Profile of Myracrodruon urundeuva Volatile Compounds Ease of Extraction and Biodegradability and In Silico Evaluation of Their Interactions with COX-1 and iNOS
Abstract
:1. Introduction
2. Results
2.1. Solid Phase Microextraction Allows Fine Profiling of the Aroeira Seeds Volatiles
2.2. Comparative Virtual Docking Points for Significant Distinctions between Hits for COX-1 and iNOScs, with iNOSas Presenting an “Intermediary” Behavior between Both Sites
2.3. Volume and Hydrophobicity as Major Components for the COX-1 Affinity, Followed by Inos and with iNOScs Presenting a More Complex and Less Predictable Behavior
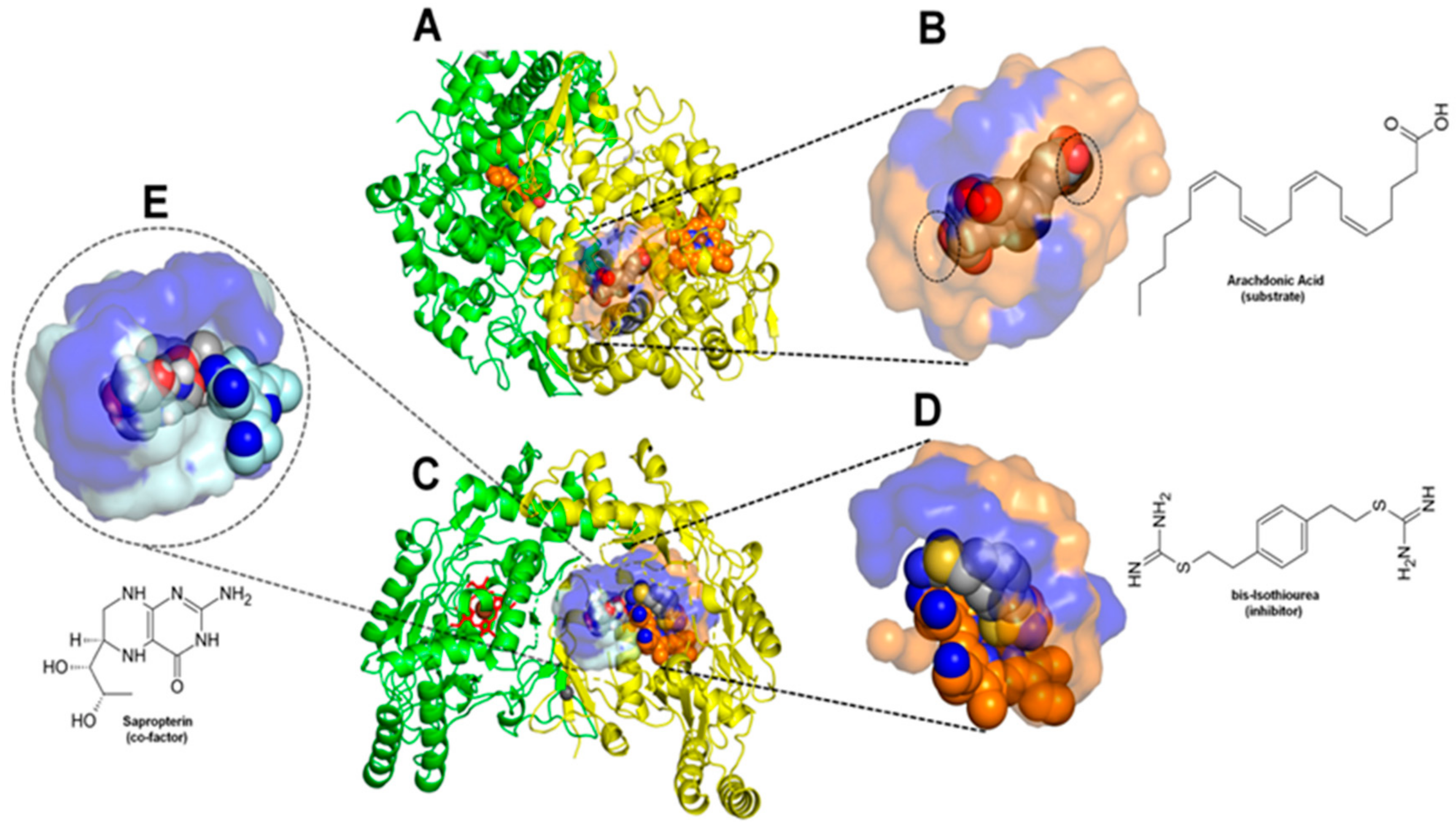
2.4. Structural Interpretation of the Differential Selectivity for COX-1 and iNOS Active/Cofactor Sites for the Different Ligand Classes on Aroeira Seeds
2.5. Two Salicylate Derivatives on Aroeira Seeds Seems to Be Promissor for Direct, or after Modification, Suicide Inhibition of the COX-1 Enzyme
3. Material and Methods
3.1. Gas Chromatography—Mass Spectrometry
3.2. Volatile Compound Identification
3.3. Virtual Docking Assays
3.4. Molecular Descriptors and Statistical Analysis
3.5. Estimation of the Major Contacts Involved in Ligand-Target Interactions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nunes, Y.R.F.; Fagundes, M.; Almeida, H.S.; Veloso, M.D.M. Aspectos ecológicos da aroeira (Myracrodruon urundeuva Allemão-Anacardiaceae): Fenologia e germinação de sementes. Rev. Árvore Viçosa 2008, 32, 233–243. [Google Scholar] [CrossRef]
- Feliciano, A.L.P.; Maragon, L.C.; Holanda, A.C. Morfologia de sementes, de plântulas e de plantas jovens de aroeira (Myracrodruon urundeuva Allemão). Rev. Biol. Ciências Terra Paraíba 2008, 8, 110–118. [Google Scholar]
- Virgens, I.O.; Castro, R.D.; Fernandez, L.G.; Pelacani, C.R. Comportamento fsiológico de sementes de Myracrodruon urundeuva fr. all. (Anacardiaceae) submetidas a fatores abióticos. Ciência Florest. St. Maria RS 2012, 22, 681–692. [Google Scholar] [CrossRef] [Green Version]
- Araújo, R.D.I. Atividade Antimicrobiana e Citotóxica de Óleo Essencial e Extratos Orgânicos Provenientes da Myracrodruon urundeuva Fr. Allem. (Aroeira-do-Sertão). Dissertação de Mestrado, Universidade Federal do Rio Grande do Norte, Natal, RN, Brasil, 2017. [Google Scholar]
- Aquino, N.C.; Araújo, M.R.; Silveira, R.E. Intraspecific Variation of the Volatile Chemical Composition of Myracrodruon urundeuva Fr. Allem. (“Aroeira-do-Sertão”): Characterization of Six Chemotypes. J. Braz. Chem. Soc. 2017, 28, 907–912. [Google Scholar] [CrossRef]
- Medeiros, A.C.S.; Smith, R.; Probert, R.J.; Sader, R. Comportamento fisiológico de sementes de aroeira (Myracrodruon urundeuva Fr. All.), em condições de armazenamento. Bol. Pesq. Fl. Colombo 2000, 40, 77. [Google Scholar]
- Silva, P.D.D. Caracterização de Compostos Fenólicos por Espectrometria de Massas e Potencial Antioxidante das Cascas de Myracrodruon urundeuva (Aroeira-do-Sertão) do Cariri Paraibano. Dissertação de Mestrado, Universidade Federal da Paraíba, Sumé, PB, Brasil, 2018. [Google Scholar]
- Ferreira, P.M.P.; Farias, D.F.; Viana, M.P.; Souza, P.M.; Vasconcelos, I.M.; Soares, B.M.; Pessoa, C.; Costa-Lotufo, L.V.; Moraes, M.O.; Carvalho, A.F.U. Study of the antiproliferative potential of seed extracts from Northeastern Brazilian plants. Biomed. Sci. An. Acad. Bras. Cienc. 2011, 83, 1045–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, A.M.S.; Oliveira, J.T.A.; Rocha, C.Q.; Ferreira, A.T.S.; Perales, J.; Zanatta, A.C.; Vilegas, W.; Silva, C.R.; Costa-Junior, L.M. Myracrodruon urundeuva seed exudates proteome and anthelmintic activity against Haemonchus contortus. PLoS ONE 2018, 13, e0200848. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.C.; Oliveira, R.C. Phytotherapy medicines in dentistry: Evidence and perspectives on the use of “Aroeira-do-sertão” (Myracrodruon Urundeuva Allemão). Rev. Bras. Plantas Med. 2014, 16, 283–289. [Google Scholar] [CrossRef]
- Lica, I.C.L.; Soares, A.; de Mesquita, L.S.S.; Malik, S. Biological properties and pharmacological potential of plant exudates. Food Res. Int. 2018, 105, 1039–1053. [Google Scholar] [CrossRef]
- Carvalho, M.G.; Melo, A.G.N.; Aragão, C.F.S.; Raffin, F.N.; Moura, T.F.A.L. Schinus terebinthifolius Raddi: Chemical composition, biological properties and toxicity. Rev. Bras. Pl. Med. Botucatu 2013, 15, 158–169. [Google Scholar] [CrossRef]
- Ceruks, M.; Romoff, P.; Favero, A.O.; Lago, J.H.G. Constituintes fenólicos polares de Schinus terebinthifolius Raddi (Anacardiacea). Rev. Química Nova São Paulo 2007, 30, 597–599. [Google Scholar] [CrossRef] [Green Version]
- Jain, M.K.; Yu, B.Z.; Rogers, J.M.; Smith, A.E.; Boger, E.T.; Ostrander, R.L.; Rheingold, A.L. Specifi c competiti ve inhibitor of secreted phospholipase A2 from berries of Schinus terebinthifolius. Phytochemistry 1995, 39, 537–547. [Google Scholar] [CrossRef]
- Santos, B.O.; Augusti, R.; Melo, J.O.F.; Takahashi, J.A.; Araújo, R.L.B. Optimization of extraction conditions of volatile compounds from pequi peel (Caryocar brasiliense Camb.) using HS-SPME. Res. Soc. Dev. 2020, 9, e919974893. [Google Scholar] [CrossRef]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef] [Green Version]
- Aponso, M.; Patti, A.; Bennett, L.E. Dose-related effects of inhaled essential oils on behavioural measures of anxiety and depression and biomarkers of oxidative stress. J. Ethnopharmacol. 2020, 250, 112469. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Z.A.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G. Essential Oils: Extraction Techniques, Pharmaceutical and Therapeutic potential Review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.L.; De Almeida, R.; Verícimo, M.A.; De Macedo, H.W.; Castro, H.C. Atividades terapêuticas do óleo essencial de melaleuca (Melaleuca alternifolia) Uma revisão de literatura. Braz. J. Health Rev. 2019, 2, 6011–6021. [Google Scholar] [CrossRef]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, medicinal and toxicological significance ofEucalyptusleaf essential oil: A review. J. Sci. Food Agric. 2017, 98, 833–848. [Google Scholar] [CrossRef]
- Farrar, A.J.; Farrar, F.C. Clinical Aromatherapy. Nurs. Clin. N. Am. 2020, 55, 489–504. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Jafarikukhdan, A.; Hosseini, A.; Armand, R. The Application of Medicinal Plants in Traditional and Modern Medicine: A Review of Thymus vulgaris. Int. J. Clin. Med. 2015, 6, 635–642. [Google Scholar] [CrossRef] [Green Version]
- Yuan, R.; Zhang, D.; Yang, J.; Wu, Z.; Luo, C.; Han, L.; Yang, F.; Lin, J.; Yang, M. Review of aromatherapy essential oils and their mechanism of action against migraines. J. Ethnopharmacol. 2020, 265, 113326. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, J.T.; Shropshire, B.C.; Nagy, T.R.; Chambers, K.D.; Li, Y.; Korach, K.S. Essential Oils and Health. Yale J. Biol. Med. 2020, 93, 291–305. [Google Scholar] [PubMed]
- Asif, M.; Saleem, M.; Saadullah, M.; Yaseen, H.S.; Al Zarzour, R. COVID-19 and therapy with essential oils having antiviral, anti-inflammatory, and immunomodulatory properties. Inflammopharmacology 2020, 28, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Gu, Y.; Wang, C.; Zhang, J.; Zhang, J.; Wang, G.; Wang, F. A Systematic Review of the Anti-Inflammatory and Immunomodulatory Properties of 16 Essential Oils of Herbs. Evid. Based Complement. Altern. Med. 2020, 2020, 8878927. [Google Scholar] [CrossRef]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. J. Clin. Ther. 2009, 14, 141–143. [Google Scholar]
- Borges, R.S.; Ortiz, B.L.S.; Pereira, A.C.M.; Keita, H.; Carvalho, J.C.T. Rosmarinus officinalis essential oil: A review of its phytochemistry, anti-inflammatory activity, and mechanisms of action involved. J. Ethnopharmacol. 2018, 229, 29–45. [Google Scholar] [CrossRef]
- Peterfalvi, A.; Miko, E.; Nagy, T.; Reger, B.; Simon, D.; Miseta, A.; Czéh, B.; Szereday, L. Much More Than a Pleasant Scent: A Review on Essential Oils Supporting the Immune System. Molecules 2019, 24, 4530. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, Y.G.; Bueno, F.C.; Júnior, A.H.D.O.; Mazzinghy, A.C.D.C.; Mendonça, H.D.O.P.; de Oliveira, A.F.; De Melo, A.C.; Reina, L.D.C.B.; Augusti, R.; Melo, J.O.F. Profile of the volatile organic compounds of pink pepper and black pepper. Sci. Electron. Arch. 2021, 14, 39–43. [Google Scholar] [CrossRef]
- Silva, M.R.; Bueno, G.H.; Araujo, R.L.B.; Lacerda, I.C.A.; Freitas, L.G.; Morais, H.A.; Augustini, R.; Melo, J.O.F. Evaluation of the Influence of Extraction Conditions on the Isolation and Identification of Volatile Compounds from Cagaita (Eugenia dysenterica) Using HS SPME/GC-MS. J. Braz. Chem. Soc. 2019, 30, 379–387. [Google Scholar] [CrossRef]
- Nascimento, P.T.; Fadini, M.A.M.; Rocha, M.S.; Souza, C.S.F.; Barros, B.A.; Melo, J.O.F.; Von Pinho, R.G.; Valicente, F.H. Olfactory response of Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) to volatiles induced by transgenic maize. Bull. Entomol. Res. 2021, 111, 674–687. [Google Scholar] [CrossRef]
- García, Y.M.; Ramos, A.L.C.C.; de Oliveira, A.H., Jr.; de Paula, A.C.C.F.F.; de Melo, A.C.; Andrino, M.A.; Silva, M.R.; Augusti, R.; de Araújo, R.L.B.; de Lemos, E.E.P.; et al. Physicochemical Characterization and Paper Spray Mass Spectrometry Analysis of Myrciaria floribunda (H. West ex Willd.) O. Berg Accessions. Molecules 2021, 26, 7206. [Google Scholar] [CrossRef] [PubMed]
- García, Y.M.; de Lemos, E.E.P.; Augusti, R.; Melo, J.O.F. Otimização da extração e identificação dos compostos voláteis de Myrciaria floribunda. Rev. Ciência Agronômica 2021, 52, e20207199. [Google Scholar] [CrossRef]
- García, Y.M.; Ramos, A.L.C.C.; de Paula, A.C.C.F.F.; do Nascimento, M.H.; Augusti, R.; de Araújo, R.L.B.; de Lemos, E.E.P.; Melo, J.O.F. Chemical Physical Characterization and Profile of Fruit Volatile Compounds from Different Accesses of Myrciaria floribunda (H. West Ex Wild.) O. Berg through Polyacrylate Fiber. Molecules 2021, 26, 5281. [Google Scholar] [CrossRef] [PubMed]
- Mariano, A.P.X.; Ramos, A.L.C.C.; de Oliveira Júnior, A.H.; García, Y.M.; de Paula, A.C.C.F.F.; Silva, M.R.; Augusti, R.; de Araújo, R.L.B.; Melo, J.O.F. Optimization of Extraction Conditions and Characterization of Volatile Organic Compounds of Eugenia klotzschiana O. Berg Fruit Pulp. Molecules 2022, 27, 935. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.F.; Huri, D.A.; Snyder, S.H. Inducible Nitric Oxide Synthase Binds, S-Nitrosylates, and Activates Cyclooxygenase-2. Science 2005, 310, 1966–1970. [Google Scholar] [CrossRef] [Green Version]
- Attiq, A.; Jalil, J.; Husain, K.; Ahmad, W. Raging the War Against Inflammation With Natural Products. Front. Pharmacol. 2018, 9, 1–27. [Google Scholar] [CrossRef]
- Zidar, N.; Odar, K.; Glavac, D.; Jerse, M.; Zupanc, T.; Stajer, D. Cyclooxygenase in normal human tissues—Is COX-1 really a constitutive isoform, and COX-2 an inducible isoform? J. Cell. Mol. Med. 2008, 13, 3753–3763. [Google Scholar] [CrossRef] [Green Version]
- Rouzer, C.A.; Marnett, L.J. Cyclooxygenases: Structural and functional insights. J. Lipid Res. 2009, 50, S29–S34. [Google Scholar] [CrossRef] [Green Version]
- Moro, M.G.; Sanchez, P.K.V.; Lupepsa, A.C.; Baller, E.M.; Franco, G.C.N. Biología de la ciclooxigenasa en la función renal–Revisión de la literatura. Rev. Colomb. Nefrol. 2017, 4, 27. [Google Scholar] [CrossRef]
- Jaradat, N.; Al-Lahham, S.; Abualhasan, M.N.; Bakri, A.; Zaide, H.; Hammad, J.; Hussein, F.; Issa, L.; Mousa, A.; Speih, R. Chemical Constituents, Antioxidant, Cyclooxygenase Inhibitor, and Cytotoxic Activities of Teucrium pruinosum Boiss. Essential Oil. BioMed. Res. Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Salaria, D.; Rolta, R.; Sharma, N.; Patel, C.N.; Ghosh, A.; Dev, K.; Sourirajan, A.; Kumar, V. In Vitro and in silico antioxidant and anti-inflammatory potential of essential oil of Cymbopogon citratus (DC.) Stapf. of North-Western Himalaya. J. Biomol. Struct. Dyn 2021, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Al-Maharik, N.; Jaradat, N.; Qneibi, M.; Abualhasan, M.N.; Emwas, N. Glechoma curviflora Volatile Oil from Palestine: Chemical Composition and Neuroprotective, Antimicrobial, and Cyclooxygenase Inhibitory Activities. Evid.-Based Complement. Altern. Med. 2020, 2020, 4195272. [Google Scholar] [CrossRef] [PubMed]
- Rollinger, J.M.; Haupt, S.; Stuppner, H.; Langer, T. Combining Ethnopharmacology and Virtual Screening for Lead Structure Discovery: COX-Inhibitors as Application Example. J. Chem. Inf. Comput. Sci. 2004, 44, 480–488. [Google Scholar] [CrossRef]
- Batlouni, M. Anti-inflamatórios não esteroides: Efeitos cardiovasculares, cérebro-vasculares e renais. Arq. Bras. Cardiol. 2010, 94, 556–563. [Google Scholar] [CrossRef] [Green Version]
- Cerqueira, N.F.; Yoshida, W.B. Óxido nÌtrico: Revisão. Acta Cir. Bras. 2002, 17, 417–423. Available online: http://www.scielo.br/acb (accessed on 28 December 2021). [CrossRef] [Green Version]
- Da Silva, C.B.; Ceron, C.S.; Mendes, A.S.; de Martinis, B.S.; Castro, M.M.; Tirapelli, C.R. Inducible nitric oxide synthase (iNOS) mediates ethanol-induced redox imbalance and upregulation of inflammatory cytokines in the kidney. Can. J. Physiol. Pharmacol. 2021, 99, 1016–1025. [Google Scholar] [CrossRef]
- De Oliveira, G.A.; Cheng, R.Y.; Ridnour, L.A.; Basudhar, D.; Somasundaram, V.; McVicar, D.W.; Monteiro, H.P.; Wink, D.A. Inducible Nitric Oxide Synthase in the Carcinogenesis of Gastrointestinal Cancers. Antioxid. Redox Signal. 2017, 26, 1059–1077. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Ghasemi, A.; Kashfi, K. Type 2 Diabetes and Cancer: The Nitric Oxide Connection. Crit. Rev. Oncog. 2019, 24, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gupta, A.K. Nitric oxide: Role in tumour biology and iNOS/NO-based anticancer therapies. Cancer Chemother. Pharmacol. 2011, 67, 1211–1224. [Google Scholar] [CrossRef] [PubMed]
- Sahebnasagh, A.; Saghafi, F.; Negintaji, S.; Hu, T.; Shabani-Boroujeni, M.; Safdari, M.; Ghaleno, H.R.; Miao, L.; Qi, Y.; Wang, M.; et al. Nitric Oxide and Immune Responses in Cancer: Searching for New Therapeutic Strategies. Curr. Med. Chem. 2021, 28, 1. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2019, 40, 158–189. [Google Scholar] [CrossRef] [PubMed]
- Özenver, N.; Efferth, T. Small molecule inhibitors and stimulators of inducible nitric oxide synthase in cancer cells from natural origin (phytochemicals, marine compounds, antibiotics). Biochem. Pharmacol. 2020, 176, 113792. [Google Scholar] [CrossRef] [PubMed]
- Tabanca, N.; Nalbantsoy, A.; Kendra, P.E.; Demirci, F.; Demirci, B. Chemical Characterization and Biological Activity of the Mastic Gum Essential Oils of Pistacia lentiscus var. chia from Turkey. Molecules 2020, 25, 2136. [Google Scholar] [CrossRef]
- Prabhakar, S.S. Tetrahydrobiopterin reverses the inhibition of nitric oxide by high glucose in cultured murine mesangial cells. Am. J. Physiol. Physiol. 2001, 281, F179–F188. [Google Scholar] [CrossRef]
- Maruca, A.; Moraca, F.; Rocca, R.; Molisani, F.; Alcaro, F.; Gidaro, M.C.; Alcaro, S.; Costa, G.; Ortuso, F. Chemoinformatic Database Building and in Silico Hit-Identification of Potential Multi-Targeting Bioactive Compounds Extracted from Mushroom Species. Molecules 2017, 22, 1571. [Google Scholar] [CrossRef] [Green Version]
- Lagunin, A.A.; Goel, R.K.; Gawande, D.Y.; Pahwa, P.; Gloriozova, T.A.; Dmitriev, A.V.; Ivanov, S.M.; Rudik, A.V.; Konova, V.I.; Pogodin, P.V.; et al. Chemo- and bioinformatics resources for in silico drug discovery from medicinal plants beyond their traditional use: A critical review. Nat. Prod. Rep. 2014, 31, 1585–1611. [Google Scholar] [CrossRef] [PubMed]
- Salvemini, D.; Kim, S.F.; Mollace, V. Reciprocal regulation of the nitric oxide and cyclooxygenase pathway in pathophysiology: Relevance and clinical implications. Am. J. Physiol. Integr. Comp. Physiol. 2013, 304, R473–R487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maldonado-Rojas, W.; Olivero-Verbel, J. Food-Related Compounds That Modulate Expression of Inducible Nitric Oxide Synthase May Act as Its Inhibitors. Molecules 2012, 17, 8118–8135. [Google Scholar] [CrossRef] [Green Version]
- Maia, J.G.S.; Silva, M.H.L.; Andrade, E.H.A.; Zoghbi, M.G.B.; Carreira, L.M.M. Essential oils from Astronium urundeuva (Allemao) Engl. And, A. fraxinifolium Schott ex Spreng. Flavour Fragr. J. 2002, 17, 72–74. [Google Scholar] [CrossRef]
- Cole, E.; Dos Santos, R.; Lacerda, V., Jr.; Martins, J.; Greco, S.; Neto, A.C. Chemical composition of essential oil from ripe fruit of Schinus terebinthifolius Raddi and evaluation of its activity against wild strains of hospital origin. Braz. J. Microbiol. 2014, 45, 821–828. [Google Scholar] [CrossRef] [Green Version]
- Elshafie, H.S.; Ghanney, N.; Mang, S.M.; Ferchichi, A.; Camele, I. An In Vitro attempt for controlling severe phytopathogens and human pathogens using essential oils from mediterranean plants of genus Schinus. J. Med. Food 2016, 19, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zardecki, C.; Dutta, S.; Goodsell, D.S.; Lowe, R.; Voigt, M.; Burley, S.K. PDB-101: Educational resources supporting molecular explorations through biology and medicine. Protein Sci. 2022, 31, 129–140. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium UniProt: A hub for protein information. Nucleic Acids Res. 2014, 43, D204–D212. [CrossRef]
- Guex, N.; Peitsch, M.C.; Schewde, P.T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: A historical perspective. Electrophoresis 2009, 30, S162–S173. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Olson AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- The PyMOL Molecular Graphics System; Version 2.0; Schrödinger, LLC.: New York, NY, USA, 2010.
- Morandat, F.; Hill, B.; Osvald, L.; Vitek, J. Evaluating the Design of the R Language; Springer: Berlin/Heidelberg, Germany, 2012; Volume 7313, pp. 104–131. [Google Scholar] [CrossRef]
- Mweene, P.; Muzaza, G. Implementation of Interactive Learning Media on Chemical Materials. J. Educ. Verkenn. 2020, 1, 8–13. [Google Scholar] [CrossRef]
- Marpaung, D.N.; Pongkendek, J.J.; Azzajjad, M.F.; Sukirno, S. Analysis of Student Motivation using Chemsketch on Hydrocarbon Topic in SMA Negeri 2 Merauke. J. Appl. Sci. Eng. Technol. Educ. 2021, 3, 69–73. [Google Scholar] [CrossRef]
- Ragno, R. www.3d-qsar.com: A web portal that brings 3-D QSAR to all electronic devices—The Py-CoMFA web application as tool to build models from pre-aligned datasets. J. Comput. Mol. Des. 2019, 33, 855–864. [Google Scholar] [CrossRef]
- Araújo, B.M.; Coelho, A.L.; Silveira, S.A.; Romanelli, J.P.; de Melo-Minardi, R.; Silveira, C.H. GAPIN: Grouped and Aligned Protein Interface Networks. bioRxiv 2019, 3, 520833. [Google Scholar] [CrossRef]








| N° | Volatile Organic Compoud | CAS | Formula | Sampled Trees | ||||
|---|---|---|---|---|---|---|---|---|
| A.1 | A.2 | A.3 | A.4 | A.5 | ||||
| MONOTERPENES | ||||||||
| 1 | α-Pinene a,b,c,d,e,f,g,i,j,l,m,n | 7785-70-8 | C10H16 | 22.6% | ND | ND | ND | ND |
| 2 | 3-Carene a,b,c,d,f,g,i,k,l | 13466-78-9 | C10H16 | 11.0% | 34.8% | 46.5% | 49.9% | 55.2% |
| 3 | Camphene f,i,l,m,n | 79-92-5 | C10H16 | ND | ND | 0.5% | 0.6% | 0.5% |
| 4 | α-Campholenal f | 4501-58-0 | C10H16O | ND | 7.7% | ND | 4.6% | ND |
| 5 | Camphenol,6- | 3570-04-5 | C10H16O | 14.9% | ND | 4.2% | ND | 5.7% |
| 6 | D-Limonene a,b,c,d,e,f,g,h,l,n | 5989-27-5 | C10H16 | ND | 23.0% | ND | ND | ND |
| 7 | m -Mentha-6,8-diene® | 1461-27-4 | C10H16 | ND | ND | 30.7% | ND | ND |
| 8 | p-Mentha-1,4(8)—diene d,f,k | 586-62-9 | C10H16 | 1.0% | 1.3% | 0.9% | 2.9% | ND |
| 9 | p-1,8-diene,(S) | 5989-54-8 | C10H16 | 5% | ND | ND | 10.4% | 21.1% |
| SESQUITERPENES | ||||||||
| 10 | β -Guaiene k | 88-84-6 | C15H24 | 0.8% | 0.2% | |||
| 11 | Caryophyllene b,c,d,e,f,g,i,j,m,n | 87-44-5 | C15H24 | 11.4% | 6.0% | 1.9% | 0.6% | 0.3% |
| 12 | Cedr-8(15) ene | 11028-42-5 | C15H24 | 1.7% | 1.2% | 0.6% | 2.6% | ND |
| 13 | Elemene d,f,m,n | 339154-9 | C15H24 | 0.2% | ND | ND | ND | ND |
| 14 | β-Chamigrene | 18431-82-8 | C15H24 | ND | ND | ND | ND | 0.4% |
| 15 | Guaia-1(5),11-diene | 3691-12-1 | C15H24 | ND | ND | 0.4% | ND | ND |
| 16 | Patchoulene | 1405-16-9 | C15H24 | ND | 0.6% | ND | ND | ND |
| 17 | 1H-Benzocycloheptene,2,4aα,5,6,7,8,9aα-octahydro-3,5,5-trimethyl-9-methylene- | 3853-83-6 | C15H24 | 1% | 0.8% | ND | 0.8% | ND |
| OTHER CLASSES | ||||||||
| 18 | Ethylcaproate | 123-66-0 | C8H16O2 | 4.0% | ND | 0.8% | ND | ND |
| 19 | Cis- Geranylacetone | 3879-26-3 | C13H22O | 27.2% | ND | 25.4% | 24.3% | 10.3% |
| 20 | o-Anisic acid, methylester | 606-45-1 | C9H10O3 | ND | 0.5% | ND | ND | 0.7% |
| 21 | Salicylic acid, methyl, methylester e | 119-36-8 | C8H8O3 | ND | 2.3% | ND | 2.0% | 5.6% |
| 22 | Trans-Geranylacetone | 3796-70-1 | C13H22O | ND | 21.6% | ND | ND | ND |
| 23 | Hexanoic acid 1-cyclopentylethylester | NA | C13H24O2 | ND | ND | ND | 0.6% | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueiredo, Y.G.; Corrêa, E.A.; de Oliveira Junior, A.H.; Mazzinghy, A.C.d.C.; Mendonça, H.d.O.P.; Lobo, Y.J.G.; García, Y.M.; Gouvêia, M.A.d.S.; de Paula, A.C.C.F.F.; Augusti, R.; et al. Profile of Myracrodruon urundeuva Volatile Compounds Ease of Extraction and Biodegradability and In Silico Evaluation of Their Interactions with COX-1 and iNOS. Molecules 2022, 27, 1633. https://doi.org/10.3390/molecules27051633
Figueiredo YG, Corrêa EA, de Oliveira Junior AH, Mazzinghy ACdC, Mendonça HdOP, Lobo YJG, García YM, Gouvêia MAdS, de Paula ACCFF, Augusti R, et al. Profile of Myracrodruon urundeuva Volatile Compounds Ease of Extraction and Biodegradability and In Silico Evaluation of Their Interactions with COX-1 and iNOS. Molecules. 2022; 27(5):1633. https://doi.org/10.3390/molecules27051633
Chicago/Turabian StyleFigueiredo, Yuri G., Eduardo A. Corrêa, Afonso H. de Oliveira Junior, Ana C. d. C. Mazzinghy, Henrique d. O. P. Mendonça, Yan J. G. Lobo, Yesenia M. García, Marcelo A. d. S. Gouvêia, Ana C. C. F. F. de Paula, Rodinei Augusti, and et al. 2022. "Profile of Myracrodruon urundeuva Volatile Compounds Ease of Extraction and Biodegradability and In Silico Evaluation of Their Interactions with COX-1 and iNOS" Molecules 27, no. 5: 1633. https://doi.org/10.3390/molecules27051633
APA StyleFigueiredo, Y. G., Corrêa, E. A., de Oliveira Junior, A. H., Mazzinghy, A. C. d. C., Mendonça, H. d. O. P., Lobo, Y. J. G., García, Y. M., Gouvêia, M. A. d. S., de Paula, A. C. C. F. F., Augusti, R., Reina, L. D. C. B., da Silveira, C. H., de Lima, L. H. F., & Melo, J. O. F. (2022). Profile of Myracrodruon urundeuva Volatile Compounds Ease of Extraction and Biodegradability and In Silico Evaluation of Their Interactions with COX-1 and iNOS. Molecules, 27(5), 1633. https://doi.org/10.3390/molecules27051633








