Supported Ionic Liquids Used as Chromatographic Matrices in Bioseparation—An Overview
Abstract
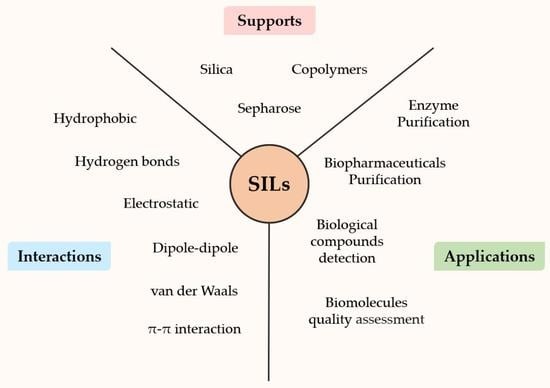
:1. Introduction
2. Biomolecules Purification by Preparative Chromatography
3. Ionic Liquids
3.1. Supported Ionic Liquids (SILs) in Analytical Methods
3.2. ILs as Ligands of Chromatographic Supports
4. Silica Supported Ionic Liquids—SSILs
4.1. Single Cation Immobilization
4.1.1. Immobilization via Halogenated Silane Groups
4.1.2. Functionalization via Modified Stöber Method Incorporating Functional Alcohols
4.1.3. Immobilization via Thiol-Containing Silanes
4.1.4. Immobilization via Amine- or Hydroxyl-Containing Silanes
4.2. Multi-Cation Immobilization
4.3. Single Anion Immobilization
4.3.1. Immobilization via Lewis Acidic Chloroaluminate Ionic Liquids
4.3.2. Immobilization via Functional Silanes
4.4. Co-Immobilization of the Anions and Cations
4.5. Cation or Anion Immobilization Using Zwitterionic ILs
5. Polymer-Supported Ionic Liquids—PSILs
5.1. ILs Immobilization after Co-Polymerization of Vinyl-Functional Polymers
5.2. ILs Immobilization onto Agarose Polymers via Steglich Esterification
5.3. ILs Immobilization onto Agarose Polymers after Cyanogen Halide Activation
5.4. ILs Immobilization onto Agarose Polymers after Epichlorohydrin Activation
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nikolin, B.; Imamović, B.; Medanhodzić-Vuk, S.; Sober, M. High perfomance liquid chromatography in pharmaceutical analyses. Bosn. J. Basic Med. Sci. 2004, 4, 5–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosing, H.; Man, W.Y.; Doyle, E.; Bult, A.; Beijnen, J.H. Bioanalytical liquid chromatographic method validation. a review of current practices and procedures. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 329–354. [Google Scholar] [CrossRef]
- Cramer, S.M.; Jayaraman, G. Preparative chromatography in biotechnology. Curr. Opin. Biotechnol. 1993, 4, 217–225. [Google Scholar] [CrossRef]
- Jungbauer, A. Preparative chromatography of biomolecules. J. Chromatogr. A 1993, 639, 3–16. [Google Scholar] [CrossRef]
- Guiochon, G. Preparative liquid chromatography. J. Chromatogr. A 2002, 965, 129–161. [Google Scholar] [CrossRef]
- Ward, W.W.; Swiatek, G. Protein Purification. Curr. Anal. Chem. 2009, 5, 85–105. [Google Scholar] [CrossRef]
- Valente, J.F.A.; Queiroz, J.A.; Sousa, F. Dilemma on plasmid DNA purification: Binding capacity vs selectivity. J. Chromatogr. A 2021, 1637, 461848. [Google Scholar] [CrossRef]
- Martins, R.; Queiroz, J.A.; Sousa, F. Ribonucleic acid purification. J. Chromatogr. A 2014, 1355, 1–14. [Google Scholar] [CrossRef]
- Lowe, C.R.; Lowe, A.R.; Gupta, G. New developments in affinity chromatography with potential application in the production of biopharmaceuticals. J. Biochem. Biophys. Methods 2001, 49, 561–574. [Google Scholar] [CrossRef]
- Łącki, K.M.; Riske, F.J. Affinity Chromatography: An Enabling Technology for Large-Scale Bioprocessing. Biotechnol. J. 2020, 15, e1800397. [Google Scholar] [CrossRef]
- Owczarek, B.; Gerszberg, A.; Hnatuszko-Konka, K. A Brief Reminder of Systems of Production and Chromatography-Based Recovery of Recombinant Protein Biopharmaceuticals. Biomed. Res. Int. 2019, 2019, 4216060. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Garcia, L.; Martín, L.; Mangues, R.; Ferrer-Miralles, N.; Vázquez, E.; Villaverde, A. Recombinant pharmaceuticals from microbial cells: A 2015 update. Microb. Cell Factories 2016, 15, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, J.R.; Chien, J. Trends in translational medicine and drug targeting and delivery: New insights on an old concept—targeted drug delivery with antibody–drug conjugates for cancers. J. Pharm. Sci. 2014, 103, 71–77. [Google Scholar] [PubMed] [Green Version]
- Arakawa, T.; Santarelli, X. Binding and Elution Properties of Mixed-Mode Chromatography and Its Applications for Purification. Curr. Protein Pept. Sci. 2019, 20, 3. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, K.; Gao, D.; Fu, Q.; Zeng, J.; Zhou, D.; Wang, L.; Xia, Z. Mixed-mode liquid chromatography with a stationary phase co-functionalized with ionic liquid embedded C18 and an aryl sulfonate group. J. Chromatogr. A 2018, 1564, 137–144. [Google Scholar] [CrossRef]
- Shi, X.; Qiao, L.; Xu, G. Recent development of ionic liquid stationary phases for liquid chromatography. J. Chromatogr. A 2015, 1420, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pietruszka, N.; Galaev, I.Y.; Kumar, A.; Brzozowski, Z.K.; Mattiasson, B. New Polymers Forming Aqueous Two-Phase Polymer Systems. Biotechnol. Prog. 2000, 16, 408–415. [Google Scholar] [CrossRef]
- Pei, Y.; Wang, J.; Wu, K.; Xuan, X.; Lu, X. Ionic liquid-based aqueous two-phase extraction of selected proteins. Sep. Purif. Technol. 2009, 64, 288–295. [Google Scholar] [CrossRef]
- Ayyar, B.V.; Arora, S.; Murphy, C.; O’Kennedy, R. Affinity chromatography as a tool for antibody purification. Methods 2012, 56, 116–129. [Google Scholar] [CrossRef]
- Subramanian, S.; Ross, P.D. Dye-Ligand Affinity Chromatography: The Interaction of Cibacron Blue F3GA® with Proteins and Enzyme. Crit. Rev. Biochem. 1984, 16, 169–205. [Google Scholar] [CrossRef]
- Zou, H.; Luo, Q.; Zhou, D. Affinity membrane chromatography for the analysis and purification of proteins. J. Biochem. Biophys. Methods 2001, 49, 199–240. [Google Scholar] [CrossRef]
- Bo, H.; Chen, J.; Liang, T.; Li, S.; Shao, H.; Huang, S. Chromatographic purification of adenoviral vectors on anion-exchange resins. Eur. J. Pharm. Sci. 2015, 67, 119–125. [Google Scholar] [CrossRef]
- Silva-Santos, A.R.; Alves, C.P.A.; Prazeres, D.M.F.; Azevedo, A.M. A process for supercoiled plasmid DNA purification based on multimodal chromatography. Sep. Purif. Technol. 2017, 182, 94–100. [Google Scholar] [CrossRef]
- Hirsch, D.B.; Álvarez, L.M.M.; Urtasun, N.; Baieli, M.F.; Lázaro-Martínez, J.M.; Glisoni, R.J.; Miranda, M.V.; Cascone, O.; Wolman, F.J. Lactoferrin purification and whey protein isolate recovery from cheese whey using chitosan mini-spheres. Int. Dairy J. 2020, 109, 104764. [Google Scholar] [CrossRef]
- Diogo, M.M.; Queiroz, J.A.; Prazeres, D.M.F. Studies on the retention of plasmid DNA and Escherichia coli nucleic acids by hydrophobic interaction chromatography. Bioseparation 2001, 10, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.M.; Eusébio, D.; Queiroz, J.A.; Sousa, F.; Sousa, A. The use of size-exclusion chromatography in the isolation of supercoiled minicircle DNA from Escherichia coli lysate. J. Chromatogr. A 2020, 1609, 460444. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.M.; Queiroz, J.A.; Sousa, F.; Sousa, A. Minicircle DNA purification: Performance of chromatographic monoliths bearing lysine and cadaverine ligands. J. Chromatogr. B 2019, 1118–1119, 7–16. [Google Scholar] [CrossRef]
- Pereira, P.; Sousa, Â.; Queiroz, J.; Correia, I.; Figueiras, A.; Sousa, F. Purification of pre-miR-29 by arginine-affinity chromatography. J. Chromatogr. B 2014, 951, 16–23. [Google Scholar] [CrossRef]
- Azevedo, G.M.; Valente, J.F.A.; Sousa, A.; Pedro, A.Q.; Pereira, P.; Sousa, F.; Queiroz, J.A. Effect of Chromatographic Conditions on Supercoiled Plasmid DNA Stability and Bioactivity. Appl. Sci. 2019, 9, 5170. [Google Scholar] [CrossRef] [Green Version]
- Kuchenbuch, A.; Giernoth, R. Ionic liquids beyond simple solvents: Glimpses at the state of the art in organic chemistry. ChemistryOpen 2015, 4, 677. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [Green Version]
- Shukla, S.K.; Pandey, S.; Pandey, S. Applications of ionic liquids in biphasic separation: Aqueous biphasic systems and liquid–liquid equilibria. J. Chromatogr. A 2018, 1559, 44–61. [Google Scholar] [CrossRef] [PubMed]
- E Silva, F.A.; Pereira, J.F.; Kurnia, K.A.; Ventura, S.P.; Silva, A.M.; Rogers, R.D.; Coutinho, J.A.; Freire, M.G. Temperature dependency of aqueous biphasic systems: An alternative approach for exploring the differences between Coulombic-dominated salts and ionic liquids. Chem. Commun. 2017, 53, 7298–7301. [Google Scholar] [CrossRef]
- Han, D.; Row, K.H. Recent Applications of Ionic Liquids in Separation Technology. Molecules 2010, 15, 2405–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventura, S.P.; e Silva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A. Ionic-liquid-mediated extraction and separation processes for bioactive compounds: Past, present, and future trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.G.; Claudio, A.F.M.; Araujo, J.M.; Coutinho, J.A.; Marrucho, I.M.; Lopes, J.N.C.; Rebelo, L.P.N. Aqueous biphasic systems: A boost brought about by using ionic liquids. Chem. Soc. Rev. 2012, 41, 4966–4995. [Google Scholar] [CrossRef]
- Sintra, T.E.; Nasirpour, M.; Siopa, F.; Rosatella, A.A.; Gonçalves, F.; Coutinho, J.A.; Afonso, C.A.; Ventura, S.P. Ecotoxicological evaluation of magnetic ionic liquids. Ecotoxicol. Environ. Saf. 2017, 143, 315–321. [Google Scholar] [CrossRef]
- Taha, M.; Quental, M.V.; e Silva, F.A.; Capela, E.V.; Freire, M.G.; Ventura, S.P.; Coutinho, J.A. Good’s buffer ionic liquids as relevant phase-forming components of self-buffered aqueous biphasic systems. J. Chem. Technol. Biotechnol. 2017, 92, 2287–2299. [Google Scholar] [CrossRef]
- Zhang, M.; Liang, X.; Jiang, S.; Qiu, H. Preparation and applications of surface-confined ionic-liquid stationary phases for liquid chromatography. Trac Trends Anal. Chem. 2014, 53, 60–72. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Y.; Zhu, L.; Lan, J.; Xie, R.; You, J. A Concept of Supported Amino Acid Ionic Liquids and Their Application in Metal Scavenging and Heterogeneous Catalysis. J. Am. Chem. Soc. 2007, 129, 13879–13886. [Google Scholar] [CrossRef]
- Trujillo-Rodríguez, M.J.; Nan, H.; Varona, M.; Emaus, M.N.; Souza, I.D.; Anderson, J.L. Advances of Ionic Liquids in Analytical Chemistry. Anal. Chem. 2019, 91, 505–531. [Google Scholar] [CrossRef] [PubMed]
- Nawała, J.; Dawidziuk, B.; Dziedzic, D.; Gordon, D.; Popiel, S. Applications of ionic liquids in analytical chemistry with a particular emphasis on their use in solid-phase microextraction. Trac Trends Anal. Chem. 2018, 105, 18–36. [Google Scholar] [CrossRef]
- Pletnev, I.; Smirnova, S.; Shvedene, N. New directions in using ionic liquids in analytical chemistry. 1: Liquid–liquid extraction. J. Anal. Chem. 2019, 74, 625–658. [Google Scholar] [CrossRef]
- Pino, V.; Afonso, A.M. Surface-bonded ionic liquid stationary phases in high-performance liquid chromatography—A review. Anal. Chim. Acta 2012, 714, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Vidal, L.; Riekkola, M.-L.; Canals, A. Ionic liquid-modified materials for solid-phase extraction and separation: A review. Anal. Chim. Acta 2012, 715, 19–41. [Google Scholar] [CrossRef]
- Jandera, P.; Janás, P. Recent advances in stationary phases and understanding of retention in hydrophilic interaction chromatography. A review. Anal. Chim. Acta 2017, 967, 12–32. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, X.; Pei, Y.; Wang, J.; He, M. Design of environmentally friendly ionic liquid aqueous two-phase systems for the efficient and high activity extraction of proteins. Green Chem. 2012, 14, 2941–2950. [Google Scholar] [CrossRef]
- Qiu, H.; Wang, L.; Liu, X.; Jiang, S. Preparation and characterization of silica confined ionic liquids as chromatographic stationary phases through surface radical chain-transfer reaction. Analyst 2009, 134, 460–465. [Google Scholar] [CrossRef]
- Bi, W.; Zhou, J.; Row, K.H. Separation of xylose and glucose on different silica-confined ionic liquid stationary phases. Anal. Chim. Acta 2010, 677, 162–168. [Google Scholar] [CrossRef]
- Qiao, L.; Wang, S.; Li, H.; Shan, Y.; Dou, A.; Shi, X.; Xu, G. A novel surface-confined glucaminium-based ionic liquid stationary phase for hydrophilic interaction/anion-exchange mixed-mode chromatography. J. Chromatogr. A 2014, 1360, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.; Pereira, P.; Pedro, A.; Martins, J.; Trindade, T.; Queiroz, J.; Freire, M.; Sousa, F. Improved ionic-liquid-functionalized macroporous supports able to purify nucleic acids in one step. Mater. Today Bio 2020, 8, 100086. [Google Scholar] [CrossRef]
- Qiu, H.; Jiang, S.; Takafuji, M.; Ihara, H. Polyanionic and polyzwitterionic azobenzene ionic liquid-functionalized silica materials and their chromatographic applications. Chem. Commun. 2013, 49, 2454–2456. [Google Scholar] [CrossRef]
- Qiu, H.; Zhang, M.; Chen, J.; Gu, T.; Takafuji, M.; Ihara, H. Anionic and cationic copolymerized ionic liquid-grafted silica as a multifunctional stationary phase for reversed-phase chromatography. Anal. Methods 2014, 6, 469–475. [Google Scholar] [CrossRef]
- Qiao, L.; Dou, A.; Shi, X.; Li, H.; Shan, Y.; Lu, X.; Xu, G. Development and evaluation of new imidazolium-based zwitterionic stationary phases for hydrophilic interaction chromatography. J. Chromatogr. A 2013, 1286, 137–145. [Google Scholar] [CrossRef]
- Qiu, H.; Jiang, S.; Liu, X. N-Methylimidazolium anion-exchange stationary phase for high-performance liquid chromatography. J. Chromatogr. A 2006, 1103, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Auler, L.M.; Silva, C.R.; Collins, K.E.; Collins, C.H. New stationary phase for anion-exchange chromatography. J. Chromatogr. A 2005, 1073, 147–153. [Google Scholar] [CrossRef]
- Almeida, H.F.D.; Neves, M.C.; Trindade, T.; Marrucho, I.M.; Freire, M.G. Supported ionic liquids as efficient materials to remove non-steroidal anti-inflammatory drugs from aqueous media. Chem. Eng. J. 2020, 381, 122616. [Google Scholar] [CrossRef]
- Liu, H.; Chen, J.; Li, Z.; Takafuji, M.; Ihara, H.; Qiu, H. A new route for synthesis of N-methylimidazolium-grafted silica stationary phase and reevaluation in hydrophilic interaction liquid chromatography. Talanta 2017, 164, 137–140. [Google Scholar] [CrossRef]
- Bi, W.; Row, K.H. Novel bi-functional amino-imidazolium silica confined stationary phase for liquid chromatography. J. Liq. Chromatogr. Relat. Technol. 2010, 33, 1459–1475. [Google Scholar] [CrossRef]
- Yang, B.; Liu, H.; Chen, J.; Guan, M.; Qiu, H. Preparation and evaluation of 2-methylimidazolium-functionalized silica as a mixed-mode stationary phase for hydrophilic interaction and anion-exchange chromatography. J. Chromatogr. A 2016, 1468, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Stalcup, A.; Martire, D.; Sander, L.C.; Wise, S.A. Synthesis and characterization of novel bonded phases for reversed-phase liquid chromatography. Chromatographia 1989, 27, 405–411. [Google Scholar] [CrossRef]
- Wang, Q.; Baker, G.A.; Baker, S.N.; Colón, L.A. Surface confined ionic liquid as a stationary phase for HPLC. Analyst 2006, 131, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Tian, M.; Row, K.H. Solid-phase extraction of liquiritin and glycyrrhizin from licorice using porous alkyl-pyridinium polymer sorbent. Phytochem. Anal. 2010, 21, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.; Zhao, K.-L.; Yang, F.; Tian, L.; Yang, Y.; Bai, Q. Protein separation using a novel silica-based RPLC/IEC stationary phase modified with N-methylimidazolium ionic liquid. Chin. Chem. Lett. 2015, 26, 988–992. [Google Scholar] [CrossRef]
- Wang, Z.; Ge, H.; Wang, X.; Ye, C.; Fan, S. Mono and co-immobilization of imidazolium ionic liquids on silica: Effects of the substituted groups on the adsorption behavior of 2,4-dinitrophenol. Rsc Adv. 2019, 9, 32425–32434. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Chen, J.; Gu, T.; Qiu, H.; Jiang, S. Novel imidazolium-embedded and imidazolium-spaced octadecyl stationary phases for reversed phase liquid chromatography. Talanta 2014, 126, 177–184. [Google Scholar] [CrossRef]
- Wang, Z.; Ye, C.; Wang, H. Preparation of amino functionalized imidazolium-modified silicas by different coupling agents for removal of 2,4-dinitrophenol from aqueous solutions. Int. J. Environ. Sci. Technol. 2016, 13, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Qiao, X.; Zhang, L.; Zhang, N.; Wang, X.; Qin, X.; Yan, H.; Liu, H. Imidazolium embedded C8 based stationary phase for simultaneous reversed-phase/hydrophilic interaction mixed-mode chromatography. J. Chromatogr. A 2015, 1400, 107–116. [Google Scholar] [CrossRef]
- Qiao, L.; Lv, W.; Chang, M.; Shi, X.; Xu, G. Surface-bonded amide-functionalized imidazolium ionic liquid as stationary phase for hydrophilic interaction liquid chromatography. J. Chromatogr. A 2018, 1559, 141–148. [Google Scholar] [CrossRef]
- Hu, K.; Zhang, W.; Yang, H.; Cui, Y.; Zhang, J.; Zhao, W.; Yu, A.; Zhang, S. Calixarene ionic liquid modified silica gel: A novel stationary phase for mixed-mode chromatography. Talanta 2016, 152, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.W.H.; Toenjes, S.; Gates, B.D. Altering Surface Charge of Silica Nanoparticles through Co-condensation of Choline Chloride and Tetraethyl Orthosilicate (TEOS). Mrs Adv. 2016, 1, 2115–2123. [Google Scholar] [CrossRef]
- Carpenter, A.W.; Worley, B.V.; Slomberg, D.L.; Schoenfisch, M.H. Dual Action Antimicrobials: Nitric Oxide Release from Quaternary Ammonium-Functionalized Silica Nanoparticles. Biomacromolecules 2012, 13, 3334–3342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donato, K.Z.; Matějka, L.; Mauler, R.S.; Donato, R.K. Recent Applications of Ionic Liquids in the Sol-Gel Process for Polymer–Silica Nanocomposites with Ionic Interfaces. Colloids Interfaces 2017, 1, 5. [Google Scholar] [CrossRef] [Green Version]
- Hermanson, G.T. Chapter 13—Silane Coupling Agents. In Bioconjugate Techniques, 3rd ed.; Hermanson, G.T., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 535–548. [Google Scholar]
- Zhou, Z.; Li, X.; Chen, X.; Hao, X. Synthesis of ionic liquids functionalized β-cyclodextrin-bonded chiral stationary phases and their applications in high-performance liquid chromatography. Anal. Chim. Acta 2010, 678, 208–214. [Google Scholar] [CrossRef]
- Tian, M.; Yan, H.; Row, K.H. Solid-phase extraction of tanshinones from Salvia Miltiorrhiza Bunge using ionic liquid-modified silica sorbents. J. Chromatogr. B 2009, 877, 738–742. [Google Scholar] [CrossRef]
- Qiao, L.; Shi, X.; Lu, X.; Xu, G. Preparation and evaluation of surface-bonded tricationic ionic liquid silica as stationary phases for high-performance liquid chromatography. J. Chromatogr. A 2015, 1396, 62–71. [Google Scholar] [CrossRef]
- Valkenberg, M.H.; deCastro, C.; Hölderich, W.F. Immobilisation of ionic liquids on solid supports. Green Chem. 2002, 4, 88–93. [Google Scholar] [CrossRef]
- Dou, Q.; Liu, L.; Yang, B.; Lang, J.; Yan, X. Silica-grafted ionic liquids for revealing the respective charging behaviors of cations and anions in supercapacitors. Nat. Commun. 2017, 8, 2188. [Google Scholar] [CrossRef]
- Fukumoto, K.; Yoshizawa, M.; Ohno, H. Room Temperature Ionic Liquids from 20 Natural Amino Acids. J. Am. Chem. Soc. 2005, 127, 2398–2399. [Google Scholar] [CrossRef]
- Shen, A.; Guo, Z.; Cai, X.; Xue, X.; Liang, X. Preparation and chromatographic evaluation of a cysteine-bonded zwitterionic hydrophilic interaction liquid chromatography stationary phase. J. Chromatogr. A 2012, 1228, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Fontanals, N.; Ronka, S.; Borrull, F.; Trochimczuk, A.W.; Marcé, R.M. Supported imidazolium ionic liquid phases: A new material for solid-phase extraction. Talanta 2009, 80, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Tian, M.; Row, K.H. Solid-phase extraction of matrine and oxymatrine from Sophora Flavescens Ait using amino-imidazolium polymer. J. Sep. Sci. 2010, 33, 1739–1745. [Google Scholar] [CrossRef]
- Tian, M.; Bi, W.; Row, K.H. Molecular imprinting in ionic liquid-modified porous polymer for recognitive separation of three tanshinones from Salvia miltiorrhiza Bunge. Anal. Bioanal. Chem. 2011, 399, 2495–2502. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.P.; Ramos, S.S.; Sousa, F.; Boto, R.E.F.; Almeida, P. A Benzothiazolium Salt as Chromatography Ligand for Protein Purification. Chromatographia 2014, 77, 1597–1605. [Google Scholar] [CrossRef]
- Axéan, R.; Ernback, S. Chemical Fixation of Enzymes to Cyanogen Halide Activated Polysaccharide Carriers. Eur. J. Biochem. 1971, 18, 351–360. [Google Scholar] [CrossRef]
- Bethell, G.S.; Ayers, J.S.; Hancock, W.S.; Hearn, M.T. A novel method of activation of cross-linked agaroses with 1,1’-carbonyldiimidazole which gives a matrix for affinity chromatography devoid of additional charged groups. J. Biol. Chem. 1979, 254, 2572–2574. [Google Scholar] [CrossRef]
- Matsumoto, I.; Mizuno, Y.; Seno, N. Activation of Sepharose with epichlorohydrin and subsequent immobilization of ligand for affinity adsorbent. J. Biochem. 1979, 85, 1091–1098. [Google Scholar] [CrossRef]
- Platis, D.; Labrou, N.E. Affinity chromatography for the purification of therapeutic proteins from transgenic maize using immobilized histamine. J Sep Sci 2008, 31, 636–645. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; e Silva, F.A.; Gonçalves, A.M.M.; Pereira, J.L.; Gonçalves, F.; Coutinho, J.A.P. Ecotoxicity analysis of cholinium-based ionic liquids to Vibrio fischeri marine bacteria. Ecotoxicol. Environ. Saf. 2014, 102, 48–54. [Google Scholar] [CrossRef]
- Petkovic, M.; Ferguson, J.L.; Gunaratne, H.Q.N.; Ferreira, R.; Leitão, M.C.; Seddon, K.R.; Rebelo, L.P.N.; Pereira, C.S. Novel biocompatible cholinium-based ionic liquids—toxicity and biodegradability. Green Chem. 2010, 12, 643–649. [Google Scholar] [CrossRef]
- Taha, M.; e Silva, F.A.; Quental, M.V.; Ventura, S.P.M.; Freire, M.G.; Coutinho, J.A.P. Good’s buffers as a basis for developing self-buffering and biocompatible ionic liquids for biological research. Green Chem. 2014, 16, 3149–3159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, D.-J.; Cheng, Z.; Chen, F.-F.; Li, Z.-M.; Hu, N.; Chen, X.-S. Synthesis and Thermophysical Properties of Biocompatible Cholinium-Based Amino Acid Ionic Liquids. J. Chem. Eng. Data 2013, 58, 1542–1548. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Wang, J.; Xue, X.; Xi, X.; Chu, Q.; Dong, G.; Wei, Y. Amino acid-based ionic liquid surface modification of magnetic nanoparticles for the magnetic solid-phase extraction of heme proteins. Rsc Adv. 2016, 6, 105550–105557. [Google Scholar] [CrossRef]




| Bioproduct | Used Matrices | References |
|---|---|---|
| Adenoviral vector | Fractogel TMAE | [22] |
| sc pDNA | CaptoTM adhere resin | [23] |
| Lactoferrin | Sulfanilic acid-modified chitosan mini-spheres | [24] |
| Nucleic Acids | Sepharose CL-6B treated with 1,4-butanediol diglycidyl ether | [25] |
| mcDNA | Sephacryl S-1000 SF matrix | [26] |
| mcDNA | Cadaverine modified monolith | [27] |
| pre-miRNA-29 | L-arginine–Sepharose 4B gel | [28] |
| sc pDNA | Histidine-agarose, arginine-macroporous, Histidine-monolith | [29] |
| Type of Ligand | Application | Analytes | Type of Sample | Type of Immobilization | References |
|---|---|---|---|---|---|
| C8, C10, Naph, C4-Ph | RPLC | polycyclic aromatic hydrocarbons | mixture solutions | via halogenated silane groups | [62] |
| Py | AEX-LC | organic compounds/aromatic hydrocarbons; inorganic anions | mixture solutions | via halogenated silane groups (heterogeneous process) | [57] |
| MIm | AEX-LC | inorganic anions | mixture solutions | via halogenated silane groups (heterogeneous process) | [56] |
| MPIm, BPIm | RPLC | aromatic carboxylic acids | mixture solutions | via halogenated silane groups (homogeneous process) | [63] |
| EMIm | SPE | liquiritin and glycyrrhizic acid | licorice extract | via halogenated silane groups (heterogeneous process) | [64] |
| Im, Mim, EMIm, ImBF4, ImNTf2 | LC | xylose and glucose | mixture standard solution and a solution of enzymatically hydrolyzed water | via halogenated silane groups and further modifications | [50] |
| NIm | HPLC | aromatic organic compounds; alkaloids | mixture solutions | via halogenated silane groups and further modifications | [60] |
| Im, MIm, EMIm | SPE | Lactic acid | Fermentation broth | via halogenated silane groups (heterogeneous process) | [64] |
| MIm | RPLC/IEX | proteins | mixture solution and egg white | via halogenated silane groups (heterogeneous process) | [65] |
| 2-MIm, 1-MIm | HILIC/AEX-LC | Sulfonamides; nucleosides/nucleobases; vitamins; saccharides; inorganic anions | mixture solutions | via halogenated silane groups (heterogeneous process) | [61] |
| MIm | HILIC | Sulfonamides; nucleosides/nucleobases | mixture solutions | via halogenated silane groups (heterogeneous process) | [59] |
| BIm, NaphIm, AIm | SPE | 2,4-dinitrophenol | aqueous solutions | via halogenated silane groups | [66] |
| C18Im, MIm+C18 | RPLC | alkylbenzenes, alkylnaphthalenes and PAHs | mixture solutions | via halogenated silane groups (heterogeneous process) | [67] |
| P3NIm, SP3NIm | SPE | 2,4-dinitrophenol | aqueous solutions | via halogenated silane group; via thiol-containing silane (“thiol-ene” click reaction) | [68] |
| SC8ImLac | HILIC/RPLC | PAHs, anilines, and high polar compounds | milk powder, Trichoderma sp. extract | via thiol-containing silane (“thiol-ene” click reaction) | [69] |
| SNGlu | HILIC/AEX-LC | nucleotides and flavonoids | mixture solutions | via thiol-containing silane (“thiol-ene” click reaction) | [52] |
| SONIm | HILIC | nucleosides, amino acids, organic acids, flavonoids, etc. | flavonoids mixture, soybean flavonoids, and urine | via thiol-containing silane (“thiol-ene” click reaction) | [70] |
| SImCalix | RPLC/HILIC/AEX-LC | alkyl benzenes, phenols, nucleosides, and anions | mixture solutions | via thiol-containing silane (“thiol-ene” click reaction) | [71] |
| Matrix | IL (Ligand) | Application | Samples | Refs. |
|---|---|---|---|---|
| VBC-DVB copolymer | imidazolium trifluoroacetate | SPE by anion exchange | acidic analytes from real water samples | [83] |
| PS-PVP copolymer | aminopropyl-imidazolium | SPE | bioactive compounds from Sophora Flavescens Ait | [84] |
| MI-PS-PVP copolymer | imidazolium, methylimidazolium, carboxyl-imidazolium, amino-imidazolium, cyano-imidazolium chloride | SPE | tanshinones from Salvia miltiorrhiza Bunge | [85] |
| PVPB copolymer | alkyl-pyridinium chloride | SPE | liquiritin and glycyrrhizin from Licorice | [46,64] |
| Sepharose CL-6B | benzothiazolium bromide | multi-modal chromatography | protein solutions of RNase, α-chymotrypsin and BSA | [86] |
| Toyopearl® AF-Epoxy-650M | 1-methyl-3-propylimidazolium | multi-modal chromatography | three types of nucleic acids from complex bacterial lysates | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardo, S.C.; Carapito, R.; Neves, M.C.; Freire, M.G.; Sousa, F. Supported Ionic Liquids Used as Chromatographic Matrices in Bioseparation—An Overview. Molecules 2022, 27, 1618. https://doi.org/10.3390/molecules27051618
Bernardo SC, Carapito R, Neves MC, Freire MG, Sousa F. Supported Ionic Liquids Used as Chromatographic Matrices in Bioseparation—An Overview. Molecules. 2022; 27(5):1618. https://doi.org/10.3390/molecules27051618
Chicago/Turabian StyleBernardo, Sandra C., Rita Carapito, Márcia C. Neves, Mara G. Freire, and Fani Sousa. 2022. "Supported Ionic Liquids Used as Chromatographic Matrices in Bioseparation—An Overview" Molecules 27, no. 5: 1618. https://doi.org/10.3390/molecules27051618
APA StyleBernardo, S. C., Carapito, R., Neves, M. C., Freire, M. G., & Sousa, F. (2022). Supported Ionic Liquids Used as Chromatographic Matrices in Bioseparation—An Overview. Molecules, 27(5), 1618. https://doi.org/10.3390/molecules27051618