Melatonin in Brassicaceae: Role in Postharvest and Interesting Phytochemicals
Abstract
:1. Introduction
2. Brassicaceae Plants and Melatonin Studies
3. Postharvest Application of Melatonin in Brassicaceae
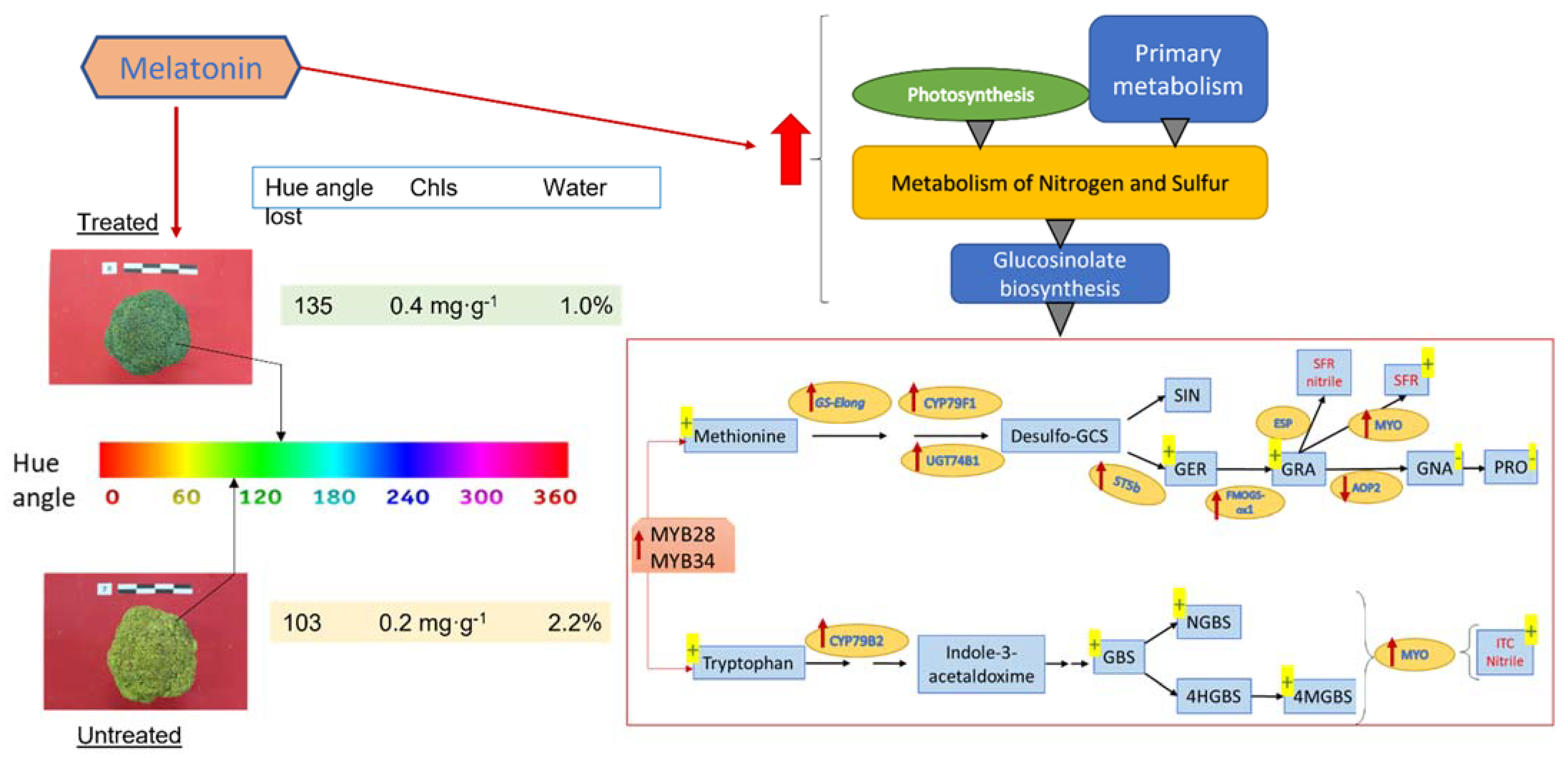
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 4HGBS | 4-hydroxyglucobrassicin |
| 4MGBS | 4-methoxyglucobrassicin |
| ABA | abscisic acid |
| AOP2 | 2-oxoglutarate-dependent dioxygenase |
| BR | brassinosteroids |
| C4H | cinnamic acid 4-hydroxylase |
| CAT | catalase |
| CHI | chalcone isomerase |
| Chl | chlorophylls |
| CHS | chalcone synthase |
| CYP79B1,2 | cytochome P450 |
| CYP79F1 | dihomomethionine N-hydroxylase |
| CYP83A1 | cytochome P450 |
| DFR | dihydroflavonol 4-reductase |
| EC | electroconductivity |
| ESP | epithiospecifier protein |
| F3H: | flavanone 3-hydroxylase |
| FMOGS-OX1 | flavin monooxygenase |
| FW | fresh weight |
| GBS | glucobrassicin |
| GER | glucoerucin |
| GNA | gluconapin |
| GRA | glucoraphanin |
| GS-Elong | glucosinolate biosynthesis enzyme |
| IAA | indolyl-3-acetic acid (auxin) |
| ITC | isothiocyanates |
| JA | jasmonic acid |
| MDA | malondialdehyde |
| MYB28/34 | regulator of glucosinolate biosynthesis |
| MYO | myrosinase |
| NGBS | neoglucobrassicin |
| PAL | phenylalanine ammonia-lyase |
| POD | peroxidase |
| PRO | progoitrin |
| RBOH | respiratory burst oxidase |
| ROS | reactive oxygen species |
| SFR | sulforaphane |
| SIN | sinigrin |
| SOD | superoxide dismutase |
| ST5b | sulfotransferase |
| TAA | total antioxidant activity |
| TCA | tricarboxylic acid cycle (Krebs cycle) |
| TGG1 | myrosinase |
| UFGT, UDP-glucose | flavonoid 3-O-glucosyltransferase |
| UGT74B1 | UDP-glycosyltransferase |
References
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of Melatonin, a Pineal Factor That Lightens Melanocytes. J. Am. Chem Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Heinzelmann, R.V. Structure of Melatonin. J. Am. Chem. Soc. 1959, 81, 6084–6085. [Google Scholar] [CrossRef]
- Majidinia, M.; Reiter, R.J.; Shakouri, S.K.; Yousefi, B. The Role of Melatonin, a Multitasking Molecule, in Retarding the Processes of Ageing. Ageing Res. Rev. 2018, 47, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Socaciu, A.I.; Ionut, R.; Socaciu, M.A.; Ungur, A.P.; Bârsan, M.; Chiorean, A.; Socaciu, C.; Râjnoveanu, A.G. Melatonin, an Ubiquitous Metabolic Regulator: Functions, Mechanisms and Effects on Circadian Disruption and Degenerative Diseases. Rev. Endocr. Metab. Disord. 2020, 21, 465–478. [Google Scholar] [CrossRef]
- Herxheimer, A. Jet Lag. Clin. Evid. 2005, 13, 2178–2183. [Google Scholar]
- Xie, Z.; Chen, F.; Li, W.A.; Geng, X.; Li, C.; Meng, X.; Feng, Y.; Liu, W.; Yu, F. A Review of Sleep Disorders and Melatonin. Neurol. Res. 2017, 39, 559–565. [Google Scholar] [CrossRef]
- Vadnie, C.A.; McClung, C.A. Circadian Rhythm Disturbances in Mood Disorders: Insights into the Role of the Suprachiasmatic Nucleus. Neural Plast. 2017, 2017, 1504507. [Google Scholar] [CrossRef] [Green Version]
- Blume, C.; Angerer, M.; Raml, M.; del Giudice, R.; Santhi, N.; Pichler, G.; Kunz, A.B.; Scarpatetti, M.; Trinka, E.; Schabus, M. Healthier Rhythm, Healthier Brain? Integrity of Circadian Melatonin and Temperature Rhythms Relates to the Clinical State of Brain-Injured Patients. Eur. J. Neurol. 2019, 26, 1051–1059. [Google Scholar] [CrossRef] [Green Version]
- Di Bella, G.; Mascia, F.; Gualano, L.; Di Bella, L. Melatonin Anticancer Effects: Review. Int. J. Mol. Sci. 2013, 14, 2410–2430. [Google Scholar] [CrossRef] [Green Version]
- Alghamdi, B.S. The Neuroprotective Role of Melatonin in Neurological Disorders. J. Neurosci. Res. 2018, 96, 1136–1149. [Google Scholar] [CrossRef]
- Cardinali, D.; Brown, G.; Pandi-Perumal, S.R. Can Melatonin Be a Potential “Silver Bullet” in Treating COVID-19 Patients? Diseases 2020, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Okeke, E.S.; Ogugofor, M.O.; Nkwoemeka, N.E.; Nweze, E.J.; Okoye, C.O. Phytomelatonin: A Potential Phytotherapeutic Intervention on COVID-19-Exposed Individuals. Microbes Infect. 2021, 24, 104886. [Google Scholar] [CrossRef] [PubMed]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in Edible Plants Identified by Radioimmunoassay and by HPLC-MS. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Hattori, A.; Migitaka, H.; Iigo, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of Melatonin in Plants and Its Effects on Plasma Melatonin Levels and Binding to Melatonin Receptors in Vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar]
- Kolar, J.; Machackova, I.; Illnerova, H.; Prinsen, E.; van Dongen, W.; van Onckelen, H. Melatonin in Higher Plant Determined by Radioimmunoassay and Liquid Chromatography-Mass Spectrometry. Biol. Rhythm. Res. 1995, 26, 406–409. [Google Scholar]
- Arnao, M.B. Phytomelatonin: Discovery, Content, and Role in Plants. Adv. Bot. 2014, 2014, 815769. [Google Scholar] [CrossRef]
- Vivien-Roels, B.; Pávet, P. Melatonin: Presence and Formation in Invertebrates. Experientia 1993, 49, 642–647. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of Melatonin in Plants: A Review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant Growth Regulator and/or Biostimulator during Stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef]
- Lv, Y.; Pan, J.; Wang, H.; Reiter, R.; Li, X.; Zongmin, M.; Zhang, J.; Yao, Z.; Zhao, D.; Yu, D. Melatonin Inhibits Seed Germination by Crosstalk with Abscisic Acid, Gibberellin, and Auxin in Arabidopsis. J. Pineal Res. 2021, 70, e12736. [Google Scholar] [CrossRef]
- Hernández-Ruiz, J.; Cano, A.; Arnao, M.B. Melatonin: Growth-Stimulating Compound Present in Lupin Tissues. Planta 2004, 220, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin Promotes Adventitious- and Lateral Root Regeneration in Etiolated Hypocotyls of Lupinus albus L. J. Pineal Res. 2007, 42, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Growth Activity, Rooting Capacity, and Tropism: Three Auxinic Precepts Fulfilled by Melatonin. Acta Physiol. Plant 2017, 39, 127. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a Chemical Substance or as Phytomelatonin Rich-Extracts for Use as Plant Protector and/or Biostimulant in Accordance with EC Legislation. Agronomy 2019, 9, 570. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin in Flowering, Fruit Set and Fruit Ripening. Plant Reprod. 2020, 33, 77–87. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Is Phytomelatonin a New Plant Hormone? Agronomy 2020, 10, 95. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A New Plant Hormone and/or a Plant Master Regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Sharif, R.; Xie, C.; Zhang, H.; Arnao, M.B.; Ali, M.; Ali, Q.; Muhammad, I.; Shalmani, A.; Nawaz, M.; Chen, P.; et al. Melatonin and Its Effects on Plant Systems. Molecules 2018, 23, 2352. [Google Scholar] [CrossRef] [Green Version]
- Moustafa-Farag, M.; Almoneafy, A.; Mahmoud, A.; Elkelish, A.; Arnao, M.B.; Li, L.; Ai, S. Melatonin and Its Protective Role against Biotic Stress Impacts on Plants. Biomolecules 2020, 10, 54. [Google Scholar] [CrossRef] [Green Version]
- Moustafa-Farag, M.; Mahmoud, A.; Arnao, M.B.; Sheteiwy, M.; Dafea, M.; Soltan, M.; Elkelish, A.; Hasanuzzaman, M.; Ai, S. Melatonin-Induced Water Stress Tolerance in Plants: Recent Advances. Antioxidants 2020, 9, 809. [Google Scholar] [CrossRef]
- Menhas, S.; Yang, X.; Hayat, K.; Aftab, T.; Bundschuh, J.; Arnao, M.B.; Zhou, Y.; Zhou, P. Exogenous Melatonin Enhances Cd Tolerance and Phytoremediation Efficiency by Ameliorating Cd-Induced Stress in Oilseed Crops: A Review. J. Plant Growth Regul. 2021. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Regulatory Role of Melatonin in the Redox Network of Plants and Plant Hormone Relationship in Stress. In Hormones and Plant Response; Gupta, D.K., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 235–272. ISBN 978-3-030-77477-6. [Google Scholar]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin Against Environmental Plant Stressors: A Review. Curr. Protein Pept. Sci. 2021, 22, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Mora-Poblete, F.; Arnao, M.B.; Naz, S.; Anwar, M.; Altaf, M.M.; Shahid, S.; Shakoor, A.; et al. Phytomelatonin: An Overview of the Importance and Mediating Functions of Melatonin against Environmental Stresses. Physiol. Plant. 2021, 172, 820–846. [Google Scholar] [CrossRef] [PubMed]
- Buttar, Z.A.; Wu, S.N.; Arnao, M.B.; Wang, C.; Ullah, I.; Wang, C. Melatonin Suppressed the Heat Stress-Induced Damage in Wheat Seedlings by Modulating the Antioxidant Machinery. Plants 2020, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin in Its Relationship to Plant Hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a Regulatory Hub of Plant Hormone Levels and Action in Stress Situations. Plant Biol. 2021, 23, 7–19. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J.; Cano, A.; Reiter, R.J. Melatonin and Carbohydrate Metabolism in Plant Cells. Plants 2021, 10, 1917. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Hernández-Ruiz, J. Phytomelatonin: An Unexpected Molecule with Amazing Performances in Plants. J. Exp. Bot. 2022, erac009. [Google Scholar] [CrossRef]
- Al-Shehbaz, I.A. A Generic and Tribal Synopsis of the Brassicaceae (Cruciferae). TAXON 2012, 61, 931–954. [Google Scholar] [CrossRef]
- Kapusta-Duch, J.; Kopeć, A.; Piatkowska, E.; Borczak, B.; Leszczyńska, T. The Beneficial Effects of Brassica Vegetables on Human Health. Rocz. Państwowego Zakładu Hig. 2012, 63, 389–395. [Google Scholar]
- Chen, Q.; Qi, W.B.; Reiter, R.J.; Wei, W.; Wang, B.M. Exogenously Applied Melatonin Stimulates Root Growth and Raises Endogenous IAA in Roots of Etiolated Seedling of Brassica juncea. J. Plant Physiol. 2009, 166, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Di, H.; Li, Z.; Wang, Y.; Zhang, Y.; Bian, J.; Xu, J.; Zheng, Y.; Gong, R.; Li, H.; Zhang, F.; et al. Melatonin Treatment Delays Senescence and Maintains the Postharvest Quality of Baby Mustard (Brassica juncea var. gemmifera). Front. Plant Sci. 2022, 12, 817861. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Long, W.; Zeng, L.; Ding, X.; Cheng, Y.; Zhang, X.; Zou, X. Melatonin-Induced Transcriptome Variation of Rapeseed Seedlings under Salt Stress. Int. J. Mol. Sci. 2019, 20, 5355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.; Zhao, Y.; Yu, X.; Felix, K.; Han, H.; Guan, R.; Wang, R.; Shen, W. Nitric Oxide Is Required for Melatonin-Enhanced Tolerance against Salinity Stress in Rapeseed (Brassica napus L.) Seedlings. Int. J. Mol. Sci. 2018, 19, 1912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.; Rizwan, M.; Fahad, S.; Xu, Z.; Hu, L. Seed Priming with Melatonin Coping Drought Stress in Rapeseed by Regulating Reactive Oxygen Species Detoxification: Antioxidant Defense System, Osmotic Adjustment, Stomatal Traits and Chloroplast Ultrastructure Perseveration. Ind. Crops Prod. 2019, 140, 111597. [Google Scholar] [CrossRef]
- Jia, C.; Yu, X.; Zhang, M.; Liu, Z.; Zou, P.; Ma, J.; Xu, Y. Application of Melatonin-Enhanced Tolerance to High-Temperature Stress in Cherry Radish (Raphanus sativus L. var. radculus Pers). J. Plant Growth Regul. 2020, 39, 631–640. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Kuran, H.; Marciniak, K.; Janas, K.M. Presowing Seed Treatment with Melatonin Protects Red Cabbage Seedlings against Toxic Copper Ion Concentrations. J. Pineal Res. 2008, 45, 24–31. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Q.; Li, H.; Cao, Y.; Zhang, H.; Li, S.; Zhang, L.; Qi, Y.; Ren, S.; Zhao, B.; et al. Melatonin Improved Anthocyanin Accumulation by Regulating Gene Expressions and Resulted in High Reactive Oxygen Species Scavenging Capacity in Cabbage. Front. Plant Sci. 2016, 7, 197. [Google Scholar] [CrossRef]
- Tan, X.-L.; Fan, Z.-Q.; Kuang, J.-F.; Lu, W.-J.; Reiter, R.J.; Lakshmanan, P.; Su, X.-G.; Zhou, J.; Chen, J.-Y.; Shan, W. Melatonin Delays Leaf Senescence of Chinese Flowering Cabbage by Suppressing ABFs-Mediated Abscisic Acid Biosynthesis and Chlorophyll Degradation. J. Pineal Res. 2019, 67, e12570. [Google Scholar] [CrossRef]
- Tan, X.-L.; Zhao, Y.-T.; Shan, W.; Kuang, J.-F.; Lu, W.-J.; Su, X.-G.; Tao, N.-G.; Lakshmanan, P.; Chen, J.-Y. Melatonin Delays Leaf Senescence of Postharvest Chinese Flowering Cabbage through ROS Homeostasis. Food Res. Int. 2020, 138, 109790. [Google Scholar] [CrossRef]
- Tan, X.-L.; Fan, Z.-Q.; Zeng, Z.-X.; Shan, W.; Kuang, J.-F.; Lu, W.-J.; Su, X.-G.; Tao, N.-G.; Lakshmanan, P.; Chen, J.-Y.; et al. Exogenous Melatonin Maintains Leaf Quality of Postharvest Chinese Flowering Cabbage by Modulating Respiratory Metabolism and Energy Status. Postharvest Biol. Technol. 2021, 177, 111524. [Google Scholar] [CrossRef]
- Teng, Z.; Yu, Y.; Zhu, Z.; Hong, S.B.; Yang, B.; Zang, Y. Melatonin Elevated Sclerotinia Sclerotiorum Resistance via Modulation of ATP and Glucosinolate Biosynthesis in Brassica Rapa Ssp. Pekinensis. J. Proteom. 2021, 243, 104264. [Google Scholar] [CrossRef] [PubMed]
- Gul, M.; Khan, D.; Wani, A.; Bhat, S.; Mir, S.; Malik, A.; Kumar, A.; Narayan, S.; Stephen, K.; Lone, A. Foliar Application of Melatonin Modulates the Growth and Photosynthetic Pigments in Broccoli Cv. Palam Samridhi. SKUAST J. Res. 2018, 20, 193–198. [Google Scholar]
- Yin, Y.; Liu, Y.; Chao, C.; Yang, Z.; Luo, Z.; Fang, W. ITRAQ-Based Proteomic and Physiological Analyses of Broccoli Sprouts in Response to Exogenous Melatonin with ZnSO4 Stress. RSC Adv. 2021, 11, 12336–12347. [Google Scholar] [CrossRef]
- Oloumi, H.; Nasibi, F.; Mozaffari, H. Investigation of the Growth Rate and Secondary Metabolites Content of Lepidium Sativum under Exogenous Melatonin Treatment. Novo Biol. Reper. 2018, 5, 144–154. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Bhattacharjee, P. Ultrasonication-Assisted Extraction of a Phytomelatonin-Rich, Erucic Acid-Lean Nutraceutical Supplement from Mustard Seeds: An Antioxidant Synergy in the Extract by Reductionism. J. Food Sci. Technol. 2020, 57, 1278–1289. [Google Scholar] [CrossRef]
- Jayarajan, S.; Sharma, R.R. Melatonin: A Blooming Biomolecule for Postharvest Management of Perishable Fruits and Vegetables. Trends Food Sci. Technol. 2021, 116, 318–328. [Google Scholar] [CrossRef]
- Wang, S.Y.; Shi, X.C.; Wang, R.; Wang, H.L.; Liu, F.; Laborda, P. Melatonin in Fruit Production and Postharvest Preservation: A Review. Food Chem. 2020, 320, 126642. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Mukherjee, S.; Flores, F.B.; Arnao, M.B.; Luo, Z.; Corpas, F.J. Functions of Melatonin During Postharvest of Horticultural Crops. Plant Cell Physiol. 2021, pcab175. [Google Scholar] [CrossRef]
- Zhu, L.; Hu, H.; Luo, S.; Wu, Z.; Li, P. Melatonin Delaying Senescence of Postharvest Broccoli by Regulating Respiratory Metabolism and Antioxidant Activity. Trans. Chin. Soc. Agric. Eng. 2018, 34, 300–308. [Google Scholar] [CrossRef]
- Luo, F.; Cai, J.H.; Zhang, X.; Tao, D.B.; Zhou, X.; Zhou, Q.; Zhao, Y.B.; Wei, B.D.; Cheng, S.C.; Ji, S.J. Effects of Methyl Jasmonate and Melatonin Treatments on the Sensory Quality and Bioactive Compounds of Harvested Broccoli. RSC Adv. 2018, 8, 41422–41431. [Google Scholar] [CrossRef] [Green Version]
- Miao, H.; Zeng, W.; Zhao, M.; Wang, J.; Wang, Q. Effect of Melatonin Treatment on Visual Quality and Health-Promoting Properties of Broccoli Florets under Room Temperature. Food Chem. 2020, 319, 126498. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Liu, C.; Wang, J.; Younas, S.; Zheng, H.; Zheng, L. Melatonin Immersion Affects the Quality of Fresh-Cut Broccoli (Brassica oleracea L.) during Cold Storage: Focus on the Antioxidant System. J. Food Process. Preserv. 2020, 44, e14691. [Google Scholar] [CrossRef]
- Wei, L.; Liu, C.; Zheng, H.; Zheng, L. Melatonin Treatment Affects the Glucoraphanin-Sulforaphane System in Postharvest Fresh-Cut Broccoli (Brassica oleracea L.). Food Chem. 2020, 307, 125562. [Google Scholar] [CrossRef]
- Wu, C.; Cao, S.; Xie, K.; Chi, Z.; Wang, J.; Wang, H.; Wei, Y.; Shao, X.; Zhang, C.; Xu, F.; et al. Melatonin Delays Yellowing of Broccoli during Storage by Regulating Chlorophyll Catabolism and Maintaining Chloroplast Ultrastructure. Postharvest Biol. Technol. 2021, 172, 111378. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Protective Effect of Melatonin against Chlorophyll Degradation during the Senescence of Barley Leaves. J. Pineal Res. 2009, 46, 58–63. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Zhang, Z.W.; Chen, Y.E.; Ding, C.B.; Yuan, S.; Reiter, R.J.; Yuan, M. Melatonin: A Potential Agent in Delaying Leaf Senescence. Crit. Rev. Plant Sci. 2021, 40, 1–22. [Google Scholar] [CrossRef]
- Rodman, J.E.; Soltis, P.S.; Soltis, D.E.; Sytsma, K.J.; Karol, K.G. Parallel Evolution of Glucosinolate Biosynthesis Inferred from Congruent Nuclear and Plastid Gene Phylogenies. Am. J. Bot. 1998, 85, 997–1006. [Google Scholar] [CrossRef] [Green Version]
- Fahey, J.; Zalcmann, A.; Talalay, P. The Chemical Diversity and Distribution of Glucosinolates and Isothiocyanates among Plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Selmar, D. Biosynthesis of Cyanogenic Glycosides, Glucosinolates and Non-Protein Amino Acids. In Annual Plant Reviews: Biochemistry of Plant Secondary Metabolism; Wink, M., Ed.; Blackwell Pub.: New York, NY, USA, 2010; Volume 2, pp. 92–181. ISBN 978-1-4443-2050-3. [Google Scholar]
- Halkier, B.A.; Gershenzon, J. Biology and Biochemistry of Glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [Green Version]
- Herr, I.; Büchler, M.W. Dietary Constituents of Broccoli and Other Cruciferous Vegetables: Implications for Prevention and Therapy of Cancer. Cancer Treat. Rev. 2010, 36, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Mandrich, L.; Caputo, E. Brassicaceae-Derived Anticancer Agents: Towards a Green Approach to Beat Cancer. Nutrients 2020, 12, 868. [Google Scholar] [CrossRef] [Green Version]
- Ku, K.M.; Choi, J.H.; Kim, H.S.; Kushad, M.M.; Jeffery, E.H.; Juvik, J.A. Methyl Jasmonate and 1-Methylcyclopropene Treatment Effects on Quinone Reductase Inducing Activity and Post-Harvest Quality of Broccoli. PLoS ONE 2013, 8, e77127. [Google Scholar] [CrossRef] [PubMed]
- Moustafa-Farag, M.; Elkelish, A.; Dafea, M.; Khan, M.; Arnao, M.B.; Abdelhamid, M.T.; El-Ezz, A.A.; Almoneafy, A.; Mahmoud, A.; Awad, M.; et al. Role of Melatonin in Plant Tolerance to Soil Stressors: Salinity, PH and Heavy Metals. Molecules 2020, 25, 5359. [Google Scholar] [CrossRef] [PubMed]
| Plant Species/Common Name | Melatonin Treatment | Response/Effect | References | |
|---|---|---|---|---|
 | B. juncea L. Indian mustard seedlings | 0.01–0.5 µM | ↑ IAA, root growth | [42] |
| B. juncea var. gemmifera Baby lateral buds | 100 µM | ↑ total phenols and glucosinolates, vit. C, carotenoids, ↓ weight loss, Chl loss | [43] | |
 | B. napus var. oleracea Rapeseed seedlings | 50 µM | ↑ growth, salt stress tolerance, IAA, ABA, BR and JA signaling factors, JA and BR levels | [44] |
| 0.01–100 µM | ↑ salt tolerance, growth, redox balance | [45] | ||
| 500 µM | ↑ drought tolerance, germination, Chl level, stoma size, redox balance | [46] | ||
 | Raphanus sativus var. radculus Cherry radish seedlings | 50–290 µM | ↑ IAA, growth, heat stress tolerance, biomass, Chl levels, protein content, solid soluble content, redox balance | [47] |
 | B. oleracea var. rubrum Red cabbage seedlings | 1–100 µM | ↑ germination, growth, Cu tolerance | [48] |
 | B. oleracea var. album and rubrum White and red cabbage seedlings | 0.1–1 mM | ↑ growth, anthocyanins, redox balance | [49] |
 | B. rapa var. parachinensis Chinese flowering cabbage (Choy Sum) seedlings | 100 µM | ↑ shelf life, energy level, ↓ ABA level, senescence factors, ABA biosynthesis genes, ABA-transcription factors, Chl-degrading genes, ROS, MDA, RBOH | [50,51,52] |
 | B. rapa var. pekinensis Chinese cabbage | 50–100 µM | ↑ sclerotinia rot tolerance, thiamine, ATP, glucosinolates, antioxidant enzymes | [53] |
 | B. oleracea var. italica Broccoli seedlings | 60 ppm | ↑ growth, photosynthesis, biomass, Chl and carotenoid levels | [54] |
| 10 µM | ↑ growth, Zn tolerance, glucosinolate biosynthesis genes, myrosinase, isothiocyanate, sulforaphane, ↓ EC, MDA | [55] | ||
 | Lepidum sativum L. Gardencress seedlings | 5–100 µM | ↑ growth, Chl, carotenoid, anthocyanin and phenol levels | [56] |
 | Mustard seeds | - | To obtain phytomelatonin | [57] |
| Plant Material/Ta | Melatonin Treatment | Response/Effect | Ref. |
|---|---|---|---|
| Intact florets, 20 °C | 100 µM immersed | ↑ shelf life, Chls, ATP, ADP, SOD, CAT, POD ↓ yellow index, senescence, respiration rate, TCA, AMP, ROS | [61] |
| Intact florets, 20 °C | 100 µM sprayed | ↑ shelf life, Chls, flavonoids, carotenoids ↓ yellow index, senescence, bitterness, astringency, sulfur-volatiles, sulforaphane | [62] |
| Intact florets, 20 °C | 1, 50, 500 µM immersed (5 min) | ↑ shelf life, visual quality, Chls, carotenoids, vit. C, phenols, TAA, total glucosinolates, glucoraphanin, glucosinolate biosynthesis genes | [63] |
| Small cut florets, 4 °C | 10, 100, 500 µM immersed (10 min) | ↑ shelf life, Hue angle, Chls, FW, vit. C, TAA, phenols, flavonoids (rutin, quercetin, epicatechin), SOD, CAT ↓ yellow index, senescence, POD, MDA, ROS | [64] |
| Small cut florets, 4 °C | 100 µM immersed (10 min) | ↑ total glucosinolates, sulforaphane and glucoraphanin content, glucosinolate biosynthesis genes, myrosinase | [65] |
| Intact florets, 20 °C | 100 µM immersed (30 min) | ↑ shelf life, Chls, chloroplast integrity ↓ yellow index, Chl-degrading enzymes and genes | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Ruiz, J.; Ruiz-Cano, D.; Giraldo-Acosta, M.; Cano, A.; Arnao, M.B. Melatonin in Brassicaceae: Role in Postharvest and Interesting Phytochemicals. Molecules 2022, 27, 1523. https://doi.org/10.3390/molecules27051523
Hernández-Ruiz J, Ruiz-Cano D, Giraldo-Acosta M, Cano A, Arnao MB. Melatonin in Brassicaceae: Role in Postharvest and Interesting Phytochemicals. Molecules. 2022; 27(5):1523. https://doi.org/10.3390/molecules27051523
Chicago/Turabian StyleHernández-Ruiz, Josefa, Domingo Ruiz-Cano, Manuela Giraldo-Acosta, Antonio Cano, and Marino B. Arnao. 2022. "Melatonin in Brassicaceae: Role in Postharvest and Interesting Phytochemicals" Molecules 27, no. 5: 1523. https://doi.org/10.3390/molecules27051523
APA StyleHernández-Ruiz, J., Ruiz-Cano, D., Giraldo-Acosta, M., Cano, A., & Arnao, M. B. (2022). Melatonin in Brassicaceae: Role in Postharvest and Interesting Phytochemicals. Molecules, 27(5), 1523. https://doi.org/10.3390/molecules27051523









