Phytochemical and In Silico ADME/Tox Analysis of Eruca sativa Extract with Antioxidant, Antibacterial and Anticancer Potential against Caco-2 and HCT-116 Colorectal Carcinoma Cell Lines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Profiling of E. sativa
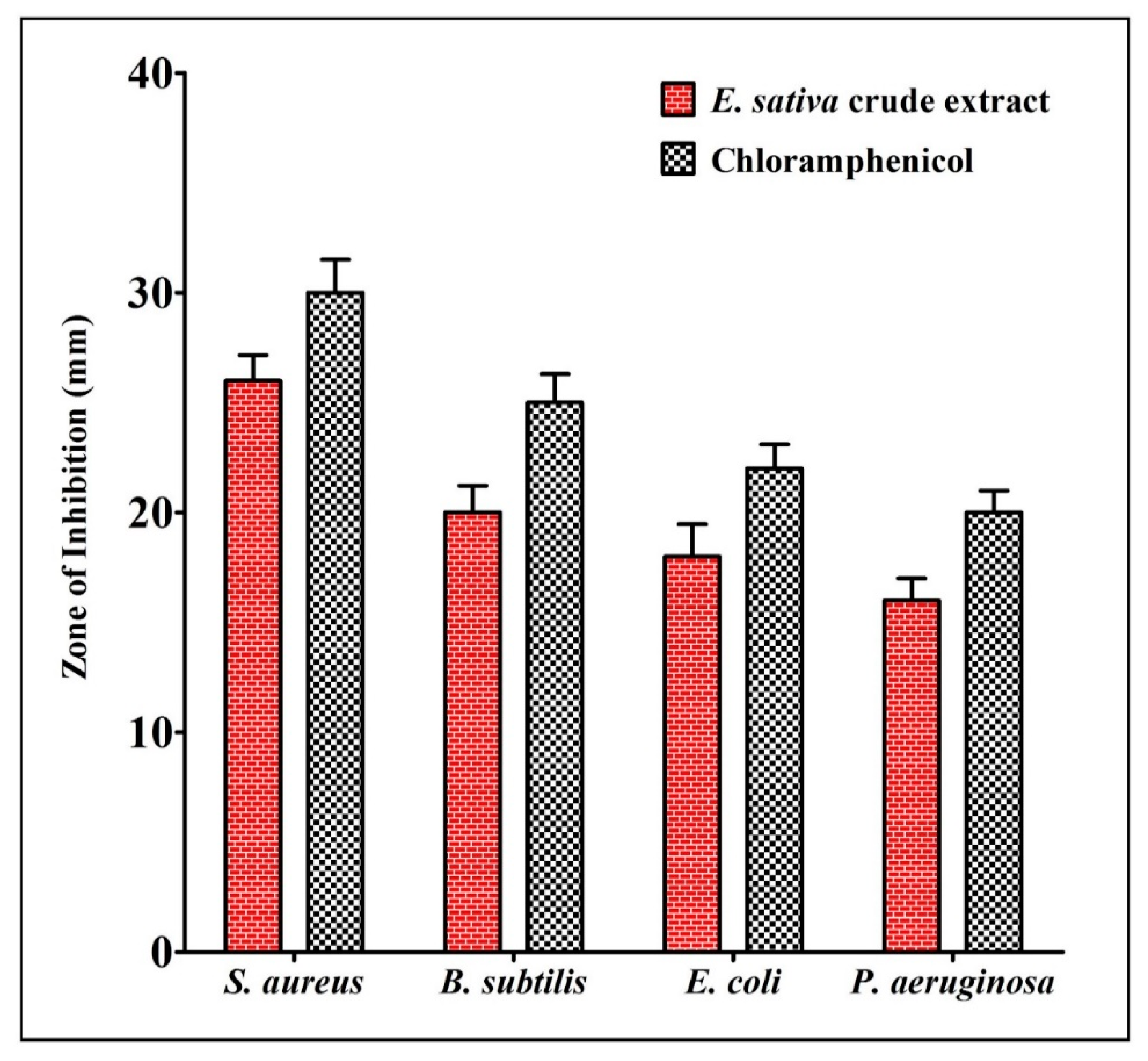
2.2. Antibacterial Activity of E. sativa Crude Extract
2.3. The Antioxidant Activity of E. sativa Crude Extract
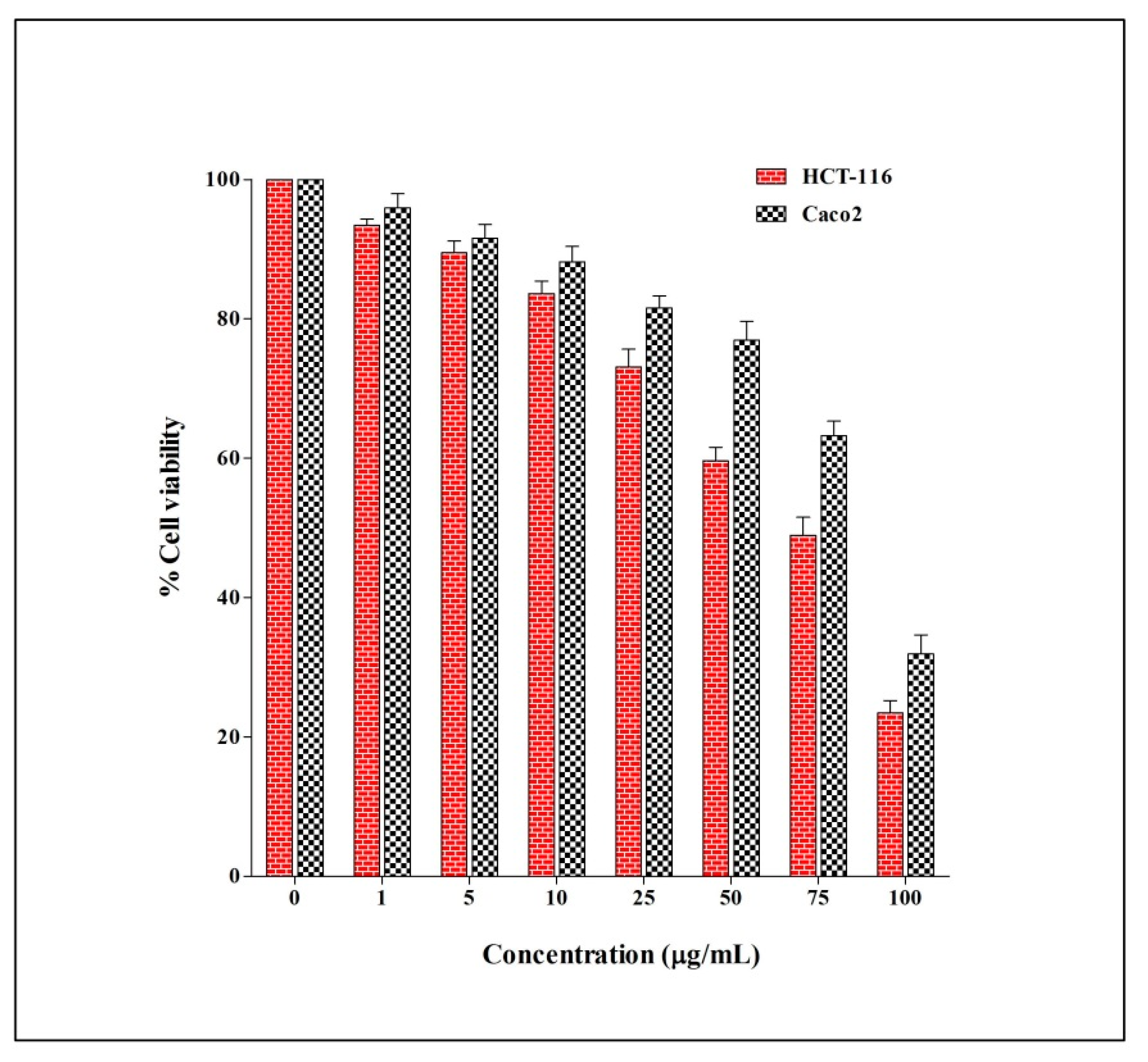
2.4. The Anticancer Activity of E. sativa Crude Extract
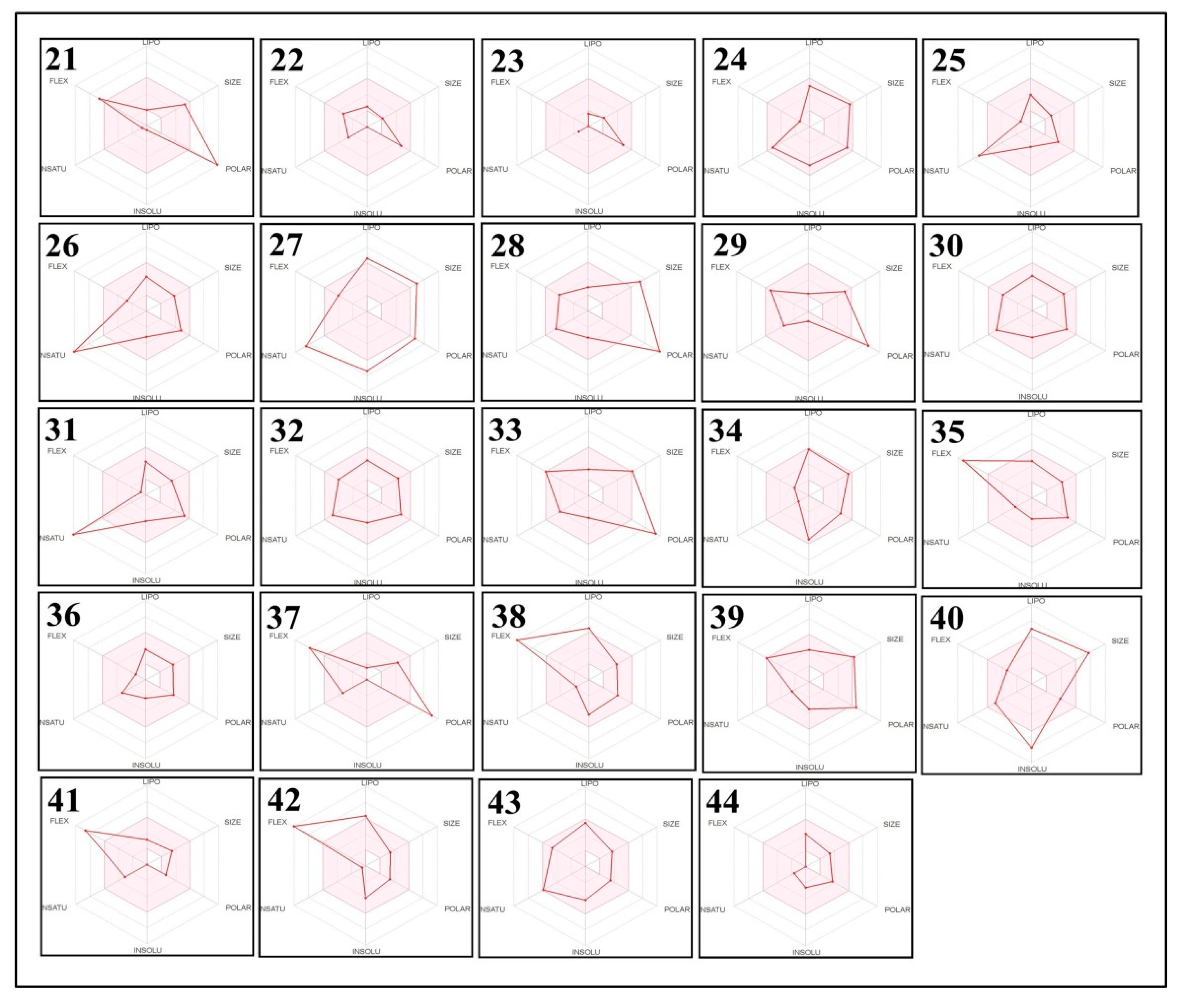
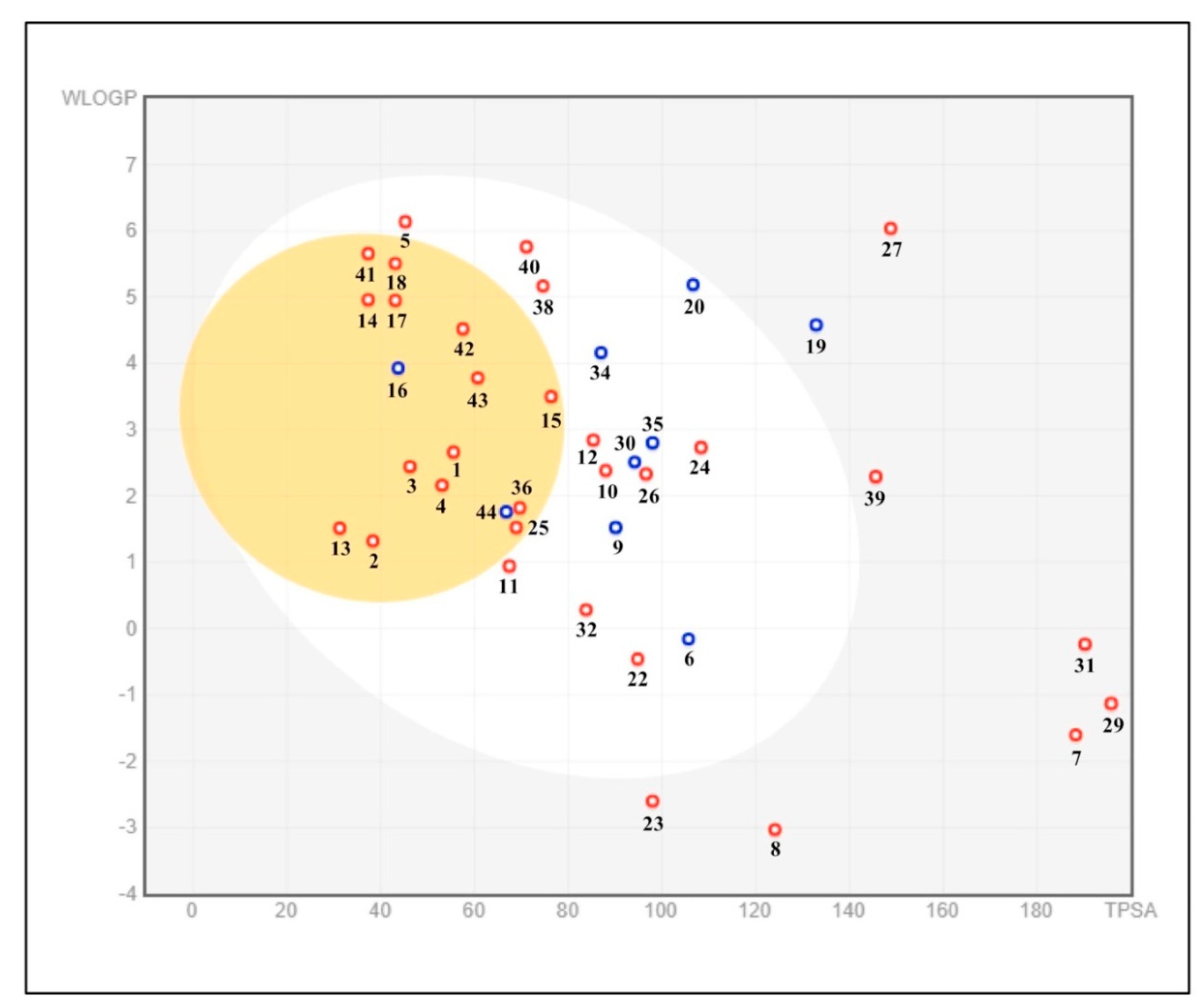
2.5. The Pharmacokinetic and Toxicity (ADMET) Profiles of the Identified Phytoconstituents from E. sativa Ethanolic Crude Extract
3. Materials and Methods
3.1. Media and Chemicals
3.2. Plant Material and Extraction Preparation
3.3. High Resolution Liquid Chromatograph Mass Spectrometery Analysis
3.4. Antibacterial Assay
3.5. Antioxidant Assays
3.5.1. DPPH Scavenging Activity
3.5.2. Hydrogen Peroxide Scavenging Activity
3.6. Anticancer Assay (MTT Assay)
3.7. ADMET Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gulfraz, M.; Sadiq, A.; Tariq, H.; Imran, M.; Qureshi, R. Phytochemical analysis and antibacterial activity of Eruca sativa seed. Pak. J. Bot. 2011, 43, 1351–1359. [Google Scholar]
- Alqasoumi, S.; Al-Sohaibani, M.; Al-Howiriny, T.; Al-Yahya, M.; Rafatullah, S. Rocket “Eruca sativa”: A salad herb with potential gastric anti-ulcer activity. World J. Gastroenterol. 2009, 15, 1958–1965. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Jalil, T.Z. Phytochemicals Screening by GC/MS and Determination of Some Flavonol in Cultivated Iraqi Eruca sativa Dried Leaves Extract and its Biological Activity as Antioxidant. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1722–1730. [Google Scholar]
- Nurzyńska-Wierdak, R. Nutritional and energetic value of Eruca sativa Mill. leaves. Acta Sci. Pol. Hortorum Cultus 2015, 14, 191–199. [Google Scholar]
- Di Gioia, F.; Avato, P.; Serio, F.; Argentieri, M.P. Glucosinolate profile of Eruca sativa, Diplotaxis tenuifolia and Diplotaxis erucoides grown in soil and soilless systems. J. Food Compos. Anal. 2018, 69, 197–204. [Google Scholar] [CrossRef]
- Jirovetz, L.; Smith, D.; Buchbauer, G. Aroma compound analysis of Eruca sativa (Brassicaceae) SPME headspace leaf samples using GC, GC-MS, and olfactometry. J. Agric. Food Chem. 2002, 50, 4643–4646. [Google Scholar] [CrossRef]
- Nazif, N.M.; Habib, A.A.E.; Tawfik, W.A.M.; Hassan, R.A. Chemical composition and cytotoxic activity of Eruca sativa L. Seeds cultivated in Egypt. Asian J. Chem. 2010, 22, 2407–2416. [Google Scholar]
- Hussein, S.A. Phytochemical Study of Vsome Medicinal Compounds Present in Hedera helix L. Plant Cultivated in Iraq. Master’s Thesis, Baghdad University, Baghdad, Iraq, 2014. [Google Scholar]
- Ashraf, S.A.; Al-Shammari, E.; Hussain, T.; Tajuddin, S.; Panda, B.P. In-vitro antimicrobial activity and identification of bioactive components using GC-MS of commercially available essential oils in Saudi Arabia. J. Food Sci. Technol. 2017, 54, 3948–3958. [Google Scholar] [CrossRef]
- Sastry, E.V.D. Taramira (Eruca sativa) and its improvement A review. Agric. Rev. 2003, 24, 235–249. [Google Scholar]
- Bukhsh, E.; Malik, S.; Ahmad, S. Estimation of Nutritional Value and Trace elements Content of Carthamus oxyacantha, Eruca stiva and Plantago ovata. Pak. J. Bot. 2007, 39, 1181–1187. [Google Scholar]
- Yehuda, H.; Khatib, S.; Sussan, I.; Musa, R.; Vaya, J.; Tamir, S. Potential skin antiinflammatory effects of 4-methylthiobutylisothiocyanate (MTBI) isolated from rocket (Eruca sativa) seeds. BioFactors 2009, 35, 295–305. [Google Scholar] [CrossRef]
- Melchini, A.; Traka, M.H. Biological profile of erucin: A new promising anticancer agent from cruciferous vegetables. Toxins 2010, 2, 593–612. [Google Scholar] [CrossRef] [Green Version]
- Koubaa, M.; Driss, D.; Bouaziz, F.; Ghorbel, R.; Chaabouni Ellouz, S. Antioxidant and antimicrobial activities of solvent extract obtained from rocket (Eruca sativa L.) flowers. Free Radic. Antioxid. 2015, 5, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Allen, D.R.; McWhinney, B.C. Quadrupole Time-of-Flight Mass Spectrometry: A Paradigm Shift in Toxicology Screening Applications. Clin. Biochem. Rev. 2019, 40, 135–146. [Google Scholar] [CrossRef]
- Elkhalifa, A.E.O.; Alshammari, E.; Adnan, M. Okra (Abelmoschus Esculentus) as a Potential Dietary Medicine with Nutraceutical Importance for Sustainable Health Applications. Molecules 2021, 26, 696. [Google Scholar] [CrossRef]
- Blažević, I.; Mastelić, J. Free and bound volatiles of rocket (Eruca sativa Mill.). Flavour Fragr. J. 2008, 23, 278–285. [Google Scholar] [CrossRef]
- Villatoro-Pulido, M.; Priego-Capote, F.; Álvarez-Sánchez, B.; Saha, S.; Philo, M.; Obregón-Cano, S.; De Haro-Bailón, A.; Font, R.; Del Río-Celestino, M. An approach to the phytochemical profiling of rocket [Eruca sativa (Mill.) Thell]. J. Sci. Food Agric. 2013, 93, 3809–3819. [Google Scholar] [CrossRef] [PubMed]
- Michael, H.; Shafik, R.; Rasmy, G. Studies on the chemical constituents of fresh leaf of Eruca sativa extract and its biological activity as anticancer agent in vitro. J. Med. Plants Res. 2011, 5, 1184–1191. [Google Scholar]
- Bennett, R.N.; Rosa, E.A.; Mellon, F.A.; Kroon, P.A. Ontogenic profiling of glucosinolates, flavonoids, and other secondary metabolites in Eruca sativa (salad rocket), Diplotaxis erucoides (wall rocket), Diplotaxis tenuifolia (wild rocket), and Bunias orientalis (Turkish rocket). J. Agric. Food Chem. 2006, 54, 4005–4015. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.; Wagstaff, C. Glucosinolates, myrosinase hydrolysis products, and flavonols found in rocket (Eruca sativa and Diplotaxis tenuifolia). J. Agric. Food Chem. 2014, 62, 4481–4492. [Google Scholar] [CrossRef] [PubMed]
- Uğur, A.; Süntar, I.; Aslan, S.; Orhan, I.E.; Kartal, M.; Sekeroğlu, N.; Eşiyok, D.; Sener, B. Variations in fatty acid compositions of the seed oil of Eruca sativa Mill. caused by different sowing periods and nitrogen forms. Pharmacogn. Mag. 2010, 6, 305–308. [Google Scholar]
- Elfakir, C.; Dreux, M. Simultaneous analysis of intact and desulfated glucosinolates with a porous graphitized carbon column. J. Chromatogr. A 1996, 727, 71–82. [Google Scholar] [CrossRef]
- Miyazawa, M.; Maehara, T.; Kurose, K. Composition of the essential oil from the leaves of Eruca sativa. Flavour Fragr. J. 2002, 17, 187–190. [Google Scholar] [CrossRef]
- Hussein, Z.F. Study the Effect of Eruca sativa Leaves Extract on Male Fertility in Albino Mice. J. Al-Nahrain Univ. -Sci. 2013, 16, 143–146. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, L. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta Pharmacol. Sin. 2007, 28, 1343–1354. [Google Scholar] [CrossRef]
- Arora, R.; Sharma, D.; Kumar, R.; Singh, B.; Vig, A.P.; Arora, S. Evaluating extraction conditions of glucosinolate hydrolytic products from seeds of Eruca sativa (Mill.) Thell. using GC-MS. J. Food Sci. 2014, 79, C1964–C1969. [Google Scholar] [CrossRef]
- Cavaiuolo, M.; Ferrante, A. Nitrates and glucosinolates as strong determinants of the nutritional quality in rocket leafy salads. Nutrients 2014, 6, 1519–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoobchandani, M.; Ojeswi, B.K.; Ganesh, N.; Srivastava, M.M.; Gabbanini, S.; Matera, R.; Iori, R.; Valgimigli, L. Antimicrobial properties and analytical profile of traditional Eruca sativa seed oil: Comparison with various aerial and root plant extracts. Food Chem. 2010, 120, 217–224. [Google Scholar] [CrossRef]
- Qaddoumi, S.Q.; El-Banna, N. Antimicrobial Activity of Arugula (Eruca sativa) Leaves on Some Pathogenic Bacteria. Int. J. Biol. 2019, 11, 10. [Google Scholar] [CrossRef]
- Rizwana, H.; Alwhibi, M.; Khan, F.A.; Soliman, D.A. Chemical Composition and Antimicrobial Activity of Eruca sativa Seeds Against Pathogenic Bacteria and Fungi. J. Anim. Plant Sci. 2016, 26, 1859–1871. [Google Scholar]
- Kurutas, E.B.; Ciragil, P.; Gul, M.; Kilinc, M. The Effects of Oxidative Stress in Urinary Tract Infection. Mediat. Inflamm. 2005, 2005, 528064. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Stefanis, L.; Burke, R.E.; Greene, L.A. Apoptosis in neurodegenerative disorders. Curr. Opin. Neurol. 1997, 10, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35 Pt 5, 1147–1150. [Google Scholar] [CrossRef]
- Kishore, S. Evaluation of Antioxidant Activity and Total Phenolic Content of Eruca sativa L., Seeds. Int. J. Toxicol. Pharm. Res. 2016, 8, 146–151. [Google Scholar]
- Maia, M.L.; Correia-Sá, L.; Coelho, A.; Barroso, M.F.; Domingues, V.F.; Delerue-Matos, C. Eruca sativa: Benefits as antioxidants source versus risks of already banned pesticides. J. Environ. Sci. Health Part B 2015, 50, 338–345. [Google Scholar] [CrossRef]
- Adnan, M.; Siddiqui, A.J.; Hamadou, W.S.; Patel, M.; Ashraf, S.A.; Jamal, A.; Awadelkareem, A.M.; Sachidanandan, M.; Snoussi, M.; De Feo, V. Phytochemistry, Bioactivities, Pharmacokinetics and Toxicity Prediction of Selaginella repanda with Its Anticancer Potential against Human Lung, Breast and Colorectal Carcinoma Cell Lines. Molecules 2021, 26, 768. [Google Scholar] [CrossRef]
- Azarenko, O.; Jordan, M.A.; Wilson, L. Erucin, the major isothiocyanate in arugula (Eruca sativa), inhibits proliferation of MCF7 tumor cells by suppressing microtubule dynamics. PLoS ONE 2014, 9, e100599. [Google Scholar] [CrossRef] [Green Version]
- Melchini, A.; Costa, C.; Traka, M.; Miceli, N.; Mithen, R.; De Pasquale, R.; Trovato, A. Erucin, a new promising cancer chemopreventive agent from rocket salads, shows anti-proliferative activity on human lung carcinoma A549 cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2009, 47, 1430–1436. [Google Scholar] [CrossRef]
- Jakubikova, J.; Bao, Y.; Sedlak, J. Isothiocyanates induce cell cycle arrest, apoptosis and mitochondrial potential depolarization in HL-60 and multidrug-resistant cell lines. Anticancer. Res. 2005, 25, 3375–3386. [Google Scholar] [PubMed]
- Soundararajan, P.; Kim, J.S. Anti-Carcinogenic Glucosinolates in Cruciferous Vegetables and Their Antagonistic Effects on Prevention of Cancers. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Lombardo, F.; Gifford, E.; Shalaeva, M.Y. In silico ADME prediction: Data, models, facts and myths. Mini Rev. Med. Chem. 2003, 3, 861–875. [Google Scholar] [CrossRef]
- Gleeson, M.P.; Hersey, A.; Hannongbua, S. In-silico ADME models: A general assessment of their utility in drug discovery applications. Curr. Top. Med. Chem. 2011, 11, 358–381. [Google Scholar] [CrossRef]
- DiMasi, J.A.; Hansen, R.W.; Grabowski, H.G. The price of innovation: New estimates of drug development costs. J. Health Econ. 2003, 22, 151–185. [Google Scholar] [CrossRef] [Green Version]
- Hodgson, J. ADMET--turning chemicals into drugs. Nat. Biotechnol. 2001, 19, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Darvas, F.; Keseru, G.; Papp, A.; Dormán, G.; Urge, L.; Krajcsi, P. In Silico and Ex silico ADME approaches for drug discovery. Curr. Top. Med. Chem. 2002, 2, 1287–1304. [Google Scholar] [CrossRef]
- Heaney, R.K.; Fenwick, G.R. Identifying toxins and their effects: Glucosinolates. In Natural Toxicants in Food: Progress and Prospects; Watson, D.H., Ed.; Ellis Horwood: Chichester, UK, 1987; pp. 76–109. [Google Scholar]
- Brown, P.D.; Tokuhisa, J.G.; Reichelt, M.; Gershenzon, J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 2003, 62, 471–481. [Google Scholar] [CrossRef]
- Zhang, Y.; Talalay, P. Anticarcinogenic activities of organic isothiocyanates: Chemistry and mechanisms. Cancer Res. 1994, 54 (Suppl. S7), 1976s–1981s. [Google Scholar]
- Brown, P.D.; Morra, M.J. Glucosinolate-containing plant tissues as bioherbicides. J. Agric. Food Chem. 1995, 43, 3070–3074. [Google Scholar] [CrossRef]
- Fahey, J.W.; Talalay, P. Antioxidant functions of sulforaphane: A potent inducer of Phase II detoxication enzymes. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1999, 37, 973–979. [Google Scholar] [CrossRef]
- Vig, A.P.; Rampal, G.; Thind, T.S.; Arora, S. Bio-protective effects of glucosinolates–A review. LWT-Food Sci. Technol. 2009, 42, 1561–1572. [Google Scholar] [CrossRef]
- Fimognari, C.; Hrelia, P. Sulforaphane as a promising molecule for fighting cancer. Mutat. Res. 2007, 635, 90–104. [Google Scholar] [CrossRef]
- Adnan, M.; Patel, M.; Deshpande, S.; Alreshidi, M.; Siddiqui, A.J.; Reddy, M.N.; Emira, N.; De Feo, V. Effect of Adiantum philippense Extract on Biofilm Formation, Adhesion With Its Antibacterial Activities Against Foodborne Pathogens, and Characterization of Bioactive Metabolites: An in vitro-in silico Approach. Front. Microbiol. 2020, 11, 823. [Google Scholar] [CrossRef]
- Muzaffer, U.; Paul, V.I. Phytochemical analysis, in vitro antioxidant and antimicrobial activities of male flower of Juglans regia L. Int. J. Food Prop. 2018, 21, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Adnan, M.; Patel, M.; Reddy, M.N.; Alshammari, E. Formulation, evaluation and bioactive potential of Xylaria primorskensis terpenoid nanoparticles from its major compound xylaranic acid. Sci. Rep. 2018, 8, 1740. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Aslan, M. Appraisal of scopolamine-induced antiamnesic effect in mice and in vitro antiacetylcholinesterase and antioxidant activities of some traditionally used Lamiaceae plants. J. Ethnopharmacol. 2009, 122, 327–332. [Google Scholar] [CrossRef]
- Ruch, R.J.; Cheng, S.J.; Klaunig, J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 1989, 10, 1003–1008. [Google Scholar] [CrossRef]
- Reddy, M.N.; Adnan, M.; Alreshidi, M.M.; Saeed, M.; Patel, M. Evaluation of Anticancer, Antibacterial and Antioxidant Properties of a Medicinally Treasured Fern Tectaria coadunata with its Phytoconstituents Analysis by HR-LCMS. Anti-Cancer Agents Med. Chem. 2020, 20, 1845–1856. [Google Scholar] [CrossRef]
- Lombardi, V.R.; Carrera, I. In Vitro Screening for Cytotoxic Activity of Herbal Extracts. Evid.-Based Complementary Altern. Med. 2017, 2017, 2675631. [Google Scholar] [CrossRef] [PubMed]
- Kadri, A.; Aouadi, K. In vitro antimicrobial and α-glucosidase inhibitory potential of enantiopure cycloalkylglycine derivatives: Insights into their in silico pharmacokinetic, druglikeness, and medicinal chemistry properties. J. Appl. Pharm. Sci. 2020, 10, 107–115. [Google Scholar]
- Othman, I.M.M.; Gad-Elkareem, M.A.M.; Hassane Anouar, E.; Aouadi, K.; Kadri, A.; Snoussi, M. Design, synthesis ADMET and molecular docking of new imidazo[4,5-b]pyridine-5-thione derivatives as potential tyrosyl-tRNA synthetase inhibitors. Bioorg. Chem. 2020, 102, 104105. [Google Scholar] [CrossRef]
- Ghannay, S.; Kadri, A.; Aouadi, K. Synthesis, in vitro antimicrobial assessment, and computational investigation of pharmacokinetic and bioactivity properties of novel trifluoromethylated compounds using in silico ADME and toxicity prediction tools. Mon. Chem.-Chem. Mon. 2020, 151, 267–280. [Google Scholar] [CrossRef]

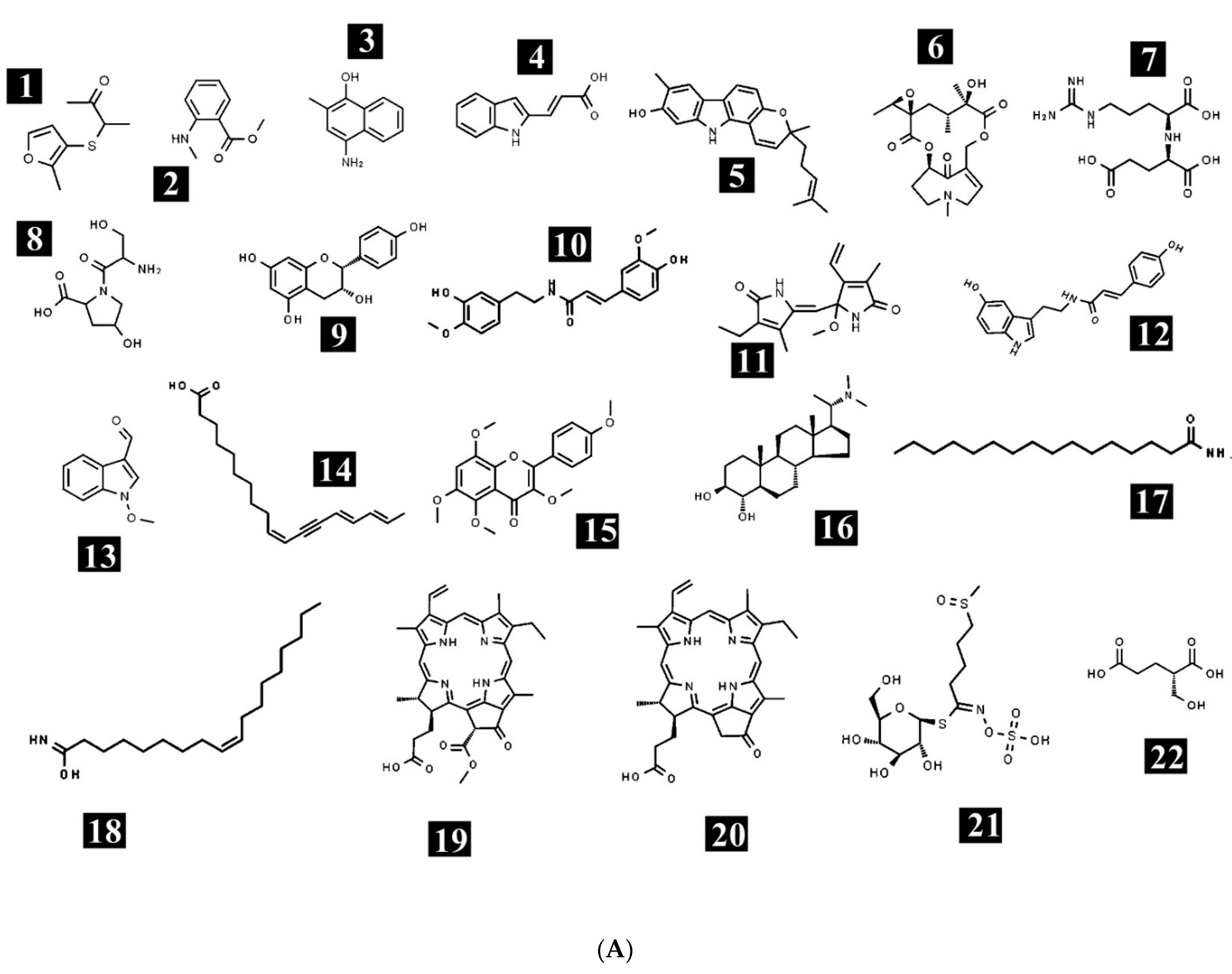



| Compound Number | Analysis Mode | Name | Class | Formula | Mass | m/z | RT |
|---|---|---|---|---|---|---|---|
| 1 | Positive | (+/−)-3-[(2-methyl-3-furyl)thio]-2-butanone | Furans-aryl thioethers | C9H12O2S | 184.0576 | 185.0649 | 0.816 |
| 2 | Positive | Methyl N-methylanthranilate | Methyl ester | C9H11NO2 | 165.078 | 166.0852 | 2.06 |
| 3 | Positive | 4-Amino-2-methyl-1-naphthol | Vitamin | C11H11NO | 173.0859 | 174.0937 | 2.55 |
| 4 | Positive | Indoleacrylic acid | Indoles | C11H9NO2 | 187.0623 | 188.0696 | 3.189 |
| 5 | Positive | Pyrafoline D | Carbazoles | C23H25NO2 | 347.1928 | 348.2001 | 3.705 |
| 6 | Positive | Petasitenine | Spiro-epoxide | C19H27NO7 | 381.1773 | 382.1846 | 4.232 |
| 7 | Positive | Nopaline | Amino acid | C11H20N4O6 | 304.1444 | 305.152 | 5.05 |
| 8 | Positive | Serinyl-Hydroxyproline | Dipeptide | C8H14N2O5 | 218.0894 | 219.0974 | 5.443 |
| 9 | Positive | Afzelechin | Flavonoid | C15H14O5 | 274.079 | 275.0873 | 6.004 |
| 10 | Positive | N-trans-Feruloyl-4-O-methyldopamine | Cinnamamides | C19H21NO5 | 343.1368 | 344.145 | 6.22 |
| 11 | Positive | (±)-Rollipyrrole | Pyrrolines | C16H20N2O3 | 288.1463 | 289.1536 | 6.853 |
| 12 | Positive | N6-cis-p-Coumaroylserotonin | N-acylserotonins | C19H18N2O3 | 322.1306 | 323.1379 | 7.024 |
| 13 | Positive | 1-Methoxy-1H-indole-3-carboxaldehyde | Indoles | C10H9NO2 | 175.0625 | 176.0697 | 7.09 |
| 14 | Positive | (10Z,14E,16E)-10,14,16-Octadecatrien-12-ynoic acid | Fatty acids | C18H26O2 | 274.192 | 275.1991 | 8.672 |
| 15 | Positive | 3,4′,5,6,8-Pentamethoxyflavone | Flavonoids | C20H20O7 | 372.1191 | 373.1264 | 11.085 |
| 16 | Positive | Terminaline | Corticosteroid | C23H41NO2 | 363.3122 | 364.3195 | 12.545 |
| 17 | Positive | Palmitic amide | Fatty amide | C16H33NO | 255.2554 | 256.2627 | 16.876 |
| 18 | Positive | Oleamide | Fatty amide | C18H35NO | 281.2705 | 282.2778 | 17.242 |
| 19 | Positive | Pheophorbide a | - | C35H36N4O5 | 592.2665 | 593.2738 | 17.474 |
| 20 | Positive | Pyropheophorbide a | - | C33H34N4O3 | 534.2615 | 535.2688 | 17.918 |
| 21 | Negative | Glucoraphanin | Thia-glucosinolic acid | C12H23NO10S3 | 437.0439 | 436.0367 | 1.124 |
| 22 | Negative | (S)-2-(Hydroxymethyl)glutarate | - | C6H10O5 | 162.0501 | 161.0429 | 1.202 |
| 23 | Negative | 2-Deoxy-scyllo-inosose | Cyclohexanone | C6H10O5 | 162.0502 | 161.0431 | 1.485 |
| 24 | Negative | Artomunoxanthentrione epoxide | Pyranoxanthones | C26H22O8 | 462.1337 | 461.1265 | 3.084 |
| 25 | Negative | Fraxidin | Hydroxycoumarins | C11H10O5 | 222.0532 | 221.0463 | 3.883 |
| 26 | Negative | N-(6-Oxo-6H-dibenzo[b,d]pyran-3-yl)maleamic acid | Coumarins | C17H11NO5 | 309.067 | 354.0659 | 4.262 |
| 27 | Negative | Sciadopitysin | Flavonoid | C33H24O10 | 580.1389 | 625.1373 | 5.015 |
| 28 | Negative | Rutin | Flavonoid | C27H30O16 | 610.1504 | 609.1432 | 5.015 |
| 29 | Negative | 5′-Butyrylphosphoinosine | - | C14H19N4O9P | 418.0875 | 463.0856 | 5.735 |
| 30 | Negative | Evoxine | Alkaloid | C18H21NO6 | 347.1374 | 392.136 | 6.06 |
| 31 | Negative | Kaempferol 3-O-β-d-galactoside | - | C21H20O11 | 448.0983 | 447.0911 | 6.223 |
| 32 | Negative | Lactucin | Gamma butyrolactones | C15H16O5 | 276.1009 | 321.0994 | 6.227 |
| 33 | Negative | 1,4-Dimethoxyglucobrassicin | Indole glucosinolate | C18H24N2O11S2 | 508.0794 | 507.0722 | 6.294 |
| 34 | Negative | Pubesenolide | - | C28H42O5 | 458.2963 | 457.2891 | 7.746 |
| 35 | Negative | Corchorifatty acid F | Fatty acid | C18H32O5 | 328.2228 | 327.2156 | 8.681 |
| 36 | Negative | Linifolin A | Terpenoid | C17H20O5 | 304.1319 | 349.1304 | 8.903 |
| 37 | Negative | N2-(2-Carboxymethyl-2-hydroxysuccinoyl)arginine | Amino acid | C12H20N4O8 | 348.1297 | 393.1286 | 10.612 |
| 38 | Negative | 9Z-Octadecenedioic acid | Fatty acid | C18H32O4 | 312.2278 | 311.2204 | 11.488 |
| 39 | Negative | Trilobolide | Terpenoid | C27H38O10 | 522.2548 | 521.2495 | 12.359 |
| 40 | Negative | Thalidasine | Alkaloid | C39H44N2O7 | 652.3179 | 711.3339 | 12.591 |
| 41 | Negative | α-linolenic acid | Fatty acid | C18H30O2 | 278.2223 | 277.2151 | 16.479 |
| 42 | Negative | 16-Hydroxy hexadecanoic acid | Fatty acid | C16H32O3 | 272.2328 | 271.2256 | 16.487 |
| 43 | Negative | 4-(3-Hydroxy-7-phenyl-6-heptenyl)-1,2-benzenediol | - | C19H22O3 | 298.1575 | 297.1505 | 17.381 |
| 44 | Negative | (6beta,8betaOH)-6,8-Dihydroxy-7(11)-eremophilen-12,8-olide | Terpenoids | C15H22O4 | 266.1527 | 265.1458 | 17.524 |
| Entry | 01 | 02 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug-Likeness | ||||||||||||||||||||
| Lipinski | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Bioavailability score | 0.55 | 0.55 | 0.55 | 0.56 | 0.55 | 0.55 | 0.11 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.85 | 0.55 | 0.55 | 0.55 | 0.55 | 0.56 | 0.56 |
| Absorption | ||||||||||||||||||||
| Water solubility | −2.128 | −1.264 | −2.845 | −3.419 | −5.033 | −3.47 | −2.892 | −2.236 | −3.254 | −3.438 | −3.044 | −3.73 | −1.522 | −5.475 | −4.782 | −3.24 | −6.511 | −7.074 | −4.432 | −4.517 |
| Caco2 permeability | 1.638 | 1.747 | 1.183 | 0.903 | 1.159 | 0.639 | −0.55 | −0.382 | 1.077 | 1.031 | 0.684 | 0.812 | 1.86 | 1.597 | 1.245 | 1.142 | 1.525 | 1.55 | 0.538 | 0.603 |
| Intestinal absorption (human) | 95.166 | 93.334 | 91.703 | 91.239 | 90.172 | 83.228 | 0.00 | 29.521 | 91.482 | 90.302 | 94.956 | 90.379 | 97.487 | 94.648 | 98.581 | 88.19 | 90.399 | 90.218 | 70.994 | 83.4 |
| Skin Permeability | −2.164 | −2.165 | −2.743 | −2.717 | −2.752 | −2.777 | −2.735 | −2.735 | −2.735 | −2.786 | −3.917 | −2.738 | −2.107 | −2.717 | −2.672 | −3.106 | −2.565 | −2.725 | −2.735 | −2.734 |
| P-glycoprotein substrate | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | No | Yes | No | No | No | No |
| P-glycoprotein I inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No | Yes | Yes | No | No | No | No |
| P-glycoprotein II inhibitor | No | No | No | No | Yes | No | No | No | No | No | No | No | No | No | Yes | No | No | No | Yes | Yes |
| Distribution | ||||||||||||||||||||
| VDss (human) | 0.0006 | −0.15 | 0.322 | −0.905 | 0.19 | 0.353 | 0.007 | −0.842 | 0.562 | 0.053 | −0.067 | 0.053 | 0.088 | −0.702 | −0.228 | −0.266 | 0.319 | 0.281 | −0.678 | −0.434 |
| BBB permeability | 0.22 | −0.087 | 0.333 | −0.746 | 0.163 | −0.701 | −1.329 | −0.793 | −0.818 | −0.834 | −0.567 | −0.808 | 0.094 | −0.036 | −1.026 | 0.182 | −0.332 | −0.389 | −0.888 | −0.722 |
| CNS permeability | −2.759 | −1.785 | −1.873 | −2.411 | −1.361 | −3.053 | −4.282 | −4.104 | −2.473 | −2.682 | −2.992 | −2.326 | −2.126 | −1.387 | −3.022 | −2.298 | −1.813 | −1.651 | −2.116 | −1.779 |
| Metabolism | ||||||||||||||||||||
| CYP2D6 substrate | No | No | No | No | No | No | No | No | No | No | No | Yes | No | No | No | No | No | No | No | No |
| CYP3A4 substrate | No | No | Yes | No | Yes | No | No | No | No | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| CYP1A2 inhibitor | No | No | Yes | No | Yes | No | No | No | No | No | No | Yes | Yes | Yes | Yes | No | Yes | Yes | No | No |
| CYP2C19 inhibitor | No | No | No | No | Yes | No | No | No | No | No | No | Yes | No | No | Yes | No | No | No | No | No |
| CYP2C9 inhibitor | No | No | No | No | Yes | No | No | No | No | No | No | Yes | No | No | Yes | No | No | No | No | No |
| CYP2D6 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | Yes | No | No | No | No |
| CYP3A4 inhibitor | No | No | No | No | Yes | No | No | No | No | No | No | Yes | No | Yes | Yes | No | No | No | No | No |
| Excretion | ||||||||||||||||||||
| Total clearance | 0.371 | 0.75 | 0.302 | 0.644 | 0.343 | 0.627 | −0.171 | 0.339 | 0.255 | 0.271 | 0.583 | 0.454 | 0.297 | 1.917 | 0.769 | 0.206 | 1.837 | 1.959 | 0.135 | 0.213 |
| Renal OCT2 substrate | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Toxicity (Compound Number) | ||||||||||||||||||||
| AMES toxicity | No | No | Yes | No | Yes | Yes | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Hepatotoxicity | No | No | No | No | Yes | Yes | No | No | No | No | No | Yes | No | Yes | No | No | No | No | Yes | Yes |
| hERG I inhibitors | No | No | No | No | No | No | No | No | No | Yes | No | No | No | No | No | No | No | No | No | No |
| Skin Sensitization | Yes | Yes | Yes | No | Yes | No | No | No | No | No | No | No | Yes | Yes | No | No | Yes | Yes | No | No |
| Entry | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug-Likeness | ||||||||||||||||||||
| Lipinski | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Bioavailability score | 0.11 | 0.56 | 0.55 | 0.56 | 0.55 | 0.56 | 0.55 | 0.17 | 0.11 | 0.55 | 0.17 | 0.55 | 0.11 | 0.55 | 0.56 | 0.55 | 0.11 | 0.85 | 0.55 | 0.55 |
| Absorption | ||||||||||||||||||||
| Water solubility | −2.338 | −0.839 | −1.509 | −3.683 | −2.659 | −3.633 | −3.02 | −2.892 | −2.848 | −3.23 | −2.863 | −2.337 | −2.811 | −5.093 | −3.539 | −3.27 | −2.654 | −3.298 | −4.682 | −4.011 |
| Caco2 permeability | −0.675 | −0.386 | −0.181 | 0.879 | 0.487 | 0.129 | −0.229 | −0.949 | −0.616 | 1.195 | 0.306 | 0.484 | −0.622 | 0.876 | 0.747 | 1.336 | −0.53 | 0.252 | 0.315 | 0.468 |
| Intestinal absorption (human) | 0.00 | 25.105 | 40.251 | 99.42 | 95.178 | 66.637 | 98.322 | 23.446 | 28.681 | 94.251 | 48.052 | 58.69 | 10.102 | 94.476 | 41.903 | 100.00 | 0.00 | 93.188 | 100.00 | 94.002 |
| Skin permeability | −2.735 | −2.735 | −3.121 | −2.889 | −3.023 | −2.734 | −2.735 | −2.735 | −2.735 | −2.806 | −2.735 | −4.388 | −2.735 | −3.641 | −2.728 | −3.337 | −2.735 | −2.735 | −2.833 | −2.735 |
| P-glycoprotein substrate | Yes | No | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | No | Yes | No | Yes | Yes |
| P-glycoprotein I inhibitor | No | No | No | Yes | No | No | Yes | No | No | No | No | No | No | Yes | No | No | No | No | Yes | Yes |
| P-glycoprotein II inhibitor | No | No | No | No | No | No | Yes | No | No | No | No | No | No | Yes | No | No | No | No | No | Yes |
| Distribution | ||||||||||||||||||||
| VDss (human) | −0.572 | −1.009 | −0.026 | −0.234 | −0.056 | −0.929 | −1.284 | −1.663 | 0.683 | −0.15 | 1.444 | −0.098 | −0.83 | −0.367 | −1.174 | −0.003 | −1.001 | −1.452 | −0.384 | −0.297 |
| BBB permeability | −1.774 | −0.879 | −0.611 | −0.845 | −0.254 | −0.424 | −1.851 | −1.889 | −2.225 | −0.823 | −1.514 | −0.169 | −1.874 | −0.568 | −1.032 | −0.293 | −1.353 | −0.441 | −0.739 | −0.171 |
| CNS permeability | −3.935 | −3.155 | −3.205 | −3.099 | −2.462 | −2.326 | −3.239 | −5.178 | −4.043 | −3.272 | −3.908 | −3.031 | −4.424 | −2.094 | −3.545 | −2.892 | −4.274 | −3.007 | −3.479 | −2.421 |
| Metabolism | ||||||||||||||||||||
| CYP2D6 substrate | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| CYP3A4 substrate | No | No | No | Yes | No | Yes | Yes | No | No | No | No | No | No | Yes | No | Yes | No | Yes | No | Yes |
| CYP1A2 inhibitor | No | No | No | No | No | No | No | No | No | Yes | No | No | No | No | No | No | No | No | No | No |
| CYP2C19 inhibitor | No | No | No | No | No | No | Yes | No | No | No | No | No | No | No | No | No | No | No | No | No |
| CYP2C9 inhibitor | No | No | No | No | No | No | Yes | No | No | No | No | No | No | No | No | No | No | No | No | No |
| CYP2D6 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| CYP3A4 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | Yes |
| Excretion | ||||||||||||||||||||
| Total clearance | 0.394 | 0.794 | 0.581 | -0.119 | 0.716 | 0.76 | -0.833 | -0.369 | 0.486 | 0.638 | 0.462 | 0.368 | 0.507 | 0.567 | 2.019 | 0.417 | -0.043 | 1.835 | 0.82 | 0.693 |
| Renal OCT2 substrate | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Toxicity (Compound Number) | ||||||||||||||||||||
| AMES toxicity | No | No | No | No | No | No | No | No | No | No | No | Yes | No | No | No | Yes | No | No | Yes | No |
| Hepatotoxicity | No | No | No | No | No | Yes | Yes | No | No | Yes | No | No | Yes | Yes | No | No | No | No | No | No |
| hERG I inhibitors | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Skin sensitization | No | No | No | No | No | No | No | No | No | No | No | No | No | No | Yes | No | No | Yes | No | No |
| Entry | 41 | 42 | 43 | 44 | ||||||||||||||||
| Drug-Likeness | ||||||||||||||||||||
| Lipinski | Yes | Yes | Yes | Yes | ||||||||||||||||
| Bioavailability score | 0.85 | 0.85 | 0.55 | 0.55 | ||||||||||||||||
| Absorption | ||||||||||||||||||||
| Water solubility | −5.787 | −4.518 | −3.487 | −3.338 | ||||||||||||||||
| Caco2 permeability | 1.577 | −1.458 | −1.108 | 0.955 | ||||||||||||||||
| Intestinal absorption (human) | 92.836 | 91.169 | 90.306 | 95.298 | ||||||||||||||||
| Skin permeability | −2.722 | −2.719 | −2.736 | −3.799 | ||||||||||||||||
| P-glycoprotein substrate | No | No | Yes | No | ||||||||||||||||
| P-glycoprotein I inhibitor | No | No | No | No | ||||||||||||||||
| P-glycoprotein II inhibitor | No | No | Yes | No | ||||||||||||||||
| Distribution | ||||||||||||||||||||
| VDss (human) | −0.617 | −0.796 | 0.635 | −0.148 | ||||||||||||||||
| BBB permeability | −0.115 | −0.36 | 0.014 | −0.315 | ||||||||||||||||
| CNS permeability | −1.547 | −2.99 | −2.236 | −2.252 | ||||||||||||||||
| Metabolism | ||||||||||||||||||||
| CYP2D6 substrate | No | No | No | No | ||||||||||||||||
| CYP3A4 substrate | Yes | Yes | Yes | No | ||||||||||||||||
| CYP1A2 inhibitor | Yes | No | Yes | No | ||||||||||||||||
| CYP2C19 inhibitor | No | No | Yes | No | ||||||||||||||||
| CYP2C9 inhibitor | No | No | Yes | No | ||||||||||||||||
| CYP2D6 inhibitor | No | No | No | No | ||||||||||||||||
| CYP3A4 inhibitor | Yes | No | No | No | ||||||||||||||||
| Excretion | ||||||||||||||||||||
| Total clearance | 1.991 | 1.786 | 0.136 | −1.018 | ||||||||||||||||
| Renal OCT2 substrate | No | No | No | Yes | ||||||||||||||||
| Toxicity (Compound Number) | ||||||||||||||||||||
| AMES toxicity | No | No | No | Yes | ||||||||||||||||
| Hepatotoxicity | Yes | No | No | No | ||||||||||||||||
| hERG I inhibitors | No | No | No | No | ||||||||||||||||
| Skin sensitization | Yes | Yes | No | No | ||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awadelkareem, A.M.; Al-Shammari, E.; Elkhalifa, A.E.O.; Adnan, M.; Siddiqui, A.J.; Snoussi, M.; Khan, M.I.; Azad, Z.R.A.A.; Patel, M.; Ashraf, S.A. Phytochemical and In Silico ADME/Tox Analysis of Eruca sativa Extract with Antioxidant, Antibacterial and Anticancer Potential against Caco-2 and HCT-116 Colorectal Carcinoma Cell Lines. Molecules 2022, 27, 1409. https://doi.org/10.3390/molecules27041409
Awadelkareem AM, Al-Shammari E, Elkhalifa AEO, Adnan M, Siddiqui AJ, Snoussi M, Khan MI, Azad ZRAA, Patel M, Ashraf SA. Phytochemical and In Silico ADME/Tox Analysis of Eruca sativa Extract with Antioxidant, Antibacterial and Anticancer Potential against Caco-2 and HCT-116 Colorectal Carcinoma Cell Lines. Molecules. 2022; 27(4):1409. https://doi.org/10.3390/molecules27041409
Chicago/Turabian StyleAwadelkareem, Amir Mahgoub, Eyad Al-Shammari, Abd Elmoneim O. Elkhalifa, Mohd Adnan, Arif Jamal Siddiqui, Mejdi Snoussi, Mohammad Idreesh Khan, Z R Azaz Ahmad Azad, Mitesh Patel, and Syed Amir Ashraf. 2022. "Phytochemical and In Silico ADME/Tox Analysis of Eruca sativa Extract with Antioxidant, Antibacterial and Anticancer Potential against Caco-2 and HCT-116 Colorectal Carcinoma Cell Lines" Molecules 27, no. 4: 1409. https://doi.org/10.3390/molecules27041409
APA StyleAwadelkareem, A. M., Al-Shammari, E., Elkhalifa, A. E. O., Adnan, M., Siddiqui, A. J., Snoussi, M., Khan, M. I., Azad, Z. R. A. A., Patel, M., & Ashraf, S. A. (2022). Phytochemical and In Silico ADME/Tox Analysis of Eruca sativa Extract with Antioxidant, Antibacterial and Anticancer Potential against Caco-2 and HCT-116 Colorectal Carcinoma Cell Lines. Molecules, 27(4), 1409. https://doi.org/10.3390/molecules27041409













