Oxazolidine Nitroxide Transformation in a Coordination Sphere of the Ln3+ Ions
Abstract
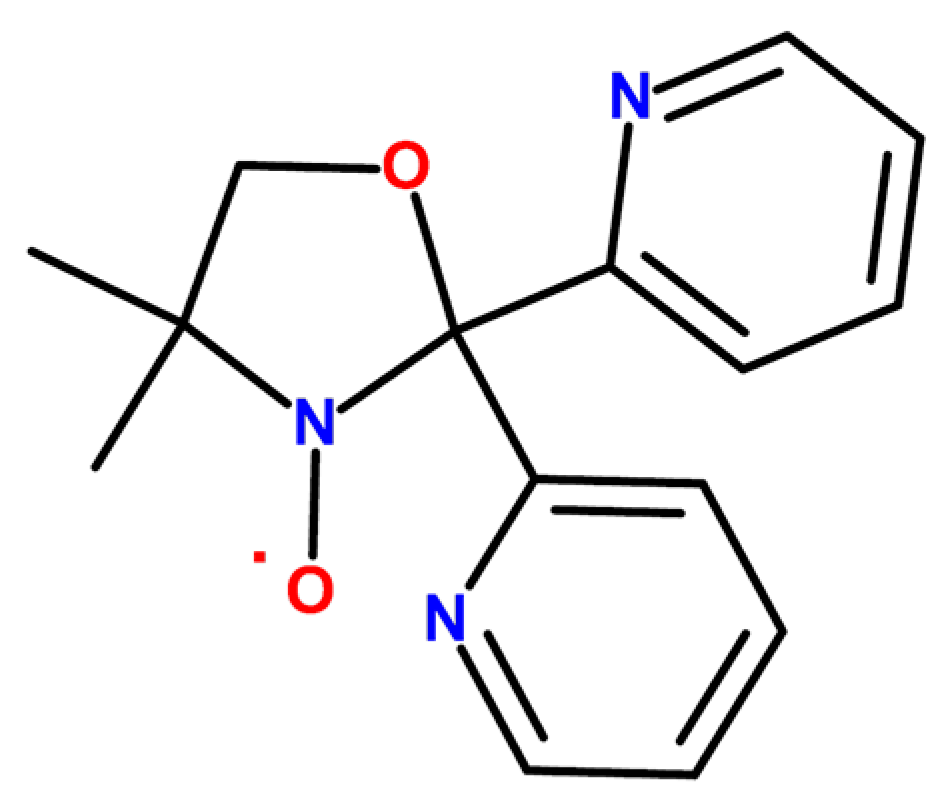
:1. Introduction
2. Results and Discussion
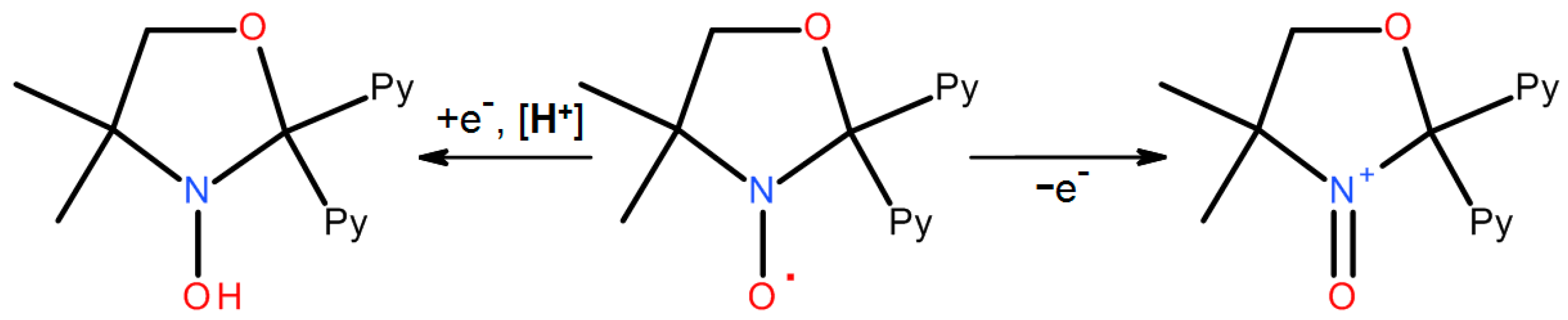
2.1. Influence of Moisture and Acidity
2.2. Transformations in the Presence of Coordination Metal Ions
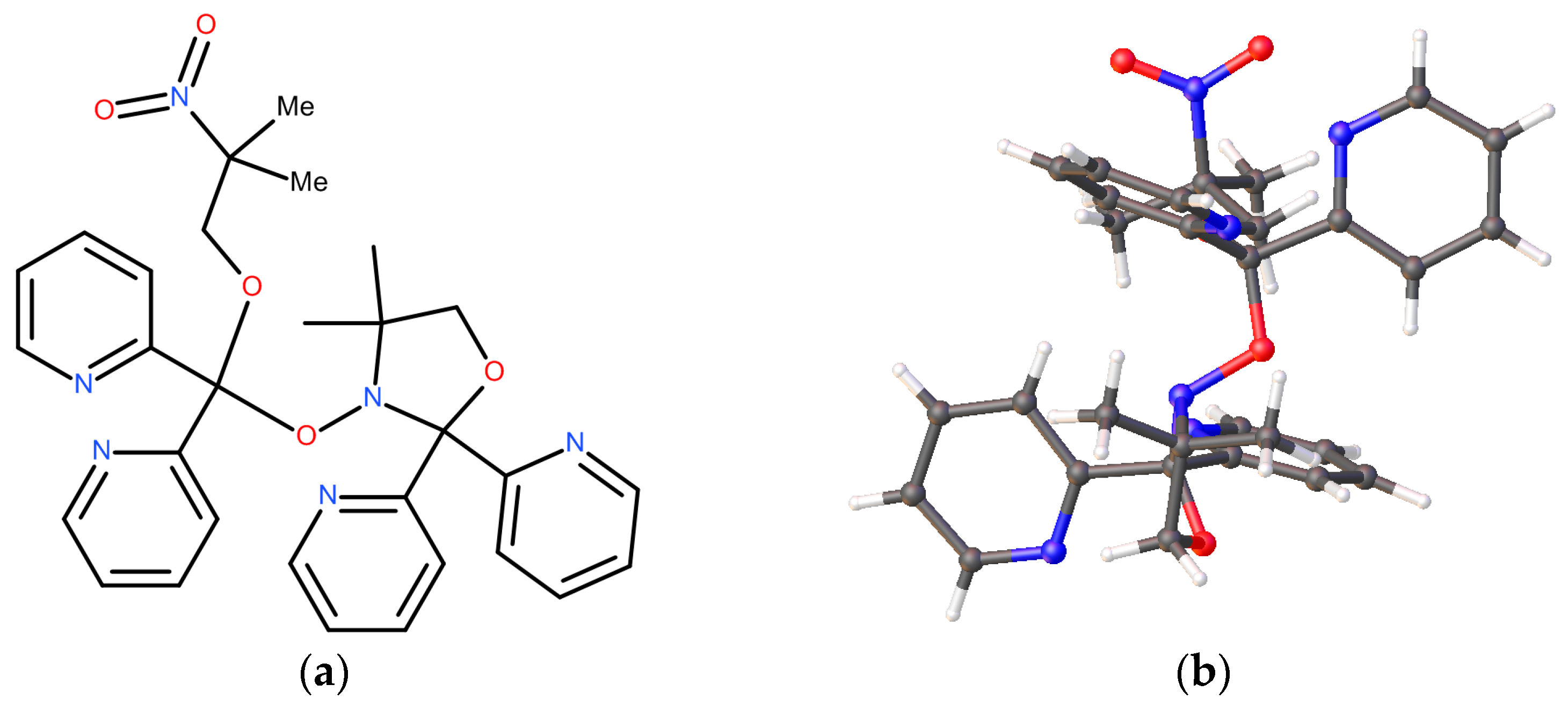
2.2.1. Tridentate Ligand Formation
2.2.2. Tetradentate Ligand Formation
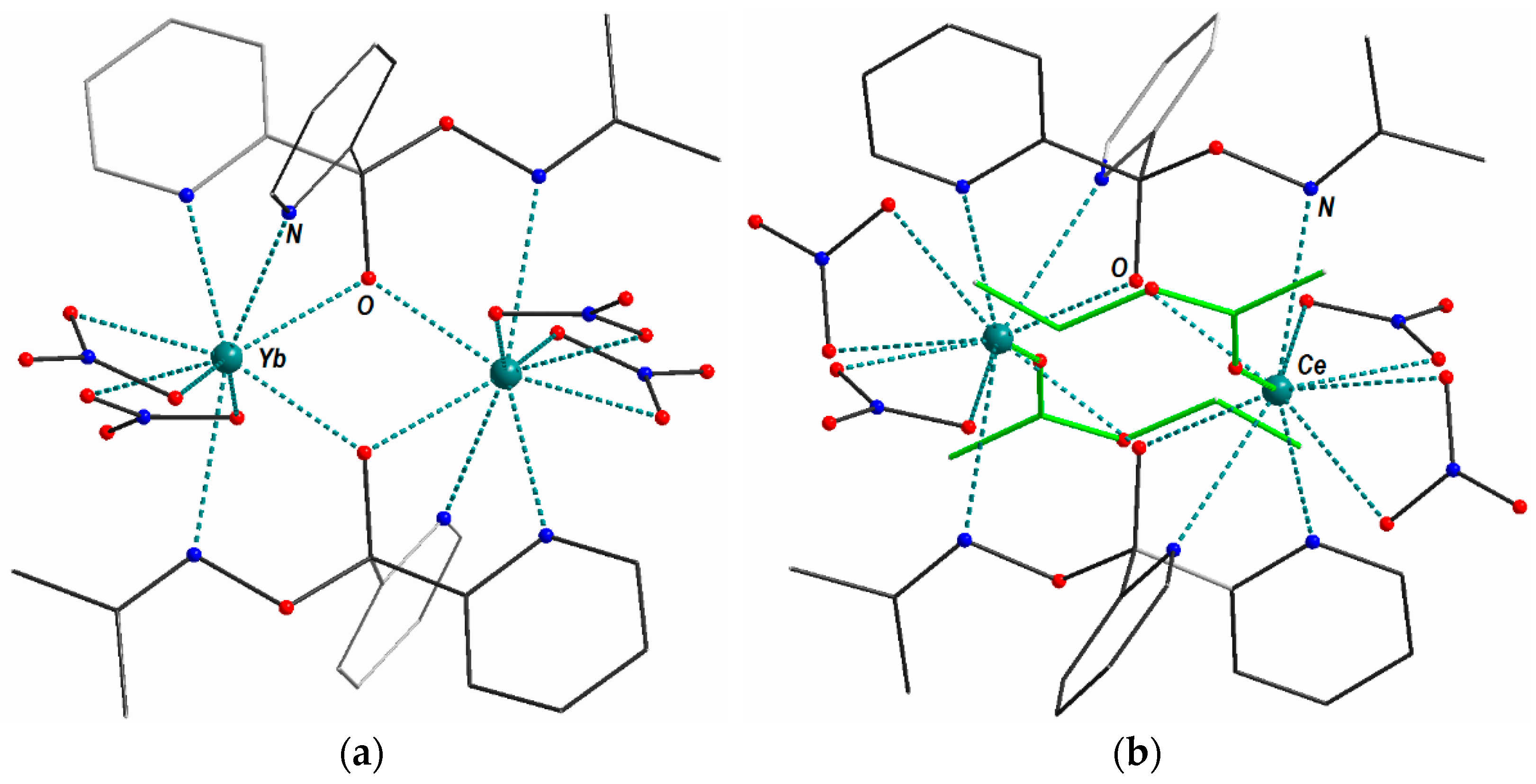
2.2.3. Crystallographic Characterization of Rad and Its Diamagnetic Precursor
2.2.4. The Crystallographic Characterization of 6, a Rad Transformation Product Obtained from the Mother Liquor Remaining after the Recrystallization Process
3. Experimental Section
3.1. Materials and Methods
3.2. Single-Crystal X-ray Crystallography Data Collection and Refinement
3.3. Synthesis
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sieklucka, B.; Pinkowicz, D. (Eds.) Molecular Magnetic Materials; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; ISBN 9783527694228. [Google Scholar]
- Coronado, E.; Gatteschi, D. Trends and Challenges in Molecule-Based Magnetic Materials. J. Mater. Chem. 2006, 16, 2513–2515. [Google Scholar] [CrossRef]
- Demir, S.; Jeon, I.-R.; Long, J.R.; Harris, T.D. Radical Ligand-Containing Single-Molecule Magnets. Coord. Chem. Rev. 2015, 289–290, 149–176. [Google Scholar] [CrossRef] [Green Version]
- Vostrikova, K.E. High-Spin Molecules Based on Metal Complexes of Organic Free Radicals. Coord. Chem. Rev. 2008, 252, 1409–1419. [Google Scholar] [CrossRef]
- Dei, A.; Gatteschi, D.; Pécaut, J.; Poussereau, S.; Sorace, L.; Vostrikova, K. Crystal Field and Exchange Effects in Rare Earth Semiquinone Complexes. Comptes Rendus L’Academie Sci. Ser. IIC Chem. 2001, 4, 135–141. [Google Scholar] [CrossRef]
- Görller-Walrand, C.; Binnemans, K. Chapter 155. Rationalization of Crystal-Field Parametrization. In Handbook on the Physics and Chemistry of Rare Earths; Gschneidner, K.A., Jr., Eyring, L., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 23, pp. 123–283. ISBN 978-0-444-82507-0. [Google Scholar]
- Sørensen, M.A.; Hansen, U.B.; Perfetti, M.; Pedersen, K.S.; Bartolomé, E.; Simeoni, G.G.; Mutka, H.; Rols, S.; Jeong, M.; Zivkovic, I.; et al. Chemical Tunnel-Splitting-Engineering in a Dysprosium-Based Molecular Nanomagnet. Nat. Commun. 2018, 9, 1292. [Google Scholar] [CrossRef]
- Bonde, N.A.; Petersen, J.B.; Sørensen, M.A.; Nielsen, U.G.; Fåk, B.; Rols, S.; Ollivier, J.; Weihe, H.; Bendix, J.; Perfetti, M. Importance of Axial Symmetry in Elucidating Lanthanide–Transition Metal Interactions. Inorg. Chem. 2020, 59, 235–243. [Google Scholar] [CrossRef]
- Ito, A.; Nakano, Y.; Urabe, M.; Tanaka, K.; Shiro, M. Structural and Magnetic Studies of Copper(II) and Zinc(II) Coordination Complexes Containing Nitroxide Radicals as Chelating Ligands. Eur. J. Inorg. Chem. 2006, 2006, 3359–3368. [Google Scholar] [CrossRef]
- Gass, I.A.; Gartshore, C.J.; Lupton, D.W.; Moubaraki, B.; Nafady, A.; Bond, A.M.; Boas, J.F.; Cashion, J.D.; Milsmann, C.; Wieghardt, K.; et al. Anion Dependent Redox Changes in Iron Bis-Terdentate Nitroxide {NNO} Chelates. Inorg. Chem. 2011, 50, 3052–3064. [Google Scholar] [CrossRef]
- Gass, I.A.; Tewary, S.; Nafady, A.; Chilton, N.F.; Gartshore, C.J.; Asadi, M.; Lupton, D.W.; Moubaraki, B.; Bond, A.M.; Boas, J.F.; et al. Observation of Ferromagnetic Exchange, Spin Crossover, Reductively Induced Oxidation, and Field-Induced Slow Magnetic Relaxation in Monomeric Cobalt Nitroxides. Inorg. Chem. 2013, 52, 7557–7572. [Google Scholar] [CrossRef]
- Gass, I.A.; Tewary, S.; Rajaraman, G.; Asadi, M.; Lupton, D.W.; Moubaraki, B.; Chastanet, G.; Létard, J.-F.; Murray, K.S. Solvate-Dependent Spin Crossover and Exchange in Cobalt(II) Oxazolidine Nitroxide Chelates. Inorg. Chem. 2014, 53, 5055–5066. [Google Scholar] [CrossRef]
- Gass, I.A.; Asadi, M.; Lupton, D.W.; Moubaraki, B.; Bond, A.M.; Guo, S.-X.; Murray, K.S. Manganese(II) Oxazolidine Nitroxide Chelates: Structure, Magnetism, and Redox Properties. Aust. J. Chem. 2014, 67, 1618. [Google Scholar] [CrossRef]
- Pedersen, A.H.; Geoghegan, B.L.; Nichol, G.S.; Lupton, D.W.; Murray, K.S.; Martínez-Lillo, J.; Gass, I.A.; Brechin, E.K. Hexahalorhenate(IV) Salts of Metal Oxazolidine Nitroxides. Dalt. Trans. 2017, 46, 5250–5259. [Google Scholar] [CrossRef] [Green Version]
- Gass, I.A.; Lu, J.; Asadi, M.; Lupton, D.W.; Forsyth, C.M.; Geoghegan, B.L.; Moubaraki, B.; Cashion, J.D.; Martin, L.L.; Bond, A.M.; et al. Use of the TCNQF42− Dianion in the Spontaneous Redox Formation of [FeIII(L−)2][TCNQF4−]. Chempluschem 2018, 83, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Perfetti, M.; Caneschi, A.; Sukhikh, T.; Vostrikova, K.E. Lanthanide Complexes with a Tripodal Nitroxyl Radical Showing Strong Magnetic Coupling. Inorg. Chem. 2020, 59, 16591–16598. [Google Scholar] [CrossRef] [PubMed]
- Rey, P.; Caneschi, A.; Sukhikh, T.S.; Vostrikova, K.E. Tripodal Oxazolidine-N-Oxyl Diradical Complexes of Dy3+ and Eu3+. Inorganics 2021, 9, 91. [Google Scholar] [CrossRef]
- Gass, I.A.; Lu, J.; Ojha, R.; Asadi, M.; Lupton, D.W.; Geoghegan, B.L.; Moubaraki, B.; Martin, L.L.; Bond, A.M.; Murray, K.S. [FeII(L•)2][TCNQF4•−]2: A Redox-Active Double Radical Salt. Aust. J. Chem. 2019, 72, 769–777. [Google Scholar] [CrossRef]
- Andrews, P.C.; Beck, T.; Forsyth, C.M.; Fraser, B.H.; Junk, P.C.; Massi, M.; Roesky, P.W. Templated Assembly of a Μ6-CO32– Dodecanuclear Lanthanum Dibenzoylmethanide Hydroxido Cluster with Concomitant Formation of Phenylglyoxylate. Dalt. Trans. 2007, 5651. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Kolybalov, D.S.; Pylova, E.K.; Bashirov, D.A.; Komarov, V.Y.; Kuratieva, N.V.; Smolentsev, A.I.; Fitch, A.N.; Konchenko, S.N. A Fresh Look at the Structural Diversity of Dibenzoylmethanide Complexes of Lanthanides. New J. Chem. 2019, 43, 9934–9942. [Google Scholar] [CrossRef]
- Grant, K.; Kassai, M. Major Advances in the Hydrolysis of Peptides and Proteins by Metal Ions and Complexes. Curr. Org. Chem. 2006, 10, 1035–1049. [Google Scholar] [CrossRef]
- Keana, J.F.W.; Keana, S.B.; Beetham, D. New Versatile Ketone Spin Label. J. Am. Chem. Soc. 1967, 89, 3055–3056. [Google Scholar] [CrossRef]
- Keana, J.F.W. Newer Aspects of the Synthesis and Chemistry of Nitroxide Spin Labels. Chem. Rev. 1978, 78, 37–64. [Google Scholar] [CrossRef]
- Bergmann, E.D. The Oxazolidines. Chem. Rev. 1953, 53, 309–352. [Google Scholar] [CrossRef]
- Rosantsev, E.G. Free Nitroxide Radicals, 1st ed.; Plenum Press: New York, NY, USA, 1970; ISBN 978-0306303968. [Google Scholar]
- Sen, V.D.; Golubev, V.A. Kinetics and Mechanism for Acid-Catalyzed Disproportionation of 2,2,6,6-Tetramethylpiperidine-1-Oxyl. J. Phys. Org. Chem. 2009, 22, 138–143. [Google Scholar] [CrossRef]
- Tikhonov, I.V.; Sen’, V.D.; Borodin, L.I.; Pliss, E.M.; Golubev, V.A.; Rusakov, A.I. Effect of the Structure of Nitroxyl Radicals on the Kinetics of Their Acid-Catalyzed Disproportionation. J. Phys. Org. Chem. 2014, 27, 114–120. [Google Scholar] [CrossRef]
- Bobbitt, J.M.; BrüCkner, C.; Merbouh, N. Oxoammonium- and Nitroxide-Catalyzed Oxidations of Alcohols. In Organic Reactions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 103–424. [Google Scholar]
- Hay, R.W. Reactions of Coordinated Ligands. In Reaction Mechanisms of Metal Complexes; Elsevier: Amsterdam, The Netherlands, 2000; pp. 140–163. [Google Scholar]
- Wang, H.-S.; Chen, Y.; Hu, Z.-B.; Yin, C.-L.; Zhang, Z.; Pan, Z.-Q. Modulation of the Directions of the Anisotropic Axes of Dy III Ions through Utilizing Two Kinds of Organic Ligands or Replacing Dy III Ions by Fe III Ions. CrystEngComm 2019, 21, 5429–5439. [Google Scholar] [CrossRef]
- Gieren, A.; Lamm, V. [1,1-Bis(1-Phenylethylidenaminooxy)Ethyl]Lbenzol. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1978, 34, 3837–3840. [Google Scholar] [CrossRef]
- Hierso, J.-C.; Ellis, D.D.; Spek, A.L.; Bouwman, E.; Reedijk, J. Cobalt-Assisted Condensation of 2-Butanone Oxime and Acetone: Synthesis and X-ray Structure of the Novel Acetaldiimine Complex [CoI2{((CH3CH2)(CH3)C=NO)2C(CH3)2}]. Eur. J. Inorg. Chem. 2000, 2000, 2459–2462. [Google Scholar] [CrossRef]
- Kukushkin, V.Y.; Belsky, V.K.; Tudela, D. Unusual Reactivity Mode of Coordinated Oximes: Platinum(IV)-Assisted Ring Closure by Reaction with Acetone. Inorg. Chem. 1996, 35, 510–513. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of Silver and Molybdenum Microfocus X-Ray Sources for Single-Crystal Structure Determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. PLATON SQUEEZE: A Tool for the Calculation of the Disordered Solvent Contribution to the Calculated Structure Factors. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rey, P.; Smolentsev, A.I.; Vostrikova, K.E. Oxazolidine Nitroxide Transformation in a Coordination Sphere of the Ln3+ Ions. Molecules 2022, 27, 1626. https://doi.org/10.3390/molecules27051626
Rey P, Smolentsev AI, Vostrikova KE. Oxazolidine Nitroxide Transformation in a Coordination Sphere of the Ln3+ Ions. Molecules. 2022; 27(5):1626. https://doi.org/10.3390/molecules27051626
Chicago/Turabian StyleRey, Philippe, Anton I. Smolentsev, and Kira E. Vostrikova. 2022. "Oxazolidine Nitroxide Transformation in a Coordination Sphere of the Ln3+ Ions" Molecules 27, no. 5: 1626. https://doi.org/10.3390/molecules27051626
APA StyleRey, P., Smolentsev, A. I., & Vostrikova, K. E. (2022). Oxazolidine Nitroxide Transformation in a Coordination Sphere of the Ln3+ Ions. Molecules, 27(5), 1626. https://doi.org/10.3390/molecules27051626






