Polyopes affinis Suppressed IFN-γ- and TNF-α-Induced Inflammation in Human Keratinocytes via Down-Regulation of the NF-κB and STAT1 Pathways
Abstract
1. Introduction
2. Results
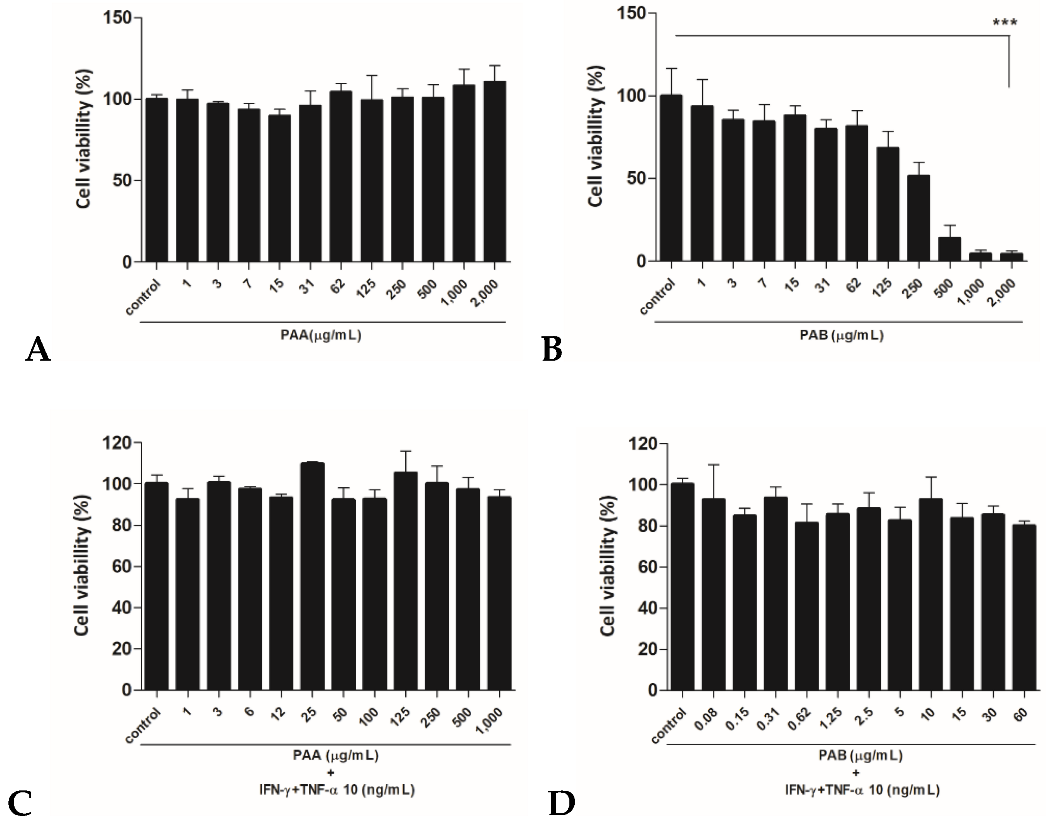
2.1. Cytotoxic Effects of PAA and PAB in HaCaT Cells
2.2. Effects of PAA and PAB on IFN-γ- and TNF-α-stimulated TARC/CCL17 and MDC/CCL22mRNA Expression in HaCaT Cells
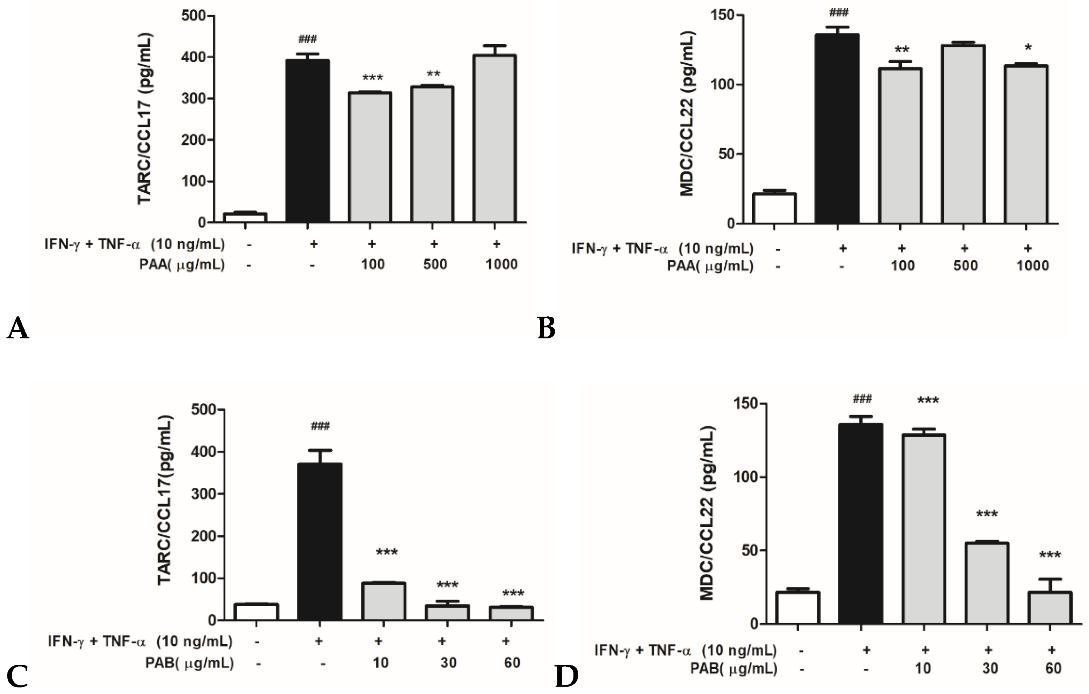
2.3. Effects of PAA and PAB on IFN-γ- and TNF-α-Stimulated TARC/CCL17 and MDC/CCL22 Secretory Proteins in HaCaT Cells
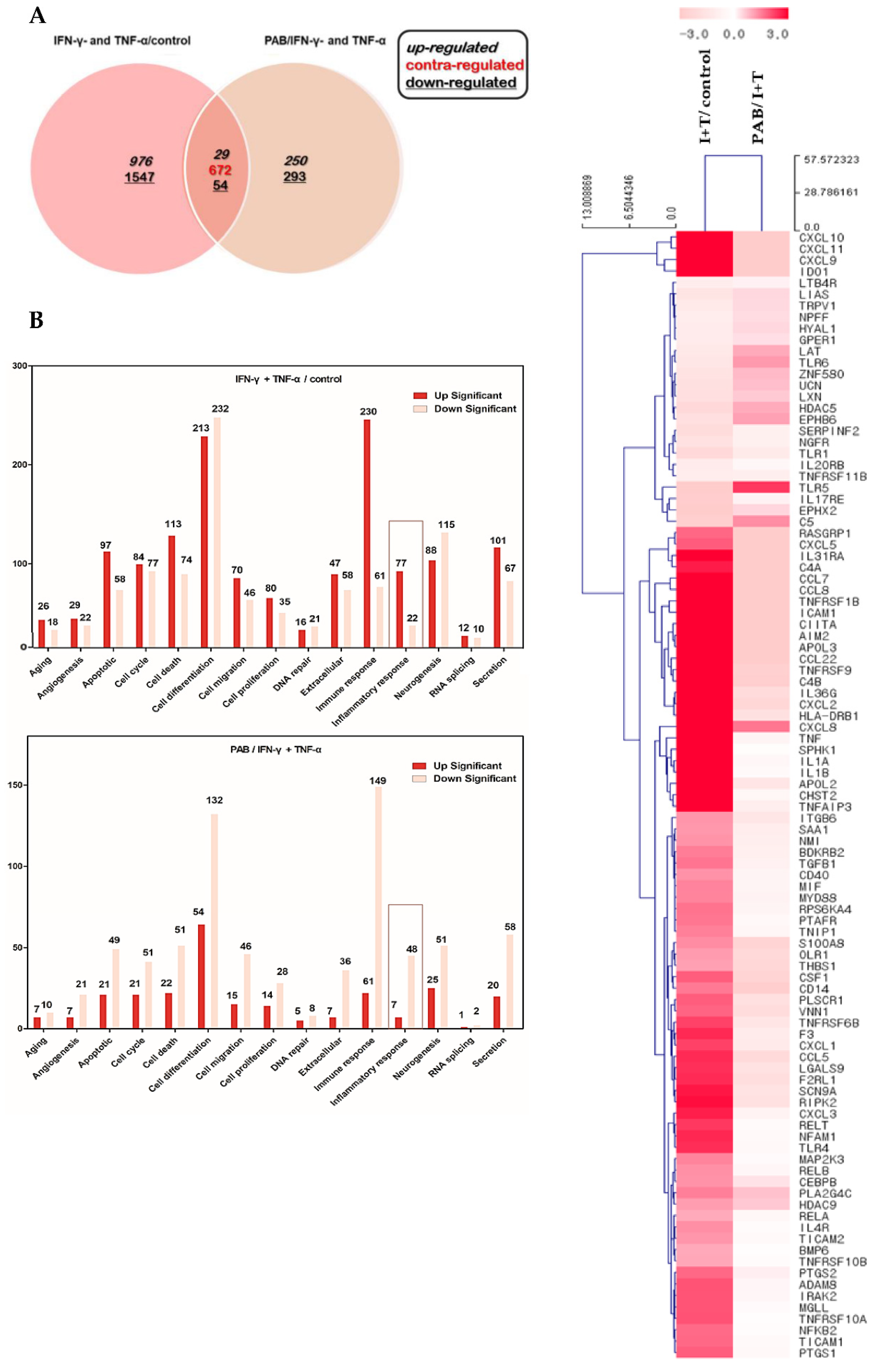
2.4. Effect of PAB on the Gene Expression Profile of IFN-γ- and TNF-α-Stimulated HaCaT Cells
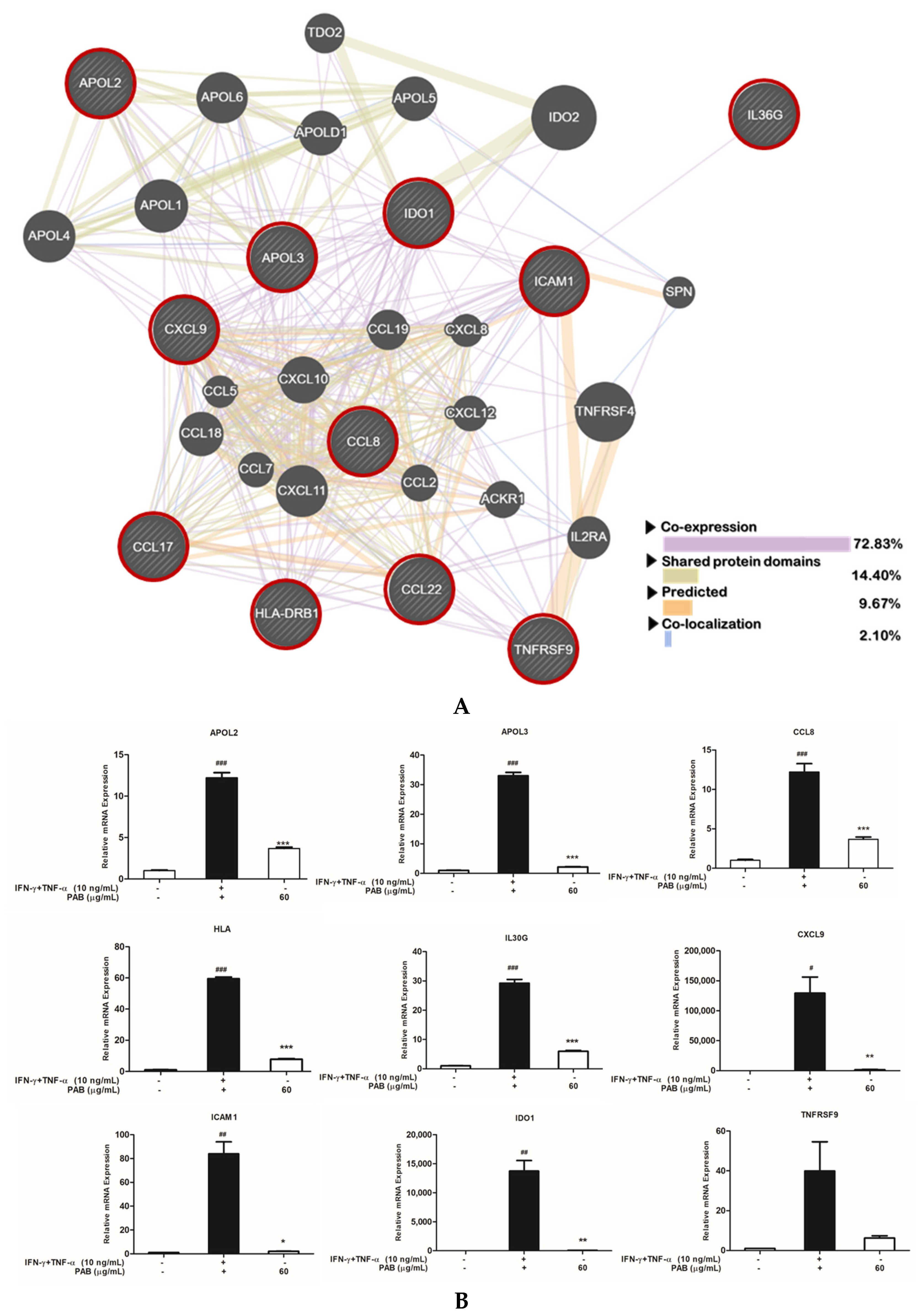
2.5. Nine Classified Genes and Interconnected Networks with TARC/CCL17 and MDC/CCL22
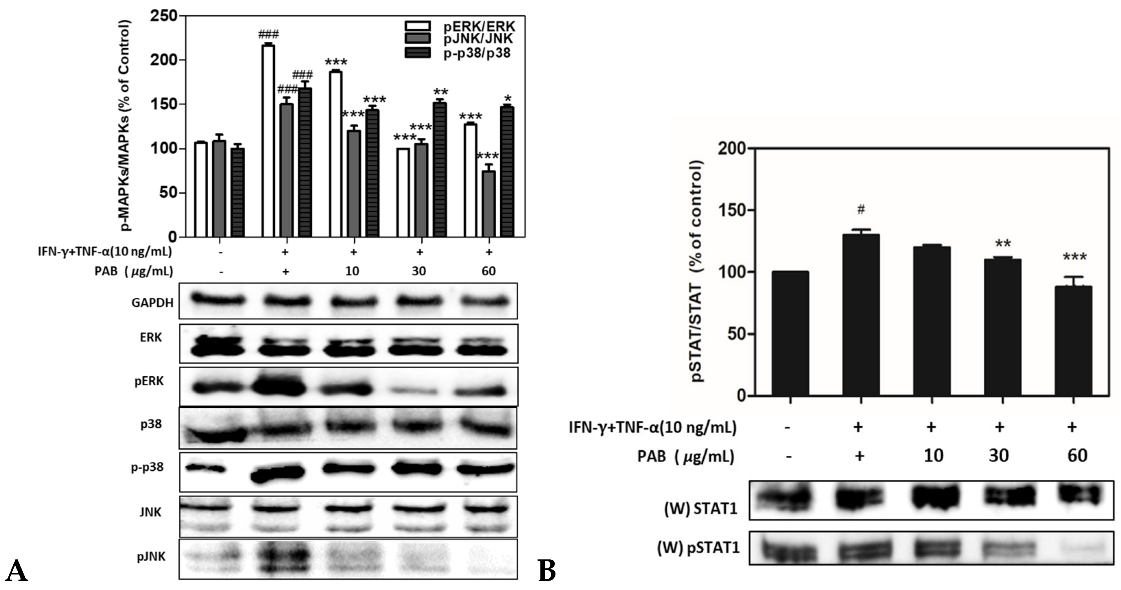
2.6. Effect of PAB Regulation on Inflammation Related MAPKs and STAT1 Signaling Pathways in IFN-γ and TNF-α-Induced HaCaT Cells
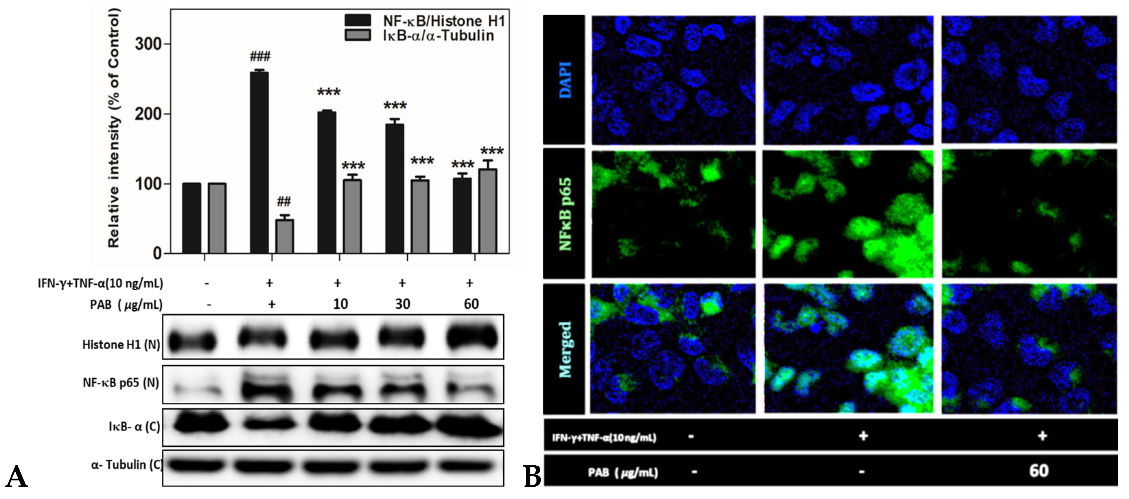
2.7. Effect of PAB on NF-κB Translocation in IFN-γ- and TNF-α-Induced HaCaT Cells
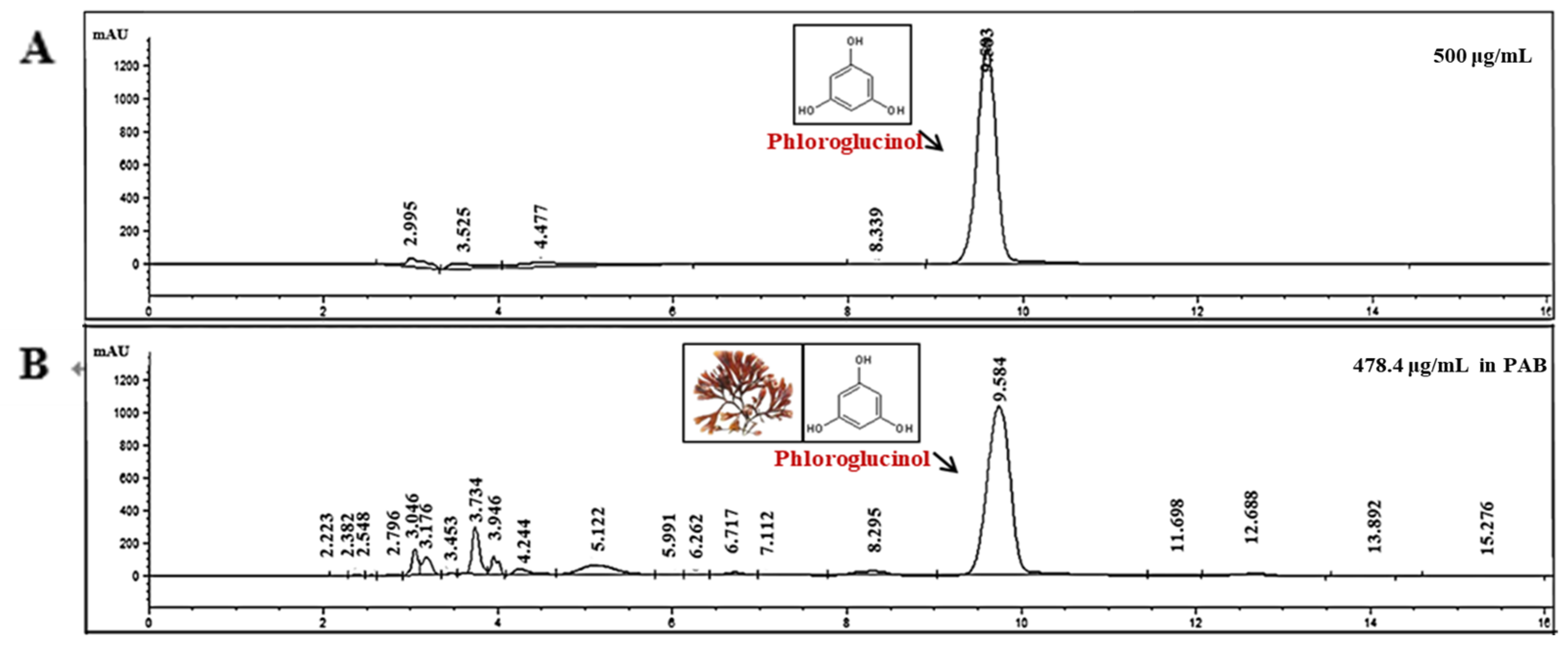
2.8. Identification of Anti-AD Compounds in PAB
3. Discussion
4. Materials and Methods
4.1. Preparation of Seaweed Materials
4.2. Cell Culture and Treatment
4.3. Cell Viability
4.4. RNA Isolation and cDNA Synthesis
4.5. Library Preparation and Sequencing
4.6. Quantitative Real-Time PCR
4.7. ELISA
4.8. Western Blotting
4.9. Separation of Cytosolic and Nuclear Protein Extracts
4.10. Immunofluorescence
4.11. HPLC Analysis
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Murai, U.; Yamagishi, K.; Kishida, R.; Iso, H. Impact of seaweed intake on health. Eur. J. Clinic. Nutr. 2021, 75, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-J.; Kim, J.; Han, G.; Kim, S.-K.; Kim, H.-G.; Yeo, I.; Ryu, B.; Park, J.-W. Laminaria japonica Combined with Probiotics Improves Intestinal Microbiota: A Randomized Clinical Trial. J. Med. Food 2014, 17, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.Y.; Hwang, J.H.; Yang, S.H.; Um, J.I.; Hong, K.W.; Kang, K.; Pan, C.H.; Hwang, K.T.; Kim, S.M. Anti-Obesity Effect of Standardized Extract of Microalga Phaeodactylum tricornutum Containing Fucoxanthin. Mar. Drugs 2019, 17, 311. [Google Scholar] [CrossRef] [PubMed]
- Gille, A.; Stojnic, B.; Derwenskus, F.; Trautmann, A.; Schmid-Staiger, U.; Posten, C.; Briviba, K.; Palou, A.; Bonet, M.L.; Ribot, J. A Lipophilic Fucoxanthin-Rich Phaeodactylum tricornutum Extract Ameliorates Effects of Diet-Induced Obesity in C57BL/6J Mice. Nutrients 2019, 11, 796. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M.; Adamczak, A. Fucoxanthin—An Antibacterial Carotenoid. Antioxidants 2019, 8, 239. [Google Scholar] [CrossRef]
- Seo, G.-Y.; Ha, Y.; Park, A.-H.; Kwon, O.W.; Kim, Y.-J. Leathesia difformis Extract Inhibits α-MSH-Induced Melanogenesis in B16F10 Cells via Down-Regulation of CREB Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 536. [Google Scholar] [CrossRef]
- Barbosa, M.; Lopes, G.; Andrade, P.B.; Valentão, P. Bioprospecting of brown seaweeds for biotechnological applications: Phlorotannin actions in inflammation and allergy network. Trends Food Sci. Technol. 2019, 86, 153–171. [Google Scholar] [CrossRef]
- Asanka Sanjeewa, K.K.; Jayawardena, T.U.; Kim, H.S.; Kim, S.Y.; Shanura Fernando, I.P.; Wang, L.; Abetunga, D.T.U.; Kim, W.S.; Lee, D.S.; Jeon, Y.J. Fucoidan isolated from Padina commersonii inhibit LPS-induced inflammation in macrophages blocking TLR/NF-kappaB signal pathway. Carbohydr. Polym. 2019, 224, 115195. [Google Scholar] [CrossRef]
- Gil, T.Y.; Kang, Y.M.; Eom, Y.J.; Hong, C.H.; An, H.J. Anti-Atopic Dermatitis Effect of Seaweed Fulvescens Extract via Inhibiting the STAT1 Pathway. Mediat. Inflamm. 2019, 2019, 3760934. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Fernando, I.P.S.; Kim, S.-Y.; Kim, W.-S.; Ahn, G.; Jee, Y.; Jeon, Y.-J. Ecklonia cava (Laminariales) and Sargassum horneri (Fucales) synergistically inhibit the lipopolysaccharide-induced inflammation via blocking NF-κB and MAPK pathways. Algae 2019, 34, 45–56. [Google Scholar] [CrossRef]
- Lee, D.S.; Park, W.S.; Heo, S.J.; Cha, S.H.; Kim, D.; Jeon, Y.J.; Park, S.G.; Seo, S.K.; Choi, J.S.; Park, S.J.; et al. Polyopes affinis alleviates airway inflammation in a murine model of allergic asthma. J. Biosci. 2011, 36, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Batra, R.; Bluhm, R.; Boekhout, T.; Dawson, T.L., Jr. Skin diseases associated with Malassezia species. J. Am. Acad. Dermatol. 2004, 51, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Hensel, P.; Santoro, D.; Favrot, C.; Hill, P.; Griffin, C. Canine atopic dermatitis: Detailed guidelines for diagnosis and allergen identification. BMC Vet. Res. 2015, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Fenner, J.; Silverberg, N.B. Skin diseases associated with atopic dermatitis. Clin. Dermatol. 2018, 36, 631–640. [Google Scholar] [CrossRef]
- Lee, K.-S.; Chun, S.-Y.; Lee, M.-G.; Kim, S.; Jang, T.-J.; Nam, K.-S. The prevention of TNF-α/IFN-γ mixture-induced inflammation in human keratinocyte and atopic dermatitis-like skin lesions in Nc/Nga mice by mineral-balanced deep sea water. Biomed. Pharmacother. 2018, 97, 1331–1340. [Google Scholar] [CrossRef]
- Choi, J.H.; Jin, S.W.; Park, B.H.; Kim, H.G.; Khanal, T.; Han, H.J.; Hwang, Y.P.; Choi, J.M.; Chung, Y.C.; Hwang, S.K.; et al. Cultivated ginseng inhibits 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in NC/Nga mice and TNF-α/IFN-γ-induced TARC activation in HaCaT cells. Food Chem. Toxic. 2013, 56, 195–203. [Google Scholar] [CrossRef]
- Lim, H.-S.; Seo, C.-S.; Jin, S.-E.; Yoo, S.-R.; Lee, M.-Y.; Shin, H.-K.; Jeong, S.-J. Ma Huang Tang Suppresses the Production and Expression of Inflammatory Chemokines via Downregulating STAT1 Phosphorylation in HaCaT Keratinocytes. Evid. -Based Complement. Altern. Med. 2016, 2016, 7831291. [Google Scholar] [CrossRef]
- The Gene Ontology Consortium. Expansion of the Gene Ontology knowledgebase and resources. Nucleic Acids Res. 2016, 45, D331–D338. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Gene. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Deng, W.; Liang, P.; Zheng, Y.; Su, Z.; Gong, Z.; Chen, J.; Feng, P.; Chen, J. Differential gene expression in HaCaT cells may account for the various clinical presentation caused by anthropophilic and geophilic dermatophytes infections. Mycoses 2020, 63, 21–29. [Google Scholar] [CrossRef]
- Cho, S.; Yang, H.; Jeon, Y.J.; Lee, C.J.; Jin, Y.H.; Baek, N.I.; Kim, D.; Kang, S.M.; Yoon, M.; Yong, H.; et al. Phlorotannins of the edible brown seaweed Ecklonia cava Kjellman induce sleep via positive allosteric modulation of gamma-aminobutyric acid type A-benzodiazepine receptor: A novel neurological activity of seaweed polyphenols. Food Chem. 2012, 132, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Ta, Q.V.; Mendis, E.; Rajapakse, N.; Jung, W.K.; Byun, H.G.; Jeon, Y.J.; Kim, S.K. Phlorotannins in Ecklonia cava extract inhibit matrix metalloproteinase activity. Life Sci. 2006, 79, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-X.; Wijesekara, I.; Li, Y.; Kim, S.-K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Wijesekara, I.; Yoon, N.Y.; Kim, S.K. Phlorotannins from Ecklonia cava (Phaeophyceae): Biological activities and potential health benefits. Biofactors 2010, 36, 408–414. [Google Scholar] [CrossRef]
- Lee, J.H.; Ko, J.Y.; Oh, J.Y.; Kim, C.Y.; Lee, H.J.; Kim, J.; Jeon, Y.J. Preparative isolation and purification of phlorotannins from Ecklonia cava using centrifugal partition chromatography by one-step. Food Chem. 2014, 158, 433–437. [Google Scholar] [CrossRef]
- Liao, W.; Goh, F.Y.; Betts, R.J.; Kemeny, D.M.; Tam, J.; Bay, B.H.; Wong, W.S.F. A novel anti-apoptotic role for apolipoprotein L2 in IFN-γ-induced cytotoxicity in human bronchial epithelial cells. J. Cell. Phys. 2011, 226, 397–406. [Google Scholar] [CrossRef]
- Yang, S.; Wen, X.U.; Lin, F.; Zhou, M.; Ai’e, X.U. Effects of tacrolimus on the secretion of chemokines CXCL9 and CXCL10 by γ-interferon-simulated HaCaT cells. Chin. J. Dermatol. 2018, 51, 375–378. [Google Scholar]
- Hudemann, C.; Maglie, R.; Llamazares-Prada, M.; Beckert, B.; Didona, D.; Tikkanen, R.; Schmitt, T.; Hashimoto, T.; Waschke, J.; Hertl, M. Human Desmocollin 3—Specific IgG Antibodies Are Pathogenic in a Humanized HLA Class II Transgenic Mouse Model of Pemphigus. J. Investig. Dermatol. 2021, 142, 915–923. [Google Scholar] [CrossRef]
- Bissonnette, R.; Maari, C.; Forman, S.; Bhatia, N.; Lee, M.; Fowler, J.; Tyring, S.; Pariser, D.; Sofen, H.; Dhawan, S.; et al. The oral Janus kinase/spleen tyrosine kinase inhibitor ASN002 demonstrates efficacy and improves associated systemic inflammation in patients with moderate-to-severe atopic dermatitis: Results from a randomized double-blind placebo-controlled study. Br. J. Dermatol. 2019, 181, 733–742. [Google Scholar] [CrossRef]
- Brunner, P.M.; Suárez-Fariñas, M.; He, H.; Malik, K.; Wen, H.-C.; Gonzalez, J.; Chan, T.C.-C.; Estrada, Y.; Zheng, X.; Khattri, S.; et al. The atopic dermatitis blood signature is characterized by increases in inflammatory and cardiovascular risk proteins. Sci. Rep. 2017, 7, 8707. [Google Scholar] [CrossRef]
- Lin, Z.-M.; Ma, M.; Li, H.; Qi, Q.; Liu, Y.-T.; Yan, Y.-X.; Shen, Y.-F.; Yang, X.-Q.; Zhu, F.-H.; He, S.-J. Topical administration of reversible SAHH inhibitor ameliorates imiquimod-induced psoriasis-like skin lesions in mice via suppression of TNF-α/IFN-γ-induced inflammatory response in keratinocytes and T cell-derived IL-17. Pharmacol. Res. 2018, 129, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Youn, G.S.; Kwon, D.-J.; Ju, S.M.; Choi, S.Y.; Park, J. Curcumin ameliorates TNF-α-induced ICAM-1 expression and subsequent THP-1 adhesiveness via the induction of heme oxygenase-1 in the HaCaT cells. BMB Rep. 2013, 46, 410. [Google Scholar] [CrossRef] [PubMed]
- González-Navajas, J.M.; Lee, J.; David, M.; Raz, E. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 2012, 12, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Dai, Y.-W.; Peng, H.-L.; Kang, C.-W.; Kuo, C.-Y.; Liou, C.-J. Phloretin ameliorates chemokines and ICAM-1 expression via blocking of the NF-κB pathway in the TNF-α-induced HaCaT human keratinocytes. Int. Immunopharm. 2015, 27, 32–37. [Google Scholar] [CrossRef]
- Coelho, A.L.; Hogaboam, C.M.; Kunkel, S.L. Chemokines provide the sustained inflammatory bridge between innate and acquired immunity. Cytokine Growth Factor Rev. 2005, 16, 553–560. [Google Scholar] [CrossRef]
- Sumiyoshi, K.; Nakao, A.; Setoguchi, Y.; Tsuboi, R.; Okumura, K.; Ogawa, H. TGF-β/Smad signaling inhibits IFN-γ and TNF-α-induced TARC (CCL17) production in HaCaT cells. J. Dermatol. Sci. 2003, 31, 53–58. [Google Scholar] [CrossRef]
- Jung, M.-r.; Lee, T.H.; Bang, M.-H.; Kim, H.; Son, Y.; Chung, D.K.; Kim, J. Suppression of thymus- and activation-regulated chemokine (TARC/CCL17) production by 3-O-β-d-glucopyanosylspinasterol via blocking NF-κB and STAT1 signaling pathways in TNF-α and IFN-γ-induced HaCaT keratinocytes. Biochem. Biophys. Res. Commun. 2012, 427, 236–241. [Google Scholar] [CrossRef]
- Qi, X.-F.; Kim, D.-H.; Yoon, Y.-S.; Li, J.-H.; Jin, D.; Teng, Y.-C.; Kim, S.-K.; Lee, K.-J. Fluvastatin inhibits expression of the chemokine MDC/CCL22 induced by interferon-γ in HaCaT cells, a human keratinocyte cell line. Br. J. Pharmacol. 2009, 157, 1441–1450. [Google Scholar] [CrossRef]
- Kagami, S.; Saeki, H.; Komine, M.; Kakinuma, T.; Nakamura, K.; Tsunemi, Y.; Sasaki, K.; Asahina, A.; Tamaki, K. CCL28 production in HaCaT cells was mediated by different signal pathways from CCL27. Exper. Dermatol. 2006, 15, 95–100. [Google Scholar] [CrossRef]
- Kanda, N.; Watanabe, S. Prolactin Enhances Interferon-γ-Induced Production of CXC Ligand 9 (CXCL9), CXCL10, and CXCL11 in Human Keratinocytes. Endocrinology 2007, 148, 2317–2325. [Google Scholar] [CrossRef]
- Ju, S.M.; Song, H.Y.; Lee, S.J.; Seo, W.Y.; Sin, D.H.; Goh, A.R.; Kang, Y.-H.; Kang, I.-J.; Won, M.-H.; Yi, J.-S.; et al. Suppression of thymus- and activation-regulated chemokine (TARC/CCL17) production by 1,2,3,4,6-penta-O-galloyl-β-d-glucose via blockade of NF-κB and STAT1 activation in the HaCaT cells. Biochem. Biophys. Res. Commun. 2009, 387, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Safdar, M.; Ozaslan, M.; Junejo, Y.; Channa, I.S. Cytotoxic and anticancer activity of a novel synthesized tet-AuNPs simultaneously activates p53 and inhibits NF-kB signaling in SKBR3 cell line. Toxic. Environ. Health Sci. 2021, 14, 69–76. [Google Scholar] [CrossRef]
- Kisseleva, T.; Bhattacharya, S.; Braunstein, J.; Schindler, C.W. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene 2002, 285, 1–24. [Google Scholar] [CrossRef]
- Goh, K.C.; Haque, S.J.; Williams, B.R.G. p38 MAP kinase is required for STAT1 serine phosphorylation and transcriptional activation induced by interferons. EMBO J. 1999, 18, 5601–5608. [Google Scholar] [CrossRef]
- Wu, J.; Lei, G.; Wang, T.; Dong, S.; Zhan, X. Esculentoside A exerts anti-oxidative stress and anti-apoptotic effects in rat experimental membranous nephropathy by regulating MAPK pathway. Mol. Cell. Toxic. 2021. [Google Scholar] [CrossRef]
- Hyun, Y.J.; Piao, M.J.; Kim, K.C.; Zheng, J.; Yao, C.W.; Cha, J.W.; Kang, H.K.; Yoo, E.S.; Koh, Y.S.; Lee, N.H. Photoprotective effect of a Polyopes affinis (Harvey) Kawaguchi and Wang (Halymeniaceae)-derived ethanol extract on human keratinocytes. Trop. J. Pharmaceut. Res. 2014, 13, 863–871. [Google Scholar] [CrossRef][Green Version]
- Kim, M.-J.; Kim, K.-B.-W.-R.; Park, S.-H.; Park, S.-Y.; Choi, H.-D.; Choi, J.-S.; Jang, M.-R.; Im, M.-H.; Ahn, D.-H. Anti-Inflammatory effect of ethanolic extract from Polyopes affinis through suppression of NF-κB and MAPK activation in LPS-stimulated RAW 264.7 cells. J. Korean Soc. Food Sci. Nutr. 2017, 46, 537–544. [Google Scholar] [CrossRef]
- Kim, H.S.; Choi, Y.H.; Hwang, H.J. Inhibitory Effects of Polyopes affinis Ethanol Extract on Melanogenesis in B16F10 Melanoma Cells. J. Life Sci. 2019, 29, 972–976. [Google Scholar] [CrossRef]
- Qin, Y. Applications of Bioactive Seaweed Substances in Functional Food Products. Bioact. Seaweeds Food Appl. 2018, 111–134. [Google Scholar] [CrossRef]
- Hermund, D.B. Antioxidant Properties of Seaweed-Derived Substances. Bioact. Seaweeds Food Appl. 2018, 201–221. [Google Scholar] [CrossRef]
- Okada, Y.; Ishimaru, A.; Suzuki, R.; Okuyama, T. A new phloroglucinol derivative from the brown alga Eisenia bicyclis: Potential for the effective treatment of diabetic complications. J. Nat. Prod. 2004, 67, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.; Li, Y.; Qian, Z.-J.; Kim, M.-M.; Kim, S.-K. Differentiation of human osteosarcoma cells by isolated phlorotannins is subtly linked to COX-2, iNOS, MMPs, and MAPK signaling: Implication for chronic articular disease. Chem. -Biol. Interact. 2009, 179, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.; Ahn, G.; Lee, W.-W.; Kang, M.-C.; Kim, E.-A.; Jeon, Y.-J. Anti-inflammatory activity of phlorotannin-rich fermented Ecklonia cava processing by-product extract in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Appl. Phycol. 2013, 25, 1207–1213. [Google Scholar] [CrossRef]
- Piao, M.J.; Kim, K.C.; Kang, K.A.; Fernando, P.D.S.M.; Herath, H.M.U.L.; Hyun, J.W. Phloroglucinol Attenuates Ultraviolet B-Induced 8-Oxoguanine Formation in Human HaCaT Keratinocytes through Akt and Erk-Mediated Nrf2/Ogg1 Signaling Pathways. Biomol. Ther. 2021, 29, 90. [Google Scholar] [CrossRef]
- Kim, Y.C.; An, R.B.; Yoon, N.Y.; Nam, T.J.; Choi, J.S. Hepatoprotective constituents of the edible brown algaEcklonia stolonifera on tacrine-induced cytotoxicity in hep G2 cells. Arch. Pharmacal Res. 2005, 28, 1376. [Google Scholar] [CrossRef]
- Quéguineur, B.; Goya, L.; Ramos, S.; Martín, M.A.; Mateos, R.; Guiry, M.D.; Bravo, L. Effect of phlorotannin-rich extracts of Ascophyllum nodosum and Himanthalia elongata (Phaeophyceae) on cellular oxidative markers in human HepG2 cells. J. Appl. Phycol. 2013, 25, 1–11. [Google Scholar] [CrossRef]
- Lee, S.-S.; Bang, M.-H.; Jeon, H.-J.; Hwang, T.; Yang, S.-A. Anti-inflammatory and Anti-allergic Effects of Phlorofucofuroeckol A and Dieckol Isolated from Ecklonia cava. J. Life Sci. 2018, 28, 1170–1178. [Google Scholar] [CrossRef]
- Kang, H.S.; Chung, H.Y.; Jung, J.H.; Son, B.W.; Choi, J.S. A new phlorotannin from the brown alga Ecklonia stolonifera. Chem. Pharmaceut. Bull. 2003, 51, 1012–1014. [Google Scholar] [CrossRef]
- Kang, H.S.; Chung, H.Y.; Kim, J.Y.; Son, B.W.; Jung, H.A.; Choi, J.S. Inhibitory phlorotannins from the edible brown algaecklonia stolonifera on total reactive oxygen species (ROS) generation. Arch. Pharm. Res. 2004, 27, 194–198. [Google Scholar] [CrossRef]
- Kang, J.; Khan, M.; Park, N.; Cho, J.; Lee, M.; Fujii, H.; Hong, Y. Antipyretic, analgesic, and anti-inflammatory activities of the seaweed Sargassum fulvellum and Sargassum thunbergii in mice. J. Ethnopharm. 2008, 116, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar] [CrossRef]
- Kim, D.; Hwang, I.; Ku, B.; Choi, E.-M. Antioxidant and skin anti-aging effects of the aqueous ethanol extract of Ginkgo biloba leaf: An in vitro study using HaCaT keratinocytes. Toxic. Environ. Health Sci. 2021, 13, 133–142. [Google Scholar] [CrossRef]
- Choi, J.; Yang, C.; Lim, W.; Song, G.; Choi, H. Antioxidant and apoptotic activity of cocoa bean husk extract on prostate cancer cells. Mol. Cell. Toxic. 2021. [Google Scholar] [CrossRef]

| Inflammatory Signature Genes | IFN-γ+TNFα /Control Fold Change | PAB /IFN-γ+TNFα Fold Change | Description |
|---|---|---|---|
| TNFRSF9 | 13.660 | 0.152 | tumor necrosis factor receptor superfamily member 9 |
| MDC/CCL22 | 12.597 | 0.090 | C-C motif chemokine ligand 22 |
| CCL8 | 69.982 | 0.013 | C-C motif chemokine ligand 8 |
| ICAM1 | 63.309 | 0.045 | intercellular adhesion molecule 1 |
| IL36G | 31.627 | 0.239 | interleukin 36, gamma |
| APOL3 | 26.531 | 0.101 | apolipoprotein L3 |
| APOL2 | 10.946 | 0.348 | apolipoprotein L2 |
| CXCL9 | 3543.936 | 0.011 | C-X-C motif chemokine ligand 9 |
| HLA-DRB1 | 34.774 | 0.287 | major histocompatibility complex, class II, DR beta 1 |
| IDO1 | 1066.824 | 0.011 | indoleamine 2,3-dioxygenase 1 |
| Gene | Forward (5′-3′) | Reverse (3′-5′) |
|---|---|---|
| GAPDH | CGT CTC CTC TGA CTT CAA CA | AGC CAA ATT CGT TGT CAT AC |
| MDC/CCL22 | CAG CRC GAG GGA CCA ATG TG | CTT GGG GTC CGA ACA GAT GG |
| TARC/CCL17 | ACT GCA CTC CTG GTT GTC CT | AAG GTT AGC AAC ACC ACG CC |
| TNFRSF9 | CAG CAT GTG TGA ACA GGA TTG | GAG GAA GAA CAG CAG GAA GAG |
| CCL8 | CTC ATG GCA GCC ACT TTC A | ATG GAA TCC CTG ACC CAT CT |
| ICAM1 | GGC TGA CGT GTG CAG TAA TA | GGG AAA GTG CCA TCC TTT AGA |
| IL36G | GAA GGG CCG TCT ATC AAT CAA | CAG TCT TGG CAC GGT AGA AA |
| APOL3 | AGG AAG ATC CAG GAG TCC ATA G | GGG TGT GAT GTC ACG CAT AA |
| APOL2 | GAC CAA GTG AGC AGA GAG AAT C | CCA CCC ACA AAC TCC TTC AT |
| CXCL9 | TTT CCT CTT GGG CAT CAT CTT | CTG ACC TGT TTC TCC CAC TTT |
| HLA-DRB1 | GCT CTG TGA GTG GTT TCT ATC C | CTG AAG TCC AGA GTG TCC TTT C |
| IDO1 | GAA ACT GGA ACT GCC TCC TAT T | GTC TTC CCA GAA CCC TTC ATA C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, Y.; Lee, W.-H.; Kim, J.K.; Jeon, H.-K.; Lee, J.; Kim, Y.-J. Polyopes affinis Suppressed IFN-γ- and TNF-α-Induced Inflammation in Human Keratinocytes via Down-Regulation of the NF-κB and STAT1 Pathways. Molecules 2022, 27, 1836. https://doi.org/10.3390/molecules27061836
Ha Y, Lee W-H, Kim JK, Jeon H-K, Lee J, Kim Y-J. Polyopes affinis Suppressed IFN-γ- and TNF-α-Induced Inflammation in Human Keratinocytes via Down-Regulation of the NF-κB and STAT1 Pathways. Molecules. 2022; 27(6):1836. https://doi.org/10.3390/molecules27061836
Chicago/Turabian StyleHa, Yuna, Won-Hwi Lee, Jang Kyun Kim, Hee-Kyung Jeon, Jongsung Lee, and Youn-Jung Kim. 2022. "Polyopes affinis Suppressed IFN-γ- and TNF-α-Induced Inflammation in Human Keratinocytes via Down-Regulation of the NF-κB and STAT1 Pathways" Molecules 27, no. 6: 1836. https://doi.org/10.3390/molecules27061836
APA StyleHa, Y., Lee, W.-H., Kim, J. K., Jeon, H.-K., Lee, J., & Kim, Y.-J. (2022). Polyopes affinis Suppressed IFN-γ- and TNF-α-Induced Inflammation in Human Keratinocytes via Down-Regulation of the NF-κB and STAT1 Pathways. Molecules, 27(6), 1836. https://doi.org/10.3390/molecules27061836








