Promoting Effect of Soluble Polysaccharides Extracted from Ulva spp. on Zea mays L. Growth
Abstract
:1. Introduction
2. Material and Methods
2.1. Algae
2.2. Extraction of Water-Soluble Polysaccharides
2.3. Physical Characterizations
Thermal Characteristics of the Extracted Polysaccharides
2.4. Chemical Characterizations
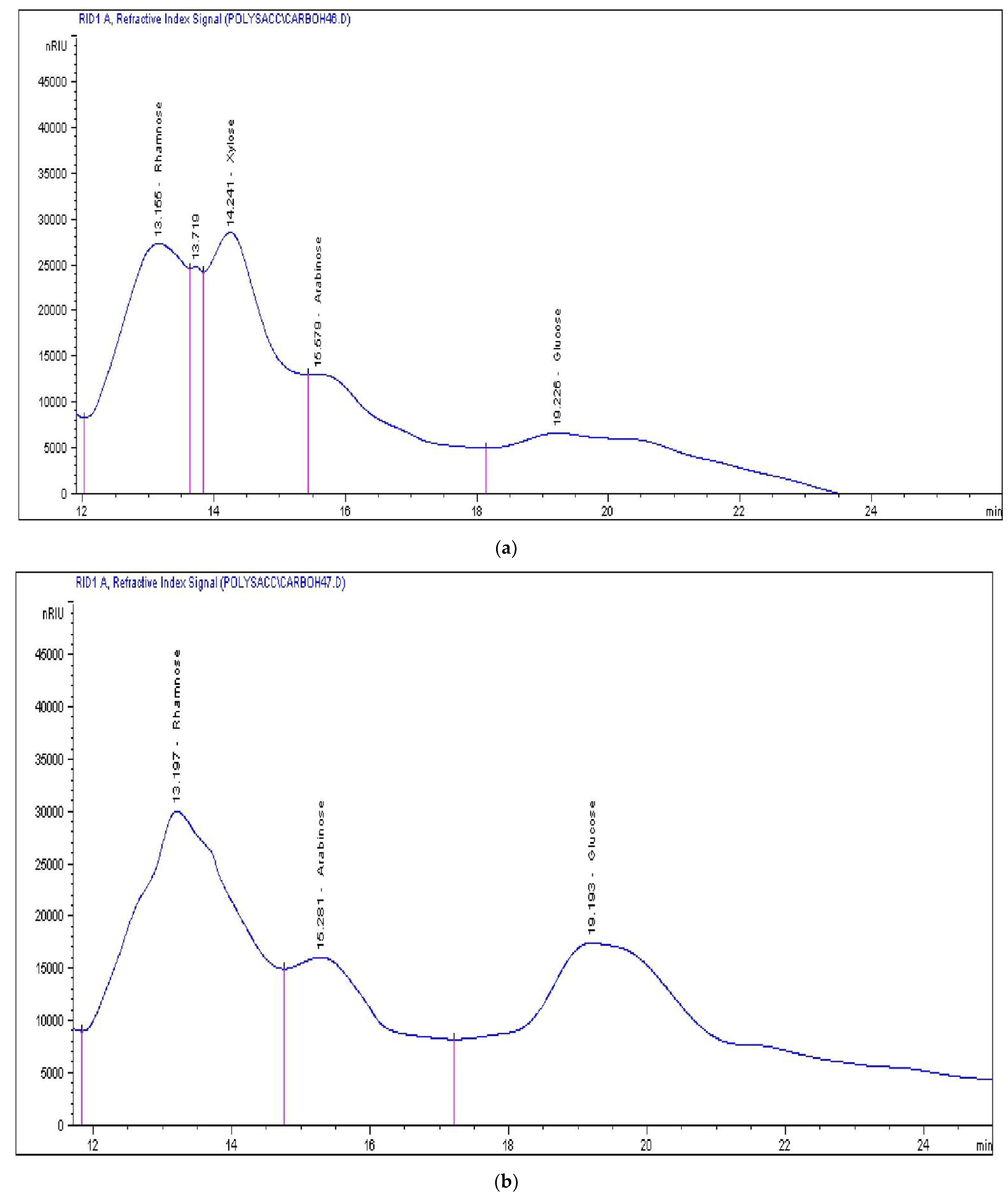
High-Performance Liquid Chromatography (HPLC)-Refractive Index Detector
2.5. Biological Activity
2.5.1. Plant Growth Analysis
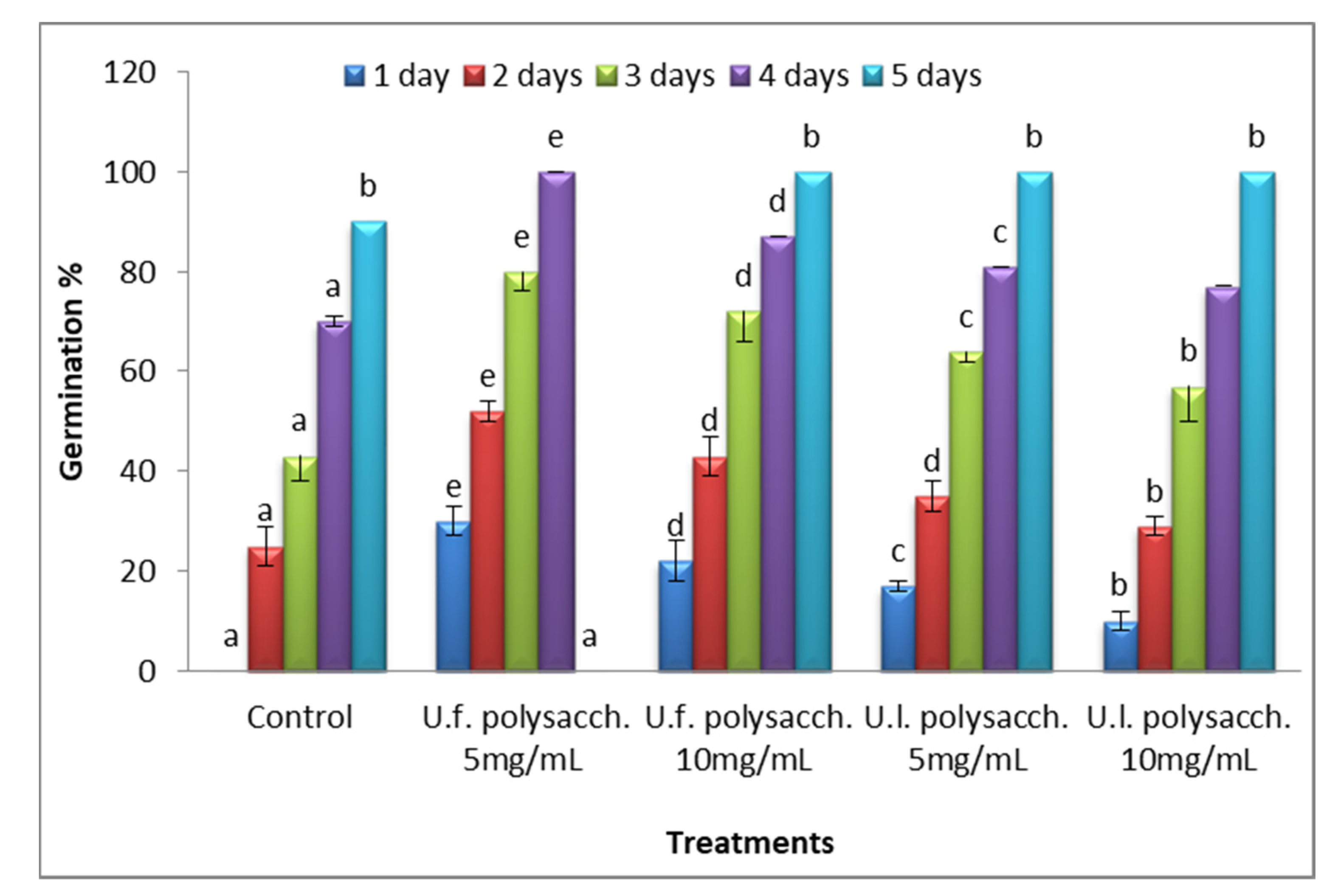
2.5.2. Determination of Germination
2.6. Phytochemical Contents
2.6.1. Total Soluble Proteins Banding Pattern for the Tested Crop Plant
2.6.2. Antioxidants
Total Ascorbic Acid Content
Catalase Activity
Peroxidase
Amylase Assay
Protease Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physical Characterization of the Extracted Polysaccharides
Thermal Characteristics of the Extracted Polysaccharides
3.2. Chemical Characterization of the Extracted Polysaccharide
3.3. Effect of Ulva’s Soluble Polysaccharide (Ulvan) on the Seed Germination and Seedling Growth of Zea Mays
3.3.1. Germination Stage
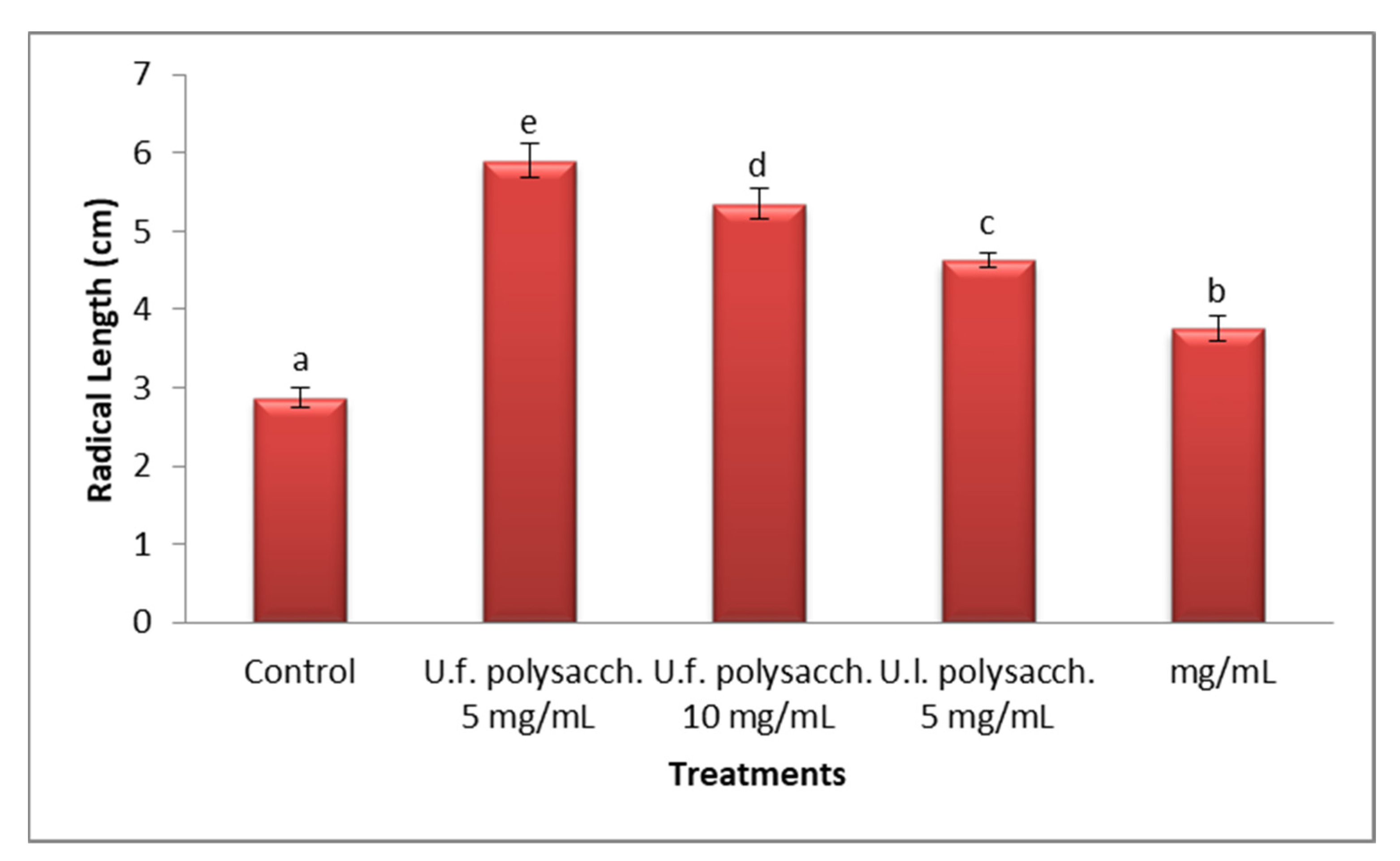
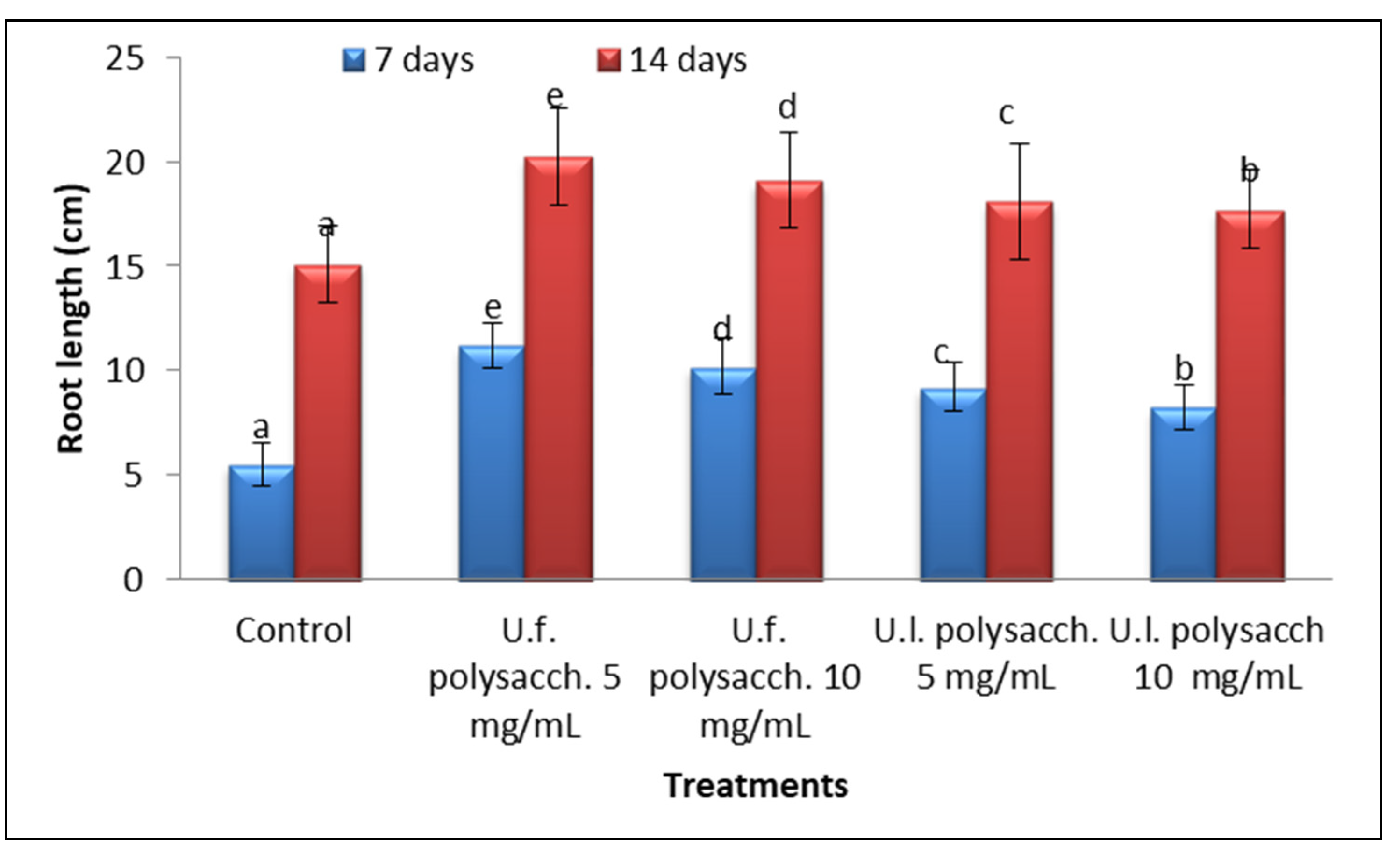
3.3.2. Radical Length
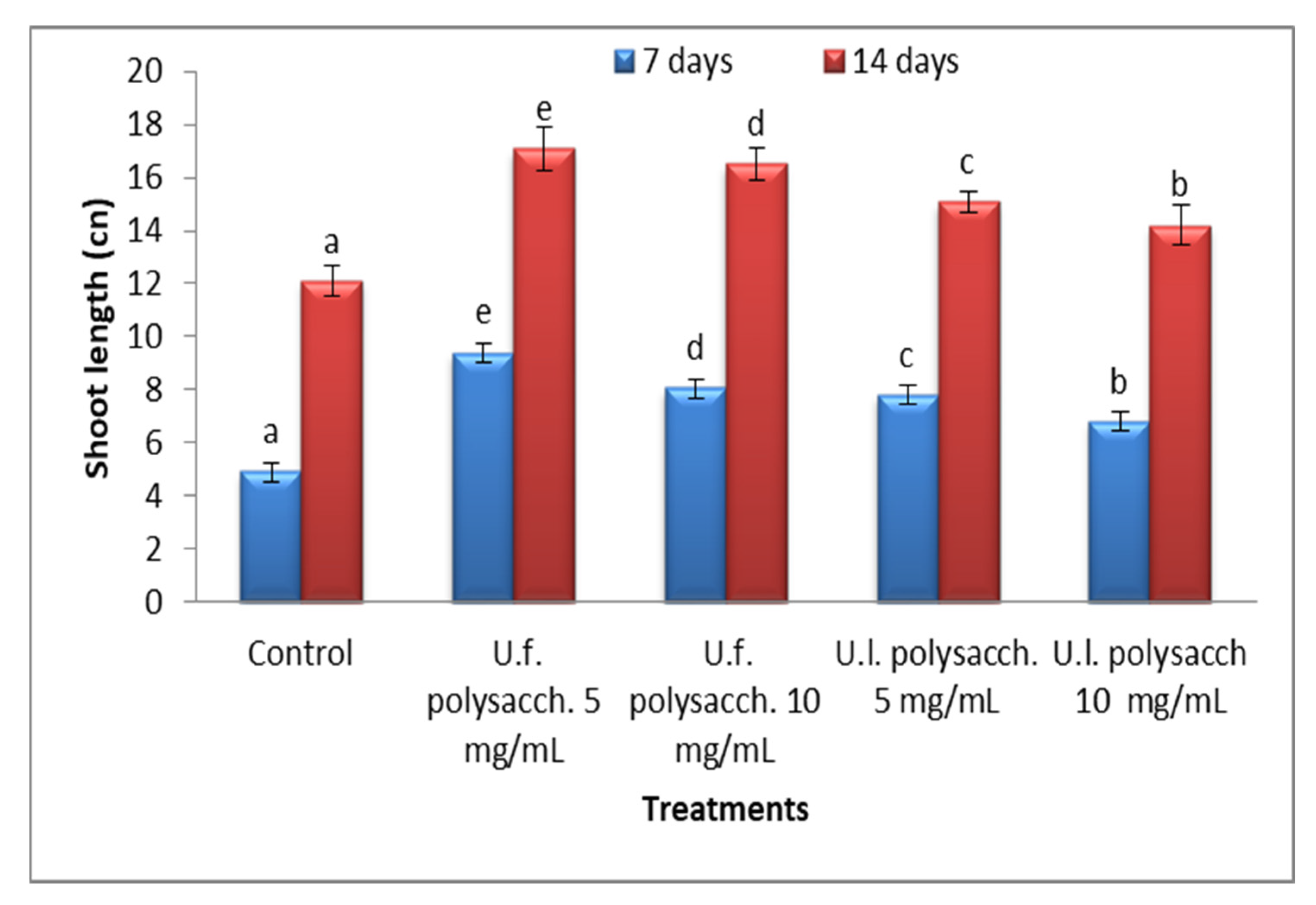
3.3.3. Morphological Criteria
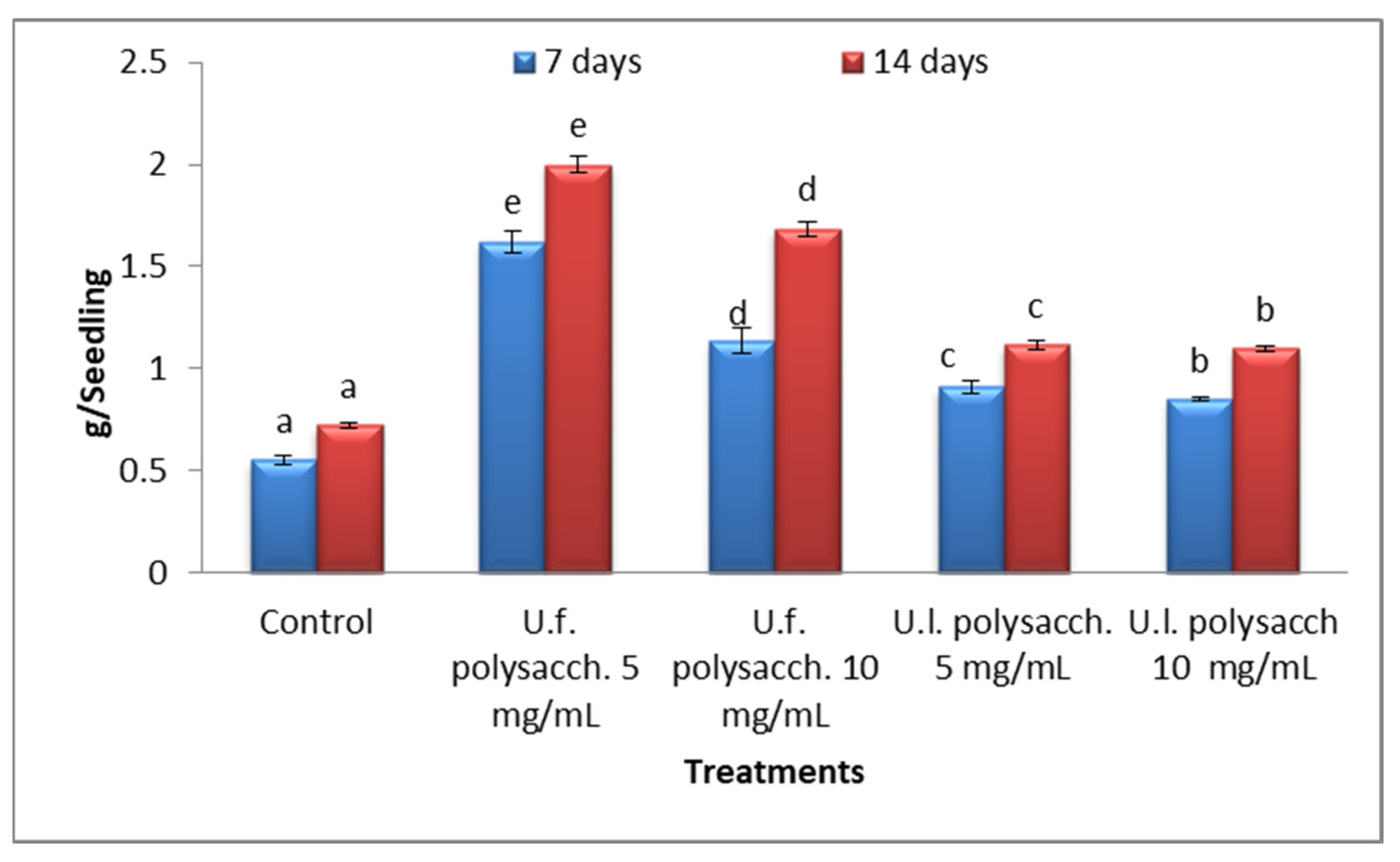
3.3.4. Fresh Weight
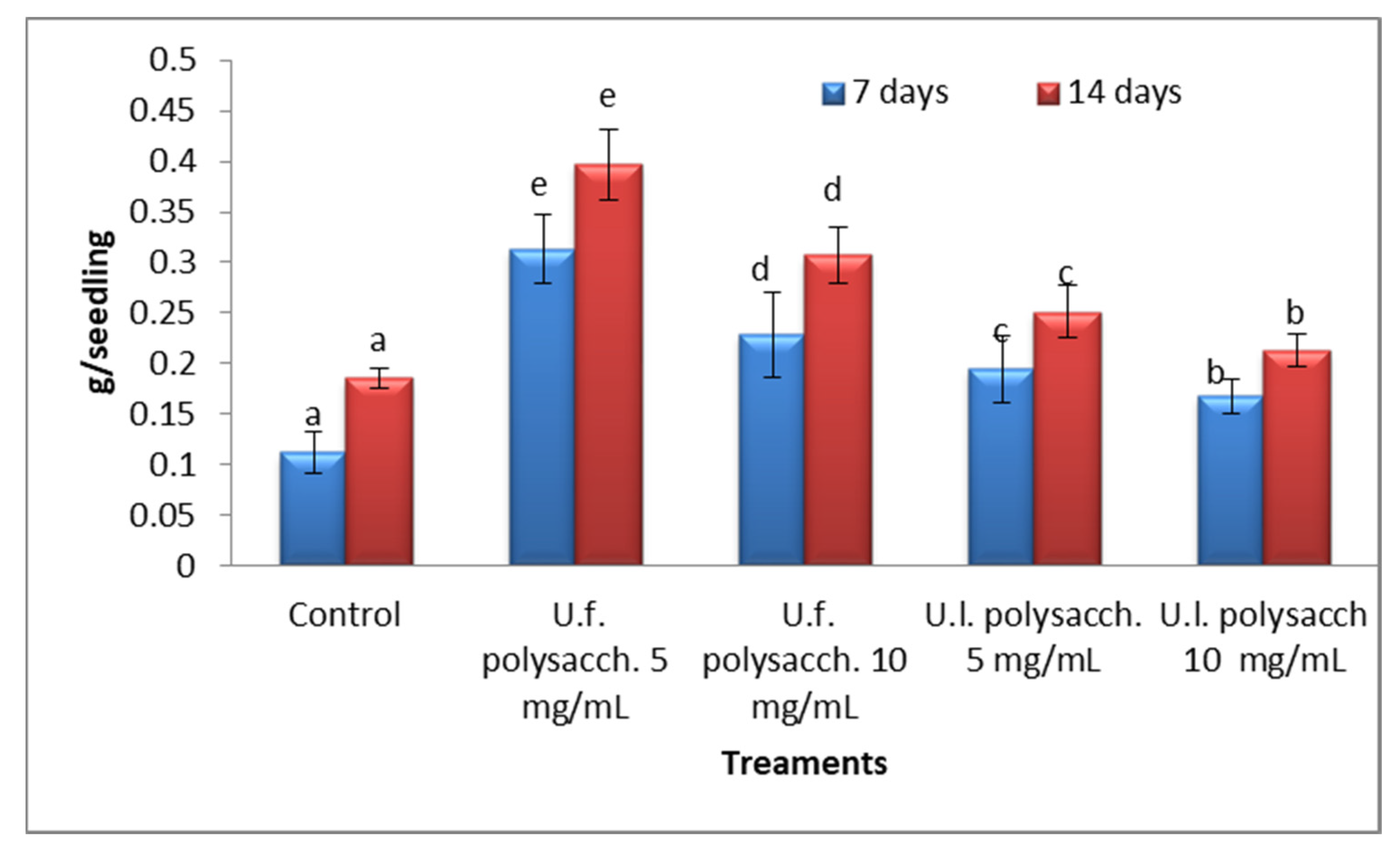
3.3.5. Dry Weight
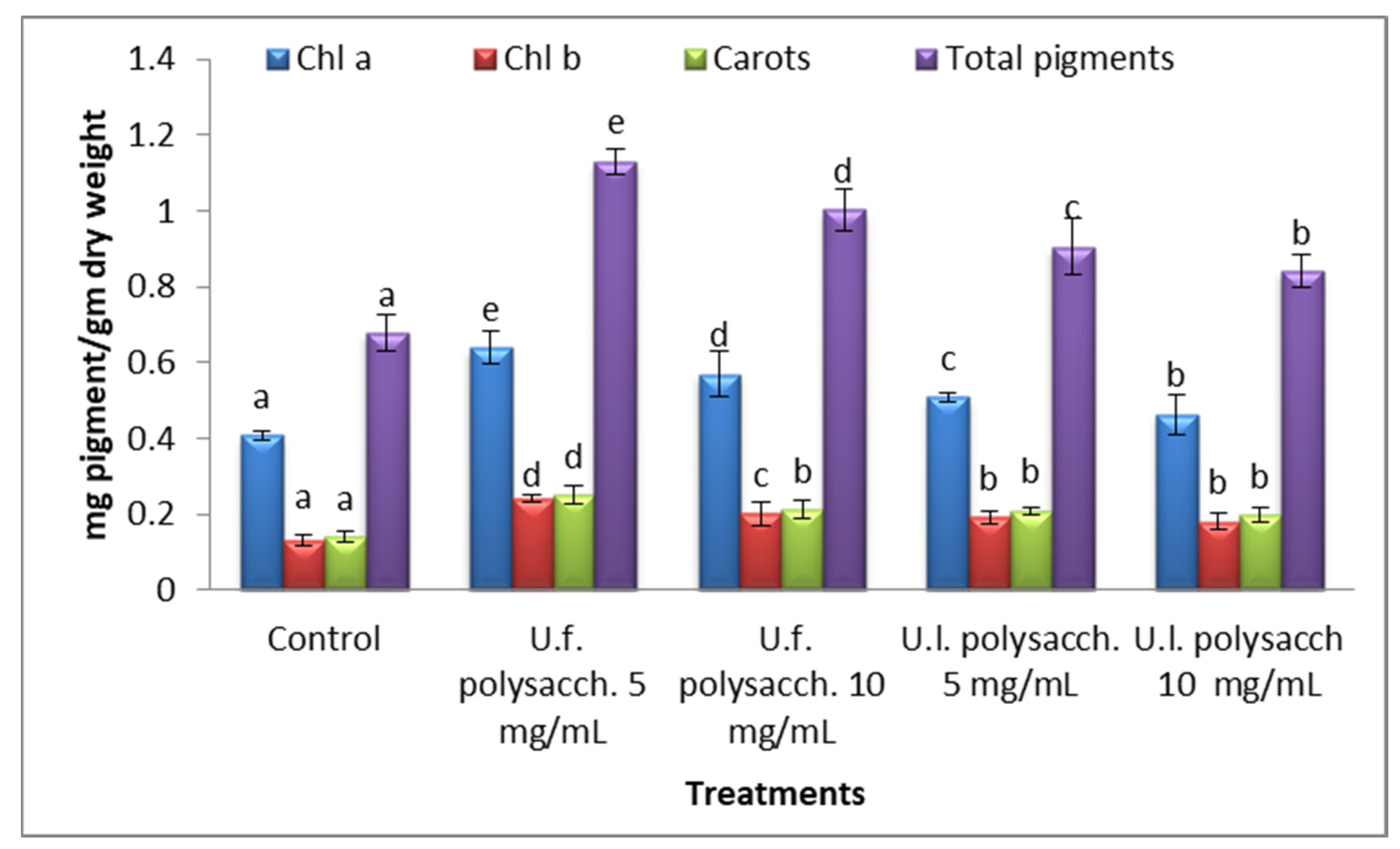
3.3.6. Changes in the Photosynthetic Pigments
3.3.7. Carbohydrate Contents
3.3.8. Changes in Enzymatic Activities
3.3.9. Protein Content
3.3.10. Protein Banding Pattern
Pattern of Total Soluble Proteins in Zea mays Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gibbon, D.; Pain, A. Crops of Drier Regions of the Tropics; Longman Publishing Limited: Singapore, 1985. [Google Scholar]
- Subramaniyan, V.; Malliga., P. Effect of Cyanopith Biofertilizer as Basal and Spray on Zea mays (Corn). Int. J. Environ. Sci. 2011, 2, 661–670. [Google Scholar]
- Schwietzke, S.; Kim, Y.; Ximenes, E.; Mosier, N.; Ladisch, M. Ethanol production from maize. In Molecular Genetic Approaches to Maize Improvement; Springer: Berlin/Heidelberg, Germany, 2009; pp. 347–364. [Google Scholar]
- Mishra, K.k.; Vikram, P.; Yadaw, R.B.; Swamy, M.B.P.; Dixit, S.; Cruz, M.T.S.; Maturan, P.; Marker, S.; Kumar, A. A locus with a consistent effect on grain yield under drought in rice. BMC Genet. 2013, 14, 12. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.A.S. Cyanobacterial Application for the Improvement of Soil Fertility. Master Thesis, Botany Deptartment, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt, 2009. [Google Scholar]
- Chiellini, E.; Cinelli, P.; Ilieva, V.; Martera, M. Biodegradable Termoplastic Composites Based on Polyvinyl Alcohol and Algae. Biomacromolecules 2008, 9, 1007–1013. [Google Scholar] [CrossRef]
- Chiellini, E.; Cinelli, P.; Ivanova, I.V.; Zimbardi, F.; Kanellopoulos, N.; de Wilde, B.; Pipino, A.; Anders, B. Hybrid Composites Based on Fibres of Marine Origin. Int. J. Mater. Prod. Technol. 2009, 36, 47–61. [Google Scholar] [CrossRef]
- Pauline, S.; Claire, J.; Elie, D.; Arsène, I. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar]
- Taboada, S.; Garcia-Fernandez, L.F.; Bueno, S.; Vazquez, J.; Cuevas, C.; Avila., C. Antitumoral activity in Antarctic and Sub-Antarctic benthic organisms. Antarct. Sci. 2010, 22, 449–507. [Google Scholar] [CrossRef]
- Divya, K.; Roja, M.N.; Padal, S.B. Influence of seaweed liquid fertilizer of Ulva lactuca on the seed germination, growth, productivity of Abelmoschus esculentus (L). Int J Pharm. Res 2015, 5, 344–346. [Google Scholar]
- Türkmen, M.; Su, A. The effect of sea lettuce (Ulva lactuca) liquid fertilizer and zeolite combinations on the development of cucumber (Cucumis sativus). Turk. J. Agric. Food Sci. Technol. 2019, 7, 1021–1027. [Google Scholar] [CrossRef] [Green Version]
- Safinaz, A.F.; Ragaa, A.H. Effect of some red marine algae as biofertilizers on growth of maize (Zea mays L.) plants. Int. Food Res. J. 2013, 20, 1629–1632. [Google Scholar]
- Dhargalkar, V.K.; Untawale, A.G. Some observations of the effect of SLF on higher plants. Indian J. Mar. Sci. 1983, 12, 210–214. [Google Scholar]
- Wajahatullah, K.U.; Rayirath, P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; MarkHodges, D.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed Extracts as Biostimulant of Plant Growth and Development. J. Plant. Growth. Regul. 2009, 28, 386–399. [Google Scholar]
- Norrie, J.; Keathley, J.P. Benefit of Ascophyllum nodosum marine-plant extract ‘Thompson seedless’ grape production. (Proceeding softhe Xth International Symposiumon Plant Bioregulators in Fruit Production. Acta Hortic. 2006, 727, 243–247. [Google Scholar] [CrossRef]
- Kloareg, B.; Quatrano, R.S. Structure of the cell walls of marine algae and ecophysiological functions of the matrixpolysaccharides. Oceanogr. Mar. Biol. Annu. Rev. 1988, 26, 259–315. [Google Scholar]
- Dixon, G.R.; Walsh, U.F. Suppressing Pythium ultimum induced damping-off in cabbage seedlings by biostimulation with proprietary liquid seaweed extracts managing soil-borne pathogens: A sound rhizosphere to improve productivity in intensive horticultural systems. Proceedings of the XXVIth Int. Hortic. Congr. Tor. Can. 2002, 635, 11–17. [Google Scholar]
- Ferreira, L.G.; Noseda, M.D.; Gonçalves, A.G.; Ducatti, D.R.B.; Fujii, M.T.; Duarte, M.E.R. Chemical structure of the complex pyruvylated and sulfated agaran from the red seaweed Palisada flagellifera (Ceramiales, Rhodophyta). Carbohydr. Res. 2012, 347, 83–94. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Jeon, Y.J. Enzyme-assistant extraction (EAE) of bioactive components: A useful approach for recovery of industrially important metabolites from seaweeds: A review. Fi-Toterapia 2012, 83, 6–12. [Google Scholar] [CrossRef]
- Vidanarachchi, J.K.; Iji, P.A.; Mikkelsen, L.L.; Sims, I.; Choct, M. Isolation and characterization of water-soluble prebiotic compounds from Australian and New Zealand plants. Carbohydr. Polym. 2009, 77, 670–676. [Google Scholar] [CrossRef]
- Ganapathy, S.G.; Sivakumar, K. Effect of foliar spray from seaweed liquid fertilizer of Ulva reticulata (Forsk) on Vigna mungo L. and their elemental composition using SEM- energy dispersive spectroscopic analysis. Asian Pac. J. Reprod. 2013, 2, 119–125. [Google Scholar]
- Bourgougnon, N.; Lahaye, M.; Chermann, J.; Kornprobst, J. Composition and antiviral activities of sulfated polysaccharide from Schizymenia dubyi (rodophyta, gigartinales). Bioorg. Med. Chem. 1993, 316, 1141–1146. [Google Scholar] [CrossRef]
- Younesikelaki, F.S.; Ebrahimzadeh, M.H.; Desfardi, M.K.; Banala, M.; Marka, R.; Nanna, R.S. Optimization of seed surface sterilization method and in vitro seed germination in Althaea officinalis (L.)-an important medicinal herb. Indian J. Sci. Technol. 2016, 9, 1–6. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, A.; Randell, R. Protein measurement with Folin-Phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am. J. Enol. Viticul. 1965, 16, 144–158. [Google Scholar]
- Stegman, B.W.; Shah, A.A.; Framchsen, H.; Elen, K. Gel Electrophoresis and Isoelectric Focusing. Pantaphor Manual; Revised ed.; Institute fur Biochemie, Biologische Bundesanstlt: Braunschweig, Germany, 1988; Chapter 3. [Google Scholar]
- Hemeida, A. Cytological and Biochemical Genetic Studies in Fishes. Ph.D. Thesis, Faculty of Agriculture Alexandria University, Alexandria, Egypt, 1994. [Google Scholar]
- Chen, G.-X.; Asada, K. Ascorbate peroxidase in tea leaves: Occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol. 1989, 30, 987–998. [Google Scholar]
- Kang, H.M.; Saltveit, M.E. Activity of enzymatic antioxidant defense systems in chilled and heat shocked cucumber seedling radicles. Physiol. Plant. 2001, 113, 548–556. [Google Scholar] [CrossRef]
- Racusen, D.; Foote, M. Protein synthesis in dark grown bean leaves. Can. J. Bot. 1965, 43, 817–824. [Google Scholar] [CrossRef]
- Monroe, J.; Preiss, J. Purification of amylase that accumulates in Arabidopsis thaliana mutants defective in starch metabolism. Plant Physiol. 1990, 94, 1033–1039. [Google Scholar] [CrossRef] [Green Version]
- Anson, M. Estimation of pepsin, trypsin, papain and cathepsin with hemoglobin. J. Gen. Physiol. 1938, 22, 79–89. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 3rd ed.; W.H. Freeman and Company: New York, NY, USA, 1995; p. 937. [Google Scholar]
- Annette, B.; Jonas, D.; Henrik, B.; Lars, N.; Michael, B.; Stiig, M.; Birgit, O.; Carlos, A.; Peter, D. Bioenergy potential of Ulva lactuca: Biomass yield, methane production and combustion. Bioresour. Technol. 2011, 102, 2595–2604. [Google Scholar]
- Lahaye, M.; Robic, A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef]
- Robic, A.; Rondeau-Mouro, C.; Sassi, J.; Lerat, Y.; Lahaye, M. Structure and interactions of ulvan in the cell wall of the marine green algae Ulva rotundata (Ulvales, Chlorophyceae). Carbohydr. Polym. 2009, 77, 206–216. [Google Scholar] [CrossRef]
- Ortiz, J.; Romero, N.; Robert, P.; Araya, J.; Lopez Hernandez, J.; Bozzo, C. Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chem. 2006, 99, 98–104. [Google Scholar] [CrossRef]
- Norziah, M.; Ching, Y. Nutritional composition of edible seaweed Gracilaria changgi. Food Chem. 2000, 68, 69–76. [Google Scholar] [CrossRef]
- Ruperez, P.; Ahrazem, O.; Leal, J. Potential antioxidant capacity of sulfated polysaccharides from the edibic marine brown seeweed Fucus vesiculosus. J. Agric. Food Chem. 2002, 50, 840–845. [Google Scholar] [CrossRef]
- Anastasakis, K.; Ross, A. Hydrothermal liquefaction of the brown macro-alga Laminaria saccharina: Effect of reaction conditions on product distribution and composition. Bioresour. Technol. 2011, 102, 4876–4883. [Google Scholar] [CrossRef]
- Alves, A.; Caridade, S.; Mano, J.; Sousa, R.; Reis, R. Extraction and physico-chemical characterization of a versatile biodegradable polysaccharide obtained from green algae. J. Carbohydr. Res. 2010, 345, 2194–2200. [Google Scholar] [CrossRef]
- Lahaye, M.; Cimadevilla, E.; Kuhlenkamp, R.; Quemener, B.; Lognoné, V.; Dion, P. Chemical composition and 13C NMR spectroscopic characterization of ulvans from Ulva (Ulvales, Chlorophyta). J. Appl. Phycol. 1999, 11, 1–7. [Google Scholar] [CrossRef]
- Crouch, I.; Staden, J. Effects of seaweed concentrate from Ecklonia maxima (Osbeck) paenfess on Melodogyre incognita infestation on tomato. J. Appl. Phycol. 1993, 5, 37–43. [Google Scholar] [CrossRef]
- Foley, E.; Chao, S.; Horvath, P.; Dogramaci, M.; Anderson, V. The transcriptomes of dormant leafy spurge seed under alternating temperature are differently affected by germination –enhancing pretreatment. J. Plant Physiol. 2012, 170, 539–547. [Google Scholar] [CrossRef]
- Booth, E. The manufacture and properties of liquid seaweed extracts. Proc. Int. Seaweed Symp. 1969, 6, 655–662. [Google Scholar]
- Bukhari, S.S.; Unttawale, A.G. Seaweeds as liquid fertilizer and foliar spray. Seaweed Res. Utiln 1978, 3, 71–78. [Google Scholar]
- Paulert, R.; Talamini, V.; Cassolato, J.; Duarte, M.; Noseda, M.; Smania, A.; Stadnik, M. Effects of sulfated polysaccharide and alcoholic extracts from green seaweed Ulva fasciata on anthracnose severity and growth of common bean (Phaseolus vulgaris L.). J. Plant Dis. Prot. 2009, 116, 263–270. [Google Scholar] [CrossRef]
- Ramarajan, S.; Raya, S.; Gandhi, A. Effect of seaweed extracts mediated changes on the germination and pigment concentration of cluster bean (var. Pusa naubahar). J. Agric. Sci. Technol. 2012, 1, 22–25. [Google Scholar]
- Demir, N.; Dural, B.; Yildirim, K. Effect of seaweed suspensions on seed germination of Tomoto, Pepper and Aubergine. J. Biol. Sci. 2006, 6, 1130–1133. [Google Scholar]
- Erulan, V.; Soundarapandian, P.; Thirumaran, G.; Ananthan, G. Studies on the effect of Sargassum polycystum (C.Agardh, 1824) Extract on the growth and biochemical composition of Cajanus cajan (L.) Mill sp. American-Eurasian. J. Agric. Environ. Sci. 2009, 6, 392–399. [Google Scholar]
- Sabale, A.; Pise, N. Effect of seaweed extracts (SWE) on germination of Trigonella foenum-graecum seeds. Bioinfolet 2010, 7, 131–132. [Google Scholar]
- Sridhar, S.; Rengasamy, R. The effect of seaweed liquid fertilizer of Ulva lactuca on Capsicum annum. Algol. Stud. 2012, 138, 75–88. [Google Scholar] [CrossRef]
- Aitkin, J.; Senn, J. Seaweed products as fertilizers and soil conditioners. Bot. Mar. 1965, 8, 144–148. [Google Scholar]
- Arumugam, R.; Anantharaman, P. Effect of seaweed liquid fertilizer on growth and pigment concentration of Abelmoschus esculentus (l) medikus. J. Agron. 2009, 2, 57–66. [Google Scholar]
- Cluz, S.; Torregrossa, C.; Jacquet, C.; Lafitte, J.; Fournier, J.; Mercier, L.; Salamagne, S.; Briand, X.; Esquerre-Tuggaye, M.; Dumas, B. Gene expression profiling and protection of Medicago truncatula against a fungal infection in response to an elicitor from green algae Ulva spp. Plant Cell Environ. 2004, 27, 917–928. [Google Scholar] [CrossRef]
- Kavipriy, R.; Dhanalakshmi, P.; Jayashree, N.; Thangaraju, N. Seaweed extract as a biostimulant for legume crop, green gram. J. Ecobiotechnol. 2011, 3, 16–19. [Google Scholar]
- El-Ansary, M.S.M.; Hamouda, R.A. Biocontrol of root-knot nematode infected banana plants by some marine algae. Russ. J. Mar. Biol. 2014, 40, 140–146. [Google Scholar] [CrossRef]
- Vijayanand, V.; Rathinavel, S. Bio-fertilizing efficiency of seaweed liquid extract of Hydroclathrus clathratus on Sorghum vulgare. Seaweed Res. Util. Assoc. 2004, 26, 181–186. [Google Scholar]
- Stephenson, W. Seaweeds in Agriculture and Horticulture. In Reteaver, Peruma Valley, 3rd ed.; Faber & Faber: London, UK, 1974; p. 241. [Google Scholar]
- Mohan, V.R.; Venkataraman Kumar, V.; Murugeswari, R.; Muthuswami, S. Effect of crude and commercial seaweed extracts on seed germination and seedling growth in Cajanus cajan L. Phykos 1994, 33, 47. [Google Scholar]
- Dhargalkar, V.; Untawale, A. Some observations of effect of seaweed liquid fertilizer on the higher plants. In Proceedings of the National Workshop on Algal Systems; Indian Society of Biotechnology: Uttar Pradesh, New Delhi, 1980; p. 65. [Google Scholar]
- Selvam, G.; Balamurugan, M.; Thinakaran, T.; Sivakumar, K. Developmental changes in the germination, growth and chlorophyllase activity of Vigna mungo L. using seaweed extract of Ulva reticulata forsskal. J. Pharm. Int. Res. 2013, 4, 252–254. [Google Scholar]
- Blunden, G.; Wildgoose, P. The effect of aqueous seaweed extract and kinetin on potato yields. J. Sci. Food Agric. 1977, 28, 121–125. [Google Scholar] [CrossRef]
- Stirk, W.; Arthur, G.; Lourens, A.; Novak, O.; Strnad, M.; Vanstaden, J. Changes in cytokinin and auxin concentrations in seaweed concentrates when stored at an elevated temperature. J. Appl. Phycol. 2004, 16, 31–39. [Google Scholar] [CrossRef]
- Christobel, G. Effect of seaweed (Sargassum wightii L.) on the germination of green gram (Phaseolus aureus L.). J. Basic Appl. Biol. 2008, 2, 105–109. [Google Scholar]
- Thirumaran, G.; Arumugam, M.; Arumugam, R.; Anantharaman, P. Effect of seaweed liquid fertilizer on growth and pigment concentration of Cyamopsis tetrogonolaba (L.) taub. Am. Eurasian J. Agron. 2009, 2, 50–56. [Google Scholar]
- Francisca, P.; Kalavathy, S. A comparative study on the efficiency of two seaweed extracts as biostimulants on Zea mays L. J. Basic Appl. Biol. 2011, 5, 252–257. [Google Scholar]
- Bograh, A.; Gingras, Y.; Tajmir, R.; Carpentier, R. The effects of spermine and spermidine on the structure of photosystem II proteins in relation to inhibition of electron transport. FEBS Lett. 1997, 402, 41–44. [Google Scholar] [CrossRef] [Green Version]
- Younis, M.; El-Shahaby, A.; Abo-Hamed, A.; Haroun, S. Plant growth, metabolism and adaptation in relation to stress conditions. XI. Modification of osmotic-stress-induced metabolic effects by GA3 or IAA in Pisum sativum plants. Acta Agron. Hung. 1991, 40, 367–375. [Google Scholar]
- Zheleva, D.; Tsonev, T.; Sergiev, I.; Karanov, E. Protective effect of exogenous polyamines against atrazine in pea plants. J. Plant Growth Regul. 1994, 13, 203–221. [Google Scholar] [CrossRef]
- Patel, R.V.; Pandya, K.Y.; Jasrai, R.T.; Brahmbhatt, N. A review: Scope of utilizing seaweed as a biofertilizer in agriculture. Int. J. Adv. Res. 2017, 5, 2046–2054. [Google Scholar] [CrossRef] [Green Version]
- El-Ansary, M.S.M.; Hamouda, R.A.; Ahmed-Farid, O.A. Bioremediation of Oxamyl Compounds by Algae: Description and Traits of Root-Knot Nematode Control. Waste Biomass Valorization. 2021, 12, 251–261. [Google Scholar] [CrossRef]
- El-Sheekh, M.; El-Saied, A. Effect of seaweed extracts on seed germination, seedling growth and some metabolic processes of faba beans (Vicia faba L.). J. Phycol. Soc. 1999, 38, 55–64. [Google Scholar]
- Abdel-Hamid, M.S.; Hamouda, R.A.E.F.; Abd El-Aal, H. Distinctive Application of the Consortium of Chlorella vulgaris and Anabaena oryzae toward different planting dates and climate change on Jerusalem Artichoke Yield. J. Plant. Growth. Regul. 2022, 41, 479–493. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Macias, A. Effects of 6-methoxy-2-benzoxazolinone on the germination and a-amylase activity in lettuce seeds. J. Plant Physiol. 2005, 162, 1304–1307. [Google Scholar] [CrossRef]
- Mikkonen, A. Activities of some peptidases and proteinases in germinating kidney bean, Phaseoulus vulgaris. Physiol. Plant. 1986, 65, 282–286. [Google Scholar] [CrossRef]
- Perata, P.; Guglielminetti, L.; Alpi, A. Mobilization of endosperm reserves in cereal seeds under anoxia. Ann. Botony. 1997, 79, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Karthik, T.; Sarkar, G.; Babu, S.; Amalraj, L.D.; Jayasri, M.A. Preparation and evaluation of liquid fertilizer from Turbinaria ornata and Ulva Reticul. Biocatal. Agric. Biotechnol. 2020, 28, 101712. [Google Scholar] [CrossRef]
- Hassanein, A.; Bassuony, F.; Baraka, D.; Khalil, R. Physiological effects of nicotinamide and ascrobic acid on Zea mays plant grown under salinity stress. I-Changes in growth, some relevant metabolic activities and oxidative defense systems. J. Agric. Biol. Sci. 2009, 5, 72–81. [Google Scholar]
- Bailly, C.; Bogatek-Leszczynska, R.; Come, D.; Corbineau, F. Changes in activities of antioxidant enzymes and lipoxygenase during growth of sunflower seedlings from seeds of different vigour. Seed Sci. Res. 2002, 12, 47–55. [Google Scholar] [CrossRef]
- Dohlert, D.; Stanley, H.; Duke, A.; Anderson, L. Beta-amylases from alfalfa roots. J. Plant. Physiol. 1982, 69, 1096–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaparzadeh, N.; Amico, D.; Khavari- Najad, A.; Izzo, R.; Navarizzo, F. Antioxidative responses of Calendula officinalis under salinity conditions. J. Plant Physiol. Biochem. 2004, 42, 695–701. [Google Scholar] [CrossRef] [PubMed]
- El-Bassiony, H. Physiological responses of wheat to salinity alleviation by nicotinamide and tryptophan. Int. J. Agric. Biol. 2005, 1560, 653–659. [Google Scholar]
- Freitas, M.; Stadnik, M. Race-specific and ulvan-induced defense responses in bean (Phaseolus vulgaris) aginst Colletrichum lindemuthianum. Physiol. Mol. Plant. Pathol. 2012, 78, 8–13. [Google Scholar] [CrossRef]
- Araújo, L.; Stadnik, M.; Borsato, L.; Valdebenito-Sanhueza, R. Potassium phosphite and ulvan in the control of ‘Gala’ leaf spot on apple. Trop. Plant. Pathol. 2008, 33, 148–152. [Google Scholar] [CrossRef] [Green Version]
- Montealegre, J.; López, C.; Stadnik, M.; Henríquez, J.; Herrera, R.; Polanco, R.; Piero, R.; Pérez, L. Control of grey rot of apple fruits by biologically active natural products. Trop. Plant. Pathol. 2010, 35, 271–276. [Google Scholar]
- Jaulneau, V.; Lafitte, C.; Jacquet, C.; Fournier, S.; Salamagne, S.; Briand, X.; Esquerré-Tugayé, M.; Dumas, B. Ulvan, a sulfated polysaccharide from green algae, activates plant immunity through the jasmonic acid signaling pathway. J. Biomed. Biotechnol. 2010, 2010. [Google Scholar] [CrossRef] [Green Version]
- Qi, H.; Zhang, Q.; Zhao, T.; Chen, R.; Zhang, H.; Niu, X.; Li, Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int. J. Biol. Macromol. 2005, 37, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Haley, S.; Perret, J.; Harris, M.; Wilson, J.; Aian, M. Free radical scavenging properties of wheat extracts. J. Agric. Food Chem. 2002, 50, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Nurhanan, A.; Rosli, W.; Mohsin, S. Total polyphenol content and free radical scavenging activity of cornsilk (Zea mays hairs). Sains Malays. 2012, 41, 1217–1221. [Google Scholar]
- Hamouda, R.A.; El-Ansary, M.S.M. Potential of Plant-Parasitic Nematode Control in Banana Plants by Microalgae as a New Approach Towards Resistance. Egypt. J. Biol. Pest Control. 2017, 27, 165–172. [Google Scholar]
- Noctor, G.; Foyer, H. Ascorbate and glutathione: Keeping active oxygen under Control. Annu. Revission Plant Physiol. Plant Mol. Biol. 1998, 42, 249–279. [Google Scholar] [CrossRef]
- Asir, S.; Saravanababu, S. Studies on the effect of seaweed extract in Oryza sativa var. Ambai-16 during senescence. Seaweed Res. Util. Assoc. 2004, 26, 11–175. [Google Scholar]
- Siddhanat, A.; Goswami, A.; Ramavat, B.; Mody, K.; Mairh, O. Water soluble polysaccharides of marine algal species of Ulva (Ulvales, Chlorophyta) of Indian waters. Indian J. Mar. Sci. 2001, 30, 166–172. [Google Scholar]
- Adamczuk, A.; Kercheva, M.; Hristova, M.; Jozefaciuk, G. Impact of Chitosan on Water Stability and Wettability of Soils. Materials 2021, 14, 7724. [Google Scholar] [CrossRef]
- Jones, H.; Davies, W. A Perspective on ABA research in the 1990s. In Abscisic Acid Physiology and Biochemistry; Davies, W., Jones, H., Eds.; Bio Scientific Publishers: Oxford, UK, 1991; pp. 1–4. [Google Scholar]




| Species | Carbohydrates | Protein | Lipids | Ash |
|---|---|---|---|---|
| Ulva lactuca | 56.8 ± 4 | 25.9 ± 2.5 | 0.59 ± 0.02 | 16.3 ± 1.3 |
| Ulva fasciata | 58.5 ± 6 | 27.7 ± 3.3 | 0.70 ± 0.01 | 12.8 ± 2.2 |
| Ulva Lactuca | Ulva Fasciata | ||||||
|---|---|---|---|---|---|---|---|
| TS (°C) | TM (°C) | TE (°C) | Loss (%) | TS (°C) | TM(°C) | TE (°C) | Loss (%) |
| 33.64 | 92.55 | 142.65 | 10.794 | 30.48 | 112.46 | 200.33 | 12.935 |
| 142.65 | 168.64 | 195.60 | 4.975 | 200.97 | 244.71 | 291.39 | 14.053 |
| 195.60 | 221.20 | 269.16 | 13.471 | 291.39 | 328.95 | 368.81 | 10.251 |
| 592.58 | 665.93 | 751.99 | 15.333 | 592.58 | 677.72 | 738.37 | 19.418 |
| Species | Xylose | Arabinose | Rhamnose | Glucose & Galactose |
|---|---|---|---|---|
| Ulva lactuca | 62.3881 | 36.0013 | 90.1297 | 75.9949 |
| Ulva fasciata | - | 33.3524 | 146.9788 | 125.9704 |
| Amylase | Protease | Catalase | Peroxidase | |
|---|---|---|---|---|
| Treatments | ||||
| Control | 100.18 ± 17 a | 60.93 ± 13 a | 18011.11 ± 300 a | 2.067 ± 0.12 a |
| U.f. polysacch. 5mg/mL | 447.34 ± 28 e | 482.81 ± 32 e | 38615.19 ± 57 e | 7.46 ± 0.21 e |
| U.f. polysacch. 10mg/mL | 370.81 ± 25 d | 447.18 ± 36 d | 34623.21 ± 55 d | 5.95 ± 0.19 d |
| U.l. polysacch. 5mg/mL | 308.57 ± 15 c | 315.93 ± 25 c | 29765.43 ± 53 c | 4.02 ± 0.09 c |
| U.l. polysacch. 10mg/mL | 223.26 ± 21 b | 248.43 ± 18 b | 25341.54 ± 51 b | 3.34 ± 0.16 b |
| p value | 0.0011 | 0.0012 | 0.002 | 0.009 |
| Treatment | Control | U. fasciata | U. fasciata | U. lactuca | U.lactuca | p Value |
|---|---|---|---|---|---|---|
| 5mg/mL | 10mg/mL | 5mg/mL | 10mg/mL | |||
| Ascorbic Acid | 0.416 ± 0.022 a | 1.658 ± 0.054 e | 1.275 ± 0.062 d | 0.996 ± 0.033 c | 0.871 ± 0.012 b | 0.0000 |
| Phenolic | 1.443 ± 0.056 a | 4.835 ± 0.13 e | 4.016 ± 0.17 d | 3.38 ± 0.081 c | 2.892 ± 0.09 b | 0.0000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamouda, R.A.; Hussein, M.H.; El-Naggar, N.E.-A.; Karim-Eldeen, M.A.; Alamer, K.H.; Saleh, M.A.; Al Masoudi, L.M.; Sharaf, E.M.; El-Azeem, R.M.A. Promoting Effect of Soluble Polysaccharides Extracted from Ulva spp. on Zea mays L. Growth. Molecules 2022, 27, 1394. https://doi.org/10.3390/molecules27041394
Hamouda RA, Hussein MH, El-Naggar NE-A, Karim-Eldeen MA, Alamer KH, Saleh MA, Al Masoudi LM, Sharaf EM, El-Azeem RMA. Promoting Effect of Soluble Polysaccharides Extracted from Ulva spp. on Zea mays L. Growth. Molecules. 2022; 27(4):1394. https://doi.org/10.3390/molecules27041394
Chicago/Turabian StyleHamouda, Ragaa A., Mervat H. Hussein, Noura El-Ahmady El-Naggar, Mohammed A. Karim-Eldeen, Khalid H. Alamer, Muneera A. Saleh, Luluah M. Al Masoudi, Eman M. Sharaf, and Reham M. Abd El-Azeem. 2022. "Promoting Effect of Soluble Polysaccharides Extracted from Ulva spp. on Zea mays L. Growth" Molecules 27, no. 4: 1394. https://doi.org/10.3390/molecules27041394






