Widely-Targeted Metabolic Profiling in Lyciumbarbarum Fruits under Salt-Alkaline Stress Uncovers Mechanism of Salinity Tolerance
Abstract
:1. Introduction
2. Results
2.1. Soil Physico–Chemical Properties along the Salt-Alkaline Stress Gradients
2.2. Effects of Salt-Alkaline Stress on Wolfberry Fruit and Leaf Morphological Traits
2.3. Metabolic Profiles of Ripe Wolfberry Fruits from the Different Salt-Alkaline Gradients
2.4. Variation of Metabolome along Salt-Alkaline Gradients in Worlfberry Fruits
2.5. Correlations between Soil Physico–Chemical Properties and Major Metabolite Classes
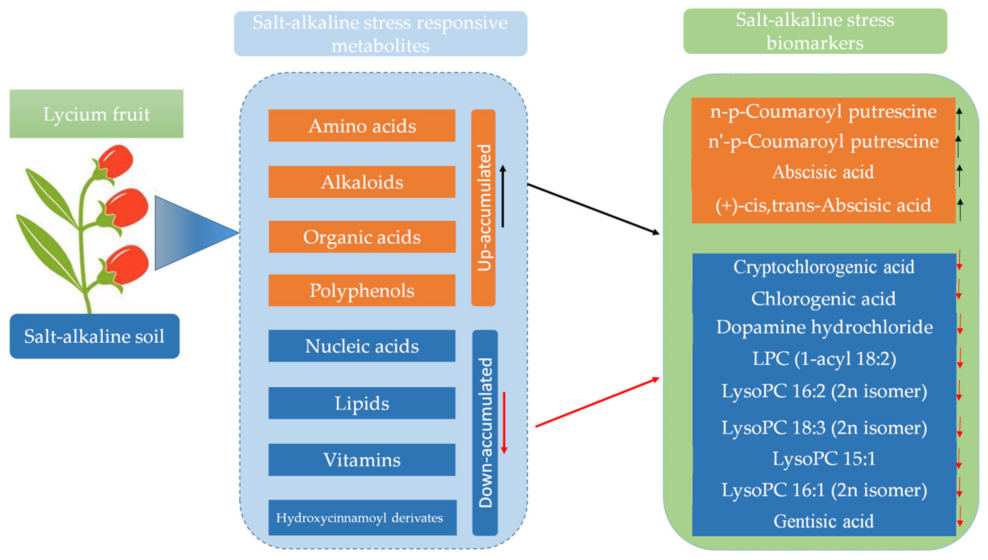
2.6. Differential Metabolites Accumulation in Wolfberry Fruits from the Different Stress Gradients
3. Discussion
3.1. High Soil Salt-Alkaline Levels Improve Ningqi1 Wolfberry Fruit Quality
3.2. Wolfberry Metabolic Alteration in Response to Salinity Stress
3.3. Key Salinity-Tolerant Biomarkers Identified in Wolfberry
4. Materials and Methods
4.1. Plant Materials, Study Site and Sampling
4.2. Soil Physico–Chemical Analyses
4.3. Fruit and Leaf Survey
4.4. Fruit Metabolome Analyses
4.4.1. Metabolome Profiling
4.4.2. Sample Preparation for Metabolites Extraction
4.4.3. Metabolites Determination and Assessment
4.5. Statistical Analyses
5. Conclusions
- The soil pH, Na+, K+, Ca2+, Mg2+ and HCO3− contents decreased proportionally across the salt-alkaline stress gradients. Based on the widely-targeted metabolomics approach, we identified 457 diverse metabolites, 53% of which were affected by salt-alkaline stress.
- Soil salt-alkaline stress enhanced metabolites accumulation in wolfberry fruits. Amino acids, alkaloids, organic acids, and polyphenols contents increased proportionally across the salt-alkaline stress gradients. In contrast, nucleic acids, lipids, hydroxycinnamoyl derivatives, organic acids and derivatives and vitamins were significantly reduced by high salt-alkaline stress. A total of 13 salt-responsive metabolites represent potential biomarkers for salt-alkaline stress tolerance in wolfberry.
- Specifically, we found that constant reductions of lipids and chlorogenic acids; up-regulation of abscisic acid and accumulation of polyamines are essential mechanisms for salt-alkaline stress tolerance in Ningqi1. Overall, we provide for the first time some extensive metabolic insights into salt-alkaline stress tolerance and key metabolite biomarkers, which may be useful for improving wolfberry tolerance to salt-alkaline stress.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xu, C.-Q.; Liu, S.; Xu, R.; Chen, J.; Qiao, H.-L.; Jin, H.-Y.; Lin, C.; Guo, K.; Cheng, H.-Z. Investigation of production status in major wolfberry producing areas of China and some suggestions. China J. Chin. Mater. Medica 2014, 39, 1979–1984. [Google Scholar]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic stress responses and microbe-mediated mitigation in plants: The omics strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [CrossRef]
- Amagase, H.; Farnsworth, N.R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar] [CrossRef]
- Yang, H.; Hu, J.; Long, X.; Liu, Z.; Rengel, Z. Salinity altered root distribution and increased diversity of bacterial communities in the rhizosphere soil of Jerusalem artichoke. Sci. Rep. 2016, 6, 20687. [Google Scholar] [CrossRef]
- Liu, X.; Wu, H.; Ji, C.; Wei, L.; Zhao, J.; Yu, J. An integrated proteomic and metabolomic study on the chronic effects of mercury in suaeda salsa under an environmentally relevant salinity. PLoS ONE 2013, 8, e64041. [Google Scholar] [CrossRef] [Green Version]
- Toscano, S.; Trivellini, A.; Cocetta, G.; Bulgari, R.; Francini, A.; Romano, D.; Ferrante, A. Effect of preharvest abiotic stresses on the accumulation of bioactive compounds in horticultural produce. Front. Plant Sci. 2019, 10, 1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Song, J. Analysis of widely targeted metabolites of the euhalophyte Suaeda salsa under saline conditions provides new insights into salt tolerance and nutritional value in halophytic species. BMC Plant Biol. 2019, 19, 388. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, H.; Lou, Q.; Liu, H.; Han, H.; Wang, Q.; Tang, Z.; Ma, Y.; Wang, H. Differential regulation of anthocyanins in green and purple turnips revealed by combined de novo transcriptome and metabolome analysis. Int. J. Mol. Sci. 2019, 20, 4387. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Kim, J.K.; Jia, C.; Yin, F.; Kim, H.J.; Akram, W.; Hu, X.; Li, X. Comparative transcriptome and metabolic profiling analysis of buckwheat (Fagopyrum Tataricum (L.) Gaertn.) under Salinity Stress. Metabolites 2019, 9, 225. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lv, J.; Liu, Z.; Wang, J.; Yang, B.; Chen, W.; Ou, L.; Dai, X.; Zhang, Z.; Zou, X. Integrative analysis of metabolome and transcriptome reveals the mechanism of color formation in pepper fruit (Capsicum annuum L.). Food Chem. 2020, 306, 125629. [Google Scholar] [CrossRef]
- Alseekh, S.; Bermudez, L.F.; De Haro, L.A.; Fernie, A.R.; Carrari, F. Crop metabolomics: From diagnostics to assisted breeding. Metabolomics 2018, 14, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zeng, X.; Xu, Q.; Mei, X.; Yuan, H.; Jiabu, D.; Sang, Z.; Nyima, T. Metabolite profiling in two contrasting Tibetan hulless barley cultivars revealed the core salt-responsive metabolome and key salt-tolerance biomarkers. AoB PLANTS 2019, 11, plz021. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; He, K.; Zhang, T.; Tang, D.; Li, R.; Jia, S. Physiological responses of Goji berry (Lycium barbarum L.) to saline-alkaline soil from Qinghai region, China. Sci. Rep. 2019, 9, 12057. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, X.; Li, Y.; Fan, Y.; Li, Y.; Cao, Y.; An, W.; Shi, Z.; Zhao, J.; Guo, S.; et al. Changes in metabolome and nutritional quality of lycium barbarum fruits from three typical growing areas of china as revealed by widely targeted metabolomics. Metabolites 2020, 10, 46. [Google Scholar] [CrossRef] [Green Version]
- Nzeuwa, I.B.Y.; Guo, B.; Zhang, T.; Wang, L.; Ji, Q.; Xia, H.; Sun, G. Comparative metabolic profiling of lycium fruits (Lycium barbarum and Lycium chinense) from different areas in china and from Nepal. J. Food Qual. 2019, 2019, 4396027. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liang, X.; Guo, S.; Li, Y.; Zhang, B.; Yin, Y.; An, W.; Cao, Y.; Zhao, J. Evaluation of Nutrients and Related Environmental Factors for Wolfberry (Lycium barbarum) Fruits Grown in the Different Areas of China. Biochem. Syst. Ecol. 2019, 86, 103916. [Google Scholar] [CrossRef]
- Qian, D.; Zhao, Y.; Yang, G.; Huang, L. Systematic review of chemical constituents in the genus lycium (Solanaceae). Molecular 2017, 22, 911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, R.; Heinrich, M.; Zou, Y.; Reich, E.; Zhang, X.; Chen, Y.; Weckerle, C. Quality variation of goji (fruits of Lycium spp.) in china: A comparative morphological and metabolomic analysis. Front. Pharmacol. 2018, 9, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, R.; Heinrich, M.; Weckerle, C.S. The genus Lycium as food and medicine: A botanical, ethnobotanical and historical review. J. Ethnopharmacol. 2018, 212, 50–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, X.; Liang, T.; Liu, Y.; Ding, G.; Zhang, F.; Ma, Z. Extraction, structural characterization, and biological functions of lycium barbarum polysaccharides: A Review. Biomolecules 2019, 9, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donno, D.; Beccaro, G.L.; Mellano, M.; Cerutti, A.; Bounous, G. Goji berry fruit (Lycium spp.): Antioxidant compound fingerprint and bioactivity evaluation. J. Funct. Foods 2015, 18, 1070–1085. [Google Scholar] [CrossRef]
- Islam, T.; Yu, X.; Badwal, T.S.; Xu, B. Comparative studies on phenolic profiles, antioxidant capacities and carotenoid con-tents of red goji berry (Lycium barbarum) and black goji berry (Lycium ruthenicum). Chem. Cent. J. 2017, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Jiang, X.; Wang, T.; Zhang, B.; Zhao, H. Lycium barbarum polysaccharide (LBP): A novel prebiotics candidate for Bifidobacterium and Lactobacillus. Front. Microbiol. 2018, 9, 1043. [Google Scholar] [CrossRef]
- Luo, Q.; Cai, Y.; Yan, J.; Sun, M.; Corke, H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2004, 76, 137–149. [Google Scholar] [CrossRef]
- Gan, L.; Zhang, S.H.; Yang, X.L.; Xu, H.B. Immunomodulation and antitumor activity by a polysaccharide–protein complex from Lycium barbarum. Int. Immunopharmacol. 2004, 4, 563–569. [Google Scholar] [CrossRef]
- Yang, M.; Gao, N.; Zhao, Y.; Liu, L.-X.; Lu, X.-J. Protective effect of Lycium barbarum polysaccharide on retinal ganglion cells in vitro. Int. J. Ophthalmol. 2011, 4, 377–379. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, X.; Pu, J.; Luo, P.; Ma, W.; Wang, J.; Wei, J.; Wang, Y.; Fei, Z. Lycium barbarum polysaccharide protects against oxygen glucose deprivation/reoxygenation-induced apoptosis and autophagic cell death via the PI3K/Akt/mTOR signaling pathway in primary cultured hippocampal neurons. Biochem. Biophys. Res. Commun. 2018, 495, 1187–1194. [Google Scholar] [CrossRef]
- Zhou, S.-F.; Cheng, J.; Zhou, Z.-W.; Sheng, H.-P.; He, L.-J.; Fan, X.-W.; He, Z.-X.; Sun, T.; Zhang, X.; Gu, L.; et al. An evidence-based update on the pharmacological activities and possible molecular targets of Lycium barbarum polysaccharides. Drug Des. Devel. Ther. 2014, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.F.; Zhang, H.; Teh, S.S.; Wang, C.W.; Zhang, Y.; Hayford, F.; Wang, L.; Ma, T.; Dong, Z.; Zhang, Y.; et al. Goji berries as a potential natural antioxidant medicine: An insight into their molecular mechanisms of action. Oxidative Med. Cell. Longev. 2019, 2437397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benchennouf, A.; Grigorakis, S.; Loupassaki, S.; Kokkalou, E. Phytochemical analysis and antioxidant activity of Lycium barbarum (Goji) cultivated in Greece. Pharm. Biol. 2016, 55, 596–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, B.; Huang, B. Mechanism of Salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 701596. [Google Scholar] [CrossRef]
- Rahneshan, Z.; Nasibi, F.; Moghadam, A.A. Effects of salinity stress on some growth, physiological, biochemical parameters and nutrients in two pistachio (Pistacia vera L.) rootstocks. J. Plant Interact. 2018, 13, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Sandhya, V.; Ali, S.Z. The production of exopolysaccharide by Pseudomonas putida GAP-P45 under various abiotic stress conditions and its role in soil aggregation. Microbiology 2015, 84, 512–519. [Google Scholar] [CrossRef]
- Guo, R.; Shi, L.; Yang, C.; Yan, C.; Zhong, X.; Liu, Q.; Xia, X.; Li, H. Comparison of ionomic and metabolites response under alkali stress in old and young leaves of cotton (Gossypium hirsutum L.) Seedlings. Front. Plant Sci. 2016, 7, 1785. [Google Scholar] [CrossRef] [Green Version]
- Shelden, M.C.; Dias, D.A.; Jayasinghe, N.S.; Bacic, A.; Roessner, U. Root spatial metabolite profiling of two genotypes of barley (Hordeum vulgare L.) reveals differences in response to short-term salt stress. J. Exp. Bot. 2016, 67, 3731–3745. [Google Scholar] [CrossRef] [Green Version]
- Kumari, A.; Parida, A.K.; Rangani, J.; Panda, A. Antioxidant activities, metabolic profiling, proximate analysis, mineral nutrient composition of salvadora persica fruit unravel a potential functional food and a natural source of pharmaceuticals. Front. Pharmacol. 2017, 8, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. WHO Global Water Supply and Sanitation Assessment 2000 Report; WHO: Geneva, Switzerland; New York, NY, USA, 2002. [Google Scholar]
- Chen, W.; Gong, L.; Guo, Z.; Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; et al. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Liu, J.-J.; Banerjee, S.; Zhou, N.; Zhao, Z.-Y.; Zhang, K.; Tian, C.-Y. Soil pH is equally important as salinity in shaping bacterial communities in saline soils under halophytic vegetation. Sci. Rep. 2018, 8, 4550. [Google Scholar] [CrossRef] [PubMed]
- Tavakkoli, E.; Rengasamy, P.; McDonald, G.K. High concentrations of Na+ and Cl− ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. J. Exp. Bot. 2010, 61, 4449–4459. [Google Scholar] [CrossRef] [PubMed]
- Goufo, P.; Moutinho-Pereira, J.; Jorge, T.; Correia, C.M.; Oliveira, M.R.; Rosa, E.A.S.; Antonio, C.; Trindade, H. Cowpea (Vigna unguiculata L. Walp.) Metabolomics: Osmoprotection as a physiological strategy for drought stress resistance and improved yield. Front. Plant Sci. 2017, 8, 586. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Chen, W.; Zhao, J.; Xi, W. Functional constituents and antioxidant activities of eight Chinese native goji geno-types. Food Chem. 2016, 200, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Byambasuren, S.-E.; Wang, J.; Gaudel, G. Medicinal value of wolfberry (Lycium barbarum L.). J. Med. Plants Stud. 2019, 7, 90–97. [Google Scholar]
- Zhang, Y.; Li, D.; Zhou, R.; Wang, X.; Dossa, K.; Wang, L.; Zhang, Y.; Yu, J.; Gong, H.; Zhang, X.; et al. Transcriptome and metabolome analyses of two contrasting sesame genotypes reveal the crucial biological pathways involved in rapid adaptive response to salt stress. BMC Plant Biol. 2019, 19, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurek, J. Introductory chapter. In Alkaloids—Their Importance in Nature and Human Life; Sciyo: Vienna, Austria, 2019; p. 7. [Google Scholar]
- Zafra-Rojas, Q.; Cruz-Cansino, N.; Delgadillo-Ramírez, A.; Alanís-García, E.; Añorve-Morga, J.; Lira, A.Q.; Castañeda-Ovando, A.; Ramírez-Moreno, E.; Organic Acids, Antioxidants, and Dietary Fiber of Mexican Blackberry (Rubus fruticosus) Residues cv Tupy. Available online: https://www.hindawi.com/journals/jfq/2018/5950761/ (accessed on 22 March 2020).
- YI, M. Influence of salt stress on primary metabolism of Zea mays L. seedlings of model genotypes. Plant Soil 1990, 123, 217–222. [Google Scholar]
- Reddy, I.N.B.L.; Kim, B.-K.; Yoon, I.-S.; Kim, K.-H.; Kwon, T.-R. Salt Tolerance in rice: Focus on mechanisms and approaches. Rice Sci. 2017, 24, 123–144. [Google Scholar] [CrossRef]
- Cui, B.; Liu, S.; Lin, X.; Wang, J.; Li, S.; Wang, Q.; Li, S. Phytochemical profile of brown rice and its nutrigenomic implications. Antioxidants 2018, 7, 71. [Google Scholar]
- Zhou, J.C.; Fu, T.T.; Sui, N.; Guo, J.R.; Feng, G.; Fan, J.L.; Song, J. The role of salinity in seed maturation of the euhalophyte Suaeda salsa. Plant Biosyst. 2016, 150, 83–90. [Google Scholar] [CrossRef]
- Widodo Patterson, J.H.; Newbigin, E.; Tester, M.; Bacic, A. RUMetabolic responses to salt stress of barley (Hordeum vulgare L.) cultivars, Sahara and Clipper, which differ in salinity tolerance. J. Exp. Bot. 2009, 60, 4089–4103. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.; Zahir, Z.A.; Asghar, H.N.; Asghar, M. Inducing salt tolerance in mung bean through coinoculation with rhizobia and plant-growth promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate deaminase. Can. J. Microbiol. 2011, 57, 578–589. [Google Scholar] [CrossRef]
- You, Z.; Zhang, Q.; Peng, Z.; Miao, X. Lipid droplets mediate salt stress tolerance in parachlorella kessleri. Plant Physiol. 2019, 181, 510–526. [Google Scholar] [CrossRef]
- Yan, K.; Cui, M.; Zhao, S.; Chen, X.; Tang, X.; Vasconcelos, M.W. Salinity stress is beneficial to the accumulation of chloro-genic acids in honeysuckle (Lonicera japonica Thunb). Front. Plant Sci. 2016, 7, 1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.; De, B. Metabolomics analysis of rice responses to salinity stress revealed elevation of serotonin, and gentisic acid levels in leaves of tolerant varieties. Plant Signal. Behav. 2017, 12, e1335845. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Zhang, H.-C.; Liu, W.-X.; Li, C.-Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef] [Green Version]
- China National Standards. Determination of the Contents of pH, Calcium, Copper, Iron, Magnesium, Manganese, Potassium, Sodium and Zinc in Forest Soils—Method Using Atomic Absorption Spectrometry; China Standard Press: Beijing, China, 2012; Ny/t 2272-2012. [Google Scholar]
- China National Standards. Determination of the Contents of Soil Salinity, Sodium Ions, Carbonates, Sulfates and Magnesium Ions, and Potassium Ions in Forest Soils—Methods; China Standard Press: Beijing, China, 2010; LY/T-2272-2012. [Google Scholar]
- Wang, L.; Sun, X.; Li, S.; Zhang, T.; Zhang, W.; Zhai, P. Application of organic amendments to a coastal saline soil in north china: Effects on soil physical and chemical properties and tree growth. PLoS ONE 2014, 9, e89185. [Google Scholar] [CrossRef] [Green Version]
- Ieggli, C.; Bohrer, D.; Nascimento, P.D.; De Carvalho, L.; Garcia, S. Determination of sodium, potassium, calcium, magnesium, zinc, and iron in emulsified egg samples by flame atomic absorption spectrometry. Talanta 2010, 80, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Texas Department of Transportation Test Procedure for Determining Chloride and Sulfate Contents in Soil. 2005. Available online: https://ftp.txdot.gov/pub/txdot-info/tta/nte/addendum2/5%20-%20book%202/nte%20book%202%20addendum%202%20redlined.pdf (accessed on 26 February 2020).
- The National Academies Press. Methods for sulfate quantification in soils: Recommended practice for stabilization of sulfate-rich subgrade soils. In Recommended Practice for Stabilization of Sulfate-Rich Subgrade Soils; Transportation Research Board: Beijing, China, 2009. [Google Scholar]
- Azad, H.A. Determination of a Mixture of Sodium Carbonate and Sodium Hydroxide by Using Double Indicator Method. Methods 2019. Available online: https://www.researchgate.net/publication/331199887_Experimental_No_08_Determination_of_a_mixture_of_sodium_carbonate_and_sodium_hydroxide_by_using_double_indicator_method (accessed on 26 February 2020).
- Wang, C.; Chang, S.; Inbaraj, B.S.; Chen, B. Isolation of carotenoids, flavonoids and polysaccharides from Lycium barbarum L. and evaluation of antioxidant activity. Food Chem. 2010, 120, 184–192. [Google Scholar] [CrossRef]
- Hsu, H.J.; Huang, R.F.; Kao, T.H.; Inbaraj, B.S.; Chen, B.H. Preparation of carotenoid extracts and nanoemulsions from Lycium barbarum L. and their effects on growth of HT-29 colon cancer cells. Nanotechnology 2017, 28, 135103. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, R.; Song, Y.; He, J.; Sun, J.; Bai, J.; An, Z.; Dong, L.; Zhan, Q.; Abliz, Z. RRLC-MS/MS-based metabonomics combined with in-depth analysis of metabolic correlation network: Finding potential biomarkers for breast cancer. Analyst 2009, 134, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. Metabo Analyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanehisa, M.S. Goto KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
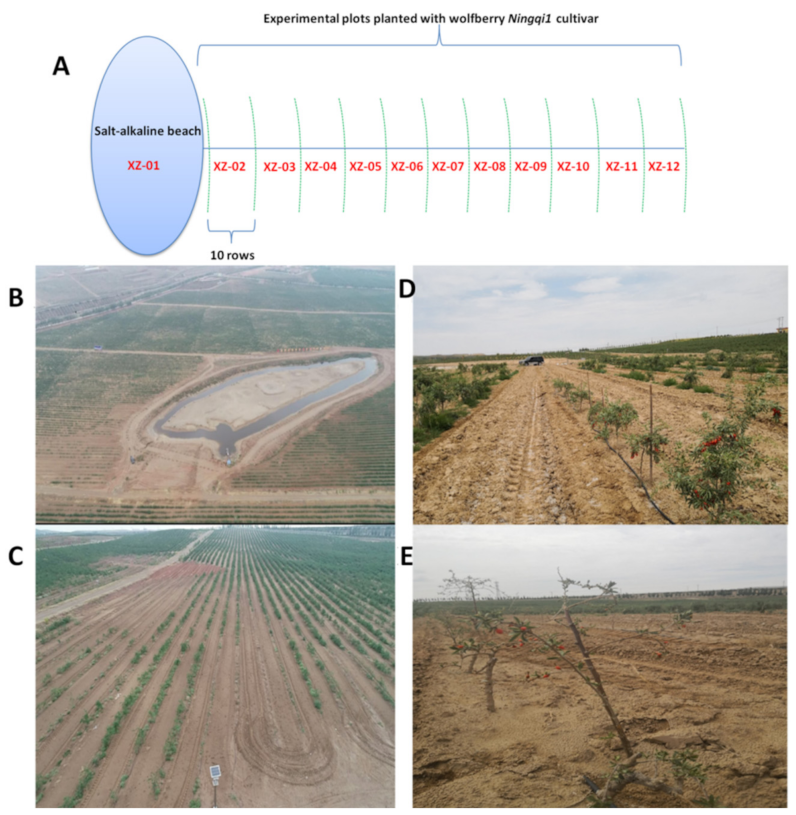
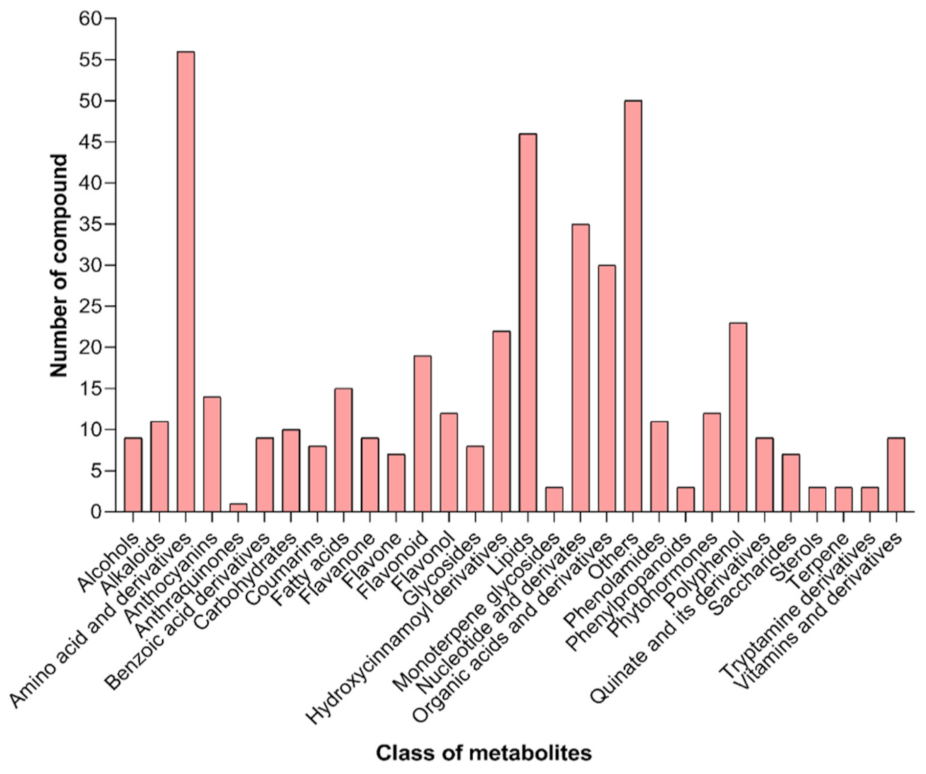

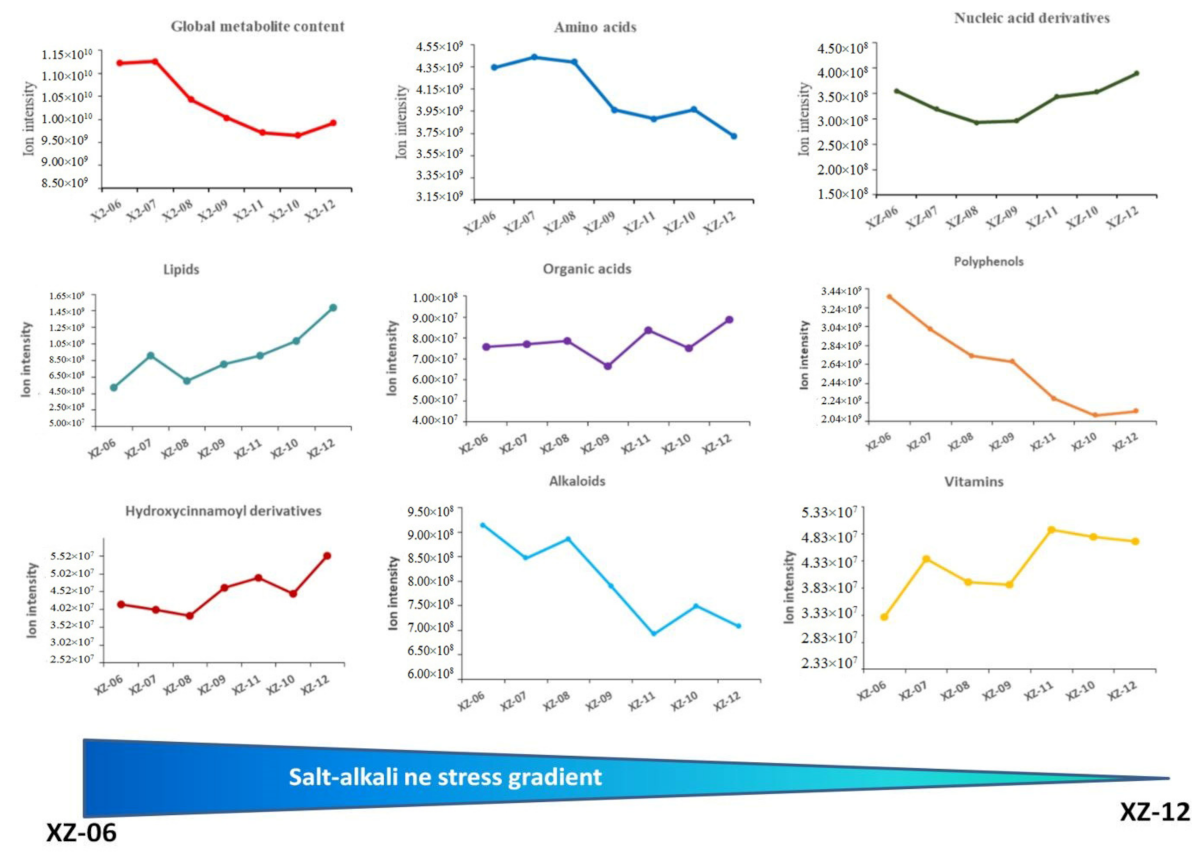
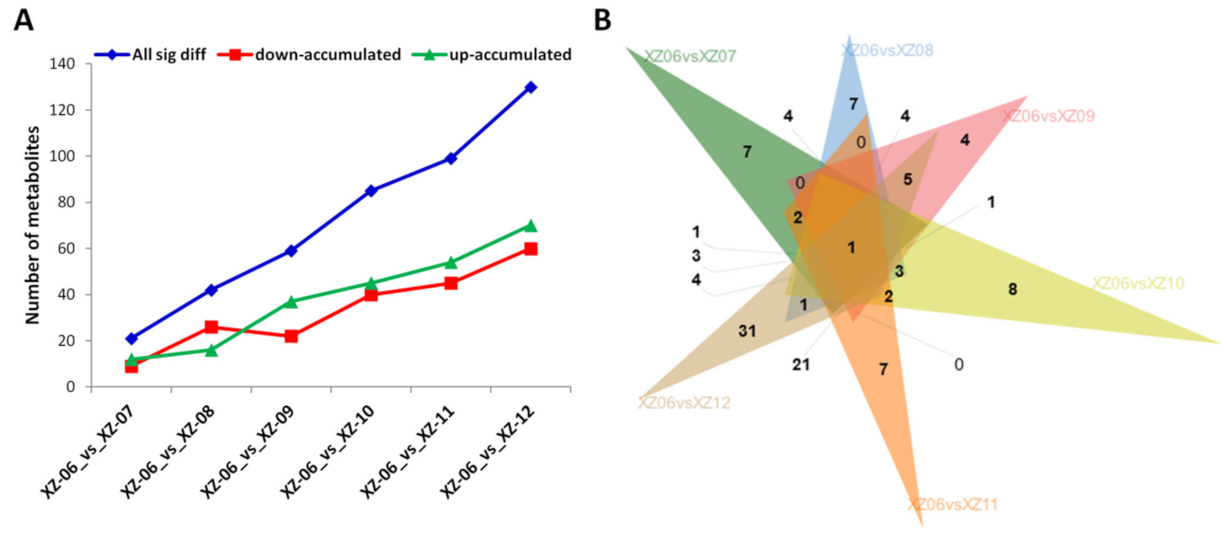
| Stress Gradients | Soil Physico–Chemical Properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | Salt | K+ | Na+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | |
| XZ-01 | 8.70 abcd | 33.90 a | 0.05 a | 5.78 a | 2.34 a | 2.11 a | 10.18 a | 6.32 a | 0.17 a |
| XZ-02 | 8.87 ab | 17.77 b | 0.03 b | 2.74 bc | 2.33 a | 1.03 b | 4.58 b | 5.07 b | 0.15 b |
| XZ-03 | 8.97 a | 12.83 c | 0.02 bc | 3.13 b | 0.61 cd | 0.86 bc | 4.22 b | 1.59 ef | 0.15 b |
| XZ-04 | 8.93 ab | 13.33 c | 0.02 bc | 3.31 b | 0.56 cd | 0.90 bc | 4.54 b | 1.53 ef | 0.16 ab |
| XZ-05 | 9.03 a | 8.93 de | 0.03 d | 2.10 cd | 0.64 cd | 0.51 d | 2.57 c | 1.53 ef | 0.12 cd |
| XZ-06 | 8.77 abcd | 10.53 cd | 0.04 b | 1.71 de | 1.95 ab | 0.54 d | 1.99 cd | 3.59 c | 0.13 c |
| XZ-07 | 8.90 ab | 10.87 cd | 0.05 a | 1.10 de | 1.64 b | 0.59 cd | 2.31 c | 3.34 cd | 0.16 ab |
| XZ-08 | 8.83 abc | 6.57 ef | 0.06 a | 1.24 ef | 1.04 c | 0.36 de | 1.36 cde | 2.30 de | 0.13 c |
| XZ-09 | 8.33 de | 5.30 efg | 0.05 a | 0.94 fg | 0.74 cd | 0.18 e | 1.02 de | 1.50 ef | 0.11 e |
| XZ-10 | 8.47 bcde | 4.00 fg | 0.06 a | 0.58 fgh | 0.67 cd | 0.18 e | 0.47 e | 1.38 ef | 0.10 ef |
| XZ-11 | 8.37 cde | 1.93 g | 0.05 a | 0.24 gh | 0.21 d | 0.09 e | 0.28 e | 0.61 f | 0.14 bc |
| XZ-12 | 8.17 e | 1.83 g | 0.05 a | 0.16 h | 0.23 d | 0.08 e | 0.23 e | 0.66 f | 0.15 b |
| Salt Stress Gradients | Leaf Traits | Fruit Traits | |||
|---|---|---|---|---|---|
| Length (cm) | Width (cm) | Weight (g) | Longitudinal Diameter (cm) | Transverse Diameter (cm) | |
| XZ-06 | 4.03 a | 0.95 a | 0.49 a | 1.25 a | 0.92 a |
| XZ-07 | 4.26 a | 1.05 a | 0.48 a | 1.43 a | 0.92 a |
| XZ-08 | 4.89 ab | 1.13 ab | 0.36 a | 1.16 a | 0.83 a |
| XZ-09 | 5.16 b | 1.35 b | 0.74 b | 1.68 b | 1.08 b |
| XZ-10 | 4.37 a | 1.22 b | 0.83 b | 1.61 b | 1.00 b |
| XZ-11 | 5.07 b | 1.33 b | 0.79 b | 1.51 b | 0.94 ab |
| XZ-12 | 5.14 b | 1.28 b | 0.86 b | 1.70 b | 1.02 b |
| Amino Acid Derivatives | Polyphenols | Hydroxycinnamoyl Derivatives | Lipids | Nucleic Acid Derivatives | Organic Acids | Vitamins | Alkaloids | |
|---|---|---|---|---|---|---|---|---|
| pH | 0.97 *** | 0.78 ** | −0.89 ** | −0.73 ** | −0.49 | −0.18 | −0.49 | 0.82 ** |
| Salt | 0.88 ** | 0.96 *** | −0.72 ** | −0.70 ** | −0.39 | −0.32 | −0.70 ** | 0.83 ** |
| K+ | −0.14 | −0.49 | 0.04 | 0.07 | −0.37 | 0.30 | 0.53 * | −0.37 |
| Na+ | 0.83 ** | 0.96 *** | −0.75 ** | −0.90 *** | −0.49 | −0.42 | −0.89 ** | 0.91 *** |
| Ca2+ | 0.85 ** | 0.97 *** | −0.68 * | −0.73 ** | −0.30 | −0.27 | −0.73** | 0.83 ** |
| Mg2+ | 0.91 *** | 0.92 *** | −0.75 ** | −0.65 * | −0.33 | −0.20 | −0.62 * | 0.83 ** |
| Cl− | 0.91 *** | 0.94 *** | −0.76 ** | −0.67 * | −0.44 | −0.35 | −0.68 * | 0.85 ** |
| SO42− | 0.89 ** | 0.96 *** | −0.72 ** | −0.72 ** | −0.34 | −0.25 | −0.71 ** | 0.85 ** |
| HCO3− | 0.29 | 0.11 | −0.13 | 0.38 | 0.30 | 0.25 | 0.07 | 0.23 |
| Metabolite Class | Compound | Log2 Fold Change | |||||
|---|---|---|---|---|---|---|---|
| XZ-06 vs. XZ-07 | XZ-06 vs. XZ-08 | XZ-06 vs. XZ-09 | XZ-06 vs. XZ-10 | XZ-06 vs. XZ-11 | XZ-06 vs. XZ-12 | ||
| Polyamine | N-p-Coumaroyl putrescine | −0.772 | −1.081 | −1.237 | −1.272 | −1.478 | −1.987 |
| Phenolamides | N′-p-Coumaroyl putrescine | −0.58 | −1.288 | −1.342 | −1.449 | −1.838 | −2.444 |
| Phytohormone | Abscisic acid | −0.991 | −2.348 | −2.376 | −2.014 | −2.304 | −2.982 |
| Phytohormones | (+)-cis,trans-Abscisic acid | −0.931 | −2.472 | −2.296 | −1.916 | −2.469 | −2.743 |
| Quinate and derivatives | Cryptochlorogenic acid | −0.269 | 1.8 | 2.209 | 1.539 | 2.253 | 3.678 |
| Quinate and derivatives | Chlorogenic acid (3-O-Caffeoylquinic acid) | −3.187 | 2.006 | 2.641 | 1.668 | 2.445 | 3.922 |
| - | Dopamine hydrochloride | −0.654 | 1.064 | 2.194 | 1.489 | 2.142 | 3.902 |
| Fatty acid | LPC(1-acyl 18:2) | 2.504 | 1.38 | 2.194 | 3.116 | 2.655 | 3.987 |
| Lipids_glycerophospholipids | LysoPC 16:2 (2n isomer) | 2.104 | 1.392 | 1.768 | 2.378 | 1.889 | 3.921 |
| Lipids_glycerophospholipids | LysoPC 18:3 (2n isomer) | 1.86 | 1.015 | 1.551 | 2.12 | 1.904 | 3.077 |
| Lipids_glycerophospholipids | LysoPC 15:1 | 2.096 | 1.023 | 1.768 | 1.822 | 1.878 | 3.604 |
| Lipids_glycerophospholipids | LysoPC 16:1 (2n isomer) | 1.987 | 1.052 | 1.618 | 2.413 | 2.445 | 2.811 |
| Benzoic acid derivatives | 2,5-dihydroxy benzoic acid O-hexoside (Gentisic acid) | 1.187 | 0.575 | 1.238 | 1.454 | 2.477 | 2.824 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Wang, Y.; Li, Y.; An, W.; He, X.; Chen, Y.; Shi, Z.; He, J.; Wan, R. Widely-Targeted Metabolic Profiling in Lyciumbarbarum Fruits under Salt-Alkaline Stress Uncovers Mechanism of Salinity Tolerance. Molecules 2022, 27, 1564. https://doi.org/10.3390/molecules27051564
Liang X, Wang Y, Li Y, An W, He X, Chen Y, Shi Z, He J, Wan R. Widely-Targeted Metabolic Profiling in Lyciumbarbarum Fruits under Salt-Alkaline Stress Uncovers Mechanism of Salinity Tolerance. Molecules. 2022; 27(5):1564. https://doi.org/10.3390/molecules27051564
Chicago/Turabian StyleLiang, Xiaojie, Yajun Wang, Yuekun Li, Wei An, Xinru He, Yanzhen Chen, Zhigang Shi, Jun He, and Ru Wan. 2022. "Widely-Targeted Metabolic Profiling in Lyciumbarbarum Fruits under Salt-Alkaline Stress Uncovers Mechanism of Salinity Tolerance" Molecules 27, no. 5: 1564. https://doi.org/10.3390/molecules27051564





