Anti-Cancer Properties of Stevia rebaudiana; More than a Sweetener
Abstract
:1. Introduction
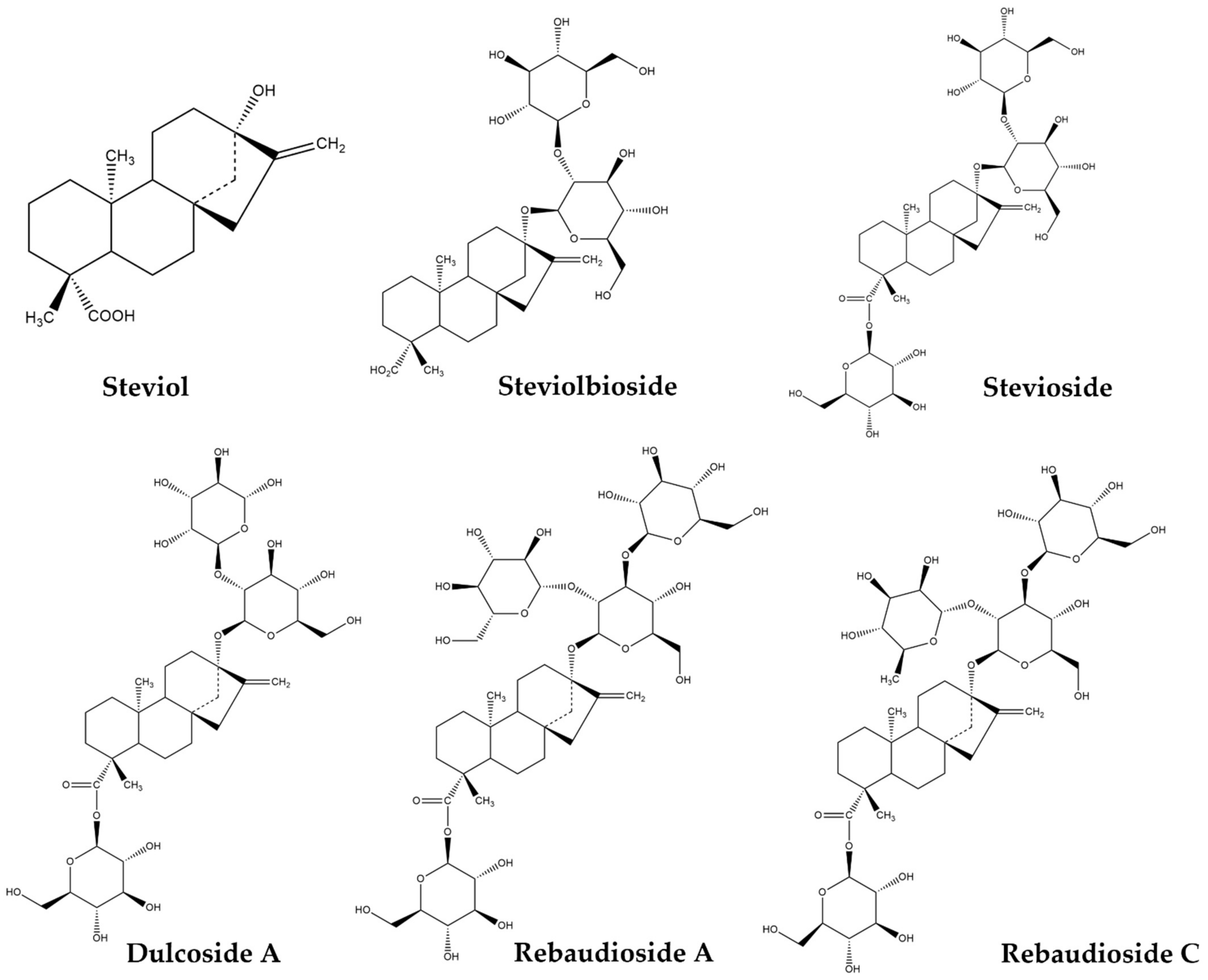
2. Steviol Glycosides: Chemical Structure and Metabolism in the Human Body
3. Antitumor Effects of Stevia rebaudiana Derivatives
3.1. Antitumor Effects of Stevia rebaudiana Derivatives in Breast Cancer
3.2. Antitumor Effects of Stevia rebaudiana Derivatives in Gastrointestinal Cancer Models
| Cell Line/Cancer Type | Compound | Effect (Cell Viability Assay) | Reference |
|---|---|---|---|
| AZ521 (Gastric) | Steviol, isosteviol derivatives | Increased cytotoxicity (MTT Assay) | [44] |
| HGC-27 (Gastric) | Steviol | Increased cytotoxicity, G1 arrest (MTT Assay) | [43] |
| MKN-45 (Gastric) | Steviol | Increased cytotoxicity, G1 arrest, apoptosis, regulation of miR-1268b and miR-23c (MTT Assay) | [43] |
| MGC-803 (Gastric) | Steviol | Increased cytotoxicity, G2 arrest (MTT Assay) | [43] |
| NUGC-3 (Gastric) | Isosteviol | Increased cytotoxicity (MTT Assay) | [45] |
| Caco-2 (Colorectal) | Aqueous extract | Decreased cell viability (MTT Assay) | [37] |
| Steviol | Increased cytotoxicity, G1 arrest (MTT Assay) | [43] | |
| HCT116 (Colorectal) | Isosteviol Triazole Conjugates | Increased cytotoxicity (MTT Assay) | [28] |
| Stevioside, ethanolic extract | Increased cytotoxicity (MTT Assay) | [46] | |
| Steviol | Cell proliferation inhibition, G1 arrest, apoptosis regulation of miR-203a-3p and miR-6088 (MTT Assay) | [43] | |
| HCT-8 (Colorectal) | Steviol | Cell proliferation inhibition, G2 arrest (MTT Assay) | [43] |
| Hep3B (Hepatocarcinoma) | Steviolbioside | Cell proliferation inhibition (MTT Assay) | [27] |
| HepG2 (Hepatocarcinoma) | Aqueous extract, stevioside, RebA | No cytotoxicity (LDH and BRDU assays) | [10] |
| Commercialized stevia, Stevioside | Increased cytotoxicity, cholesterol internalization (MTT Assay) | [47] | |
| Ammonium derivatives of steviol and isosteviol | Increased cytotoxicity (Multifunctional Cytell Cell Imaging system) | [24] | |
| ASPC-1 (Pancreatic) | Isosteviol Triazole Conjugates | Increased cytotoxicity (MTT Assay) | [28] |
| BxPC-3 (Pancreatic) | Steviolbioside | Cell proliferation inhibition (MTT Assay) | [27] |
| MiaPaCa-2 (Pancreatic) | Stevioside, ethanolic extract | Increased cytotoxicity (MTT Assay) | [46] |
3.3. Antitumor Effects of Stevia rebaudiana Derivatives in Other Solid Tumors and Blood Cancers
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Samuel, P.; Ayoob, K.T.; Magnuson, B.A.; Wölwer-Rieck, U.; Jeppesen, P.B.; Rogers, P.J.; Rowland, I.; Mathews, R. Stevia Leaf to Stevia Sweetener: Exploring Its Science, Benefits, and Future Potential. J. Nutr. 2018, 148, 1186S–1205S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, B.; López, M.D.; Martínez-López, S.; Victoriano, M.; Sharifi-Rad, J.; Martorell, M.; Rodrigues, C.F.; Martins, N. Stevia rebaudiana Bertoni bioactive effects: From in vivo to clinical trials towards future therapeutic approaches. Phytother. Res. 2019, 33, 2904–2917. [Google Scholar] [CrossRef] [PubMed]
- Momtazi-Borojeni, A.A.; Esmaeili, S.-A.; Abdollahi, E.; Sahebkar, A. A Review on the Pharmacology and Toxicology of Steviol Glycosides Extracted from Stevia rebaudiana. Curr. Pharm. Des. 2017, 23, 1616–1622. [Google Scholar] [CrossRef]
- Lemus-Mondaca, R.; Vega-Gálvez, A.; Zura-Bravo, L.; Ah-Hen, K.S. Stevia rebaudiana Bertoni, source of a high-potency natural sweetener: A comprehensive review on the biochemical, nutritional and functional aspects. Food Chem. 2012, 132, 1121–1132. [Google Scholar] [CrossRef]
- Marcinek, K.; Krejpcio, Z. Stevia Rebaudiana Bertoni-Chemical Composition and Functional Properties. Acta Sci. Pol. Technol. Aliment. 2015, 14, 145–152. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, J.C.; Moguel-Ordoñez, Y.B.; Segura-Campos, M.R. Biological activity of Stevia rebaudiana Bertoni and their relationship to health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2680–2690. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Wang, Y.; Lau, H.; Zhou, W.; Chen, C.; Tan, S. A review of stevia as a potential healthcare product: Up-to-date functional characteristics, administrative standards and engineering techniques. Trends Food Sci. Technol. 2020, 103, 264–281. [Google Scholar] [CrossRef]
- Bender, C.; Graziano, S.; Zimmermann, B.F. Study of Stevia rebaudiana Bertoni antioxidant activities and cellular properties. Int. J. Food Sci. Nutr. 2015, 66, 553–558. [Google Scholar] [CrossRef]
- Ceunen, S.; Geuns, J.M.C. Steviol Glycosides: Chemical Diversity, Metabolism, and Function. J. Nat. Prod. 2013, 76, 1201–1228. [Google Scholar] [CrossRef] [PubMed]
- Randhir, R.; Lin, Y.-T.; Shetty, K. Stimulation of phenolics, antioxidant and antimicrobial activities in dark germinated mung bean sprouts in response to peptide and phytochemical elicitors. Process Biochem. 2004, 39, 637–646. [Google Scholar] [CrossRef]
- Purkayastha, S.; Markosyan, A.; Prakash, I.; Bhusari, S.; Pugh, G.; Lynch, B.; Roberts, A. Steviol glycosides in purified stevia leaf extract sharing the same metabolic fate. Regul. Toxicol. Pharmacol. 2016, 77, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Hutapea, A.M.; Toskulkao, C.; Buddhasukh, D.; Wilairat, P.; Glinsukon, T. Digestion of Stevioside, a Natural Sweetener, by Various Digestive Enzymes. J. Clin. Biochem. Nutr. 1997, 23, 177–186. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Flavourings (FAF); Younes, M.; Aquilina, G.; Engel, K.; Fowler, P.; Fernandez, M.J.F.; Fürst, P.; Gürtler, R.; Gundert-Remy, U.; Husøy, T.; et al. Safety of a proposed amendment of the specifications for steviol glycosides (E 960) as a food additive: To expand the list of steviol glycosides to all those identified in the leaves of Stevia rebaudiana Bertoni. EFSA J. 2020, 18, e06106. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, W.; Cai, J.; Liu, W.; Akihisa, T.; Li, W.; Kikuchi, T.; Xu, J.; Feng, F.; Zhang, J. The role of metabolites of steviol glycosides and their glucosylated derivatives against diabetes-related metabolic disorders. Food Funct. 2021, 12, 8248–8259. [Google Scholar] [CrossRef]
- Magnuson, B.A.; Carakostas, M.C.; Moore, N.H.; Poulos, S.; Renwick, A.G. Biological fate of low-calorie sweeteners. Nutr. Rev. 2016, 74, 670–689. [Google Scholar] [CrossRef] [Green Version]
- Gardana, C.; Simonetti, P.; Canzi, E.; Zanchi, R.; Pietta, P. Metabolism of Stevioside and Rebaudioside A from Stevia rebaudiana Extracts by Human Microflora. J. Agric. Food Chem. 2003, 51, 6618–6622. [Google Scholar] [CrossRef]
- Geuns, J.M.; Augustijns, P.; Mols, R.; Buyse, J.G.; Driessen, B. Metabolism of stevioside in pigs and intestinal absorption characteristics of stevioside, rebaudioside A and steviol. Food Chem. Toxicol. 2003, 41, 1599–1607. [Google Scholar] [CrossRef]
- JECFA. Steviol Glycosides from Stevia rebaudiana Bertoni. In Proceedings of the 2016 82nd Joint FAO/WHO Expert Committee on Food Additives (JECFA), Geneva, Switzerland, 7–16 June 2016. [Google Scholar]
- Toyoda, K.; Matsui, H.; Shoda, T.; Uneyama, C.; Takada, K.; Takahashi, M. Assessment of the carcinogenicity of stevioside in F344 rats. Food Chem. Toxicol. 1997, 35, 597–603. [Google Scholar] [CrossRef]
- Gupta, E.; Kaushik, S.; Purwar, S.; Sharma, R.; Balapure, A.; Sundaram, S. Anticancer potential of steviol in MCF-7 human breast cancer cells. Pharmacogn. Mag. 2017, 13, 345–350. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.; Sengupta, S.; Bandyopadhyay, T.K.; Bhattacharyya, A. Stevioside Induced ROS-Mediated Apoptosis Through Mitochondrial Pathway in Human Breast Cancer Cell Line MCF-7. Nutr. Cancer 2012, 64, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Voloshina, A.D.; Sapunova, A.S.; Kulik, N.V.; Belenok, M.G.; Strobykina, I.Y.; Lyubina, A.P.; Gumerova, S.K.; Kataev, V.E. Antimicrobial and cytotoxic effects of ammonium derivatives of diterpenoids steviol and isosteviol. Bioorg. Med. Chem. 2021, 32, 115974. [Google Scholar] [CrossRef] [PubMed]
- Khare, N.; Chandra, S. Stevioside mediated chemosensitization studies and cytotoxicity assay on breast cancer cell lines MDA-MB-231 and SKBR3. Saudi J. Biol. Sci. 2019, 26, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Khodapasand, E.; Jafarzadeh, N.; Farrokhi, F.; Kamalidehghan, B.; Houshmand, M. Is Bax/Bcl-2 ratio considered as a prognostic marker with age and tumor location in colorectal cancer? Iran. Biomed. J. 2015, 19, 69–75. [Google Scholar]
- Chen, J.-M.; Ding, L.; Sui, X.-C.; Xia, Y.-M.; Wan, H.-D.; Lu, T. Production of a bioactive sweetener steviolbioside via specific hydrolyzing ester linkage of stevioside with a β-galactosidase. Food Chem. 2016, 196, 155–160. [Google Scholar] [CrossRef]
- Khaybullin, R.N.; Zhang, M.; Fu, J.; Liang, X.; Li, T.; Katritzky, A.R.; Okunieff, P.; Qi, X. Design and Synthesis of Isosteviol Triazole Conjugates for Cancer Therapy. Molecules 2014, 19, 18676–18689. [Google Scholar] [CrossRef]
- Sen, P.; Nath, A.; Bhattacharjee, C. Packed-Bed Bioreactor and Its Application in Dairy, Food, and Beverage Industry. In Current Developments in Biotechnology and Bioengineering: Bioprocesses, Bioreactors and Controls; Elsevier BV: Amsterdam, The Netherlands, 2017; ISBN 9780444636638. [Google Scholar]
- Xu, Z.; Zhao, S.-J.; Liu, Y. 1,2,3-Triazole-containing hybrids as potential anticancer agents: Current developments, action mechanisms and structure-activity relationships. Eur. J. Med. Chem. 2019, 183, 111700. [Google Scholar] [CrossRef]
- Myint, K.Z.; Wu, K.; Xia, Y.; Fan, Y.; Shen, J.; Zhang, P.; Gu, J. Polyphenols from Stevia rebaudiana (Bertoni) leaves and their functional properties. J. Food Sci. 2020, 85, 240–248. [Google Scholar] [CrossRef] [Green Version]
- Ibrahem, E.S.; Ragheb, E.M.; Yousef, F.M.; Abdel-azizand, M.F.; Alghamdi, B.A. Nutritional Value, Cytotoxic and Antimicrobial Activities of Stevia rebaudiana Leaf Extracts. J. Biochem. Technol. 2020, 11, 108–115. [Google Scholar]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry; WILEY-VCH Verlag GmbH: Weinheim, Germany, 2011; ISBN 9783527324736. [Google Scholar]
- Ertaş, A.; Öztürk, M.; Boga, M.; Topcu, G. Antioxidant and Anticholinesterase Activity Evaluation of ent-Kaurane Diterpenoids from Sideritis arguta. J. Nat. Prod. 2009, 72, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Dutra, L.M.; Bomfim, L.M.; Rocha, S.L.; Nepel, A.; Soares, M.B.; Barison, A.; Costa, E.V.; Bezerra, D.P. ent-Kaurane diterpenes from the stem bark of Annona vepretorum (Annonaceae) and cytotoxic evaluation. Bioorg. Med. Chem. Lett. 2014, 24, 3315–3320. [Google Scholar] [CrossRef] [PubMed]
- Shokrzadeh, M.; Rajabali, F.; Habibi, E.; Modanloo, M. Survey Cytotoxicity and Genotoxicity of Hydroalcoholic Extract of Stevia rebaudiana in Breast Cancer Cell Line (MCF7) and Human Fetal Lung Fibroblasts (MRC-5). J. Cancer Res. Metastasis 2018, 1, 12–17. [Google Scholar]
- Vaško, L.; Vašková, J.; Fejerčáková, A.; Mojžišová, G.; Poracova, J. Comparison of some antioxidant properties of plant extracts from Origanum vulgare, Salvia officinalis, Eleutherococcus senticosus and Stevia rebaudiana. Vitr. Cell. Dev. Biol. Anim. 2014, 50, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Tao, Z.; Zhu, B.; Zhang, W.; Liu, C.; Chen, S.; Song, M. Targeted Delivery of Drugs and Genes Using Polymer Nanocarriers for Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 9118. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Baek, K.-H. Green Nanobiotechnology: Factors Affecting Synthesis and Characterization Techniques. J. Nanomater. 2014, 2014, 219. [Google Scholar] [CrossRef] [Green Version]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ain, N.U.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef] [Green Version]
- Alijani, H.Q.; Pourseyedi, S.; Mahani, M.T.; Khatami, M. Green synthesis of zinc sulfide (ZnS) nanoparticles using Stevia rebaudiana Bertoni and evaluation of its cytotoxic properties. J. Mol. Struct. 2019, 1175, 214–218. [Google Scholar] [CrossRef]
- Chen, J.; Xia, Y.; Sui, X.; Peng, Q.; Zhang, T.; Li, J.; Zhang, J. Steviol, a natural product inhibits proliferation of the gastrointestinal cancer cells intensively. Oncotarget 2018, 9, 26299–26308. [Google Scholar] [CrossRef]
- Ukiya, M.; Sawada, S.; Kikuchi, T.; Kushi, Y.; Fukatsu, M.; Akihisa, T. Cytotoxic and Apoptosis-Inducing Activities of Steviol and Isosteviol Derivatives against Human Cancer Cell Lines. Chem. Biodivers. 2013, 10, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Mizushina, Y.; Akihisa, T.; Ukiya, M.; Hamasaki, Y.; Murakami-Nakai, C.; Kuriyama, I.; Takeuchi, T.; Sugawara, F.; Yoshida, H. Structural analysis of isosteviol and related compounds as DNA polymerase and DNA topoisomerase inhibitors. Life Sci. 2005, 77, 2127–2140. [Google Scholar] [CrossRef] [PubMed]
- López, V.; Pérez, S.; Vinuesa, A.; Zorzetto, C.; Abian, O. Stevia rebaudiana ethanolic extract exerts better antioxidant properties and antiproliferative effects in tumour cells than its diterpene glycoside stevioside. Food Funct. 2016, 7, 2107–2113. [Google Scholar] [CrossRef] [PubMed]
- Ilias, A.N.; Hamzah, H.; Ismail, I.S.; Ajat, M. Stevia: Limiting cholesterol synthesis in Hep-G2 cells. Asia Pac. J. Mol. Biol. Biotechnol. 2020, 28, 110–119. [Google Scholar] [CrossRef]
- Hazafa, A.; Rehman, K.U.; Jahan, N.; Jabeen, Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Shida, Y.; Hakariya, T.; Sakai, H. Anti-Cancer Effects of Green Tea Polyphenols Against Prostate Cancer. Molecules 2019, 24, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.Y.; Yoon, H.; Ahn, S.; Kim, D.-W.; Bae, D.-H.; Koh, D.; Lee, Y.H.; Lim, Y. Structural Properties of Polyphenols Causing Cell Cycle Arrest at G1 Phase in HCT116 Human Colorectal Cancer Cell Lines. Int. J. Mol. Sci. 2013, 14, 16970–16985. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Wu, H.; Tollefsbol, T.O. Combined Broccoli Sprouts and Green Tea Polyphenols Contribute to the Prevention of Estrogen Receptor–Negative Mammary Cancer via Cell Cycle Arrest and Inducing Apoptosis in HER2/neu Mice. J. Nutr. 2021, 151, 73–84. [Google Scholar] [CrossRef]
- Messeha, S.S.; Zarmouh, N.O.; Asiri, A.; Soliman, K.F. Rosmarinic acid-induced apoptosis and cell cycle arrest in triple-negative breast cancer cells. Eur. J. Pharmacol. 2020, 885, 173419. [Google Scholar] [CrossRef]
- Zhong, Y.; Shahidi, F. Methods for the Assessment of Antioxidant Activity in Foods. In Handbook of Antioxidants for Food Preservation; Woodhead Publishing: Cambridge, UK, 2015; ISBN 9781782420897. [Google Scholar]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef]
- Huang, B.; Song, B.-L.; Xu, C. Cholesterol metabolism in cancer: Mechanisms and therapeutic opportunities. Nat. Metab. 2020, 2, 132–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasukawa, K.; Kitanaka, S.; Seo, S. Inhibitory Effect of Stevioside on Tumor Promotion by 12-O-Tetradecanoylphorbol-13-acetate in Two-Stage Carcinogenesis in Mouse Skin. Biol. Pharm. Bull. 2002, 25, 1488–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takasaki, M.; Konoshima, T.; Kozuka, M.; Tokuda, H.; Takayasu, J.; Nishino, H.; Miyakoshi, M.; Mizutani, K.; Lee, K.-H. Cancer preventive agents. Part 8: Chemopreventive effects of stevioside and related compounds. Bioorg. Med. Chem. 2009, 17, 600–605. [Google Scholar] [CrossRef]
- Wang, G.; Hiramoto, K.; Ma, N.; Yoshikawa, N.; Ohnishi, S.; Murata, M.; Kawanishi, S. Glycyrrhizin Attenuates Carcinogenesis by Inhibiting the Inflammatory Response in a Murine Model of Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 2609. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, J.; Xia, Y.; Wang, X.; Li, J. The natural sweetener metabolite steviol inhibits the proliferation of human osteosarcoma U2OS cell line. Oncol. Lett. 2018, 15, 5250–5256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatun, M.C.S.; Muhit, A.; Hossain, J.; Al-Mansur, M.A.; Rahman, S.A. Isolation of phytochemical constituents from Stevia rebaudiana (Bert.) and evaluation of their anticancer, antimicrobial and antioxidant properties via in vitro and in silico approaches. Heliyon 2021, 7, e08475. [Google Scholar] [CrossRef]
- Salmaso, V.; Moro, S. Bridging Molecular Docking to Molecular Dynamics in Exploring Ligand-Protein Recognition Process: An Overview. Front. Pharmacol. 2018, 9, 923. [Google Scholar] [CrossRef] [Green Version]
- El-Naggar, M.; Sallam, H.A.; Shaban, S.S.; Abdel-Wahab, S.S.; Amr, A.E.-G.E.; Azab, M.E.; Nossier, E.S.; Al-Omar, M.A. Design, Synthesis, and Molecular Docking Study of Novel Heterocycles Incorporating 1,3,4-Thiadiazole Moiety as Potential Antimicrobial and Anticancer Agents. Molecules 2019, 24, 1066. [Google Scholar] [CrossRef] [Green Version]
- Begum, S.S.; Das, D.; Gour, N.K.; Deka, R.C. Computational modelling of nanotube delivery of anti-cancer drug into glutathione reductase enzyme. Sci. Rep. 2021, 11, 4950. [Google Scholar] [CrossRef]
- Blowman, K.; Magalhães, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer Properties of Essential Oils and Other Natural Products. Evid. Based Complement. Altern. Med. 2018, 2018, 3149362. [Google Scholar] [CrossRef]
- Russo, R.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Exploitation of Cytotoxicity of Some Essential Oils for Translation in Cancer Therapy. Evid. Based Complement. Altern. Med. 2015, 2015, 397821. [Google Scholar] [CrossRef] [Green Version]
- Pól, J.; Hohnová, B.; Hyötyläinen, T. Characterisation of Stevia rebaudiana by comprehensive two-dimensional liquid chromatography time-of-flight mass spectrometry. J. Chromatogr. A 2007, 1150, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.S.; Agnihotri, V.K.; Kumar, D.; Pal, P.K.; Koundal, R.; Kumar, A.; Padwad, Y.S. In Vitro Cytotoxic Activity Guided Essential Oil Composition of Flowering Twigs of Stevia rebaudiana. Nat. Prod. Commun. 2014, 9, 715–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Rojo, E.; Cariño-Cortés, R.; Berumen, L.C.; García-Alcocer, G.; Escobar-Cabrera, J. Stevia Eupatoria and Stevia Pilosa Extracts Inhibit the Proliferation and Migration of Prostate Cancer Cells. Medicina 2020, 56, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, M.; Fizazi, K.; Saad, F.; Rathenborg, P.; Shore, N.; Ferreira, U.; Ivashchenko, P.; Demirhan, E.; Modelska, K.; de Phung, B.S.; et al. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2018, 378, 2465–2474. [Google Scholar] [CrossRef] [PubMed]
- Makarem, N.; Bandera, E.V.; Lin, Y.; Jacques, P.F.; Hayes, R.B.; Parekh, N. Consumption of Sugars, Sugary Foods, and Sugary Beverages in Relation to Adiposity-Related Cancer Risk in the Framingham Offspring Cohort (1991–2013). Cancer Prev. Res. 2018, 11, 347–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, G. Food, nutrition, physical activity, and the prevention of cancer: A global perspective. Choice Rev. Online 2008, 45, 45–5024. [Google Scholar] [CrossRef]



| Solvent | Extract IC50 (μg/mL) | |
|---|---|---|
Polarity Decreases | Water | 374 |
| Methanol | 228 | |
| Ethanol | 180 | |
| Acetone | 150 | |
| Chloroform | 100 | |
| Petroleum Ether | 79 |
| Cell Line/Model | Compound | Effect (Cell Viability Assay) | Reference |
|---|---|---|---|
| F344 rats | Stevioside | Decrease in mammary adenomas | [21] |
| MCF-7 | Steviol | G2/M arrest, ROS-mediated apoptosis (SRB Assay) | [22] |
| Stevioside | G1 arrest, Bax overexpression, apoptosis (MTT Assay) | [23] | |
| Hydroalcoholic extract | Increased cytotoxicity (MTT Assay) | [36] | |
| Stevia extracts (various solvents) | Increased cytotoxicity (SRB Assay) | [32] | |
| ZnS Nanoparticles, aqueous extract | Increased cytotoxicity (MTT Assay) | [42] | |
| Ammonium derivatives of steviol and isosteviol | Increased cytotoxicity (Multifunctional Cytell Cell Imaging system) | [24] | |
| MCF-7, MDA * | Aqueous extract | Decreased cell viability (MTT Assay) | [37] |
| MDA-MB-231 | Steviolbioside | Cell proliferation inhibition (MTT Assay) | [27] |
| Isosteviol Triazole Conjugates | Increased cytotoxicity (MTT Assay) | [28] | |
| MDA-MB-231, SKBR3 | Stevioside | Cell proliferation inhibition (MTT Assay) | [25] |
| Model/Cancer Type | Compound | Effect (Cell Viability Assay) | Reference |
|---|---|---|---|
| A549 (Lung) | Steviol, isosteviol derivatives | Increased cytotoxicity (MTT Assay) | [44] |
| Isosteviol Triazole Conjugates | Increased cytotoxicity (MTT Assay) | [28] | |
| Ammonium derivatives of steviol and isosteviol | Increased cytotoxicity (Multifunctional Cytell Cell Imaging system) | [24] | |
| BALL1 (Leukemia) | Stevioside and isosteviol derivatives | Increased cytotoxicity (MTT Assay) | [45] |
| C-6 (Rat glioma) | Flowering twigs essential oils | Increased cytotoxicity (SRB Assay) | [67] |
| CHOK-1 (Chinese hamster ovary) | Flowering twigs essential oil | Increased cytotoxicity (SRB Assay) | [67] |
| HeLa (Cervix) | Isosteviol Triazole Conjugates | Increased cytotoxicity (MTT Assay) | [28] |
| Stevioside and ethanolic extract | Increased cytotoxicity (MTT Assay) | [46] | |
| Secondary metabolites from leaves (except luteolin) | Increased cytotoxicity (MTT Assay) | [60] | |
| Ammonium derivatives of steviol and isosteviol | Increased cytotoxicity (Multifunctional Cytell Cell Imaging system) | [24] | |
| HL-60 (Leukemia) | Steviol and isosteviol derivatives | Increased cytotoxicity and apoptosis (MTT Assay and Flow cytometry Analysis) | [44] |
| Isosteviol Triazole conjugates | Effect on cell proliferation (CellTiter-Glo® Luminescent Cell Viability Assay) | [28] | |
| MOLT-4 (Leukemia) | Stevioside and isosteviol derivatives | Increased cytotoxicity (MTT Assay) | [45] |
| Isosteviol Triazole conjugates | Effect on cell proliferation (CellTiter-Glo® Luminescent Cell Viability Assay) | [28] | |
| PC-3 (Prostate) | Isosteviol Triazole Conjugates | Increased cytotoxicity (MTT Assay) | [28] |
| Ammonium derivatives of steviol and isosteviol | Increased cytotoxicity (Multifunctional Cytell Cell Imaging system) | [24] | |
| T98G (Glioblastoma) | Ammonium derivatives of steviol and isosteviol | Increased cytotoxicity (Multifunctional Cytell Cell Imaging system) | [24] |
| U2OS (Osteosarcoma) | Steviol | Increased cytotoxicity, G1 arrest apoptosis (MTT Assay and Flow Cytometry Analysis) | [59] |
| Mouse skin papillomas | Stevioside, Rebaudiosides A and C and Dulcoside A | Tumor inhibition (TPA/DMBA-induced carcinogenesis) | [56] |
| Steviol, Stevioside and Isosteviol | Tumor inhibition (TPA/DMBA-induced carcinogenesis) | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iatridis, N.; Kougioumtzi, A.; Vlataki, K.; Papadaki, S.; Magklara, A. Anti-Cancer Properties of Stevia rebaudiana; More than a Sweetener. Molecules 2022, 27, 1362. https://doi.org/10.3390/molecules27041362
Iatridis N, Kougioumtzi A, Vlataki K, Papadaki S, Magklara A. Anti-Cancer Properties of Stevia rebaudiana; More than a Sweetener. Molecules. 2022; 27(4):1362. https://doi.org/10.3390/molecules27041362
Chicago/Turabian StyleIatridis, Nikos, Anastasia Kougioumtzi, Katerina Vlataki, Styliani Papadaki, and Angeliki Magklara. 2022. "Anti-Cancer Properties of Stevia rebaudiana; More than a Sweetener" Molecules 27, no. 4: 1362. https://doi.org/10.3390/molecules27041362








