3DG Functionalized Magnetic Solid Phase Extraction Materials for the Efficient Enrichment of Hexamethylenetetramine in Vermicelli
Abstract
:1. Introduction
2. Results and Discussion
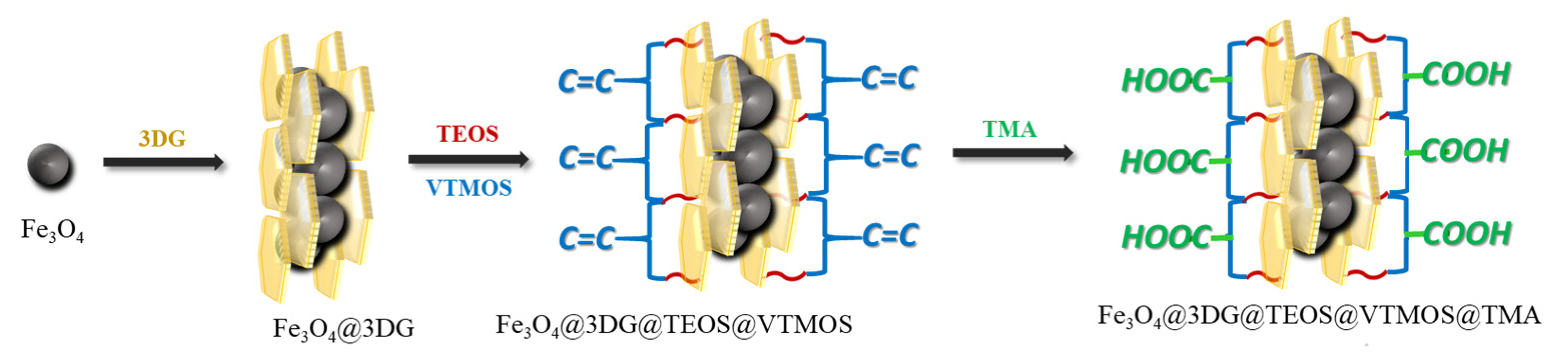
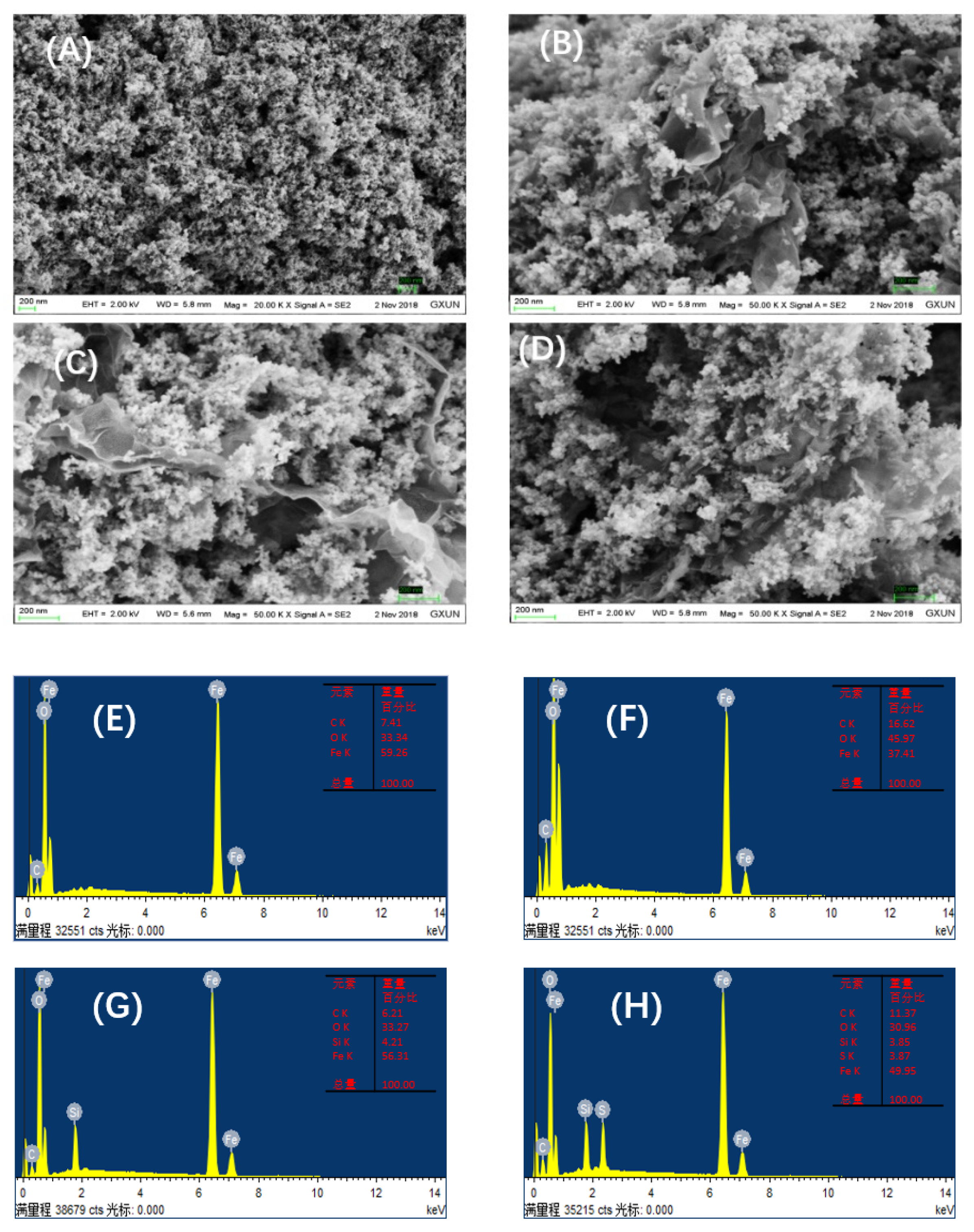
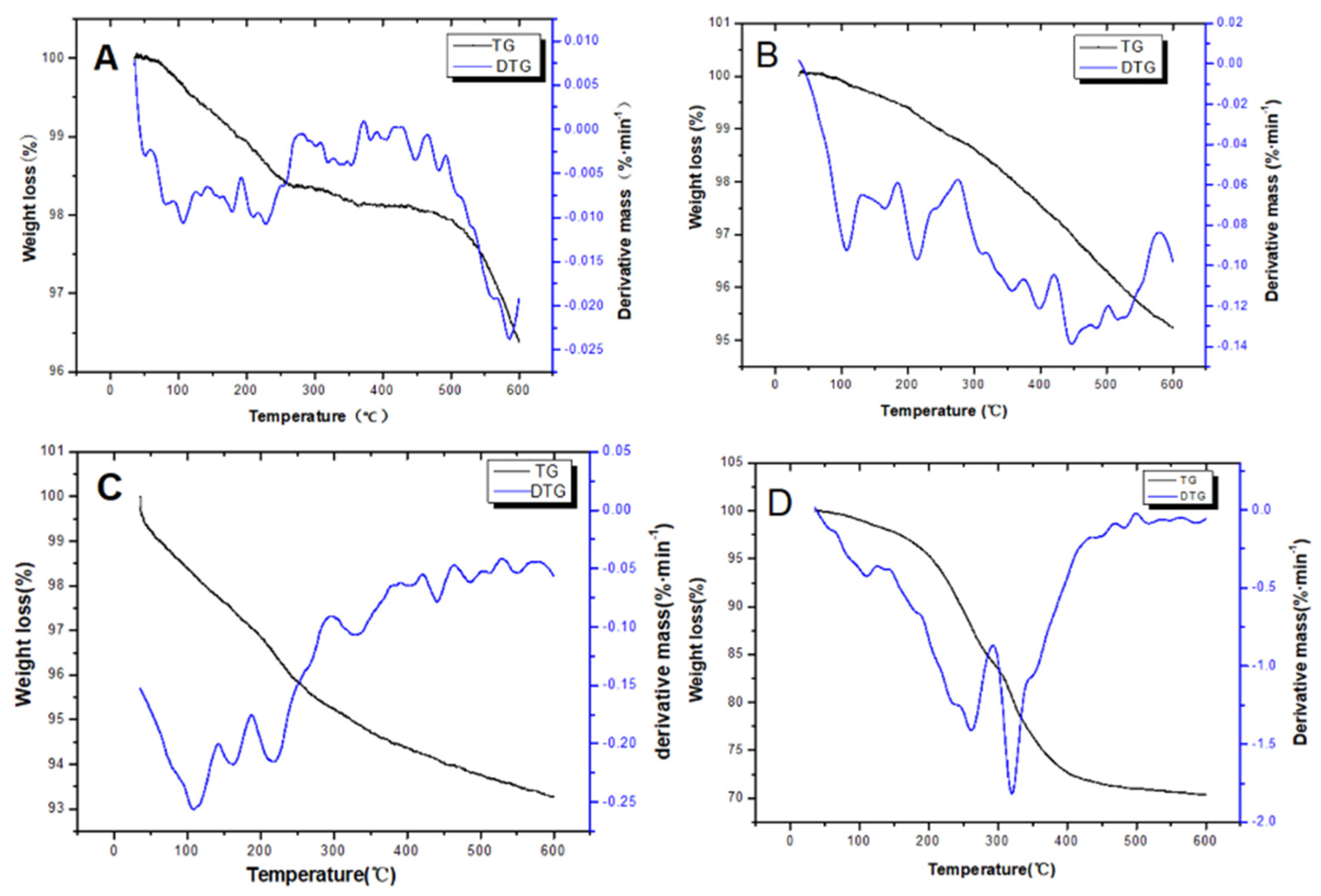
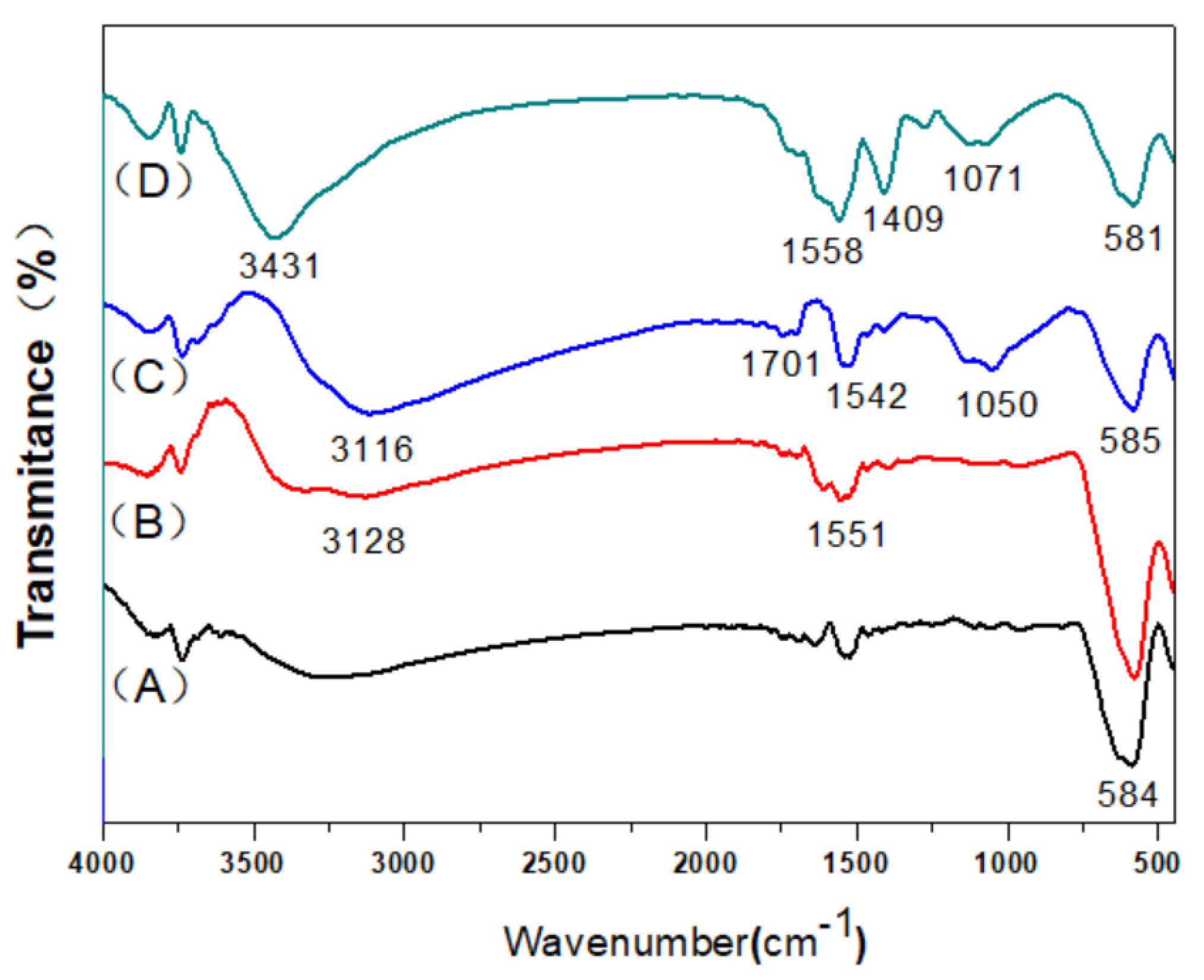
2.1. Characterizations
2.2. Study of Adsorption Properties
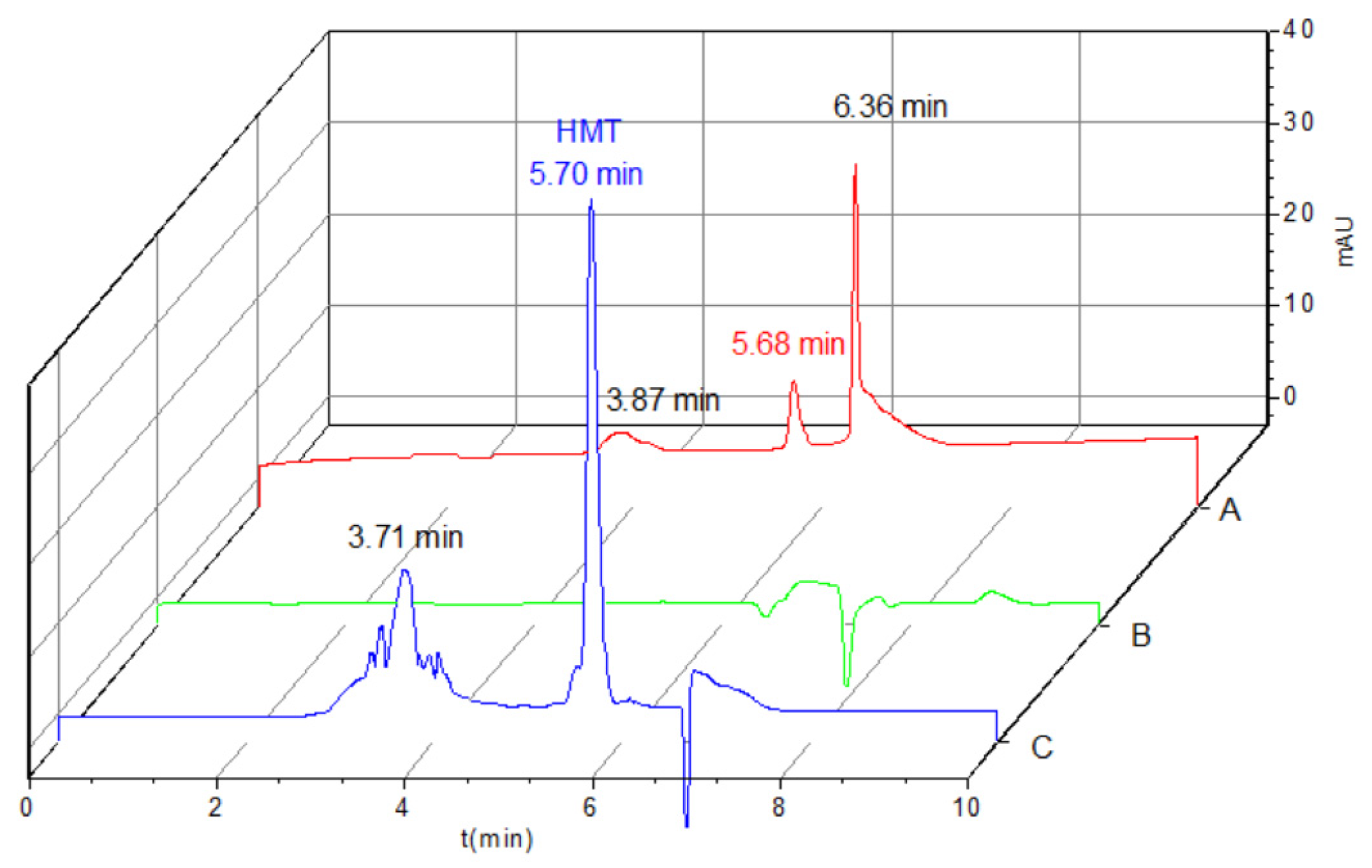
2.3. Validation of Method
2.4. Analysis of the Real Samples
3. Materials and Methods
3.1. Reagents
3.2. Apparatus
3.3. Preparation of Fe3O4@3DG@VTMOS@TMA
3.4. Adsorption Experiments
3.5. MSPE-HPLC Analysis of HMT in Vermicelli Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Caulfield, M.J.; Solomon, D.H. Studies on polyimides: Part 3. Interactions between hexamethylenetetramine and models for polyimides and novolacs. Polymer 1999, 40, 3041–3050. [Google Scholar] [CrossRef]
- Gao, X.; Li, X.; Yu, W. Flowerlike ZnO nanostructures via hexamethylenetetramine-assisted thermolysis of zinc-ethylenediamine complex. J. Phys. Chem. B 2005, 109, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Shin-Ichiro, M.; Keizo, T.; Jun, N.; Masami, W.; Genro, K.; Akihisa, T.; Hiroki, T.; Minoru, S.; Ken, O.; Yuko, K. Usefulness of Hexamethylenetetramine as an Adjuvant to Radiation and Cisplatin in the Treatment of Solid Tumors: Its Independency of p53 Status. J. Radiat. Res. 2010, 51, 27–35. [Google Scholar]
- Musher, D.M.; Griffith, D.P. Generation of Formaldehyde from Methenamine: Effect of pH and Concentration, and Antibacterial Effect. Antimicrob. Agents Chemother. 1974, 6, 708–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paranjape, S.G.; Turankar, A.V.; Sontakke, S.D. Eradication of Helicobacter pylori. Can methenamine, an antimicrobial designed to be effective at an acidic pH, meet this challenge? Med. Hypotheses 2013, 80, 507. [Google Scholar] [CrossRef]
- Papich, M.G. Saunders Handbook of Veterinary Drugs||Methenamine; Elsevier: Saint Louis, MO, USA, 2016; pp. 505–506. [Google Scholar]
- National Health Commission of the People’s Republic of China. List of food additives that may be illegally added in food and misusable food additives. Modern J. Anim. Husb. Vet. Med. 2011, 5, 78–79. [Google Scholar]
- Commonwealth of Australia. Australia New Zealand Food Standards Code—Standard 1.2.4—Labelling of Ingredients. 2021. Available online: https://www.legislation.gov.au/Details/F2021C00290 (accessed on 15 March 2021).
- Xu, X.; Duhoranimana, E.; Zhang, X. Preparation and characterization of magnetic molecularly imprinted polymers for the extraction of hexamethylenetetramine in milk samples. Talanta 2017, 163, 31–38. [Google Scholar] [CrossRef]
- British Standards Institution. Materials and Articles in Contact with foodstuffs—Plastics Substances Subject to Limitation—Part 23: Determination of Formaldehyde and Hexamethylenetetramine in Food Simulants. 2005. Available online: https://shop.bsigroup.com/products/materials-and-articles-in-contact-with-foodstuffs-plastics-substances-subject-to-limitation-determination-of-formaldehyde-and-hexamethylenetetramine-in-food-simulants/standard/preview (accessed on 16 March 2021).
- Peng, M.J.; Song, A.H.; Liu, D.H.; Huang, Q.T.; Qi, Y.Q.; Huang, R.Q. Determination of thiourea dioxide in rice, flour and their products by hydrophilic chromatographic column high performance liquid chromatography. J. Food Saf. Qual. 2018, 9, 3434–3440. [Google Scholar]
- Li, W.; Fan, X.G.; Wang, X.; Tang, M.; Zuo, Y. Design of Rapid Detection System for Urotropine in Food Based on SERS. Spectrosc. Spectr. Anal. 2017, 37, 1778–1783. [Google Scholar]
- Xu, X.; Zhang, X.; Duhoranimana, E.; Zhang, Y.; Shu, P. Determination of methenamine residues in edible animal tissues by HPLC-MS/MS using a modified QuEChERS method: Validation and pilot survey in actual samples. Food Control 2016, 61, 99–104. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, H.; Cui, F.; Jiang, J. Determination of urotropine in dried beancurd sticks by ultra performance liquid chromatography-tandem mass spectrometry. Sci. Technol. Food Ind. 2014, 35, 298–300. [Google Scholar]
- Preiner, D. Optimization of SPME-Arrow-GC/MS Method for Determination of Free and Bound Volatile Organic Compounds from Grape Skins. Molecules 2021, 26, 7409. [Google Scholar]
- Fan, H.; Deng, Z.; Zhong, H.; Yao, Q. Development of new solid phase extraction techniques in the last ten years. J. Chin. Pharm. Sci. 2013, 22, 293–302. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-Assembled Graphene Hydrogel via a One-Step Hydrothermal Process. Acs Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef]
- Li, Z.; Song, B.; Wu, Z.; Lin, Z.; Yao, Y.; Moon, K.S.; Wong, C.P. 3D porous graphene with ultrahigh surface area for microscale capacitive deionization. Nano Energy 2015, 11, 711–718. [Google Scholar] [CrossRef]
- Mateos, R.; Vera, S.; Díez-Pascual, A.; Andrés, M. Graphene solid phase extraction (SPE) of synthetic antioxidants in complex food matrices. J. Food Compos. Anal. 2017, 62, 223–230. [Google Scholar] [CrossRef]
- Yan, Z.; Yao, W.; Hu, L.; Liu, D.; Wang, C.; Lee, C.S. Progress in the preparation and application of three-dimensional graphene-based porous nanocomposites. Nanoscale 2015, 7, 5563–5577. [Google Scholar] [CrossRef] [Green Version]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Xu, Y.; Xiong, X.; Cai, L.; Tang, Z.; Ye, Z. Thiol-Ene Click Chemistry. Prog. Chem. 2012, 24, 385–394. [Google Scholar]
- Gao, S.; Guo, Y.; Li, X.; Wang, X.; Wang, J.; Qian, F.; Gu, H.; Zhang, Z. Magnetic solid phase extraction of sulfonamides based on carboxylated magnetic graphene oxide nanoparticles in environmental waters. J. Chromatogr. A 2018, 1575, 1–10. [Google Scholar] [CrossRef]
- Li, N.; Jiang, H.L.; Wang, X.L.; Wang, X.; Xu, G.J.; Zhang, B.B.; Wang, L.J.; Zhao, R.S.; Lin, J.M. Recent advances in graphene-based magnetic composites for magnetic solid-phase extraction. TrAC Trends Anal. Chem. 2018, 102, 60–74. [Google Scholar] [CrossRef]
- Liu, X.Y.; Li, J.J.; Wu, Z.Y.; Wang, F.; Tan, X.C.; Lei, F.H. Selective separation and determination of glucocorticoids in cosmetics using dual-template magnetic molecularly imprinted polymers and HPLC. J. Colloid Interf. Sci. 2017, 504, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tian, Y.; Chen, X.; Li, J. Kinetic and Synergetic Effect Analysis of the Co-Combustion of Coal Blended with Polyurethane Materials. ACS Omega 2020, 5, 26005–26014. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Liang, S.; Tang, Y.; Tan, X.; Zhou, J. Influence of PVP on Solvothermal Synthesized Fe3O4/Graphene Composites as Anodes for Lithium-ion Batteries. Electrochemistry 2015, 83, 619–623. [Google Scholar] [CrossRef] [Green Version]
- Krishnamoorthy, K.; Veerapandian, M.; Zhang, L.H.; Yun, K.; Kim, S.J. Antibacterial Efficiency of Graphene Nanosheets against Pathogenic Bacteria via Lipid Peroxidation. J. Phys. Chem. C 2012, 116, 17280–17287. [Google Scholar] [CrossRef]
- Hui, Z.; Ma, Y.; Fan, Y.; Shen, J. Fourier transform infrared spectroscopy and oxygen luminescence probing combined study of modified sol-gel derived film. Thin Solid Film. 2001, 397, 95–101. [Google Scholar]
- Azizian, S. Kinetic models of sorption: A theoretical analysis. J. Colloid Interface 2004, 276, 47–52. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Sweileh, J.A.; Misef, K.Y.; El-Sheikh, A.H.; Sunjuk, M.S. Development of a new method for determination of aluminum (Al) in Jordanian foods and drinks: Solid phase extraction and adsorption of Al3+-D-mannitol on carbon nanotubes. J. Food Compos. Anal. 2014, 33, 6–13. [Google Scholar] [CrossRef]
- Wang, F.; Li, X.; Li, J.; Zhu, C.; Liu, M.; Wu, Z.; Liu, L.; Tan, X.; Lei, F. Preparation and application of a molecular capture for safety detection of cosmetics based on surface imprinting and multi-walled carbon nanotubes. J. Colloid Interface Sci. 2018, 527, 124–131. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.Y.; Li, J.J.; Su, X.M.; Wu, Z.Y.; Li, P.F.; Lei, F.H.; Tan, X.C.; Shi, Z.W. Synthesis of magnetic molecularly imprinted polymers for the selective separation and determination of metronidazole in cosmetic samples. Anal. Bioanal. Chem. 2015, 407, 3875–3880. [Google Scholar] [CrossRef]
- Xu, X.; Ma, W.; An, B.; Zhou, K.; Huang, L. Adsorption/desorption and degradation of doxycycline in three agricultural soils. Ecotoxicol. Environ. Saf. 2021, 224, 112675. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Song, A.; Huang, R.Q.; Wu, J.W.; Zeng, H.W.; Cao, X.F. Determination of methenamine in rice, flour and their products by hydrophilic chromatographic column high performance liquid chromatography. J. Food Saf. Qual. 2018, 9, 3733–3739. [Google Scholar]
- Lim, H.S.; Kim, J.I.; Ko, K.Y.; Kim, M. Determination of hexamethylenetetramine in foods by high-performance liquid chromatography (HPLC). Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2014, 31, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Molognoni, L.; Daguer, H.; De, L.A.; De, D. A multi-purpose tool for food inspection: Simultaneous determination of various classes of preservatives and biogenic amines in meat and fish products by LC-MS. Talanta 2018, 178, 1053–1066. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Polymer Chemistry Thiol—Ene Click Chemistry. Angewandte 2010, 49, 1540–1573. [Google Scholar] [CrossRef]

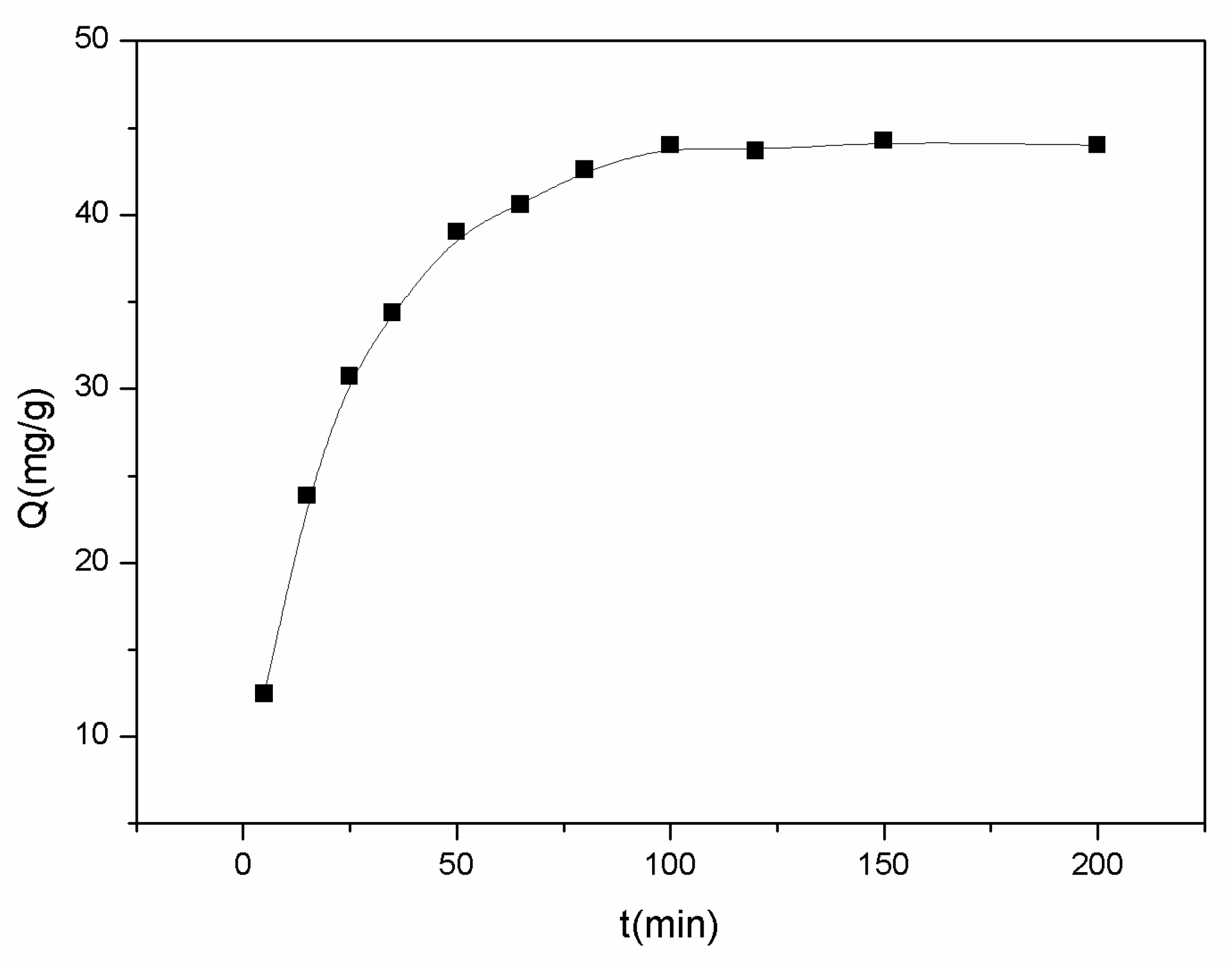

| Kinetic Model | Adsorption Equation | R2 | Qe (mg/g) |
|---|---|---|---|
| pseudo-first-order | 0.4693 | 37.75 | |
| pseudo-second-order | 0.9982 | 47.76 |
| ΔHθ | ΔSθ | ΔGθ (KJ/mol) | R2 | ||
|---|---|---|---|---|---|
| (KJ/mol) | (J/mol·K) | 293.15 K | 303.15 K | 313.15 K | |
| 18.79 | 90.03 | −8.80 | −9.38 | −10.68 | 0.9972 |
| Temperature (K) | Adsorption Isotherm | |||
|---|---|---|---|---|
| Freundlich Isotherm Parameters | ||||
| Regression Equations | Qmax (mg/g) | KL | R2 | |
| 293.15 | 1/Qe = 0.02520/Ce + 0.006767 | 147.78 | 0.27 | 0.9942 |
| 303.15 | 1/Qe = 0.02026/Ce + 0.006184 | 161.71 | 0.31 | 0.9903 |
| 313.15 | 1/Qe = 0.01602/Ce + 0.005773 | 173.22 | 0.36 | 0.9891 |
| Freundlich Isotherm Parameters | ||||
| Regression Equations | KF (mg/g) | n | R2 | |
| 293.15 | lnQe = 0.7794lnCe + 3.3809 | 29.40 | 1.28 | 0.9595 |
| 303.15 | lnQe = 0.7999lnCe + 3.5967 | 36.48 | 1.25 | 0.9606 |
| 313.15 | lnQe = 0.8019lnCe + 3.8060 | 44.97 | 1.25 | 0.9654 |
| Samples | Spiked Level | |||||
|---|---|---|---|---|---|---|
| Vermicelli | 8 μg/g | 20 μg/g | 40 μg/g | |||
| Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | |
| 1 | 89.46 | 1.95 | 85.93 | 2.07 | 91.42 | 0.70 |
| 2 | 87.44 | 1.09 | 86.43 | 1.15 | 92.69 | 0.20 |
| 3 | 86.24 | 1.64 | 83.24 | 0.56 | 91.19 | 0.43 |
| Method | LOD | LOQ | Spiked Level | Recovery | RSD | References |
|---|---|---|---|---|---|---|
| (µg/mL) | (µg/mL) | (μg/g) | (%) | (%) | ||
| HPLC | 1 | - | 4–40 | 93.5–98.7 | 1.5–4.2 | [36] |
| HPLC | 5 | 10 | 10–100 | 91.6–100.9 | 0.9–8.7 | [37] |
| SERS | 0.5 | - | 0.5–5.0 | - | 0.30–3.83 | [12] |
| LC-MS | 0.6 | 1.0 | 25–200 | 20.9–106.9 | - | [38] |
| MSPE-HPLC | 0.25 | 0.6 | 8–40 | 83.24–92.69 | 0.20–2.07 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Zhu, C.; Li, X.; Wang, F. 3DG Functionalized Magnetic Solid Phase Extraction Materials for the Efficient Enrichment of Hexamethylenetetramine in Vermicelli. Molecules 2022, 27, 1548. https://doi.org/10.3390/molecules27051548
Liu M, Zhu C, Li X, Wang F. 3DG Functionalized Magnetic Solid Phase Extraction Materials for the Efficient Enrichment of Hexamethylenetetramine in Vermicelli. Molecules. 2022; 27(5):1548. https://doi.org/10.3390/molecules27051548
Chicago/Turabian StyleLiu, Min, Chen Zhu, Xiaoyan Li, and Fang Wang. 2022. "3DG Functionalized Magnetic Solid Phase Extraction Materials for the Efficient Enrichment of Hexamethylenetetramine in Vermicelli" Molecules 27, no. 5: 1548. https://doi.org/10.3390/molecules27051548







