Multilayered Curcumin-Loaded Hydrogel Microcarriers with Antimicrobial Function
Abstract
:1. Introduction
2. Results and Discussion
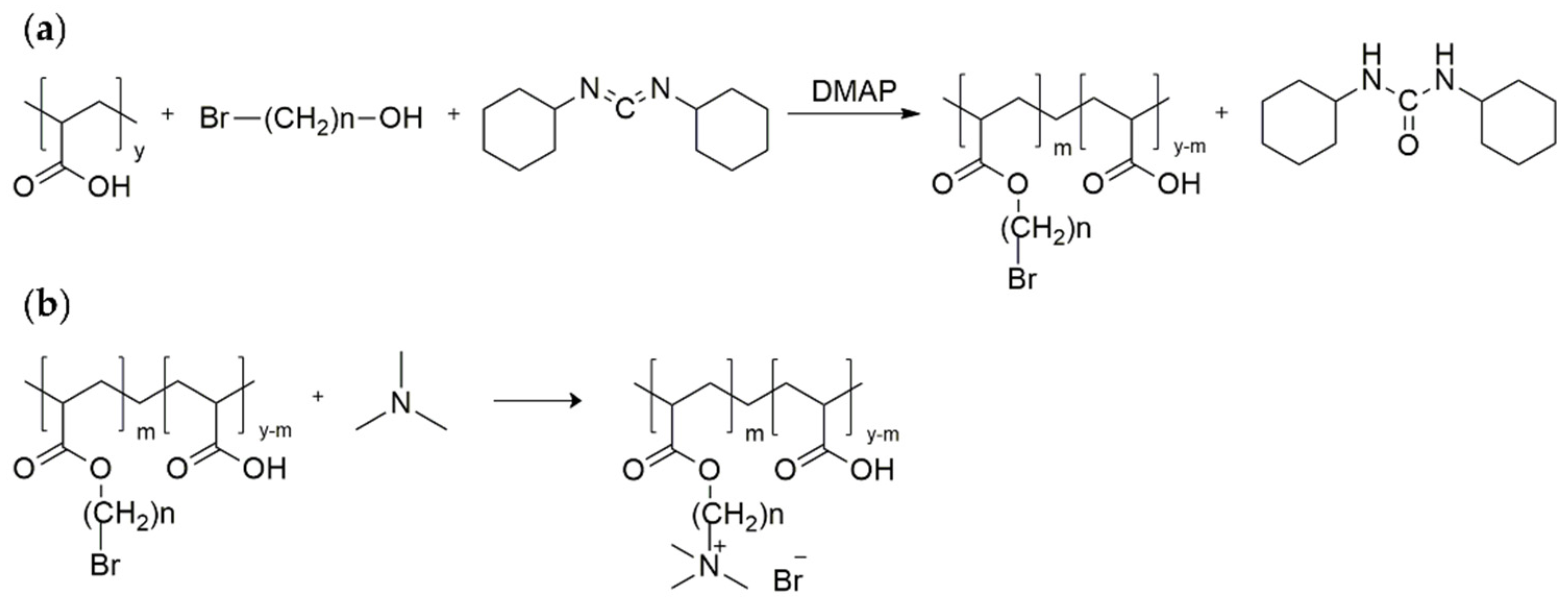
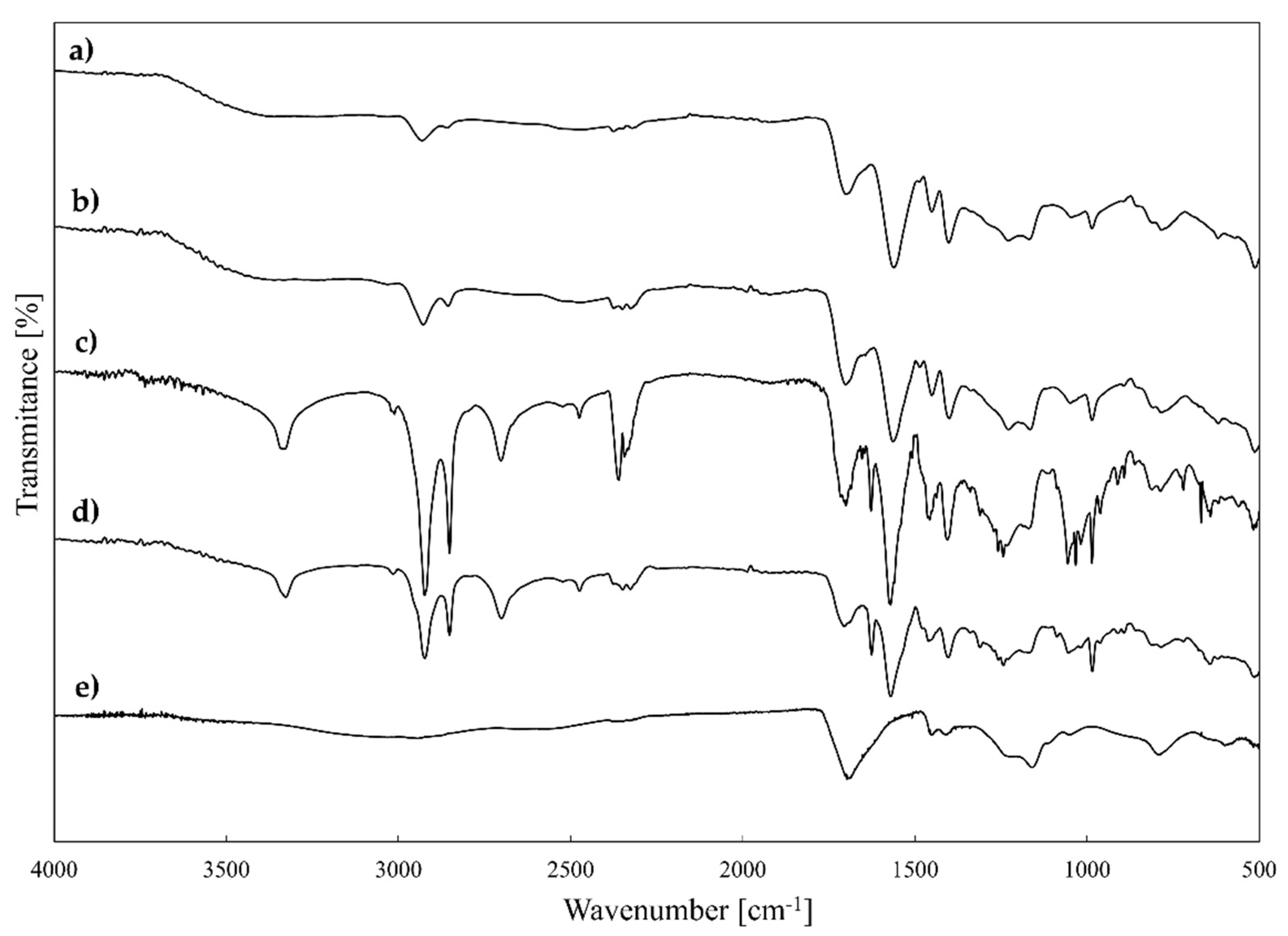
2.1. Design, Grafting, and Characterisation of Poly(acrylic acid) Containing Alkylene Quaternary Ammonium Groupings
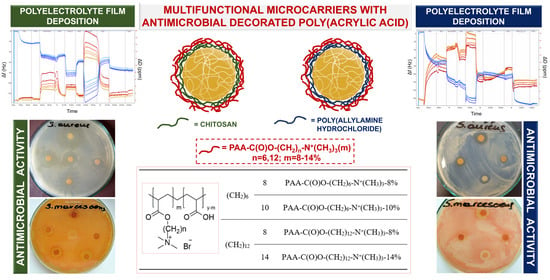
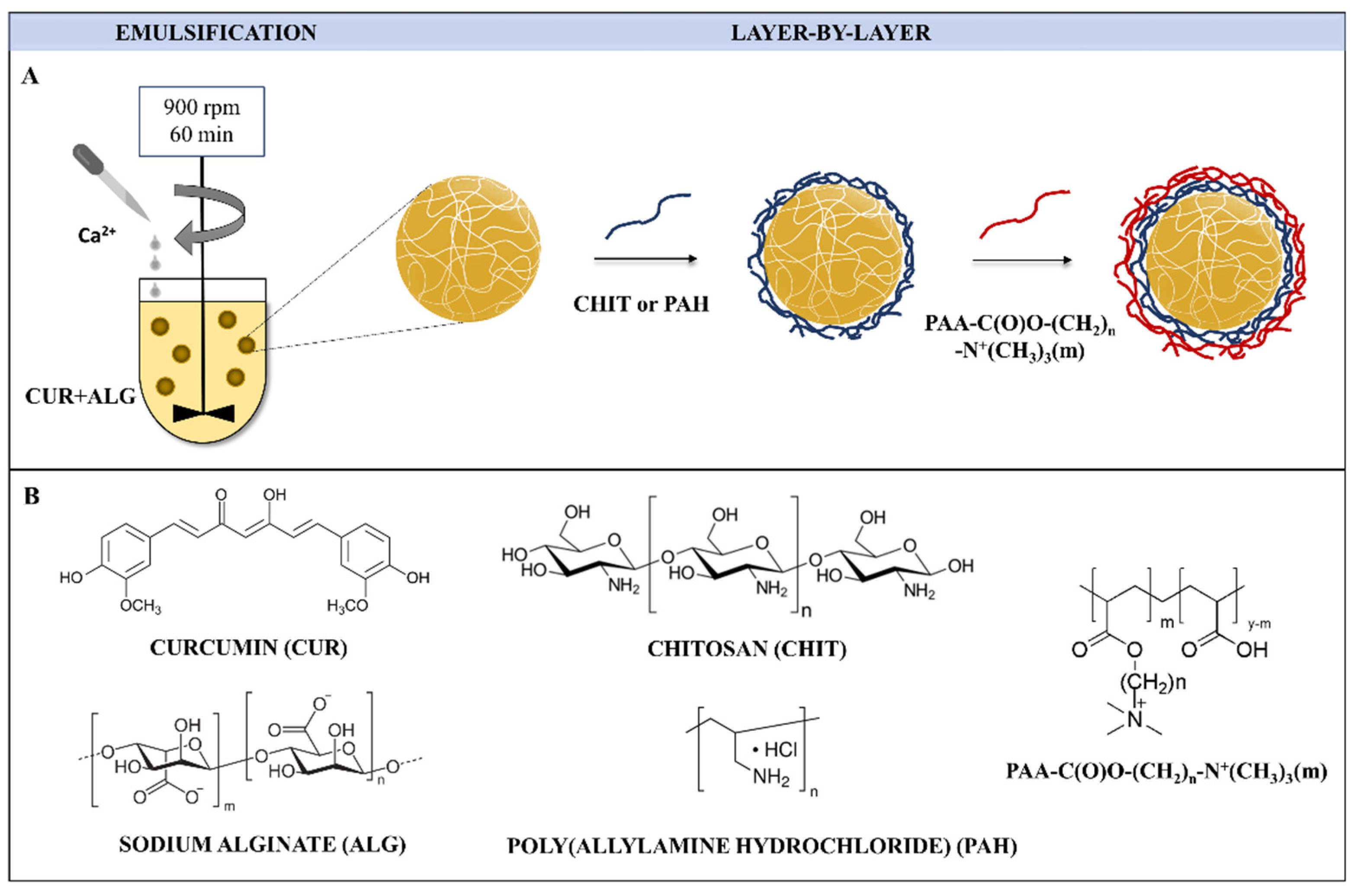
2.2. Characterisation of the Obtained Multifunctional Microcarriers
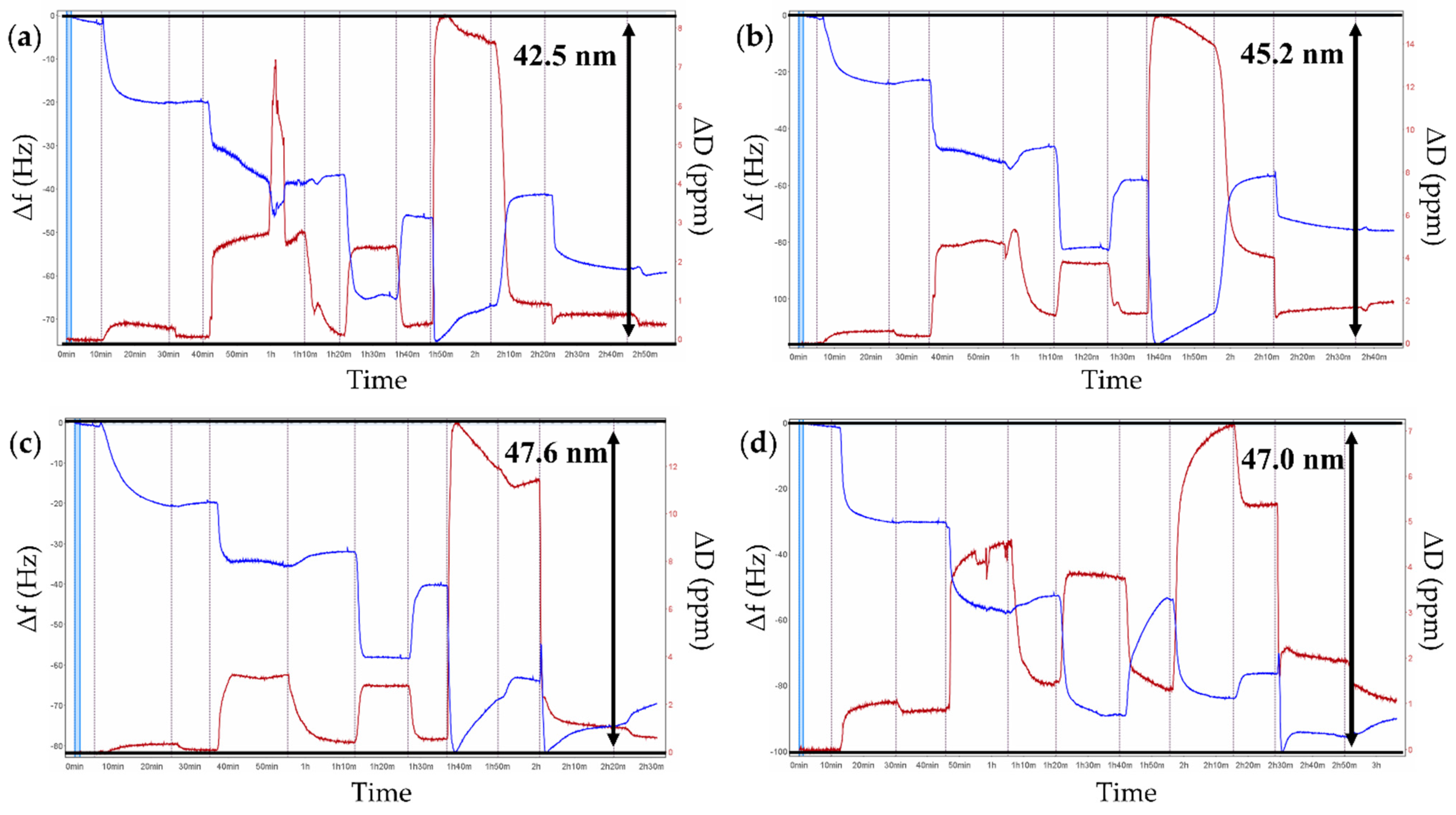
2.3. Features of the LbL Coatings Studied by QCM-D, Ellipsometry, and Zeta Potential Measurements
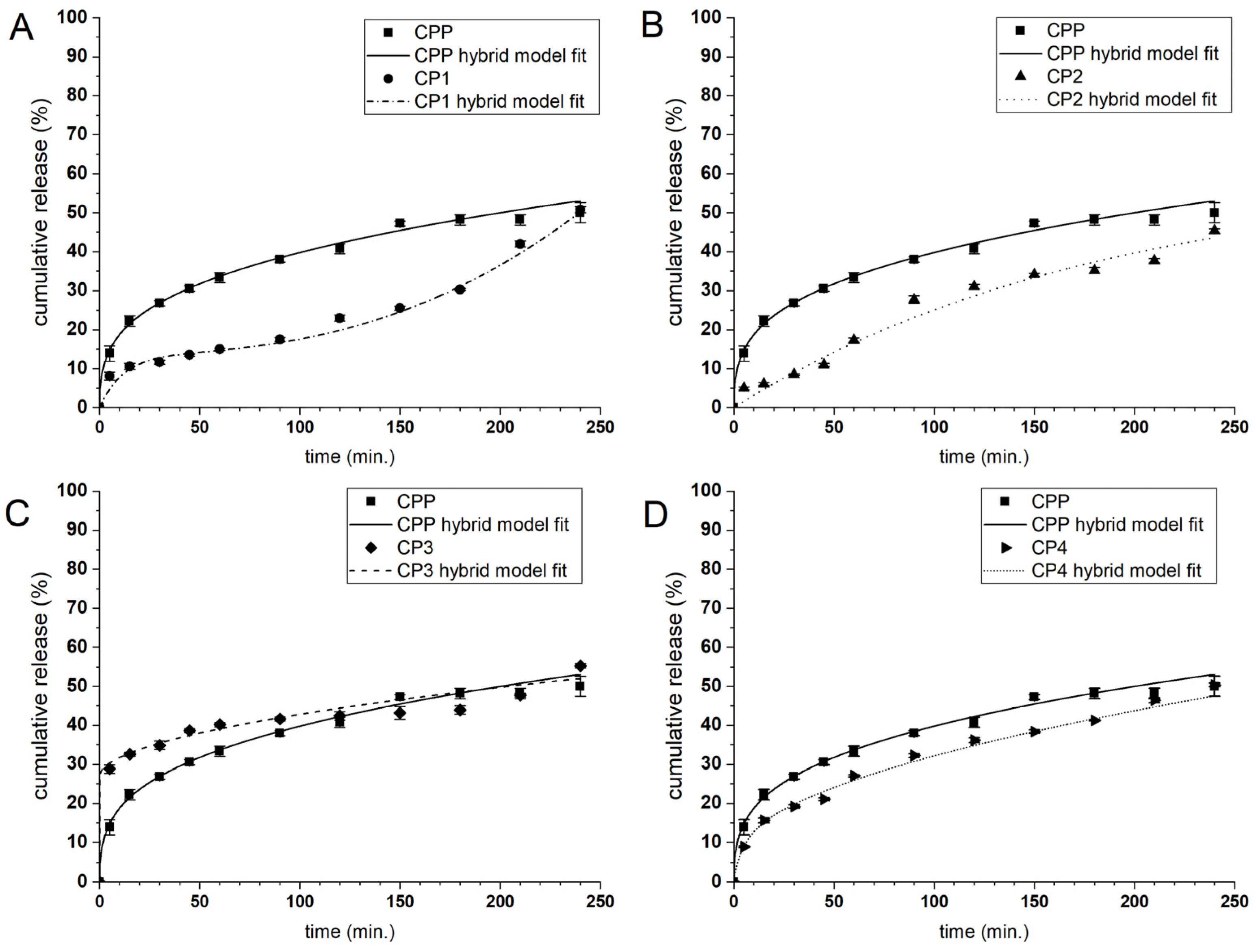
2.4. Curcumin Release Behavior
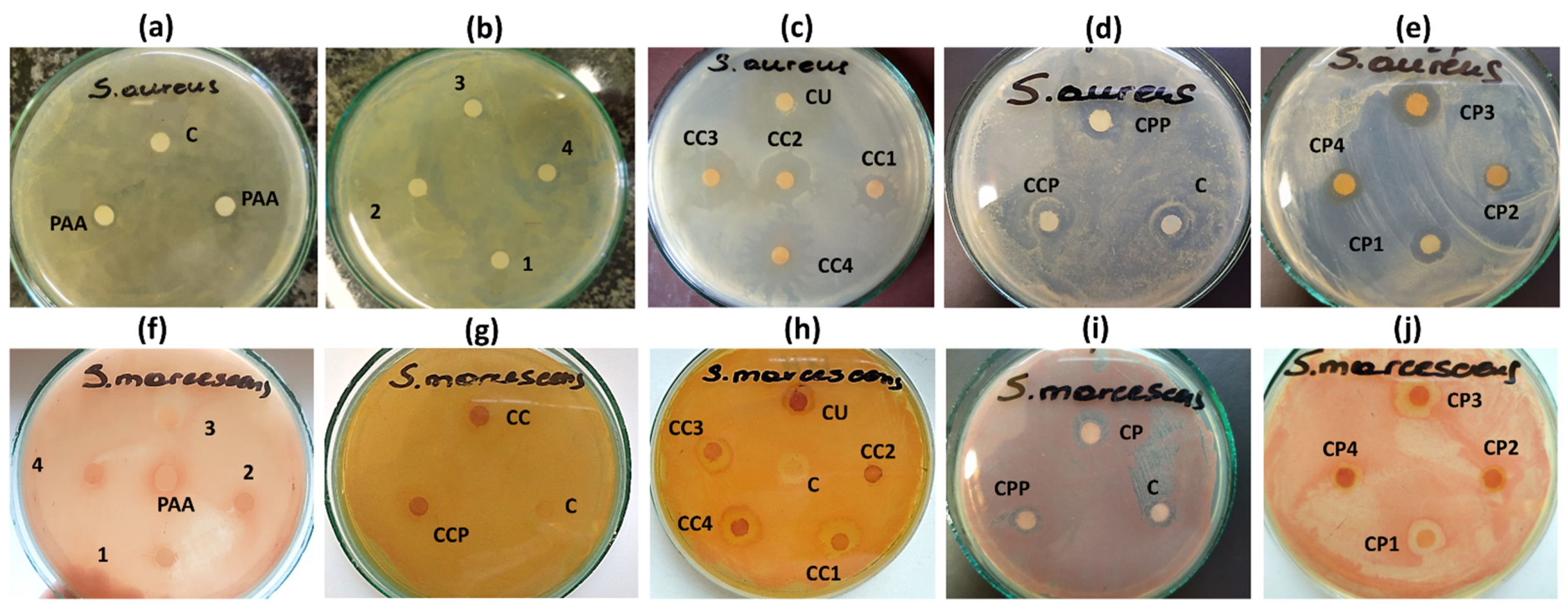
2.5. Antimicrobial Activity of Bilayer CUR-Loaded Hydrogel Microcarriers with Antimicrobial Decorated Poly(acrylic acid) as the Outer Layer
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the Poly(acrylic acid) Containing Alkylene Quaternary Ammonium Groupings
3.3. 1H NMR Spectroscopic Analysis
3.4. FTIR Spectroscopic Analysis
3.5. Formation of Microspheres
3.6. Layer-by-Layer Microparticles Coating
3.7. Morphology and Particle Size
3.8. Encapsulation Efficiency
3.9. QCM-D Analysis
3.10. Zeta Potential
3.11. Spectroscopic Ellipsometry Analysis
3.12. Release Profiles
3.13. Antimicrobial Activity—Disc Diffusion Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bansal, R.; Pathak, R.; Kumar, B.; Gautam, H.K.; Kumar, P. Enhanced Antimicrobial Activity of Amphiphilic Cationic Polymers against a Broad Range of Bacterial Strains and Skin Microbes. Colloid Polym. Sci. 2017, 295, 1177–1185. [Google Scholar] [CrossRef]
- Villalobos, V.; Leiva, Á.; Ríos, H.E.; Pavez, J.; Silva, C.P.; Ahmar, M.; Queneau, Y.; Blamey, J.M.; Chávez, F.P.; Urzúa, M.D. Inhibiting Pathogen Surface Adherence by Multilayer Polyelectrolyte Films Functionalized with Glucofuranose Derivatives. ACS Appl. Mater. Interfaces 2018, 10, 28147–28158. [Google Scholar] [CrossRef] [PubMed]
- Al-Awady, M.J.; Fauchet, A.; Greenway, G.M.; Paunov, V.N. Enhanced Antimicrobial Effect of Berberine in Nanogel Carriers with Cationic Surface Functionality. J. Mater. Chem. B 2017, 5, 7885–7897. [Google Scholar] [CrossRef]
- Ivanova, A.; Ivanova, K.; Hoyo, J.; Heinze, T.; Sanchez-Gomez, S.; Tzanov, T. Layer-By-Layer Decorated Nanoparticles with Tunable Antibacterial and Antibiofilm Properties against Both Gram-Positive and Gram-Negative Bacteria. ACS Appl. Mater. Interfaces 2018, 10, 3314–3323. [Google Scholar] [CrossRef] [PubMed]
- Decher, G.; Schlenoff, J.B. Multilayer Thin Films: Sequential Assembly of Nanocomposite Materials, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Wurster, E.C.; Elbakry, A.; Göpferich, A.; Breunig, M. Layer-by-Layer Assembled Gold Nanoparticles for the Delivery of Nucleic Acids. Methods Mol. Biol. 2013, 948, 171–182. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, Y.; Liang, J.; Libera, M.R.; Sukhishvili, S.A. Self-Defensive Antibacterial Layer-by-Layer Hydrogel Coatings with PH-Triggered Hydrophobicity. Biomaterials 2015, 45, 64–71. [Google Scholar] [CrossRef]
- Jin, Y.; Zhou, Q.; Li, Z.; Yang, Z.; Fan, H.J.S. Calcium-Cross Linked Polysaccharide Microcapsules for Controlled Release and Antimicrobial Applications. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 125025. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I.; Nazari, Z.; Mobini, P.; Mahmoudi Khatir, N. Investigation of Acyclovir-Loaded, Acrylamide-Based Hydrogels for Potential Use as Vaginal Ring. Mater. Today Commun. 2018, 16, 274–280. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I. Physical and Chemical Characterisation of Acrylamide-Based Hydrogels, Aam, Aam/NaCMC and Aam/NaCMC/MgO. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1439–1449. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I.; Nazari, Z.; Mobini, P.; Taraghdari, S.B. From Formulation of Acrylamide-Based Hydrogels to Their Optimization for Drug Release Using Response Surface Methodology. Mater. Sci. Eng. C 2018, 92, 20–25. [Google Scholar] [CrossRef]
- Budinčić, J.M.; Petrović, L.; Đekić, L.; Fraj, J.; Bučko, S.; Katona, J.; Spasojević, L. Study of Vitamin E Microencapsulation and Controlled Release from Chitosan/Sodium Lauryl Ether Sulfate Microcapsules. Carbohydr. Polym. 2021, 251, 116988. [Google Scholar] [CrossRef]
- Iqbal, M.H.; Schroder, A.; Kerdjoudj, H.; Njel, C.; Senger, B.; Ball, V.; Meyer, F.; Boulmedais, F. Effect of the Buffer on the Buildup and Stability of Tannic Acid/Collagen Multilayer Films Applied as Antibacterial Coatings. ACS Appl. Mater. Interfaces 2020, 12, 22601–22612. [Google Scholar] [CrossRef] [PubMed]
- Kougia, E.; Tselepi, M.; Vasilopoulos, G.; Lainioti, G.C.; Koromilas, N.D.; Druvari, D.; Bokias, G.; Vantarakis, A.; Kallitsis, J.K.; McPhee, D.J. Evaluation of Antimicrobial Efficiency of New Polymers Comprised by Covalently Attached and/or Electrostatically Bound Bacteriostatic Species, Based on Quaternary Ammonium Compounds. Molecules 2015, 20, 21313–21327. [Google Scholar] [CrossRef] [PubMed]
- Kiss, É.; Heine, E.T.; Hill, K.; He, Y.C.; Keusgen, N.; Pénzes, C.B.; Schnöller, D.; Gyulai, G.A.; Mendrek, A.; Keul, H.; et al. Membrane Affinity and Antibacterial Properties of Cationic Polyelectrolytes with Different Hydrophobicity. Macromol. Biosci. 2012, 12, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Farah, S.; Aviv, O.; Laout, N.; Ratner, S.; Beyth, N.; Domb, A.J. Quaternary Ammonium Poly(Diethylaminoethyl Methacrylate) Possessing Antimicrobial Activity. Colloids Surf. B Biointerfaces 2015, 128, 608–613. [Google Scholar] [CrossRef]
- Druvari, D.; Koromilas, N.D.; Lainioti, G.C.; Bokias, G.; Vasilopoulos, G.; Vantarakis, A.; Baras, I.; Dourala, N.; Kallitsis, J.K. Polymeric Quaternary Ammonium-Containing Coatings with Potential Dual Contact-Based and Release-Based Antimicrobial Activity. ACS Appl. Mater. Interfaces 2016, 8, 35593–35605. [Google Scholar] [CrossRef]
- Olea, A.F. Hydrophobic Polyelectrolytes. In Ionic Interactions in Natural and Synthetic Macromolecules; John Wiley and Sons: Hoboken, NJ, USA, 2012; pp. 211–233. [Google Scholar] [CrossRef]
- Sun, L.; Ma, S.; Wang, C.; Chi, Y.; Dong, J. Supramolecular Self-Assembly of a Polyelectrolyte Chain Based on Step-Growth Polymerization of Hydrophobic and Hydrophilic Monomers. RSC Adv. 2017, 7, 52832–52840. [Google Scholar] [CrossRef] [Green Version]
- Aricov, L.; Petkova, H.; Arabadzhieva, D.; Iovescu, A.; Mileva, E.; Khristov, K.; Stinga, G.; Mihailescu, C.F.; Anghel, D.F.; Todorov, R. Aqueous Solutions of Associative Poly(Acrylates): Bulk and Interfacial Properties. Colloids Surf. A Physicochem. Eng. Asp. 2016, 505, 138–149. [Google Scholar] [CrossRef]
- Janovák, L.; Turcsányi, Á.; Bozó, É.; Deák, Á.; Mérai, L.; Sebők, D.; Juhász, Á.; Csapó, E.; Abdelghafour, M.M.; Farkas, E.; et al. Preparation of Novel Tissue Acidosis-Responsive Chitosan Drug Nanoparticles: Characterization and in Vitro Release Properties of Ca2+ Channel Blocker Nimodipine Drug Molecules. Eur. J. Pharm. Sci. 2018, 123, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Lamch, L.; Ronka, S.; Moszynśka, I.; Warszynśki, P.; Wilk, K.A. Hydrophobically Functionalized Poly(Acrylic Acid) Comprising the Ester-Type Labile Spacer: Synthesis and Self-Organization in Water. Polymers 2020, 12, 1185. [Google Scholar] [CrossRef]
- Lamch, Ł.; Ronka, S.; Warszyński, P.; Wilk, K.A. NMR Studies of Self-Organization Behavior of Hydrophobically Functionalized Poly(4-Styrenosulfonic-Co-Maleic Acid) in Aqueous Solution. J. Mol. Liq. 2020, 308, 112990. [Google Scholar] [CrossRef]
- Wawrzyńczyk, D.; Bazylińska, U.; Lamch, Ł.; Kulbacka, J.; Szewczyk, A.; Bednarkiewicz, A.; Wilk, K.A.; Samoć, M. FÖrster Resonance Energy Transfer-Activated Processes in Smart Nanotheranostics Fabricated in a Sustainable Manner. ChemSusChem 2019, 12, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Belbekhouche, S.; Bousserrhine, N.; Alphonse, V.; Carbonnier, B. From Beta-Cyclodextrin Polyelectrolyte to Layer-by-Layer Self-Assembly Microcapsules: From Inhibition of Bacterial Growth to Bactericidal Effect. Food Hydrocoll. 2019, 95, 219–227. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Obayes, H.R.; Mohamad, A.B. Synthesis and Antioxidant Activities of Novel 5-Chlorocurcumin, Complemented by Semiempirical Calculations. Bioinorg. Chem. Appl. 2013, 2013, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wezgowiec, J.; Tsirigotis-Maniecka, M.; Saczko, J.; Wieckiewicz, M.; Wilk, K.A. Microparticles vs. Macroparticles as Curcumin Delivery Vehicles: Structural Studies and Cytotoxic Effect in Human Adenocarcinoma Cell Line (Lovo). Molecules 2021, 26. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Phonrat, B.; Tungtrongchitr, R.; Jirawatnotai, S. Reduction of Atherogenic Risk in Patients with Type 2 Diabetes by Curcuminoid Extract: A Randomized Controlled Trial. J. Nutr. Biochem. 2014, 25, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, S.; Fatima, K.; Hassanain, M.; Behera, C.; Kour, A.; Singh, A.; Luqman, S.; Sarkar, J.; Chanda, D.; Shanker, K.; et al. Antiproliferative Efficacy of Curcumin Mimics through Microtubule Destabilization. Eur. J. Med. Chem. 2018, 151, 51–61. [Google Scholar] [CrossRef]
- Khayyal, M.T.; El-Hazek, R.M.; El-Sabbagh, W.A.; Frank, J.; Behnam, D.; Abdel-Tawab, M. Micellar Solubilisation Enhances the Antiinflammatory Activities of Curcumin and Boswellic Acids in Rats with Adjuvant-Induced Arthritis. Nutrition 2018, 54, 189–196. [Google Scholar] [CrossRef]
- Salarbashi, D.; Tafaghodi, M.; Fathi, M.; Aboutorabzade, S.M.; Sabbagh, F. Development of Curcumin-Loaded Prunus Armeniaca Gum Nanoparticles: Synthesis, Characterization, Control Release Behavior, and Evaluation of Anticancer and Antimicrobial Properties. Food Sci. Nutr. 2021, 9, 6109–6119. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, F.; Yao, F.; Ma, R.; Zhang, Y.; Mao, S.; Hu, B.; Ma, G.; Zhu, Y. Study of Dietary Curcumin on the Restorative Effect of Liver Injury Induced by Carbon Tetrachloride in Common Carp, Cyprinus Carpio. Aquac. Rep. 2021, 21, 100825. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. Curcumin, a Component of Golden Spice: From Bedside to Bench and Back. In Biotechnology Advances; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 1053–1064. [Google Scholar] [CrossRef]
- Yuan, B.; Sun, M.; Bao, C.; Li, X.; Liu, Z.; Manxia, W. Dynamic Expression of Autophagy-Related Factors in Autoimmune Encephalomyelitis and Exploration of Curcumin Therapy. J. Neuroimmunol. 2019, 337, 577067. [Google Scholar] [CrossRef]
- Sahebkar, A. Are Curcuminoids Effective C-Reactive Protein-Lowering Agents in Clinical Practice? Evidence from a Meta-Analysis. In Phytotherapy Research; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2014; pp. 633–642. [Google Scholar] [CrossRef]
- Mahmood, K.; Zia, K.M.; Zuber, M.; Salman, M.; Anjum, M.N. Recent Developments in Curcumin and Curcumin Based Polymeric Materials for Biomedical Applications: A Review. Int. J. Biol. Macromol. 2015, 81, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Szczęsna, W.; Tsirigotis-Maniecka, M.; Szyk-Warszyńska, L.; Balicki, S.; Warszyński, P.; Wilk, K.A. Insight into Multilayered Alginate/Chitosan Microparticles for Oral Administration of Large Cranberry Fruit Extract. Eur. Polym. J. 2021, 160, 110776. [Google Scholar] [CrossRef]
- Tsirigotis-Maniecka, M.; Szyk-Warszyńska, L.; Maniecki, Ł.; Szczęsna, W.; Warszyński, P.; Wilk, K.A. Tailoring the Composition of Hydrogel Particles for the Controlled Delivery of Phytopharmaceuticals. Eur. Polym. J. 2021, 151, 110429. [Google Scholar] [CrossRef]
- Özkan, M.; Kumar, Y.; Keser, Y.; Hadi, S.E.; Tuncel, D. Cucurbit[7]Uril-Anchored Porphyrin-Based Multifunctional Molecular Platform for Photodynamic Antimicrobial and Cancer Therapy. ACS Appl. Bio Mater. 2019, 2, 4693–4697. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Niu, W.; Wang, M.; Chen, M.; Guo, Y.; Lei, B. Engineering a Biodegradable Multifunctional Antibacterial Bioactive Nanosystem for Enhancing Tumor Photothermo-Chemotherapy and Bone Regeneration. ACS Nano 2020, 14, 442–453. [Google Scholar] [CrossRef]
- Lamch, Ł.; Pucek, A.; Kulbacka, J.; Chudy, M.; Jastrzębska, E.; Tokarska, K.; Bułka, M.; Brzózka, Z.; Wilk, K.A. Recent Progress in the Engineering of Multifunctional Colloidal Nanoparticles for Enhanced Photodynamic Therapy and Bioimaging. Adv. Colloid Interface Sci. 2018, 261, 62–81. [Google Scholar] [CrossRef]
- Kurapati, R.; Groth, T.W.; Raichur, A.M. Recent Developments in Layer-by-Layer Technique for Drug Delivery Applications. ACS Appl. Bio Mater. 2019, 2, 5512–5527. [Google Scholar] [CrossRef]
- Séon, L.; Lavalle, P.; Schaaf, P.; Boulmedais, F. Polyelectrolyte Multilayers: A Versatile Tool for Preparing Antimicrobial Coatings. Langmuir 2015, 31, 12856–12872. [Google Scholar] [CrossRef]
- Lichter, J.A.; Rubner, M.F. Polyelectrolyte Multilayers with Intrinsic Antimicrobial Functionality: The Importance of Mobile Polycations. Langmuir 2009, 25, 7686–7694. [Google Scholar] [CrossRef]
- Kruk, T.; Szczepanowicz, K.; Kręgiel, D.; Szyk-Warszyńska, L.; Warszyński, P. Nanostructured Multilayer Polyelectrolyte Films with Silver Nanoparticles as Antibacterial Coatings. Colloids Surf. B Biointerfaces 2016, 137, 158–166. [Google Scholar] [CrossRef]
- Kim, B.S.; Park, S.W.; Hammond, P.T. Hydrogen-Bonding Layer-by-Layer-Assembled Biodegradable Polymeric Micelles as Drug Delivery Vehicles from Surfaces. ACS Nano 2008, 2, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Lichter, J.A.; van Vlietpa, K.J.; Rubner, M.F. Design of Antibacterial Surfaces and Interfaces: Polyelectrolyte Multilayers as a Multifunctional Platform. Macromolecules 2009, 42, 8573–8586. [Google Scholar] [CrossRef]
- Siedenbiedel, F.; Tiller, J.C. Antimicrobial Polymers in Solution and on Surfaces: Overview and Functional Principles. Polymers 2012, 4, 46–71. [Google Scholar] [CrossRef] [Green Version]
- Waschinski, C.J.; Herdes, V.; Schueler, F.; Tiller, J.C. Influence of Satellite Groups on Telechelic Antimicrobial Functions of Polyoxazolines. Macromol. Biosci. 2005, 5, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Tartakovsky, A.; Drutis, D.M.; Carnali, J.O. The Adsorption of Cationic and Amphoteric Copolymers on Glass Surfaces: Zeta Potential Measurements, Adsorption Isotherm Determination, and FT Raman Characterization. J. Colloid Interface Sci. 2003, 263, 408–419. [Google Scholar] [CrossRef]
- Popescu, I.; Pelin, I.M.; Ailiesei, G.L.; Ichim, D.L.; Suflet, D.M. Amphiphilic Polysaccharide Based on Curdlan: Synthesis and Behaviour in Aqueous Solution. Carbohydr. Polym. 2019, 224, 115157. [Google Scholar] [CrossRef]
- Tsirigotis-Maniecka, M.; Lamch, Ł.; Chojnacka, I.; Gancarz, R.; Wilk, K.A. Microencapsulation of Hesperidin in Polyelectrolyte Complex Microbeads: Physico-Chemical Evaluation and Release Behavior. J. Food Eng. 2017, 214, 104–116. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Tsirigotis-Maniecka, M.; Szyk-Warszyńska, L.; Lamch, Ł.; Weżgowiec, J.; Warszyński, P.; Wilk, K.A. Benefits of PH-Responsive Polyelectrolyte Coatings for Carboxymethyl Cellulose-Based Microparticles in the Controlled Release of Esculin. Mater. Sci. Eng. C 2021, 118, 111397. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarika, P.R.; James, N.R.; Anil kumar, P.R.; Raj, D.K. Preparation, Characterization and Biological Evaluation of Curcumin Loaded Alginate Aldehyde–Gelatin Nanogels. Mater. Sci. Eng. C 2016, 68, 251–257. [Google Scholar] [CrossRef]
- Sorita, G.D.; Santamaria-Echart, A.; Gozzo, A.M.; Gonçalves, O.H.; Leimann, F.v.; Bona, E.; Manrique, Y.; Fernandes, I.P.M.; Ferreira, I.C.F.R.; Barreiro, M.F. Lipid Composition Optimization in Spray Congealing Technique and Testing with Curcumin-Loaded Microparticles. Adv. Powder Technol. 2021, 32, 1710–1722. [Google Scholar] [CrossRef]
- Sundaramurthy, A.; Sundramoorthy, A.K. Polyelectrolyte Capsules Preloaded with Interconnected Alginate Matrix: An Effective Capsule System for Encapsulation and Release of Macromolecules. Int. J. Biol. Macromol. 2018, 107, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between Alginate and Chitosan Biopolymers Characterized Using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef]
- Notley, S.M.; Eriksson, M.; Wågberg, L. Visco-Elastic and Adhesive Properties of Adsorbed Polyelectrolyte Multilayers Determined In Situ with QCM-D and AFM Measurements. J. Colloid Interface Sci. 2005, 292, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Tsirigotis-Maniecka, M.; Szyk-Warszyńska, L.; Michna, A.; Warszyński, P.; Wilk, K.A. Colloidal Characteristics and Functionality of Rationally Designed Esculin-Loaded Hydrogel Microcapsules. J. Colloid Interface Sci. 2018, 530, 444–458. [Google Scholar] [CrossRef]
- Korsmqer, R.W.; Gumy, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of Solute Release from Porous Hydrophilic Polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Steigman, J.; Sussman, D. Acid-Base Reactions in Concentrated Aqueous Quaternary Ammonium Salt Solutions. I. Strong Acids and Bases, Carboxylic Acids, Amines, and Phenol. J. Am. Chem. Soc. 1967, 89, 6400–6406. [Google Scholar] [CrossRef]
- Uppu, D.S.S.M.; Samaddar, S.; Ghosh, C.; Paramanandham, K.; Shome, B.R.; Haldar, J. Amide Side Chain Amphiphilic Polymers Disrupt Surface Established Bacterial Bio-Films and Protect Mice from Chronic Acinetobacter Baumannii Infection. Biomaterials 2016, 74, 131–143. [Google Scholar] [CrossRef]



| No | Structure | (CH2)n | % Degree of Substitution (m) | Abbreviation |
|---|---|---|---|---|
| 1 | 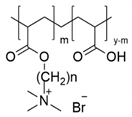 | (CH2)6 | 8 | PAA-C(O)O-(CH2)6-N+(CH3)3-8% |
| 2 | 10 | PAA-C(O)O-(CH2)6-N+(CH3)3-10% | ||
| 3 | (CH2)12 | 8 | PAA-C(O)O-(CH2)12-N+(CH3)3-8% | |
| 4 | 14 | PAA-C(O)O-(CH2)12-N+(CH3)3-14% |
| CHIT System | PAH System | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Micro-Particles | Composition of PE Coatings | MD a [µm] | PdI b | EE c [%] | Micro-Particles | Composition of PE Coatings | MD a [µm] | PdI b | EE c [%] |
| CU | uncoated | 45.9 ± 4.9 | 0.225 | 56.1 ± 3.8 | CU | uncoated | 45.9 ± 4.9 | 0.225 | 56.1 ± 3.8 |
| CC | CHIT | 48.9 ± 5.1 | 0.159 | 58.5 ± 4.8 | CP | PAH | 60.7 ± 6.1 | 0.108 | 52.2 ± 1.8 |
| CCP | CHIT/PAA | 47.3 ± 5.9 | 0.208 | 56.7 ± 6.4 | CPP | PAH/PAA | 59.0 ± 5.4 | 0.072 | 60.5 ± 2.5 |
| CC1 | CHIT/1 * | 46.2 ± 4.8 | 0.101 | 48.7 ± 2.3 | CP1 | PAH/1 * | 57.2 ± 4.0 | 0.044 | 53.8 ± 3.2 |
| CC2 | CHIT/2 * | 50.9 ± 6.0 | 0.121 | 53.9 ± 2.9 | CP2 | PAH/2 * | 56.9 ± 6.3 | 0.085 | 45.8 ± 1.9 |
| CC3 | CHIT/3 * | 50.0 ± 4.4 | 0.122 | 48.3 ± 3.0 | CP3 | PAH/3 * | 59.5 ± 5.7 | 0.066 | 44.6 ± 1.1 |
| CC4 | CHIT/4 * | 48.1 ± 5.1 | 0.103 | 55.4 ± 1.7 | CP4 | PAH/4 * | 58.9 ± 6.2 | 0.078 | 56.2 ± 2.7 |
| PE Layer | Thickness [nm] | Areal Mass [µg/cm2] | Viscosity [g/ms2] | Zeta Potential [mV] | |
|---|---|---|---|---|---|
| CHIT system | CHIT | 17.9 ± 2.2 | 1.9 ± 0.2 | 2.5 ± 0.4 | 41 ± 5.1 |
| PAA | 9.6 ± 3.0 | 1.0 ± 0.3 | 6.1 ± 0.9 | −17.3 ± 1.0 | |
| PAA-C(O)O-(CH2)6-N+(CH3)3-8% | 10.6 ± 0.1 | 1.1 ± 0.1 | 7.9 ± 1.8 | −31 ± 5 | |
| PAA-C(O)O-(CH2)6-N+(CH3)3-10% | 13.3 ± 0.5 | 1.3 ± 0.1 | 4.9 ± 2.1 | −27 ± 1.1 | |
| PAA-C(O)O-(CH2)12-N+(CH3)3-8% | 15.7 ± 3.9 | 1.7 ± 0.4 | 7.3 ± 1.0 | −32.5 ± 2.0 | |
| PAA-C(O)O-(CH2)12-N+(CH3)3-14% | 15.1 ± 1.7 | 1.6 ± 0.2 | 7.1 ± 2.7 | −36 ± 2.5 | |
| PAH system | PAH | 22.8 ± 3.4 | 2.8 ± 0.6 | 2.1 ± 0.5 | 36 ± 2.8 |
| PAA | 13.2 ± 2.5 | 1.3 ± 0.7 | 3.4 ± 0.4 | −17.3 ± 1.0 | |
| PAA-C(O)O-(CH2)6-N+(CH3)3-8% | 14.7 ± 1.3 | 1.5 ± 0.2 | 4.9 ± 0.6 | −31 ± 5 | |
| PAA-C(O)O-(CH2)6-N+(CH3)3-10% | 16.7 ± 1.7 | 1.8 ± 0.3 | 5.4 ± 1.6 | −26.7 ± 1.1 | |
| PAA-C(O)O-(CH2)12-N+(CH3)3-8% | 20.5 ± 3.5 | 2.1 ± 0.4 | 3.2 ± 0.6 | −33 ± 1.7 | |
| PAA-C(O)O-(CH2)12-N+(CH3)3-14% | 18.9 ± 4.0 | 1.9 ± 0.6 | 3.1 ± 0.9 | −36 ± 2.4 |
| Microparticles | M | k1 | k2 | n | R2 | |
|---|---|---|---|---|---|---|
| CHIT system | CU | 0.005 | 1.00 × 10−3 | 3.2 × 10−1 | 0.18 | 0.977 |
| CC | 0.005 | 1.00 × 10−3 | 3.9 × 10−1 | 0.16 | 0.980 | |
| CCP | 0.005 | 1.00 × 10−3 | 2.3 × 10−1 | 0.22 | 0.992 | |
| CC1 | 1.420 | 1.00 × 10−3 | 9.3 × 10−1 | 0.20 | 0.995 | |
| CC2 | 1.127 | 1.00 × 10−3 | 1.8 × 10−1 | 0.15 | 0.973 | |
| CC3 | 0.005 | 1.00 × 10−3 | 2.3 × 10−1 | 0.20 | 0.985 | |
| CC4 | 0.005 | 1.00 × 10−3 | 2.2 × 10−1 | 0.21 | 0.985 | |
| PAH system | CP | 1.000 | 1.94 × 10−3 | 1.4 × 10−1 | 0.18 | 0.985 |
| CPP | 0.005 | 1.00 × 10−3 | 9.2 × 10−2 | 0.31 | 0.997 | |
| CP1 | 0.005 | 1.00 × 10−3 | 5.8 × 10−3 | 0.79 | 0.953 | |
| CP2 | 0.005 | 1.00 × 10−3 | 1.1 × 10−2 | 0.67 | 0.987 | |
| CP3 | 0.005 | 1.00 × 10−3 | 1.9 × 10−1 | 0.47 | 0.969 | |
| CP4 | 0.005 | 1.00 × 10−3 | 4.2 × 10−2 | 0.45 | 0.997 | |
| Studied Sample | Zone of Inhibition (mm) | ||
|---|---|---|---|
| S. aureus | S. marcescens | ||
| Polyelectrolyte | control | - | - |
| PAA | 9 ± 0.8 | - | |
| PAA-C(O)O-(CH2)6-N+(CH3)3-8% | 12 ± 0.6 | 7 ± 0.1 | |
| PAA-C(O)O-(CH2)6-N+(CH3)3-10% | 12 ± 0.6 | 7 ± 0.1 | |
| PAA-C(O)O-(CH2)12-N+(CH3)3-8% | 10 ± 0.6 | 7 ± 0.1 | |
| PAA-C(O)O-(CH2)12-N+(CH3)3-14% | 11 ± 0.6 | 7 ± 0.1 | |
| Microparticle | control | 8 ± 0.2 | 8 ± 0.3 |
| CCP | 8 ± 0.3 | 8 ± 0.2 | |
| CC1 | 16 ± 1.0 | 12 ± 0.8 | |
| CC2 | 17 ± 0.5 | 12 ± 0.7 | |
| CC3 | 18 ± 0.6 | 11 ± 0.6 | |
| CC4 | 20 ± 0.6 | 12 ± 0.8 | |
| CPP | 8 ± 0.4 | 8 ± 0.4 | |
| CP1 | 13 ± 0.8 | 12 ± 0.7 | |
| CP2 | 11 ± 0.6 | 12 ± 0.8 | |
| CP3 | 15 ± 1.0 | 12 ± 0.6 | |
| CP4 | 12 ± 0.6 | 12 ± 0.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczęsna, W.; Tsirigotis-Maniecka, M.; Lamch, Ł.; Szyk-Warszyńska, L.; Zboińska, E.; Warszyński, P.; Wilk, K.A. Multilayered Curcumin-Loaded Hydrogel Microcarriers with Antimicrobial Function. Molecules 2022, 27, 1415. https://doi.org/10.3390/molecules27041415
Szczęsna W, Tsirigotis-Maniecka M, Lamch Ł, Szyk-Warszyńska L, Zboińska E, Warszyński P, Wilk KA. Multilayered Curcumin-Loaded Hydrogel Microcarriers with Antimicrobial Function. Molecules. 2022; 27(4):1415. https://doi.org/10.3390/molecules27041415
Chicago/Turabian StyleSzczęsna, Weronika, Marta Tsirigotis-Maniecka, Łukasz Lamch, Lilianna Szyk-Warszyńska, Ewa Zboińska, Piotr Warszyński, and Kazimiera A. Wilk. 2022. "Multilayered Curcumin-Loaded Hydrogel Microcarriers with Antimicrobial Function" Molecules 27, no. 4: 1415. https://doi.org/10.3390/molecules27041415
APA StyleSzczęsna, W., Tsirigotis-Maniecka, M., Lamch, Ł., Szyk-Warszyńska, L., Zboińska, E., Warszyński, P., & Wilk, K. A. (2022). Multilayered Curcumin-Loaded Hydrogel Microcarriers with Antimicrobial Function. Molecules, 27(4), 1415. https://doi.org/10.3390/molecules27041415