cadmium. Sci. Total Environ. 2018, 639, 608–616. [Google Scholar] [CrossRef]
- Nayak, B.K.; Adhikary, S.; Pattnaik, M.; Pal, A.K. Effect of Sulphur and zinc with combination of FYM on yield and uptake of nutrients in Mustard (Brassica juncea (L.) under Alfisols of Odisha. J. Pharmacog. Phytochem. 2020, 9, 2310–2313. [Google Scholar]
- Ulm, F.; Avelar, D.; Hobson, P.; Penha-Lopes, G.; Dias, T.; Máguas, C.; Cruz, C. Sustainable urban agriculture using compost and an open-pollinated maize variety. J. Clean. Prod. 2018, 212, 622–629. [Google Scholar] [CrossRef]
- Diekow, J.; Mielniczuk, J.; Knicker, H.; Bayer, C.; Dick, D.P.; Kogel-Knabner, I. Soil C and N stocks as affected by cropping systems and nitrogen fertilisation in a southern Brazil Acrisol managed under no-tillage for 17 years. Soil Tillage Res. 2005, 81, 87–95. [Google Scholar] [CrossRef]
- Trapet, P.; Avoscan, L.; Klinguer, A.; Pateyron, S.; Citerne, S.; Chervin, C. The Pseudomonas fluorescens siderophore pyoverdine weakens arabidopsis thaliana defense in favor of growth in iron-deficient conditions. Plant Physiol. 2016, 171, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic, V.; Oljaca, S.; Stojiljkovic, M.; Simic, M.; Dolijanovic, Z.; Kravic, N. Effect of the maize-soybean intercropping system on the potential bioavailability of magnesium, iron and zinc. Crop Pasture Sci. 2015, 66, 1118–1127. [Google Scholar] [CrossRef]
- Vanisha, K.; Atwal, A.K.; Dhaliwal, S.S.; Banga, S.K. Assessment of diverse sesame (Sesamum indicum L) germplasm for mineral composition. J. Plant Sci. Res. 2013, 2, 61–68. [Google Scholar]
- Graham, R.D.; Welch, R.M.; Bouis, H.E. Addressing micronutrient malnutrition through enhancing the nutritional quality of staple foods: Principles, perspectives and knowledge gaps. Adv. Agron. 2001, 70, 77–142. [Google Scholar]
- Jhanji, S.; Sadana, U.S.; Sekhon, N.K.; Khurana, M.P.S.; Sharma, A.; Shukla, A.K. Screening diverse wheat genotypes for manganese efficiency based on high yield and uptake efficiency. Field Crops Res. 2013, 154, 127–132. [Google Scholar] [CrossRef]
- Jegadeeswari, D.; Chitdeshwari, T.; Boominathan, P. Screening of short duration rice genotypes for zinc efficiency. J. Exp. Biol. Agric. Sci. 2019, 7, 25–33. [Google Scholar]
- Disseminating Orange-Fleshed Sweet Potato Uganda Country Report; Harvestplus: Washington, DC, USA, 2012.
- Menkir, A.; Palacios-Rojas, N.; Alamu, O.; Dias Paes, M.C.; Dhliwayo, T.; Maziya-Dixon, B.; Mengesha, W.; Ndhlela, T.; Oliveira Guimaraes, P.E.; Pixley, K.; et al. (Eds.) Vitamin A-Biofortified Maize: Exploiting Native Genetic Variation for Nutrient Enrichment; Science Brief: Biofortification Series, No. 2; CIMMYT, IITA, EMBRAPA, HarvestPlus, and Crop Trust: Bonn, Germany, 2018. [Google Scholar]
- Hindu, V.; Palacios-Rojas, N.; Babu, R.; Suwarno, W.B.; Rashid, Z.; Usha, R.; Saykhedkar, G.R.; Nair, S.K. Identification and validation of genomic regions influencing kernel zinc and iron in maize. Theor. Appl. Genet. 2018, 131, 1443–1457. [Google Scholar] [CrossRef] [PubMed]
- Trijatmiko, K.R.; Dueñas, C.; Tsakirpaloglou, N.; Torrizo, L.; Arines, F.; Adeva, C.; Balindong, J.; Oliva, N. Biofortified indica rice attains iron and zinc nutrition dietary targets in the field. Sci. Rep. 2016, 6, 19792. [Google Scholar] [CrossRef]
- Boonyaves, K.; Wu, T.Y.; Gruissem, W.; Bhullar, N.K. Enhanced grain iron levels in rice expressing an iron-regulated metal transporter, nicotianamine synthase, and ferritin gene cassette. Front. Plant Sci. 2017, 8, 130. [Google Scholar] [CrossRef]
- Banakar, R.; Alvarez-Fernandez, A.; Diaz-Benito, P.; Abadia, J.; Capell, T.; Christou, P. Phytosiderophores determine thresholds for iron and zinc accumulation in biofortified rice endosperm while inhibiting the accumulation of cadmium. J. Exp. Bot. 2017, 68, 4983–4995. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [PubMed]
- Montoya, M.; Guardia, G.; Recio, J.; Castellano-Hinojosa, A.; Ginés, C.; Bedmar, E.J.; Álvarez, J.M. Vallejo, A. Zinc-nitrogen co-fertilization influences N2O emissions and microbial communities in an irrigated maize field. Geoderma 2021, 383, 114735. [Google Scholar] [CrossRef]
- Schiavon, M.; Nardi, S.; dalla Vecchia, F. Selenium biofortification in the 21st century: Status and challenges for healthy human nutrition. Plant Soil 2020, 453, 245–270. [Google Scholar] [CrossRef] [PubMed]
- Ramkissoona, C.; Degryse, F.; Young, S.; Bailey, E.H.; McLaughlin, M.J. Effect of soil properties on time-dependent fixation (ageing) of selenite. Geoderma 2021, 383, 114741. [Google Scholar] [CrossRef]
- Gonzali, S.; Kiferle, C.; Perata, P. Iodine biofortification of crops: Agronomic biofortification, metabolic engineering and iodine bioavailability. Curr. Opin. Biotechnol. 2017, 44, 16–26. [Google Scholar] [CrossRef]
- Singh, B.R.; Timsina, Y.N.; Lind, O.C.; Cagno, S.; Janssens, K. Zinc and iron concentration as affected by nitrogen fertilization and their localization in wheat grain. Front. Plant Sci. 2018, 9, 307. [Google Scholar] [CrossRef]
- Aziz, M.Z.; Yaseen, M.; Abbas, T.; Naveed, M.; Mustafa, A.; Hamid, Y.; Saeed, Q.; Xu, M.G. Foliar application of micronutrients enhances crop stand, yield and the biofortification essential for human health of different wheat cultivars. J. Integr. Agric. 2019, 18, 1369–1378. [Google Scholar] [CrossRef]
- Zou, C.; Du, Y.; Rashid, A.; Ram, H.; Savasli, E.; Pieterse, P.; Cakmak, I. Simultaneous biofortification of wheat with zinc, iodine, selenium and iron through foliar treatment of a micronutrient cocktail in six countries. J. Agric. Food Chem. 2019, 67, 8096–8106. [Google Scholar] [CrossRef]
- Wu, C.; Dun, Y.; Zhang, Z.; Li, M.; Wu, G. Foliar application of selenium and zinc to alleviate wheat (Triticum aestivum L.) cadmium toxicity and uptake from cadmium-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 190, 110091. [Google Scholar] [CrossRef]
- Jalal, A.; Shah, S.; Carvalho Minhoto Teixeira, F. Agro-biofortification of zinc and iron in wheat grains. Gesunde Pflanz. 2020, 72, 227–236. [Google Scholar] [CrossRef]
- Chen, Y.; Jia, Z.; Liu, K.; Tian, X.; Wang, S.; Wang, S.; Li, X.; Zhao, H.; Shar, A.G. Response of exogenous zinc availability and transformation to maize straw as affected by soil organic matter. Soil Fertil. Plant Nutr. 2017, 81, 814–827. [Google Scholar] [CrossRef]
- Verma, V.; Kaur, M.; Greneche, J.M. Tailored structural, optical and magnetic properties of ternary nanohybrid Mn0.4Co0.6−xCuxFe2O4 (x = 0, 0.2, 0.4, 0.6) spinel ferrites. Ceram. Int. 2019, 45, 10865–10875. [Google Scholar] [CrossRef]
- Du, W.; Yang, J.; Peng, Q.; Liang, X.; Mao, H. Comparison study of zinc nanoparticles and zinc sulphate on wheat growth: From toxicity and zinc biofortification. Chemosphere 2019, 227, 109–116. [Google Scholar] [CrossRef]
- Hussain, B.; Li, J.; Ma, Y.; Tahir, N.; Ullah, A. Effects of Fe and Mn cations on Cd uptake by rice plant in hydroponic culture experiment. PLoS ONE 2020, 15, e0243174. [Google Scholar] [CrossRef] [PubMed]
- Kabiri, S.; Degryse, F.; Tran, D.N.H.; da Silva, R.C.; McLaughlin, M.J.; Losic, D. Graphene Oxide: A New Carrier for Slow Release of Plant Micronutrients. ACS Appl Mater. Interfaces 2017, 9, 43325–43335. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, K.; Tonev, T.; Nguyen, N.; Peltekov, A.; Mitkov, A. Impact of foliar fertilization with nanosized zinc hydroxy nitrate on maize yield and quality. Emir. J. Food Agric. 2019, 31, 597–604. [Google Scholar] [CrossRef]
- Chhipa, H. Nanofertilizers and nanopesticides for agriculture. Environ. Chem. Lett. 2017, 15, 15–22. [Google Scholar] [CrossRef]
- An, X.; Wu, Z.; Yu, J.; Ge, L.; Li, T.; Liu, X.; Yu, B. High-efficiency reclaiming phosphate from an aqueous solution by bentonite modified biochars: A slow release fertilizer with a precise rate regulation. ACS Sustain. Chem. Eng. 2020, 8, 6090–6099. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Lee, J.G.; Esposti, L.D.; Iafisco, M.; Kim, P.J.; Shin, S.G.; Adamiano, A. Synergistic release of crop nutrients and stimulants from hydroxyapatite nanoparticles functionalized with humic substances: Toward a multifunctional nanofertilizer. ACS Omega 2020, 5, 6598–6610. [Google Scholar] [CrossRef]
- Yugandhar, P.; Savithramma, N. Green synthesis of calcium carbonate nanoparticles and their effects on seed germination and seedling growth of Vigna mungo (L.) Hepper. Int. J. Adv. Res. 2013, 1, 89–103. [Google Scholar]
- Ramírez-Rodríguez, G.B.; Dal Sasso, G.; Carmona, F.J.; Miguel-Rojas, C.; Pérez de Luque, A.; Masciocchi, N.; Delgado-López, J.M. Engineering biomimetic calcium phosphate nanoparticles: A green synthesis of slow-release multinutrient (NPK) nano-fertilizers. ACS Appl. Bio Mater. 2020, 3, 1344–1353. [Google Scholar] [CrossRef]
- Singh, D.; Rajawat, M.V.S.; Kaushik, R. Beneficial role of endophytes in biofortification of Zn in wheat genotypes varying in nutrient use efficiency grown in soils sufficient and deficient in Zn. Plant Soil 2017, 416, 107–116. [Google Scholar] [CrossRef]
- Watts-Williams, S.J.; Cavagnaro, T.R. Arbuscular mycorrhizal fungi increase grain zinc concentration and modify the expression of root ZIP transporter genes in a modern barley (Hordeum vulgare) cultivar. Plant Sci. 2018, 274, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Ror, P.; Rathore, P.; Ramakrishna, W. Bacteria from native soil in combination with arbuscular mycorrhizal fungi augment wheat yield and biofortification. Plant Physiol. Biochem. 2020, 150, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Stepien, A.; Wojtkowiak, K. Effect of foliar application of Cu, Zn, and Mn on yield and quality indicators of winter wheat grain. Chil. J. Agric. Res. 2016, 76, 219–226. [Google Scholar] [CrossRef]
- Nawaz, F.; Ahmad, R.; Ashraf, M.Y. Effect of selenium foliar spray on physiological and biochemical processes and chemical constituents of wheat under drought stress. Ecotoxicol. Environ. Saf. 2015, 113, 191–200. [Google Scholar] [CrossRef]
- Aciksoz, S.B.; Ozturk, L.; Yazici, A.; Cakmak, I. Inclusion of urea in a 59Fe EDTA solution stimulated leaf penetration and translocation of 59Fe within wheat plants. Physiol. Plant. 2014, 151, 348–357. [Google Scholar] [CrossRef]
- Blasco, B.; Ríos, J.J.; Sánchez-Rodríguez, E. Study of the interactions between iodine and mineral nutrients in lettuce plants. J. Plant Nutr. 2012, 35, 1958–1969. [Google Scholar] [CrossRef]
- Wang, S.; Li, M.; Liu, K. Effects of Zn, macronutrients, and their interactions through foliar applications on winter wheat grain nutritional quality. PLoS ONE 2017, 12, e0181276. [Google Scholar] [CrossRef]
- Aciksoz, S.B.; Yazici, A.; Ozturk, L.; Cakmak, I. Biofortification of wheat with iron through soil and foliar application of nitrogen and iron fertilizers. Plant Soil 2011, 349, 215–225. [Google Scholar] [CrossRef]
- Naeem, A.; Aslam, M.; Lodhi, A. Improved potassium nutrition retrieves phosphorus-induced decrease in zinc uptake and grain zinc concentration of wheat. J. Sci. Food Agric. 2018, 98, 4351–4356. [Google Scholar] [CrossRef] [PubMed]
- Hussain, B.; Lin, Q.; Hamid, Y.; Sanaullah, M.; Di, L.; Hashmi, M.L.; Yang, X. Foliage application of selenium and silicon nanoparticles alleviates Cd and Pb toxicity in rice (Oryza sativa L.). Sci. Total Environ. 2020, 712, 136497. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, M.; Tian, X. Foliar zinc, nitrogen, and phosphorus application effects on micronutrient concentrations in winter wheat. Agron. J. 2015, 107, 61–70. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, S.; Rizwan, M.; Rehman, M.Z.; Qayyum, M.F.; Wang, H.; Rinklebe, J. Responses of wheat (Triticum aestivum) plants grown in a Cd contaminated soil to the application of iron oxide nanoparticles. Ecotoxicol. Environ. Saf. 2019, 173, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Akhtar, M.; Kamran, M.A.; Imran, M.; Riaz, M.A.; Kamran, K.; Hussain, S. Selenium biofortification in food crops: Key mechanisms and future perspectives. J. Food Compos. Anal. 2020, 93, 103615. [Google Scholar] [CrossRef]
- Yang, L.; Fan, I.; Huang, B.; Xin, J. Efficiency and mechanisms of fermented horse manure, vermicompost, bamboo biochar, and fly ash on Cd accumulation in rice. Environ. Sci. Pollut. Res. 2020, 27, 27859–27869. [Google Scholar] [CrossRef]
- Maqsood, M.A.; Schoenau, J.; Vandenberg, A. Zinc fertilization of lentil for grain yield and grain zinc concentration in ten Saskatchewan soils. J. Plant Nutr. 2015, 39, 866–874. [Google Scholar] [CrossRef]
- Yaseen, M.K.; Hussain, S. Zinc-biofortified wheat required only a medium rate of soil zinc application to attain the targets of zinc biofortification. Arch. Agron. Soil Sci. 2020, 67, 551–562. [Google Scholar] [CrossRef]
- Haider, M.U.; Hussain, M.; Farooq, M.; Nawaz, A. Soil application of zinc improves the growth, yield and grain zinc biofortification of mungbean. Soil Environ. 2018, 37, 123–128. [Google Scholar]
- Ozbahce, A.; Zengin, M. Effects of foliar and soil applications of different manganese fertilizers on yield and net return of bean. J. Plant Nutr. 2014, 37, 161–171. [Google Scholar] [CrossRef]
- Ramzan, Y.; Hafeez, M.B.; Khan, S.; Nadeem, M.; Batool, S.; Ahmad, J. Biofortification with zinc and iron improves the grain quality and yield of wheat crop. Int J. Plant Prod. 2020, 14, 501–510. [Google Scholar] [CrossRef]
- Golden, B.R.; Orlowski, J.M.; Bond, J.A. Corn injury from foliar zinc application does not affect grain yield. Agron. J. 2016, 108, 2071–2075. [Google Scholar] [CrossRef]
| Micronutrient | Crop | Reference |
|---|---|---|
| Zn | Wheat | [50] |
| Zn | Rice, Wheat | [51] |
| Zn | Cowpea | [52] |
| Zn | Lentil | [53] |
| Zn + Se | Soybean | [54] |
| Seed treatment | Micronutrient | Crop | Reference |
|---|---|---|---|
| Seed Priming | B | Wheat and Rice | [66] |
| Zn + Pseudomonas sp. MN12 | Bread-wheat | [67] | |
| Fe and Zn | Wheat and Barley | [68] | |
| Seed Coating | Zn | Wheat | [69] |
| B | Rice | [70] | |
| Mn | Bread wheat | [60] |
| Micro-Nutrient Nano-Fertilizer | Crop | Reference |
|---|---|---|
| ZnO NPs | Wheat | [113] |
| Fe3O4 | Wheat | [114] |
| Graphene oxide-Zn NPs | Wheat | [115] |
| Graphene oxide-Cu NPs | Wheat | |
| Zn5(OH)8(NO3)2·2H2O | Maize | [116] |
| Microorganisms | Micronutrient Affected | Crop | Reference |
|---|---|---|---|
| Arthrobacter sp. DS-179 and Arthrobacter sulfonivorans (DS-68) | Fe and Zn | Wheat | [122] |
| Bacillus subtilis DS-178 and Arthrobacter sp. DS-179, Enterococcus hirae DS-163 and Arthrobacter sulfonivorans DS-68 | Fe and Zn | Wheat | [106] |
| Rhizophagus irregularis | Zn | [123] | |
| PGPB and AM fungi | Zn and Fe | Wheat | [124] |
| Endophytes | Fe and Zn | Wheat | [106] |
| Micronutrient | Micronutrient and Trace Element | Type of Interaction | Crop | Reference |
|---|---|---|---|---|
| Cu | Mn | Antagonistic | [125] | |
| Fe | Se | Synergistic | Wheat and rice | [126] |
| Micro-Nutrient Involved in Interaction | Heavy-Metal Stress | Mode of Application | Crops | Reference |
|---|---|---|---|---|
| Zn | Cd | Soil + foliar | Wheat | [134] |
| Zn | Cd | Seed treatment | Wheat | [78] |
| Zn + Biochar | Cd | Wheat | ||
| Se and Si | Cd and Pb | Foliar | Brown rice | [132] |
| Zerovalent iron + Biochar | Cd | Rice | [79] | |
| Zn | Cd | Foliar | Wheat | [135] |
| Se + Zn | Cd | Foliar | Wheat | [109] |
| Micronutrient | Rate | Crop | Increase in Yield (%) | Reference |
|---|---|---|---|---|
| Zn | 62.5 kg ha−1 | Wheat | 28.6 | [50] |
| Zn | 50.0 kg ha−1 | Wheat | 50.0 | [51] |
| Zn | 50.0 kg ha−1 | Rice | 14.8 | |
| Zn | 5.0 kg ha−1 | Lentils | 40.0 | [137] |
| Zn | 12 mg kg−1 | Wheat (Zincol-2016) | 22.6 | [138] |
| Zn | 10 mg kg−1 | Mung-bean | 44.7 | [139] |
| Zn | 25 kg ha−1 | Oats | 28.9 | [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhaliwal, S.S.; Sharma, V.; Shukla, A.K.; Verma, V.; Kaur, M.; Shivay, Y.S.; Nisar, S.; Gaber, A.; Brestic, M.; Barek, V.; et al. Biofortification—A Frontier Novel Approach to Enrich Micronutrients in Field Crops to Encounter the Nutritional Security. Molecules 2022, 27, 1340. https://doi.org/10.3390/molecules27041340
Dhaliwal SS, Sharma V, Shukla AK, Verma V, Kaur M, Shivay YS, Nisar S, Gaber A, Brestic M, Barek V, et al. Biofortification—A Frontier Novel Approach to Enrich Micronutrients in Field Crops to Encounter the Nutritional Security. Molecules. 2022; 27(4):1340. https://doi.org/10.3390/molecules27041340
Chicago/Turabian StyleDhaliwal, Salwinder Singh, Vivek Sharma, Arvind Kumar Shukla, Vibha Verma, Manmeet Kaur, Yashbir Singh Shivay, Shahida Nisar, Ahmed Gaber, Marian Brestic, Viliam Barek, and et al. 2022. "Biofortification—A Frontier Novel Approach to Enrich Micronutrients in Field Crops to Encounter the Nutritional Security" Molecules 27, no. 4: 1340. https://doi.org/10.3390/molecules27041340
APA StyleDhaliwal, S. S., Sharma, V., Shukla, A. K., Verma, V., Kaur, M., Shivay, Y. S., Nisar, S., Gaber, A., Brestic, M., Barek, V., Skalicky, M., Ondrisik, P., & Hossain, A. (2022). Biofortification—A Frontier Novel Approach to Enrich Micronutrients in Field Crops to Encounter the Nutritional Security. Molecules, 27(4), 1340. https://doi.org/10.3390/molecules27041340












