Efficient Extraction of Total Polyphenols from Apple and Investigation of Its SPF Properties
Abstract
1. Introduction
2. Results
2.1. Plant Extracts’ Characterization
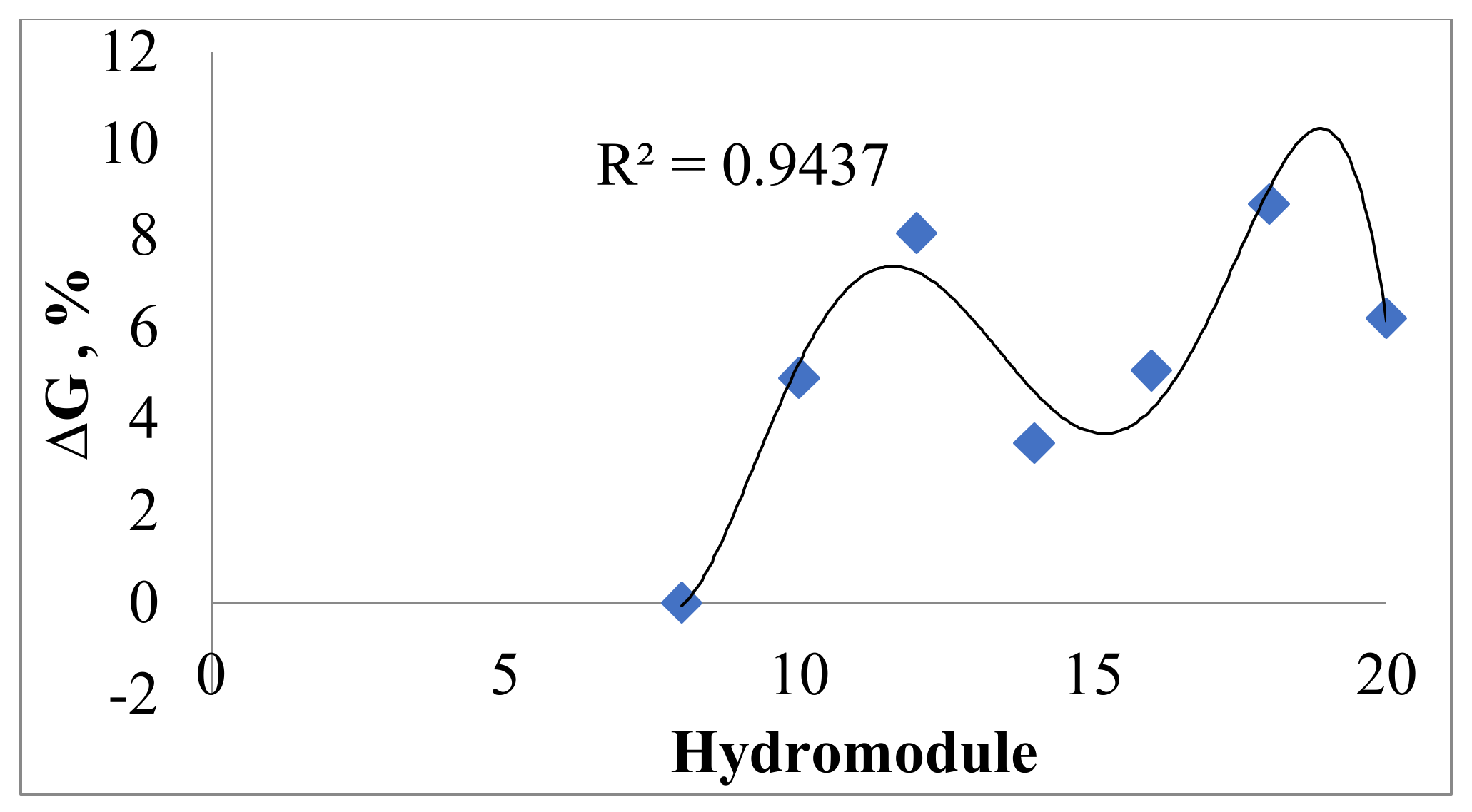
2.1.1. Single Parameter Effect on Total Polyphenols
2.1.2. Single Parameter Effect on Antioxidant Activity
2.1.3. Single Parameter Effect on SPF
2.2. Improving Apple Extracts
2.3. Emulsion Characterization
3. Materials and Methods
3.1. Polyphenolic Extract Preparation
3.1.1. Preparation of Vegetable Material
3.1.2. Determination of Hydromodule
3.1.3. Obtaining Alcoholic Extract
3.2. Characterization of the Obtained Extracts
3.2.1. Total Phenolic Content
3.2.2. Antioxidant Capacity
3.2.3. Determination of the SPF of Extracts
3.3. Incorporation of the Apple Extract in a Sunscreen Formulation
3.4. Sunscreen Characterization
3.5. Data Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Briasco, B.; Capra, P.; Mannucci, B.; Perugini, P. Stability study of sunscreens with free and encapsulated UV filters contained in plastic packaging. Pharmaceutics 2017, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Abdassah, M.; Aryani, R.; Surachman, E.; Muchtaridi, M. In-vitro assessment of effectiveness and photostability avobenzone in cream formulations by combination ethyl ascorbic acid and alpha tocopherol acetate. J. Appl. Pharm. Sci. 2015, 5, 70–74. [Google Scholar] [CrossRef][Green Version]
- Hasmeni, Z.; Ebrahimzadeh, M.A.; Khalili, M. Sun protection factor, total phenol, flavonoid contents and antioxidant activity of medicinal plants from Iran. Trop. J. Pharm. Res. 2019, 18, 1443–1448. [Google Scholar]
- Silva, V.V.; Ropke, C.D.; Almeida, R.L.; Miranda, D.V.; Kera, C.Z.; Rivelli, D.P.; Sawada, T.C.H.; Barros, S.B.M. Chemical stability and SPF determination of Pothomorphe umbellata extract gel and photostability of 4-nerolidylcathecol. Int. J. Pharm. 2005, 303, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Maske, P.P.; Lokapure, S.G.; Nimbalkar, D.; Malavi, S.; D’Souza, J.I. In vitro determination of sun protection factor and chemical stability of Rosa kordesii extract gel. J. Pharm. Res. 2013, 7, 520–524. [Google Scholar] [CrossRef]
- Napagoda, M.T.; Malkanthi, B.M.A.S.; Abayawardana, S.A.K.; Qader, M.M.; Jayasinghe, L. Photoprotective potential in some medicinal plants used to treat skin diseases in Sri Lanka. BMC Complement. Altern. Med. 2016, 16, 479. [Google Scholar] [CrossRef]
- Chtourou, Y.; Aouey, B.; Aroui, S.; Kebieche, M.; Fetoui, H. Anti-apoptotic and anti-inflammatory effects of naringin on cisplatin-induced renal injury in the rat. Chem. Biol. Interact. 2016, 243, 1–9. [Google Scholar] [CrossRef]
- Ju, S.M.; Kang, J.G.; Bae, J.S.; Pae, H.O.; Lyu, Y.S.; Jeon, B.H. The flavonoid apigenin ameliorates cisplatin-induced nephrotoxicity through reduction of p53 activation and promotion of PI3K/Akt pathway in human renal proximal tubular epithelial cells. Evid. Based Complement. Altern. Med. 2015, 2015, 186436. [Google Scholar] [CrossRef]
- Najda, A.; Bains, A.; Chawla, P.; Kumar, A.; Balant, S.; Walasek-Janusz, M.; Wach, D.; Kaushik, R. Assessment of Anti-Inflammatory and Antimicrobial Potential of Ethanolic Extract of Woodfordia fruticosa Flowers: GC-MS Analysis. Molecules 2021, 26, 7193. [Google Scholar] [CrossRef]
- Chingizova, E.A.; Skriptsova, A.; Anisimov, M.; Aminin, D. Antimicrobial activity of marine algal extracts. Int. J. Phytomed. 2017, 9, 113–122. [Google Scholar]
- Malik, A.; Najda, A.; Bains, A.; Nurzyńska-Wierdak, R.; Chawla, P. Characterization of Citrusnobilis Peel Methanolic Extract for Antioxidant, Antimicrobial, and Anti-Inflammatory Activity. Molecules 2021, 26, 4310. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, S.; Cao, S.; He, X.; Qin, L.; He, M.; Yang, Y.; Hao, J.; Mao, W. Structural characteristics and anticoagulant property in vitro and in vivo of a seaweed sulfated Rhamnan. Mar. Drugs 2018, 16, 243. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Gupta, S.; Yadav, T.C.; Pruthi, V.; Varadwaj, P.K.; Goel, N. Extrapolation of phenolic compounds as multi-target agents against cancer and inflammation. J. Biomol. Struct. Dyn. 2019, 37, 2355–2369. [Google Scholar] [CrossRef] [PubMed]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.-Y.O.; Lima, G.P.P. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability. Phenolic Compd. Biol. Act. 2017, 8, 1–24. [Google Scholar]
- Tasić-Kostov, M.; Savić, S.; Lukić, M.; Tamburić, S.; Pavlović, M.; Vuleta, G. Lactobionic acid in a natural alkylpolyglucoside-based vehicle: Assessing safety and efficacy aspects in comparison to glycolic acid. J. Cosmet. Dermatol. 2010, 9, 3–10. [Google Scholar] [CrossRef]
- Žugić, A.; Ðorđević, S.; Arsić, I.; Marković, G.; Živković, J.; Jovanović, S.; Tadić, V. Antioxidant activity and phenolic compounds in 10 selected herbs from Vrujci Spa, Serbia. Ind. Crops Prod. 2014, 52, 519–527. [Google Scholar] [CrossRef]
- Savić, S.; Tamburić, S.; Savić, M. From conventional towards new-natural surfactants in drug delivery systems design: Current status and perspectives. Expert Opin. Drug. Deliv. 2010, 7, 353–369. [Google Scholar] [CrossRef]
- Raphaelli, C.O.; Azevedo, J.G.; Pereira, E.S.; Vinholes, J.R.; Camargo, T.M.; Hoffmann, J.F.; Ribeiro, J.A.; Vizzotto, M.; Rombaldi, C.V.; Wink, M.R.; et al. Phenolic-rich apple extracts have photoprotective and anti-cancer effect in dermal cells. Phytomed. Plus 2021, 1, 100112. [Google Scholar] [CrossRef]
- Zhu, X.; Li, N.; Wang, Y.; Ding, L.; Chen, H.; Yu, Y.; Shi, X. Protective effects of quercetin on UVB irradiation-induced cytotoxicity through ROS clearance in keratinocyte cells. Oncol. Rep. 2017, 37, 209–218. [Google Scholar] [CrossRef]
- Solomon, J.S.; Umar, H.I.; Saliu, I.O.; Akinmoladun, A.C. Quercetin and catechin assuage redox imbalance and neurochemical dysfunction in rotenone-induced neurotoxicity: A comparative in vivo experiment supported by in silico study. Phytomed. Plus 2021, 1, 100077. [Google Scholar] [CrossRef]
- Giomaro, G.; Karioti, A.; Bilia, A.R.; Bucchini, A.; Giamperi, L.; Ricci, D.; Fraternale, D. Polyphenols profile and antioxidant activity of skin and pulp of a rare apple from Marche region (Italy). Chem. Cent. J. 2014, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Ranjha, M.M.A.N.; Amjad, S.; Ashraf, S.; Khawar, L.; Safdar, M.N.; Jabbar, S.; Nadeem, M.; Mahmood, S.; Murtaza, M.A. Extraction of Polyphenols from Apple and Pomegranate Peels Employing Different Extraction Techniques for the Development of Functional Date Bars. Int. J. Fruit Sci. 2020, 20, 1201–1221. [Google Scholar] [CrossRef]
- Knekt, P.; Kumpulainen, J.; Jarvinen, R.; Rissanen, H.; Heliovaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [CrossRef]
- Hyson, D.; Studebaker-Hallman, D.; Davis, P.A.; Gershwin, M.E. Apple juice consumption reduces plasma low-density lipoprotein oxidation in healthy men and women. J. Med. Food 2000, 3, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, Z.; Zuo, G.; Lim, S.S.; Yan, H. Defatted seeds of Oenothera biennis as a potential functional food ingredient for diabetes. Foods 2021, 10, 538. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Shukla, S.K.; Kumar, I.P.; Namita, I.; Afrin, F.; Sharma, R.K. Radioprotective properties of apple polyphenols: An in vitro study. Mol. Cell. Biochem. 2006, 288, 37–46. [Google Scholar] [CrossRef]
- Aprikian, O.; Duclos, V.; Guyot, S.; Besson, C.; Manach, C.; Bernalier, A.; Morand, C.; Remesy, C.; Demigne, C. Apple pectin and a polyphenol-rich apple concentrate are more effective together than separately on cecal fermentations and plasma lipids in rats. J. Nutr. 2003, 133, 1860–1865. [Google Scholar] [CrossRef]
- Eberhardt, M.V.; Lee, C.Y.; Liu, R.H. Antioxidant activity of fresh apples. Nature 2000, 405, 903–904. [Google Scholar] [CrossRef]
- Kern, M.; Tjaden, Z.; Ngiewih, Y.; Puppel, N.; Will, F.; Dietrich, H.; Pahlke, G.; Marko, D. Inhibitors of the epidermal growth factor receptor in apple juice extract. Mol. Nutr. Food Res. 2005, 49, 317–328. [Google Scholar] [CrossRef]
- Schieber, A.; Keller, P.; Streker, P.; Klaiber, I.; Carle, R. Detection of isorhamnetin glycosides in extracts of apples (Malus domestica cv. “Brettacher”) by HPLC-PDA and HPLC-APCI-MS/MS. Phytochem. Anal. 2002, 13, 87–94. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Yan, H. In situ net fishing of α-glucosidase inhibitors from evening primrose (Oenothera biennis) defatted seeds by combination of LC-MS/MS, molecular networking, affinity-based ultrafiltration, and molecular docking. Food Funct. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Yue, T.; Yuan, Y. Evolution of polyphenols and organic acids during the fermentation of apple cider. J. Sci. Food Agric. 2014, 94, 2951–2957. [Google Scholar] [CrossRef] [PubMed]
- Zucoloto, M.; Ku, K.M.; Kushad, M.M.; Sawwan, J. Bioactive compounds and quality characteristics of five apples cultivars. Cienc. Rural 2015, 45, 1972–1979. [Google Scholar] [CrossRef]
- Stojiljković, D.; Arsić, I.; Tadić, V. Extracts of wild apple fruit (Malus sylvestris (L.) Mill.. Rosaceae) as a source of antioxidant substances for use in production of nutraceuticals and cosmeceuticals. Ind. Crop Prod. 2016, 80, 165–176. [Google Scholar] [CrossRef]
- Stojiljković, D.; Arsić, I.; Tadić, V. Oil extracts of wild apple fruit as active substances in UV protection preparations. Radiat. Appl. 2016, 1, 187–192. [Google Scholar]
- Stojiljković, D.; Pavlović, D.; Arsić, I. Oxidative stress. skin aging and antioxidant therapy. Acta Fac. Med. Naissensis 2014, 31, 207–217. [Google Scholar] [CrossRef]
- Arct, J.; Pytkowska, K. Flavonoids as components of biologically active cosmeceuticals. Clin. Dermatol. 2008, 26, 347–357. [Google Scholar] [CrossRef]
- Stojiljković, D.; Tadić, V.; Stanković, M.; Roganović, S.; Arsić, I. Standardized extract of wild apple fruit in alkyl-polyglucoside-based cosmetic cream—Estimation of stability, safety, antioxidant activity and efficiency. Int. J. Cosmet. Sci. 2018, 40, 285–294. [Google Scholar] [CrossRef]
- Nešić, I.; Stojiljković, D.; Savić, S.; Tasić-Kostov, M.; Tadić, V. Stability. antioxidant activity. in vivo safety and efficacy of creams with standardized wild apple fruit extract: A comparison of conventional and biodegradable emulsifiers. Int. J. Cosmet. Sci. 2019, 41, 300–310. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berrie. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef]
- Ademosun, A.O.; Oboh, G.; Bello, F.; Ayeni, P.O. Antioxidative Properties and Effect of Quercetin and Its Glycosylated Form (Rutin) on Acetylcholinesterase and Butyrylcholinesterase Activities. J. Evid. Based Complement. Altern. Med. 2015, 21, Np11–Np17. [Google Scholar] [CrossRef]
- Gajardo, S.; Stowhas, T.; Salas, F.; Quispe, C.; Buc-calderon, P.; Benites, J. Determination of sun protection factor and antioxidant properties of six Chilean Altiplano plants. Blacpma 2016, 15, 352–363. [Google Scholar]
- Costa, S.C.C.; Detoni, C.B.; Branco, C.R.C.; Botura, M.B.; Branco, A. In vitro photoprotective effects of Marcetia taxifolia ethanolic extract and its potential for sunscreen formulations. Rev. Bras. Farmacogn. 2015, 25, 413–418. [Google Scholar] [CrossRef]
- Mejía-Giraldo, J.C.; Winkler, R.; Gallardo, C.; Sánchez-Zapata, A.M.; Puertas-Mejía, M.A. Photoprotective potential of Baccharis antioquensis (Asteraceae) as natural sunscreen. Photochem. Photobiol. 2016, 92, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, V.; Stefova, M.; Chinnici, F. Determination of the polyphenol contents in Macedonian grapes and wines by standardized spectrophotometric methods. J. Serb. Chem. Soc. 2010, 75, 45–59. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Mansur, J.S.; Breder, M.N.R.; Mansur, M.C.A.; Azulay, R.D. Determinação do fator de proteção solar por espectrofotometria. An. Bras. Dermatol. 1986, 61, 121–124. [Google Scholar]
- Opris, O.; Soran, M.L.; Lung, I.; Stegarescu, A.; Gutoiu, S.; Podea, R.; Podea, P. Optimization of extraction conditions of polyphenols, antioxidant capacity and sun protection factor from Prunus spinose fruits: Application in sunscreen formulation. J. Iran. Chem. Soc. 2021, 18, 2625–2636. [Google Scholar] [CrossRef]








| Design Point | Ethanol Concentration (%) | Temperature (°C) | Extraction Time (min) | TP (mg GA/g DW) | DPPH (mM Trolox/g DW) | SPF |
|---|---|---|---|---|---|---|
| 1 | 40.00 | 70.00 | 15.00 | 3.58 ± 0.015 | 61.15 ± 1.91 | 0.76 ± 0.0005 |
| 2 | 50.00 | 60.00 | 10.00 | 3.59 ± 0.031 | 74.41 ± 2.38 | 0.74 ± 0.010 |
| 3 | 60.00 | 50.00 | 15.00 | 3.46 ± 0.169 | 71.04 ± 5.72 | 0.72 ± 0.007 |
| 4 | 50.00 | 60.00 | 10.00 | 3.46 ± 0.169 | 71.04 ± 5.72 | 0.72 ± 0.007 |
| 5 | 40.00 | 50.00 | 15.00 | 3.49 ± 0.077 | 68.79 ± 2.22 | 0.69 ± 0.015 |
| 6 | 40.00 | 50.00 | 5.00 | 3.56 ± 0.153 | 61.83 ± 2.07 | 0.67 ± 0.003 |
| 7 | 60.00 | 70.00 | 15.00 | 3.52 ± 0.077 | 73.96 ± 13.82 | 0.70 ± 0.0008 |
| 8 | 50.00 | 60.00 | 10.00 | 3.46 ± 0.169 | 71.04 ± 5.72 | 0.72 ± 0.007 |
| 9 | 40.00 | 70.00 | 5.00 | 3.47 ± 0.031 | 52.62 ± 3.49 | 0.77 ± 0.031 |
| 10 | 60.00 | 70.00 | 5.00 | 3.55 ± 0.046 | 57.11 ± 4.77 | 0.73 ± 0.012 |
| 11 | 60.00 | 50.00 | 5.00 | 3.44 ± 0.031 | 54.19 ± 2.07 | 0.73 ± 0.015 |
| 12 | 50.00 | 60.00 | 10.00 | 3.46 ± 0.169 | 71.04 ± 5.72 | 0.72 ± 0.007 |
| 13 | 50.00 | 60.00 | 18.41 | 3.58 ± 0.107 | 62.28 ± 0.16 | 0.71 ± 0.017 |
| 14 | 50.00 | 60.00 | 1.59 | 3.36 ± 0.077 | 57.56 ± 1.27 | 0.67 ± 0.020 |
| 15 | 66.82 | 60.00 | 10.00 | 3.78 ± 0.308 | 59.36 ± 2.86 | 0.73 ± 0.020 |
| 16 | 50.00 | 43.18 | 10.00 | 3.53 ± 0.061 | 56.88 ± 1.11 | 0.75 ± 0.015 |
| 17 | 33.18 | 60.00 | 10.00 | 3.64 ± 0.169 | 66.99 ± 7.94 | 0.72 ± 0.010 |
| 18 | 50.00 | 76.82 | 10.00 | 3.82 ± 0.107 | 71.26 ± 5.88 | 0.70 ± 0.005 |
| 19 | 50.00 | 60.00 | 10.00 | 3.46 ± 0.169 | 71.04 ± 5.72 | 0.72 ± 0.007 |
| 20 | 50.00 | 60.00 | 10.00 | 3.46 ± 0.169 | 71.04 ± 5.729 | 0.72 ± 0.007 |
| Design Point | Ethanol Concentration (%) | Extraction Time (min) | TP (mg GA/g DW) | DPPH (mM Trolox/g DW) | SPF |
|---|---|---|---|---|---|
| 1 | 35.86 | 60.00 | 4.36 ± 0.061 | 57.11 ± 0.32 | 0.87 ± 0.001 |
| 2 | 40.00 | 50.00 | 4.81 ± 0.077 | 68.34 ± 5.08 | 0.98 ± 0.006 |
| 3 | 60.00 | 70.00 | 5.02 ± 0.046 | 60.25 ± 2.22 | 0.92 ± 0.009 |
| 4 | 60.00 | 50.00 | 4.12 ± 0.092 | 59.81 ± 9.53 | 1.00 ± 0.270 |
| 5 | 50.00 | 60.00 | 4.28 ± 0.015 | 56.21 ± 3.18 | 1.00 ± 0.017 |
| 6 | 50.00 | 60.00 | 4.27 ± 0.015 | 56.21 ± 3.18 | 1.00 ± 0.017 |
| 7 | 64.14 | 60.00 | 4.25 ± 0.123 | 48.35 ± 2.38 | 0.99 ± 0.065 |
| 8 | 50.00 | 74.14 | 4.12 ± 0.046 | 75.76 ± 7.47 | 0.94 ± 0.014 |
| 9 | 50.00 | 45.86 | 4.28 ± 0.031 | 54.64 ± 3.34 | 0.95 ± 0.001 |
| 10 | 50.00 | 60.00 | 4.48 ± 0.015 | 56.21 ± 3.18 | 1.01 ± 0.017 |
| 11 | 40.00 | 70.00 | 4.28 ± 0.123 | 60.25 ± 0.95 | 0.93 ± 0.017 |
| 12 | 50.00 | 60.00 | 4.28 ± 0.015 | 56.21 ± 3.18 | 1.01 ± 0.017 |
| 13 | 50.00 | 60.00 | 4.28 ± 0.015 | 56.21 ± 3.18 | 1.01 ± 0.017 |
| Extract Concentration in Cosmetic Emulsion (%) | SPF ± SE |
|---|---|
| 10 | 0.51 ± 0.007 |
| 20 | 0.64 ± 0.001 |
| 30 | 0.80 ± 0.003 |
| 40 | 0.90 ± 0.001 |
| Variable | (−1.68) | (−1) | (0) | (1) | (1.68) |
|---|---|---|---|---|---|
| Ethanol concentration (%) | 33.2 | 40 | 50 | 60 | 66.8 |
| Temperature (°C) | 43.2 | 50 | 60 | 70 | 76.8 |
| Extraction time (min) | 1.6 | 5 | 10 | 15 | 23.4 |
| Variable | (−1.41) | (−1) | (0) | (1) | (1.41) |
|---|---|---|---|---|---|
| Ethanol concentration (%) | 35.9 | 40 | 50 | 60 | 64.1 |
| Extraction time (min) | 45.9 | 50 | 60 | 70 | 74.1 |
| Ingredient | Quantity (g) | The Role of the Ingredient |
|---|---|---|
| Purified water | 35.3–65.30 | Base |
| Stearin | 3.0 | Consistent emollient factor |
| Glyceryl stearate | 2.0 | Emulsifier |
| Lanolin | 1.0 | Emollient |
| Glycerin | 1.0 | Consistency factor |
| Medole oil | 4.0 | Lubricating emollient |
| Isopropyl myristate | 5.0 | Emollient |
| Cetyl alcohol | 1.50 | Consistency factor |
| Sabowax EL-H-KOL | 5.0 | Emulsifier |
| Triethanolamine | 0.800 | Neutralizing agent |
| Microcare | 0.700 | Preservative |
| EDTA | 0.100 | Complexing agent |
| Labs-A | 0.100 | Emulsifier |
| Apple extract | 10–40 | Active principle |
| Perfume composition | 0.300 | Perfume |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opriş, O.; Lung, I.; Soran, M.-L.; Stegarescu, A.; Cesco, T.; Ghendov-Mosanu, A.; Podea, P.; Sturza, R. Efficient Extraction of Total Polyphenols from Apple and Investigation of Its SPF Properties. Molecules 2022, 27, 1679. https://doi.org/10.3390/molecules27051679
Opriş O, Lung I, Soran M-L, Stegarescu A, Cesco T, Ghendov-Mosanu A, Podea P, Sturza R. Efficient Extraction of Total Polyphenols from Apple and Investigation of Its SPF Properties. Molecules. 2022; 27(5):1679. https://doi.org/10.3390/molecules27051679
Chicago/Turabian StyleOpriş, Ocsana, Ildiko Lung, Maria-Loredana Soran, Adina Stegarescu, Tatiana Cesco, Aliona Ghendov-Mosanu, Paula Podea, and Rodica Sturza. 2022. "Efficient Extraction of Total Polyphenols from Apple and Investigation of Its SPF Properties" Molecules 27, no. 5: 1679. https://doi.org/10.3390/molecules27051679
APA StyleOpriş, O., Lung, I., Soran, M.-L., Stegarescu, A., Cesco, T., Ghendov-Mosanu, A., Podea, P., & Sturza, R. (2022). Efficient Extraction of Total Polyphenols from Apple and Investigation of Its SPF Properties. Molecules, 27(5), 1679. https://doi.org/10.3390/molecules27051679






