Regulation of Cytokines and Dihydrotestosterone Production in Human Hair Follicle Papilla Cells by Supercritical Extraction-Residues Extract of Ulmus davidiana
Abstract
:1. Introduction
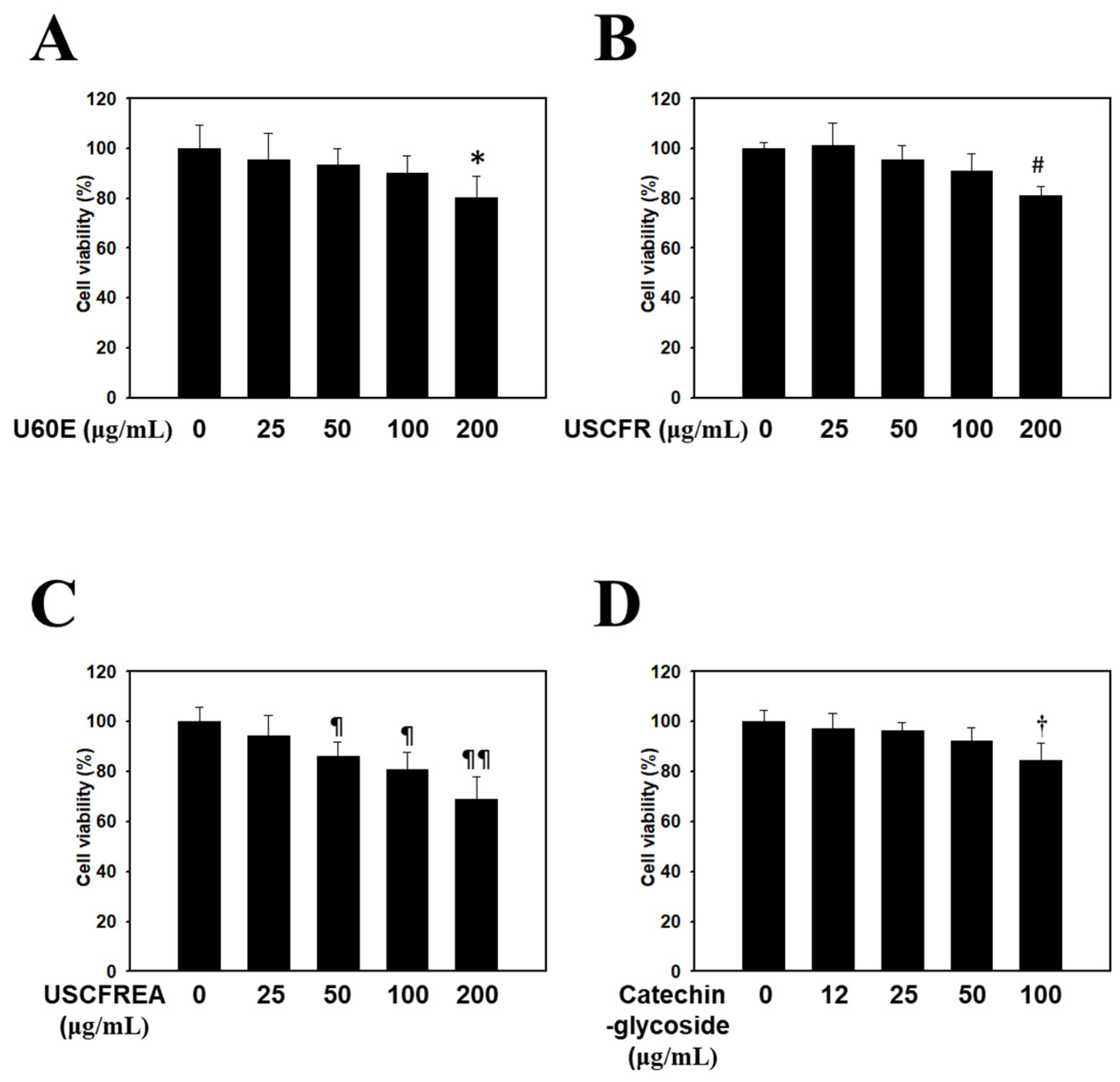
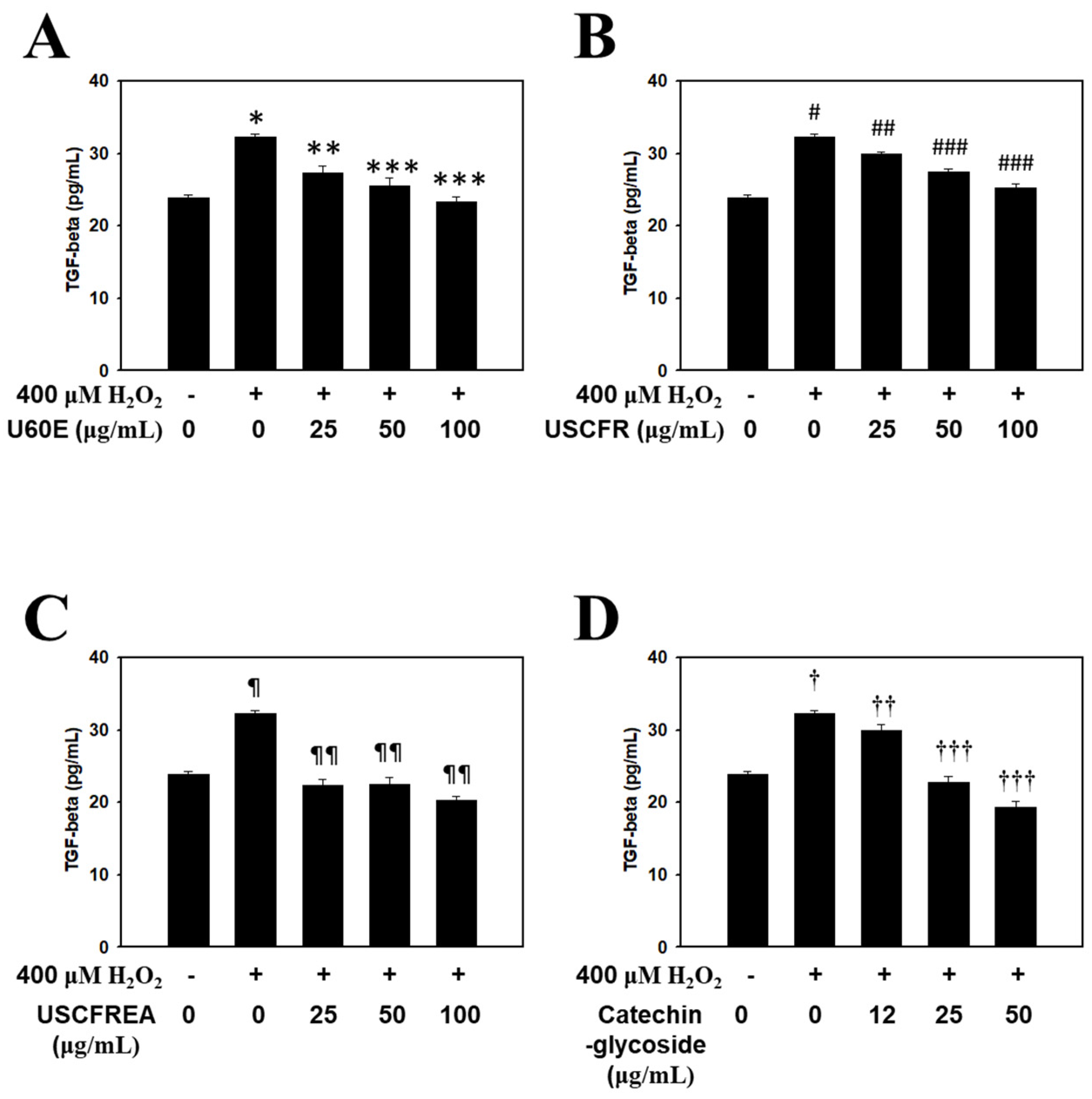
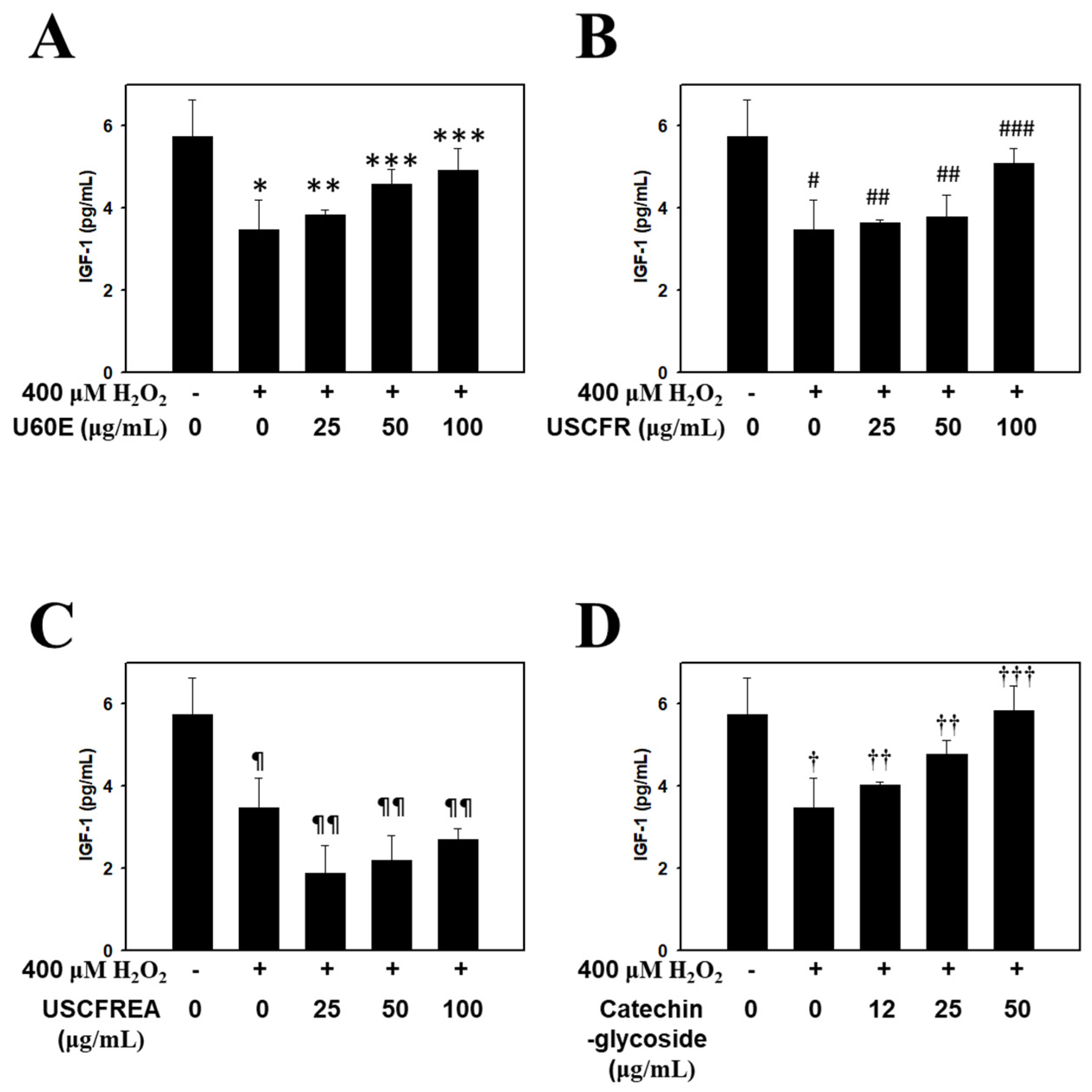
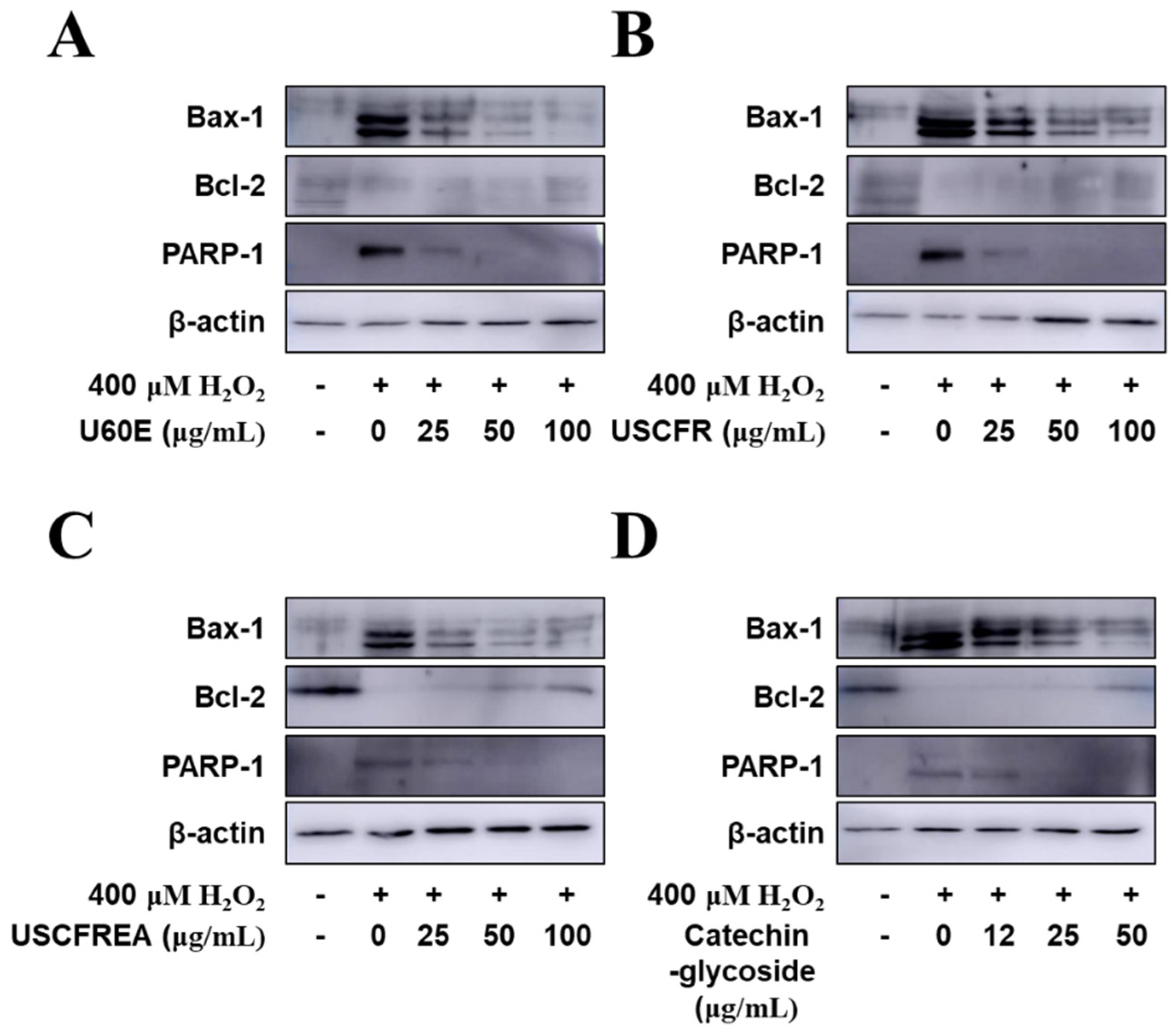
2. Results and Discussion
3. Materials and Methods
3.1. Extraction of the Supercritical Fluid Extraction-Residues Extract of Ulmus davidiana
3.2. Cell Culture
3.3. In Vitro Experiments and Enzyme-Linked Immunosorbent Assay
3.4. Western Blot Analysis
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hong, N.D.; Rho, Y.S.; Kim, N.J.; Kim, J.S. A Study on efficacy of Ulmi cortex. Korean J. Pharmacogn. 1990, 21, 217–222. [Google Scholar]
- Kim, E.-J.; Jang, M.-K.; Yoon, E.-H.; Jung, C.-Y.; Nam, D.-W.; Lee, S.-D.; Kim, K.-S. Efficacy of Pharmacopuncture Using Root Bark of Ulmus davidiana Planch in Patients With Knee Osteoarthritis: A Double-blind Randomized Controlled Trial. J. Acupunct. Meridian Stud. 2010, 3, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.H.; Lim, Y.; Kim, J.H.; Heo, W.; Lee, K.Y.; Shin, H.J.; Kim, J.K.; Lee, J.H.; Kim, Y.J. Root bark of Ulmus davidiana var. japonica restrains acute alcohol-induced hepatic steatosis onset in mice by inhibiting ROS accumulation. PLoS ONE 2017, 12, e0188381. [Google Scholar] [CrossRef] [Green Version]
- Oh, P.S.; Lee, S.J.; Lim, K.T. Glycoprotein (116 kD) isolated from Ulmus davidiana Nakai protects from injury of 12-O-tetradecanoylphorbol 13-acetate (TPA)-treated BNL CL.2 cells. Pharm. Rep. 2006, 58, 67–74. [Google Scholar]
- Eom, S.Y.; Chung, C.B.; Kim, Y.S.; Kim, J.H.; Kim, K.S.; Kim, Y.H.; Park, S.H.; Hwang, Y.I.; Kim, K.H. Cosmeceutical properties of polysaccharides from the root bark of Ulmus davidiana var. japonica. J. Cosmet. Sci. 2006, 57, 355–367. [Google Scholar]
- Lee, S.-J.; Lim, K.-T. Inhibitory effect of phytoglycoprotein on tumor necrosis factor-α and interleukin-6 at initiation stage of colon cancer in 1,2-dimethylhydrazine-treated ICR mice. Toxicol. Appl. Pharmacol. 2007, 225, 198–205. [Google Scholar] [CrossRef]
- Shin, D.-Y.; Kim, H.-S.; Min, K.-H.; Hyun, S.-S.; Kim, S.-A.; Huh, H.; Choi, E.-C.; Choi, Y.H.; Kim, J.; Choi, S.-H.; et al. Isolation of a Potent Anti-MRSA Sesquiterpenoid Quinone from Ulmus davidiana var. japonica. Chem. Pharm. Bull. 2000, 48, 1805–1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiu, R.J. Microcirculation and traditional Chinese medicine. JAMA J. Am. Med. Assoc. 1988, 260, 1755–1757. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-H.; Chung, E.-Y.; Choi, Y.-N.; Jang, H.-Y.; Kim, J.-S.; Kim, G.-B. Oral administration of Ulmus davidiana extract suppresses interleukin-1β expression in LPS-induced immune responses and lung injury. Genes Genom. 2019, 42, 87–95. [Google Scholar] [CrossRef]
- Lee, H.-S.; Jang, M.S.; Kim, J.-H.; Hong, C.-P.; Lee, E.-J.; Jeun, E.J.; Kim, C.; Kim, E.-K.; Ahn, K.-S.; Yang, B.-G.; et al. Ulmus davidiana var. japonica Nakai Upregulates Eosinophils and Suppresses Th1 and Th17 Cells in the Small Intestine. PLoS ONE 2013, 8, e76716. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, X.; Fu, C.; Liu, W.; Wang, Y.; Xu, F.; Zhang, Q.; Liu, Y.; Liu, Y. Ulmus davidiana extract improves lumbar vertebral parameters in ovariectomized osteopenic rats. Am. J. Transl. Res. 2016, 8, 298–313. [Google Scholar] [PubMed]
- Kim, M.; Park, K.H.; Choi, S.E. Protective Effects of Supercritical Fluid Extracts of Ulmus daviana var. japonica on LPS-induced Immune Responses. Korean Soc. Biotechnol. Bioeng. J. 2019, 34, 216–220. [Google Scholar]
- Jung, H.J.; Park, E.H. Anti-inflanunatory, anti-angiogenic and analgesic activities of Ulmus davidiana var. japonica. In Proceeding of the Convention of the Pharmaceutical Society of Korea, Busan, Korea, 16 April 2006; pp. 138–139. [Google Scholar]
- Jeon, G.; Ko, J.Y.; Mun, M.J.; Min, J.; Choi, S.E.; Bang, S.H. Effect of melanin reduction by extracts from Ulmus davidiana by supercritical fluid extraction. J. Toxicol. Environ. Health Sci. 2020, 12, 325–329. [Google Scholar] [CrossRef]
- Hibino, T.; Nishiyama, T. Role of TGF-β2 in the human hair cycle. J. Dermatol. Sci. 2004, 35, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. Further Clinical Evidence for the Effect of IGF-1 on Hair Growth and Alopecia. Ski. Appendage Disord. 2017, 4, 90–95. [Google Scholar] [CrossRef]
- Foitzik, K.; Lindner, G.; Mueller-Roever, S.; Maurer, M.; Botchkareva, N.; Botchkarev, V.; Handjiski, B.; Metz, M.; Hibino, T.; Soma, T.; et al. Control of murine hair follicle regression (catagen) by TGF-β1 in vivo. FASEB J. 2000, 14, 752–760. [Google Scholar] [CrossRef]
- Shin, H.; Yoo, H.G.; Inui, S.; Itami, S.; Kim, I.G.; Cho, A.-R.; Lee, D.H.; Park, W.S.; Kwon, O.; Cho, K.H.; et al. Induction of transforming growth factor-beta 1 by androgen is mediated by reactive oxygen species in hair follicle dermal papilla cells. BMB Rep. 2013, 46, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Itami, S.; Kurata, S.; Takayasu, S. Androgen Induction of Follicular Epithelial Cell Growth Is Mediated via Insulin-like Growth Factor-I from Dermal Papilla Cells. Biochem. Biophys. Res. Commun. 1995, 212, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Randall, V.A.; Jenner, T.J.; Hibberts, N.A.; De Oliveira, I.O.; Vafaee, T. Stem cell factor/c-Kit signalling in normal and androgenetic alopecia hair follicles. J. Endocrinol. 2008, 197, 11–23. [Google Scholar] [CrossRef]
- Laron, Z. Insulin-like growth factor 1 (IGF-1): A growth hormone. Mol. Pathol. 2001, 54, 311–316. [Google Scholar] [CrossRef]
- Yakar, S.; Rosen, C.J.; Beamer, W.G.; Ackert-Bicknell, C.L.; Wu, Y.; Liu, J.-L.; Ooi, G.T.; Setser, J.; Frystyk, J.; Boisclair, Y.R.; et al. Circulating levels of IGF-1 directly regulate bone growth and density. J. Clin. Investig. 2002, 110, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Weger, N.; Schlake, T. IGF-I Signalling Controls the Hair Growth Cycle and the Differentiation of Hair Shafts. J. Investig. Dermatol. 2005, 125, 873–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, H.-Y.; Hickford, J.G.H.; Palmer, B.R.; Bickerstaffe, R. Insulin-like growth factor 1 and hair growth. Dermatol. Online J. 1999, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Cobb, J.E.; Wong, N.C.; Yip, L.W.; Martinick, J.; Bosnich, R.; Sinclair, R.D.; Craig, J.M.; Saffery, R.; Harrap, S.B.; Ellis, J.A. Evidence of increased DNA methylation of the androgen receptor gene in occipital hair follicles from men with androgenetic alopecia. Br. J. Dermatol. 2011, 165, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Garza, L.A. An Overview of Alopecias. Cold Spring Harb. Perspect. Med. 2014, 4, a013615. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, M.E.; Keane, R.W.; Blume-Peytavi, U.; Mullins, D.L.; Nusbaum, B.P.; Whiting, D.; Nicholson, D.W. Androgen responsive genes as they affect hair growth. Eur. J. Dermatol. EJD 2001, 11, 304–308. [Google Scholar] [PubMed]
- Rojas-Martínez, A.; Martinez-Jacobo, L.; Villarreal-Villarreal, C.; Ortiz-López, R.; Ocampo-Candiani, J. Genetic and molecular aspects of androgenetic alopecia. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 263. [Google Scholar] [CrossRef] [PubMed]
- Springer, K.; Brown, M.; Stulberg, D.L. Common hair loss disorders. Am. Fam. Phys. 2003, 68, 93–102. [Google Scholar]
- Karrer-Voegeli, S.; Rey, F.; Reymond, M.J.; Meuwly, J.-Y.; Gaillard, R.C.; Gomez, F. Androgen Dependence of Hirsutism, Acne, and Alopecia in Women. Medicine 2009, 88, 32–45. [Google Scholar] [CrossRef]
- Lachgar; Charveron; Gall; Bonafe Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. Br. J. Dermatol. 1998, 138, 407–411. [CrossRef]
- Messenger, A.G.; Rundegren, J. Minoxidil: Mechanisms of action on hair growth. Br. J. Dermatol. 2004, 150, 186–194. [Google Scholar] [CrossRef] [PubMed]
- DeVillez, R.L.; Jacobs, J.P.; Szpunar, C.A.; Warner, M.L. Androgenetic alopecia in the female. Treatment with 2% topical minoxidil solution. Arch. Dermatol. 1994, 130, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Foley, K.A. 5% Minoxidil: Treatment for female pattern hair loss. Ski. Ther. Lett. 2014, 19, 5–7. [Google Scholar]
- Suchonwanit, P.; Thammarucha, S.; Leerunyakul, K. Minoxidil and its use in hair disorders: A review. Drug Des. Dev. Ther. 2019, 13, 2777–2786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez-Cauhe, J.; Saceda-Corralo, D.; Rodrigues-Barata, R.; Moreno-Arrones, O.M.; Ortega-Quijano, D.; Fernandez-Nieto, D.; Jaen-Olasolo, P.; Vaño-Galvan, S. Safety of low-dose oral minoxidil treatment for hair loss. A systematic review and pooled-analysis of individual patient data. Dermatol. Ther. 2020, 33, e14106. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, I.B.; Harries, M.; Rocha, V.; Thompson, J.; Wong, C.; Varkaneh, H.; Guimarães, N.; Rocha Arantes, A.; Marcolino, M. Effect of oral minoxidil for alopecia: Systematic review. Int. J. Trichol. 2020, 12, 147. [Google Scholar] [CrossRef]
- Ha, E.J.; Yun, J.-H.; Si, C.; Bae, Y.S.; Jeong, Y.-H.; Park, K.-H.; Choi, S.-E. Application of Ethanol Extracts from Alnus sibirica Fisch. ex Turcz in Hair Growth Promotion. Front. Bioeng. Biotechnol. 2021, 9, 399. [Google Scholar] [CrossRef]
- Seo, J.-H.; Lee, Y.-J.; Jo, Y.-I.; Ko, J.-Y.; Mun, M.-J.; Park, K.-H.; Choi, S.E. Anti-fungal, anti-oxidant, and anti-inflammatory effects of supercritical fluid extracts from Ulmus davidiana. J. Korea Converg. Soc. 2018, 9, 225–233. [Google Scholar] [CrossRef]
- Mun, M.-J.; Park, K.-H.; Choi, S.E. Biological activity of supercritical extraction residue 60% ethanolic extracts from Ulmus davidiana. J. Converg. Inf. Technol. 2018, 8, 29–36. [Google Scholar] [CrossRef]
- Park, K.-H.; Kim, B.-J.; Kang, J.; Nam, T.-S.; Lim, J.M.; Kim, H.T.; Park, J.K.; Kim, Y.G.; Chae, S.-W.; Kim, U.-H. Ca2+ Signaling Tools Acquired from Prostasomes Are Required for Progesterone-Induced Sperm Motility. Sci. Signal. 2011, 4, ra31. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, Y.-E.; Choi, S.-E.; Park, K.-H. Regulation of Cytokines and Dihydrotestosterone Production in Human Hair Follicle Papilla Cells by Supercritical Extraction-Residues Extract of Ulmus davidiana. Molecules 2022, 27, 1419. https://doi.org/10.3390/molecules27041419
Kwon Y-E, Choi S-E, Park K-H. Regulation of Cytokines and Dihydrotestosterone Production in Human Hair Follicle Papilla Cells by Supercritical Extraction-Residues Extract of Ulmus davidiana. Molecules. 2022; 27(4):1419. https://doi.org/10.3390/molecules27041419
Chicago/Turabian StyleKwon, Ye-Eun, Sun-Eun Choi, and Kwang-Hyun Park. 2022. "Regulation of Cytokines and Dihydrotestosterone Production in Human Hair Follicle Papilla Cells by Supercritical Extraction-Residues Extract of Ulmus davidiana" Molecules 27, no. 4: 1419. https://doi.org/10.3390/molecules27041419




