Mechanisms of Rebaudioside A Degradation and Ingredient-Sweetener Interactions in Beverages during Storage
Abstract
:1. Introduction
2. Results and Discussion
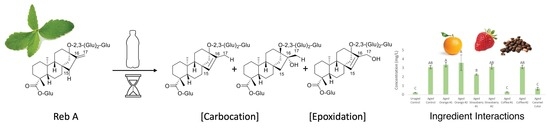
2.1. Mechanism of Reb A Degradation and Molecular Energy Calculations
2.2. Beverage Ingredient–Rebaudioside A Interactions
3. Materials and Methods
3.1. Preparation of Deuterium-Enriched Rebaudioside A Degradation Products
3.2. Solid-Phase Extraction of Rebaudioside A Degradation Products
3.3. First-Dimension Preparative Liquid Chromatography
3.4. Second-Dimension Isolation of Deuterium-labeled Compounds 1–3
3.5. Nuclear Magnetic Resonance Spectroscopy
3.6. Rebaudioside A Beverage Systems
3.7. Quantification of Rebaudioside A Degradation Products
3.8. Molecular Energy Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Grand View Research. Stevia Market Size Worth $553.7 Million by 2024; Grand View Research: San Francisco, CA, USA, 2018. [Google Scholar]
- Mintel. Global Food and Drink Trends for 2016; Mintel: London, UK, 2016. [Google Scholar]
- Mintel. The Natural Household Consumer US, June 2019; Mintel: London, UK, 2019. [Google Scholar]
- World Health Organization. Guideline: Sugars Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2015; pp. 1–49. [Google Scholar] [CrossRef]
- Johnson, R.K.; Appel, L.J.; Brands, M.; Howard, B.V.; Lefevre, M.; Lustig, R.H.; Sacks, F.; Steffen, L.M.; Wylie-Rosett, J. Dietary Sugars Intake and Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation 2009, 120, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Kinghorn, D.A. Stevia: The Genus Stevia; CRC Press: London, UK, 2002. [Google Scholar]
- Clos, J.F.; DuBois, G.E.; Prakash, I. Photostability of Rebaudioside A and Stevioside in Beverages. J. Agric. Food Chem. 2008, 56, 8507–8513. [Google Scholar] [CrossRef] [PubMed]
- Prakash, I.; Clos, J.F.; Chaturvedula, V.S.P. Stability of Rebaudioside A Under Acidic Conditions and its Degradation Products. Food Res. Int. 2012, 48, 65–75. [Google Scholar] [CrossRef]
- Chang, S.S.; Cook, J.M. Stability Studies of Stevioside and Rebaudioside A in Carbonated Beverages. J. Agric. Food Chem. 1983, 31, 409–412. [Google Scholar] [CrossRef]
- Gelinas, B.S.; Liu, Y.; Tello, E.; Peterson, D.G. Identification of Rebaudioside A Degradation Products that Impact Flavor Stability. Food Chem. 2021, in press.
- Carey, F.A.; Sundberg, R.J. Concerted Pericyclic Reactions. In Advanced Organic Chemistry; Springer: Berlin/Heidelberg, Germany, 2007; pp. 833–964. [Google Scholar]
- JECFA Caramel Colour, Class IV. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=1668 (accessed on 6 August 2020).
- Lauro, G.J.; Francis, J. Natural Food Colorants: Science and Technology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Sengar, G.; Sharma, H.K. Food Caramels: A Review. J. Food Sci. Technol. 2014, 51, 1686–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodge, J.E. Chemistry of Browning Reactions in Model Systems. J. Agric. Food Chem 1953, 1, 928–943. [Google Scholar] [CrossRef]
- Mayr, H.; Patz, M.; Gotta, M.F.; Ofial, A.R. Reactivities and Selectivities of Free and Metal-Coordinated Carbocations. Pure Appl. Chem. 1998, 70, 1993–2000. [Google Scholar] [CrossRef] [Green Version]
- Manzocco, L.; Calligaris, S.; Mastrocola, D.; Nicoli, M.C.; Lerici, C.R. Review of Non-Enzymatic Browning and Antioxidant Capacity in Processed Foods. Trends Food Sci. Technol. 2000, 11, 340–346. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant Properties of Phenolic Compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Rufián-Henares, J.A.; Morales, F.J. Functional Properties of Melanoidins: In Vitro Antioxidant, Antimicrobial And Antihypertensive Activities. Food Res. Int. 2007, 40, 995–1002. [Google Scholar] [CrossRef]




| Carbon Position | Compound 1d | Compound 2d | ||
|---|---|---|---|---|
| δC, mult | δH, (J in Hz) | δC, mult | δH, (J in Hz) | |
| 17 | 12.1, CH2D, t | 1.56, s, 2H | 20.7, CH2D, t | 1.31, s, 2H |
| 18 | 28.1, CH3 | 1.07, s, 3Hz | 27.7, CH3 | 1.25, s, 3H |
| 20 | 0.81, s, 3H | 14.9, CH3 | 14.9, CH3 | 0.91, s, 3H |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gelinas, B.S.; Tello, E.; Peterson, D.G. Mechanisms of Rebaudioside A Degradation and Ingredient-Sweetener Interactions in Beverages during Storage. Molecules 2022, 27, 1385. https://doi.org/10.3390/molecules27041385
Gelinas BS, Tello E, Peterson DG. Mechanisms of Rebaudioside A Degradation and Ingredient-Sweetener Interactions in Beverages during Storage. Molecules. 2022; 27(4):1385. https://doi.org/10.3390/molecules27041385
Chicago/Turabian StyleGelinas, Benjamin S., Edisson Tello, and Devin G. Peterson. 2022. "Mechanisms of Rebaudioside A Degradation and Ingredient-Sweetener Interactions in Beverages during Storage" Molecules 27, no. 4: 1385. https://doi.org/10.3390/molecules27041385
APA StyleGelinas, B. S., Tello, E., & Peterson, D. G. (2022). Mechanisms of Rebaudioside A Degradation and Ingredient-Sweetener Interactions in Beverages during Storage. Molecules, 27(4), 1385. https://doi.org/10.3390/molecules27041385