In Vitro Antileishmanial and Antitrypanosomal Activities of Plicataloside Isolated from the Leaf Latex of Aloe rugosifolia Gilbert & Sebsebe (Asphodelaceae)
Abstract
:1. Introduction
2. Results
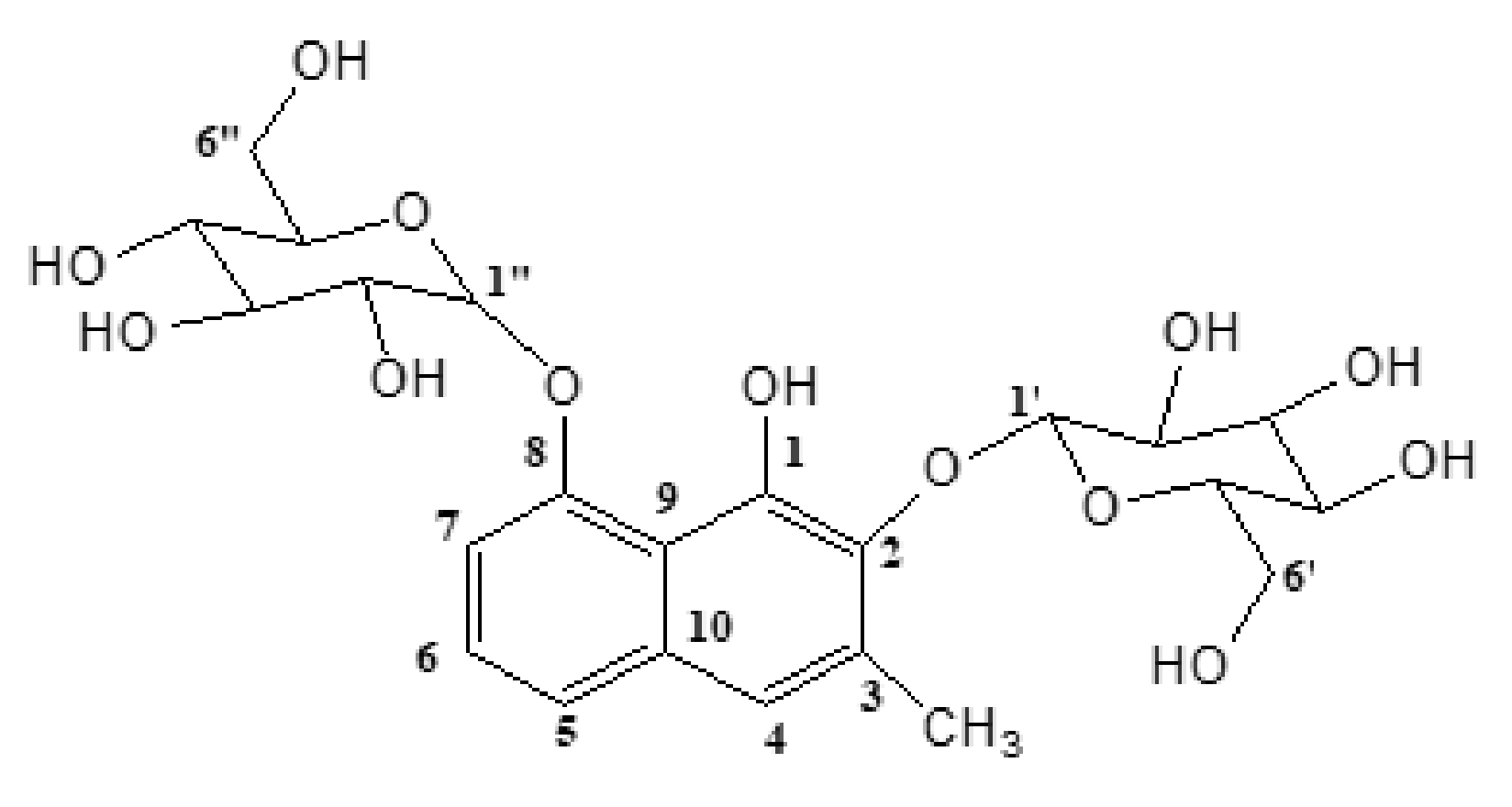
2.1. Structural Elucidation of Plicataloside
2.2. Oral Acute Toxicity
2.3. In Vitro Antitrypanosomal Activity
2.4. In Vivo Infectivity Test
2.5. In Vitro Antipromastigote Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals and Drugs
4.3. Instruments
4.4. Experimental Animals
4.5. Leaf Latex Preparation
4.6. Isolation of Plicataloside
4.7. Spectral Data Plicataloside
4.8. Acute Toxicity Study
4.9. Isolation of Trypanosoma congolense
4.10. Leishmania Test Strains
4.11. In Vitro Antitrypanosomal Activity Tests
4.12. In Vivo Infectivity Test
4.13. In Vitro Antileishmanial Activity Assay
4.14. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mello, J.; Gomes, R.A.; Vital-Fujii, D.G.; Ferreira, G.M.; Trossini, G.H.G. Fragment-based drug discovery as alternative strategy to the drug development for neglected diseases. Chem. Biol. Drug Des. 2017, 90, 1067–1107. [Google Scholar] [CrossRef]
- Ready, P.D. Epidemiology of visceral leishmaniasis. Clin. Epidemiol. 2014, 6, 147–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mckerrow, J.H. Recognition of the role of natural products as drugs to treat neglected tropical diseases by the 2015 Nobel Prize in physiology or medicine. Nat. Prod. Reports 2015, 32, 1610–1611. [Google Scholar] [CrossRef] [PubMed]
- Peacock, L.; Cook, S.; Ferris, V.; Bailey, M.; Gibson, W. The life cycle of Trypanosoma (Nannomonas) congolense in the tsetse fly. Parasit. Vectors 2012, 5, 109. [Google Scholar] [CrossRef] [Green Version]
- Enyanw, O.M.; Igwe, O.H.; Emmanue, E. In vivo antitrypanosomal activity of aqueous extract of Azadirachta indica leaves on Trypanosoma brucei brucei infected mice. Int. J. Infect. Dis. Ther. 2018, 3, 13–17. [Google Scholar]
- Flohé, L. In search of trypanocoidal drugs. In Antiparasitic and Antibacterial Drug Discovery: From Molecular Targets to Drug Candidates; Selzer, P.M., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2009; pp. 227–247. [Google Scholar]
- Cheuka, P.M.; Mayoka, G.; Mutai, P.; Chibale, K. The role of natural products in drug discovery and development against neglected tropical diseases. Molecules 2017, 22, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Junyent, J.; Pinazo, M.J.; Castro, P.; Fernández, S.; Mas, J.; Chaguaceda, C.; Pellicé, M.; Gascón, J.; Muñoz, J. Human African Trypanosomiasis in a Spanish traveler returning from Tanzania. PLoS Negl. Trop. Dis. 2017, 11, e0005324. [Google Scholar] [CrossRef] [Green Version]
- Etet, P.F.S.; Mahomoodally, M.F. New insights in staging and chemotherapy of African trypanosomiasis and possible contribution of medicinal plants. Sci. World J. 2012, 2012, 343652. [Google Scholar]
- World Health Organization. Surveillance of leishmaniasis in the WHO European region, 2016 and global leishmaniasis surveillance updated, 1998-2016. Wkly. Epidemiol. Rec. 2018, 93, 521–540. [Google Scholar]
- Mohapatra, S. Drug resistance in leishmaniasis: Newer developments. Trop. Parasitol. 2014, 4, 4–9. [Google Scholar] [CrossRef] [Green Version]
- Tedla, D.G.; Bariagabr, F.H.; Abreha, H.H. Incidence and trends of leishmaniasis and its risk factors in Humera, Western Tigray. J. Parasitol. Res. 2018, 2018, 8463097. [Google Scholar]
- Lemma, W. Zoonotic leishmaniasis and control in Ethiopia. Asian Pacific J. Trop. Med. 2018, 11, 313–319. [Google Scholar] [CrossRef]
- Menezes, J.P.; Guedes, C.E.; Petersen, A.L.; Fraga, D.B.; Veras, P.S. Advances in development of new treatment for leishmaniasis. BioMed. Res. Int. 2015, 2015, 815023. [Google Scholar] [CrossRef]
- Tiwari, N.; Gedda, M.R.; Tiwari, V.K.; Singh, S.P.; Singh, R.K. Limitations of current therapeutic options, possible drug targets and scope of natural products in control of leishmaniasis. Mini Rev. Med. Chem. 2018, 18, 26–41. [Google Scholar] [CrossRef]
- Babokhov, P.; Sanyaolu, A.O.; Oyibo, W.A.; Fagbenro-Beyioku, A.F.; Iriemenam, N.C. A current analysis of chemotherapy strategies for the treatment of human African trypanosomiasis. Pathog. Glob. Health 2013, 107, 242–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, R.S.; Patil, M.S.; Kshirsagar, S.S.; Chaudhari, P.S.; Bayas, J.P.; Oswal, R.J. Synthetic and natural products against leishmaniasis: A review. World J. Pub. Health Sci. 2012, 1, 7–22. [Google Scholar]
- Gebremedhin, A.T. The types of Aloe species and their multi-function in Southern Ethiopia the cases of Hammer district. Int. J. Environ. Sci. Nat. Resources. 2017, 3, 91–94. [Google Scholar] [CrossRef]
- Dutta, A.; Sarkar, D.; Gurib-Fakim, A.; Mandal, C.; Chatterjee, M. In vitro and in vivo activity of Aloe vera leaf exudate in experimental visceral leishmaniasis. Parasitol. Res. 2008, 102, 1235–1242. [Google Scholar] [CrossRef]
- Clarkson, C.; Maharaj, V.J.; Crouch, N.R.; Grace, O.M.; Pillay, P.; Matsabisa, M.G.; Bhagwandin, N.; Smith, N.; Folb, P.I. In vitro antiplasmodial activity of medicinal plants native to or naturalized in South Africa. J. Ethnopharmacol. 2004, 92, 177–191. [Google Scholar] [CrossRef]
- Zyl, R.V.; Viljoen, A.M. In vitro activity of Aloe extracts against Plasmodium falciparum. South Afr. J. Bot. 2002, 68, 106–110. [Google Scholar]
- Wessels, P.L.; Holzapfel, C.W.; Wyk, B.V.; Marais, W. Plicataloside, an O,O-diglycosylated naphthalene derivative from Aloe plicatilis. Phytochemistry 1996, 41, 1547–1555. [Google Scholar] [CrossRef]
- Paulos, B.; Bisrat, D.; Gedif, T.; Asres, K. Antimalarial and antioxidant activities of the leaf exudate and a naphthalene derivative from Aloe otallensis Baker. Ethiop. Pharm. J. 2011, 29, 100–107. [Google Scholar] [CrossRef]
- OECD/OCDE (Testing Guidelines 425). OECD Guidelines for the Testing of Chemicals: Acute Oral Toxicity—Up and Down Procedure (UDP); OECD Publishing: Paris, France, 2008; Volume 27. [Google Scholar]
- Tewabe, Y.; Bisrat, D.; Terefe, G.; Asres, K. Antitrypanosomal activity of aloin and its derivatives against Trypanosoma congolense field isolate. BMC Vet. Res. 2014, 10, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, K.; Iqbal, J.; Freen, M.S. Comparative study on antileishmanial and cytotoxic activity of Lawsonia inermis bark and Aloe vera leaves. Int. J. Biol. Pharm. Allied Sci. 2016, 5, 1490–1500. [Google Scholar]
- Teka, T.; Bisrat, D.; Yeshak, M.Y.; Asres, K. Antimalarial activity of the chemical constituents of the leaf latex of Aloe pulcherrima Gilbert and Sebsebe. Molecules 2016, 21, 1415. [Google Scholar] [CrossRef] [PubMed]
- Abeje, F.; Bisrat, D.; Hailu, A.; Asres, K. Phytochemistry and antileishmanial activity of the leaf latex of Aloe calidophila Reynolds. Phytother. Res. 2014, 28, 1801–1805, 2014. [Google Scholar] [CrossRef]
- Atawodi, S.E.; Joseph-Idrisu, J.; Ndidi, U.S.; Yusufu, L.M.D. Phytochemical and antitrypanosomal studies of different solvents extracts of Boswellia dalzielii. Int. J. Biol. 2011, 3, 179–184. [Google Scholar] [CrossRef]
- Atawodi, S.E.; Bulus, T.; Ibrahim, S.; Ameh, D.A.; Nok, A.J.; Mamman, M.; Galadima, M. In vitro trypanocidal effect of methanolic extract of some Nigerian savannah plants. Afr. J. Biotechnol. 2003, 2, 317–321. [Google Scholar]
- Atawodi, S.E.; Ogunbusola, F. Evaluation of anti-trypanosomal properties of four extracts of leaves, stem and root barks of Prosopis africana in laboratory animals. Nigerian Soc. Experiment. Biol. 2009, 21, 101–108. [Google Scholar]
- Tadesse, B.; Terefe, G.; Kebede, N.; Shibeshi, W. In vivo anti-trypanosomal activity of dichloromethane and methanol crude leaf extracts of Dovyalis abyssinica (Salicaceae) against Trypanosoma congolense. BMC Complement. Altern. Med. 2015, 15, 278. [Google Scholar] [CrossRef] [Green Version]
- Tesfaye, A.; Terefe, G.; Giday, M.; Shibeshi, W. In vivo anti-trypanosomal activity of the leaf extracts of Albizia schimperiana (Fabaceae) against Trypanosoma congolense infection in mice. Clin. Experiment. Pharmacol. 2015, 5, 171. [Google Scholar]
- Mergia, E.; Terefe, G.; Teklehaymanot, T.; Shibeshi, W. Evaluation of in vivo antitrypanosomal activity of aqueous and methanol leaf extracts of Clutia abyssinica (Euphorbiaceae) against Trypanosoma congolense. Austin J. Pharmacol. Ther. 2014, 2, 9. [Google Scholar] [CrossRef] [Green Version]
- Mikail, H.G. In vitro trypanocidal effect of methanolic extract of Sclerocarya birrea, Commiphora kerstingii and Khaya senegalensis. Afr. J. Biotechnol. 2009, 8, 2047–2049. [Google Scholar]
- Tewabe, Y.; Kefarge, B.; Belay, H.; Bisrat, D.; Hailu, A.; Asres, K. Antileishmanial evaluation of the leaf latex of Aloe macrocarpa, Aloin A/B, and its semisynthetic derivatives against two Leishmania species. Evid. -Based Complement. Altern. Med. 2019, 2019, 4736181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngure, P.K.; Tonui, W.K.; Ingonga, J.; Mutai, C.; Kigondu, E.; Ng, Z.; Rukunga, G.; Kimutai, A. In vitro antileishmanial activity of extracts of Warburgia ugandensis (Canellaceae), a Kenyan medicinal plan. J. Med. Plant Res. 2009, 3, 61–66. [Google Scholar]
- Mikayoulou, M.; Mayr, F.; Temml, V.; Pandian, A.; Vermaak, I.; Chen, W.; Komane, B.; Stuppner, H.; Viljoen, A. Anti-tyrosinase activity of South African Aloe species and isolated compounds plicataloside and aloesin. Fitoterapia 2021, 150, 104828. [Google Scholar] [CrossRef]
- Sepulveda-Boza, S.; Cassels, B.K. Plant metabolites active against Trypanosoma cruzi. Planta Med. 1996, 62, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Nigusse, Z.T.; Wondifraw, W.A.; Abate, S.M. Phytochemical screening of the exudate of Aloe otallensis and its effect on Leishmania donovani. J. Coastal Life Med. 2016, 4, 479–482. [Google Scholar] [CrossRef]
- Nigusse, Z.T.; Wondifraw, W.A.; Abate, S.M. Screening of Aloe otallensis exudate and its effect on Leishmania aethiopica. Pharm. Analytica Acta 2016, 7, 515. [Google Scholar]
- Glew, R.H.; Saha, A.K.; Das, S.; Remaley, A.T. Biochemistry of the Leishmania species. Microbiol. Rev. 1988, 52, 412–432. [Google Scholar] [CrossRef]
- Singh, S. New developments in diagnosis of leishmaniasis. Indian J. Med. Res. 2006, 123, 311–330. [Google Scholar] [PubMed]
- Institute for Laboratory Animal Research (ILAR). Guide for the Care and Use of Laboratory Animals; The National Academy Press: Washington, DC, USA, 1996. [Google Scholar]
- Murray, M.; Murray, P.K.; Mcintyre, E.I. An improved parasitological technique for the diagnosis of African trypanosomiasis. Trans. Royal Soc. Trop. Med. Hygiene 1977, 71, 325–331. [Google Scholar] [CrossRef]
- Uilenberg, G. Field guide for diagnosis, treatment and prevention of African animal trypanosomosis; Food and Agriculture Organization of the United Nations Publications: Rome, Italy, 1998; pp. 59–86. [Google Scholar]
- Woo, P.T. The haematocrit centrifuge technique for the diagnosis of African trypanosomiasis. Acta Trop. 1970, 27, 384–386. [Google Scholar] [PubMed]
- Habtemariam, S. In vitro antileishmanial effects of antibacterial diterpens from two Ethiopian Premna species: P. schimperi and P. oligotricha. BMC Pharmacol. 2003, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Ene, A.C.; Edeh, N.G.; Bonny-Okoli, C.; Ojiako, O.A.; Ujowundu, C.O.; Igwe, C.U. In vitro and in vivo antitrypanosomal effects of methanol and aqueous extracts of Picralima nitida. Brit. J. Pharm. Res. Int. 2014, 4, 644–653. [Google Scholar] [CrossRef]
- Onyeyili, P.A.; Aliyoo, K. In vitro and in vivo evaluation of antitrypanosomal activity of Annona muricata stem bark extracts. Herba Pol. 2015, 61, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Maikai, V.A.; Nok, J.A.; Adaudi, A.O.; Alawa, C.B.I. In vitro antitrypanosomal activity of aqueous and methanolic crude extracts of stem bark of Ximenia americana on Trypanosoma congolense. J. Med. Plant Res. 2008, 2, 55–58. [Google Scholar]
| Time (min) After Which Motility Ceased | ||||
|---|---|---|---|---|
| Concentration (mg/mL) | Latex | Plicataloside | Diminazene Diaceturate | 10% DMSO |
| 4.0 | 35 | 30 | 20 | NE |
| 2.0 | 50 | 45 | 35 | NE |
| 0.4 | >60 | 60 | 50 | NE |
| 0.1 | >60 | >60 | >60 | NE |
| Dose of Test Substance (mg/mL) Mixed with 0.02 mL of Infected Blood | Number of Mice Which Developed Infection | Infection Interval in Days (mean ± SEM) | |
|---|---|---|---|
| Latex | 4.0 | 1/5 | 24.00 |
| 2.0 | 3/5 | 14.66 ± 0. 66 | |
| 0.4 | 5/5 | 13.00 ± 0.55 | |
| 0.1 | 5/5 | 12.4 ± 0.60 | |
| Plicataloside | 4.0 | 0/5 | Ni |
| 2.0 | 0/5 | Ni | |
| 0.4 | 3/5 | 18.33 ± 0.66 | |
| 0.1 | 5/5 | 14.20 ± 0.49 | |
| Diminazene Diaceturate | 4.0 | 0/5 | Ni |
| 2.0 | 0/5 | Ni | |
| 0.4 | 2/5 | 19.50 ± 0.50 | |
| 0.1 | 5/5 | 15.20 ± 0.37 | |
| Dimethylsulfoxide | 0.1 mL | 5/5 | 11.60 ± 0.24 |
| Test Samples/Drugs | IC50; µg/mL (µM) | IC50; µg/mL(µM) |
|---|---|---|
| Against L. aethiopica | Against L. donovani | |
| Latex | 24.50 ± 0.24 | 31.21 ± 0.01 |
| Plicataloside | 14.22 ± 0.41(27.66 ± 0.80) | 18.86 ± 0.03 (36.69 ± 0.06) |
| Amphotericin B | 8.10 ± 0.11 (8.77 ± 0.12) | 7.20 ± 0.15 (7.79 ± 0.16) |
| Media alone (NC) | 0.00 | 0.00 |
| 1% Dimethyl sulfoxide (NC) | 0.00 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chemeda, G.; Bisrat, D.; Yeshak, M.Y.; Asres, K. In Vitro Antileishmanial and Antitrypanosomal Activities of Plicataloside Isolated from the Leaf Latex of Aloe rugosifolia Gilbert & Sebsebe (Asphodelaceae). Molecules 2022, 27, 1400. https://doi.org/10.3390/molecules27041400
Chemeda G, Bisrat D, Yeshak MY, Asres K. In Vitro Antileishmanial and Antitrypanosomal Activities of Plicataloside Isolated from the Leaf Latex of Aloe rugosifolia Gilbert & Sebsebe (Asphodelaceae). Molecules. 2022; 27(4):1400. https://doi.org/10.3390/molecules27041400
Chicago/Turabian StyleChemeda, Gete, Daniel Bisrat, Mariamawit Y. Yeshak, and Kaleab Asres. 2022. "In Vitro Antileishmanial and Antitrypanosomal Activities of Plicataloside Isolated from the Leaf Latex of Aloe rugosifolia Gilbert & Sebsebe (Asphodelaceae)" Molecules 27, no. 4: 1400. https://doi.org/10.3390/molecules27041400
APA StyleChemeda, G., Bisrat, D., Yeshak, M. Y., & Asres, K. (2022). In Vitro Antileishmanial and Antitrypanosomal Activities of Plicataloside Isolated from the Leaf Latex of Aloe rugosifolia Gilbert & Sebsebe (Asphodelaceae). Molecules, 27(4), 1400. https://doi.org/10.3390/molecules27041400






