The Influence of Different Bleaching Protocols on Dentinal Enzymatic Activity: An In Vitro Study
Abstract
:1. Introduction
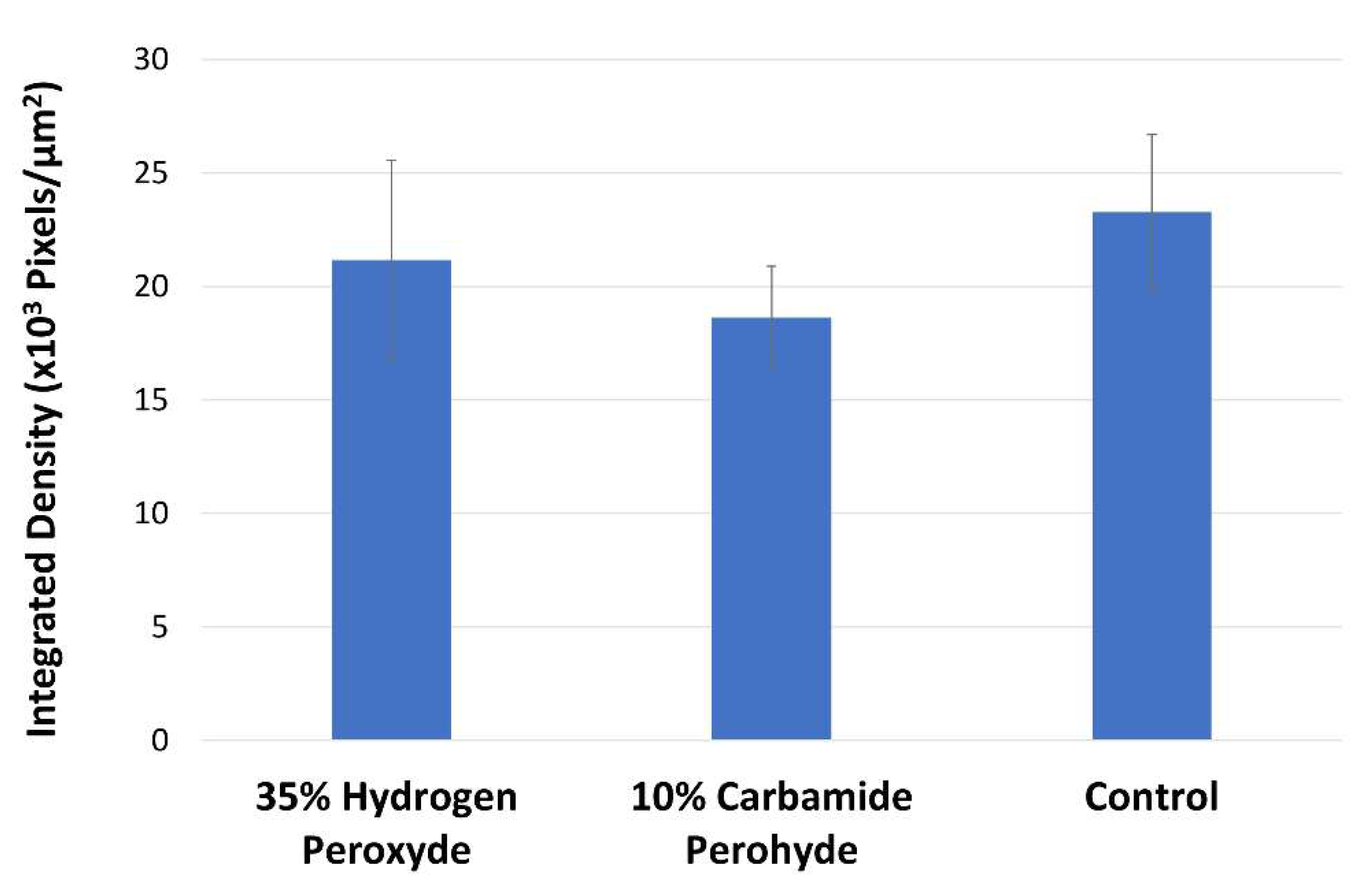
2. Results
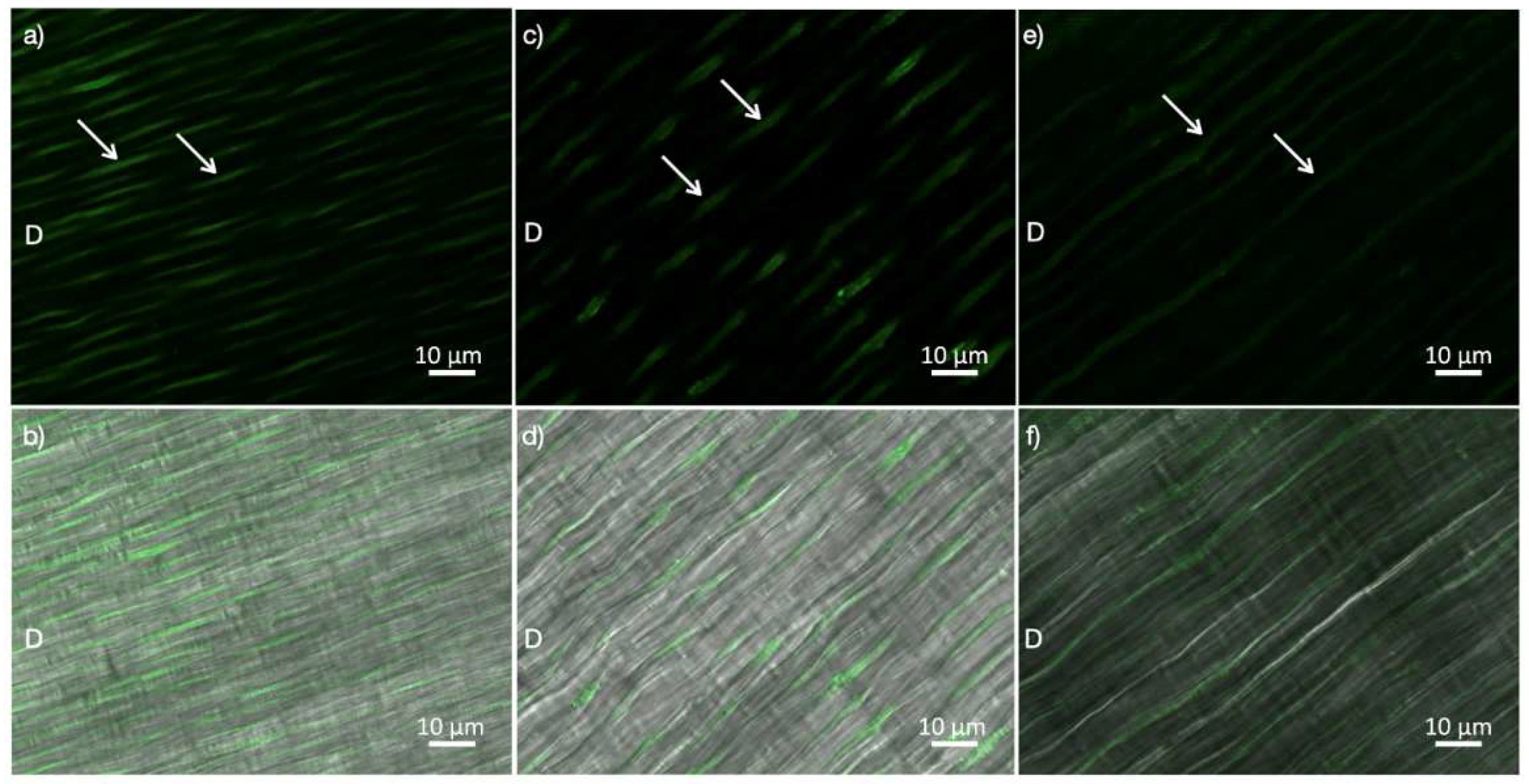
2.1. In-Situ Zymography
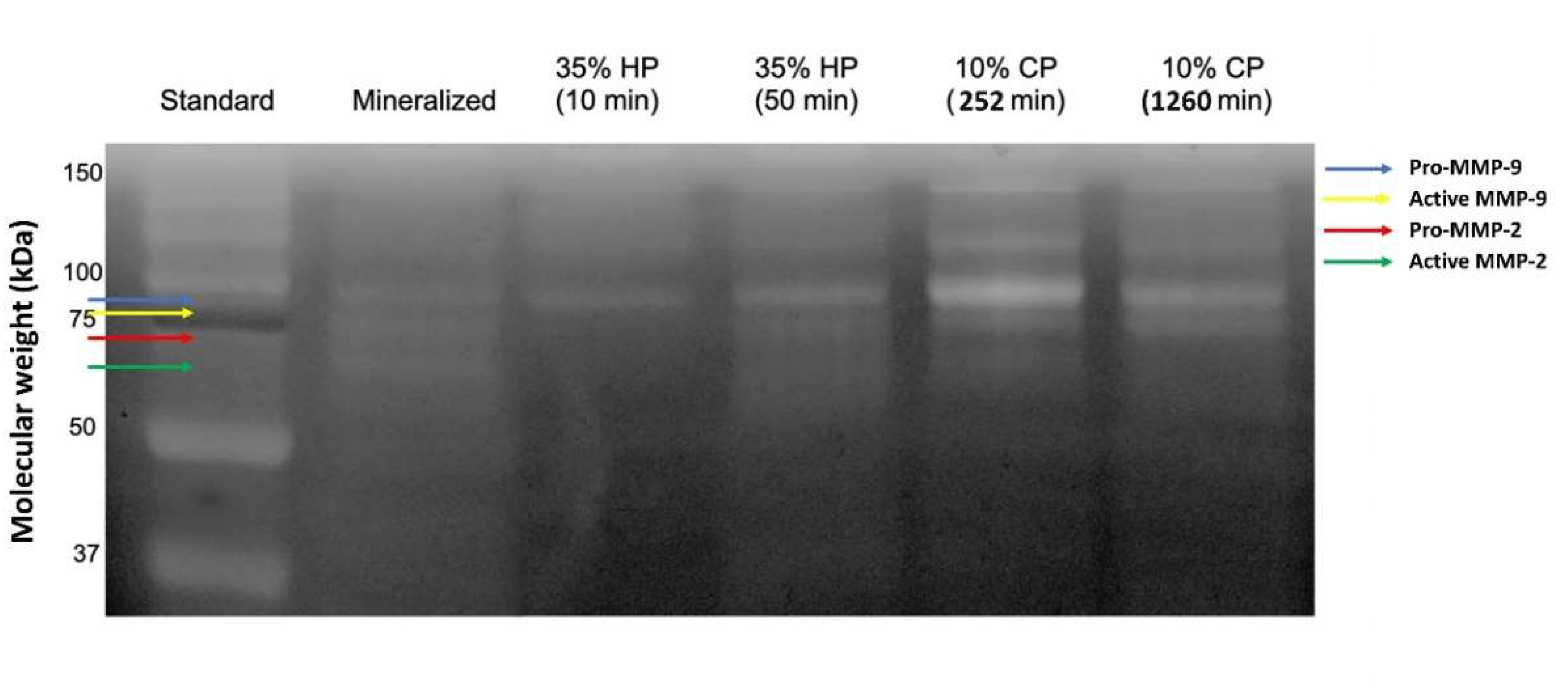
2.2. Gelatin Zymography
3. Discussion
4. Materials and Methods
4.1. In-Situ Zymography
4.2. Gelatin Zymography
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- ADA Council on Scientific Affairs. Tooth whitening/bleaching: Treatment considerations for dentists and their patients. Am. Dent. Assoc. 2010. Available online: http://www.bamatis.com/docs/HOD_whitening_rpt.pdf (accessed on 17 January 2022).
- Nutter, B.; Sharif, M.; Smith, A.; Brunton, P. A clinical study comparing the efficacy of light activated in-surgery whitening versus in-surgery whitening without light activation. J. Dent. 2013, 41S, e3–e7. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.; Roperto, R.; Akkus, A.; Akkus, O.; Palma-Dibb, R.; de Sousa-Neto, M. Effect of laser activated bleaching on the chemical stability and morphology of intracoronal dentin. Arch. Oral Biol. 2018, 86, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Joiner, A. The bleaching of teeth: A review of the literature. J. Dent. 2006, 34, 412–419. [Google Scholar] [CrossRef]
- Eimar, H.; Siciliano, R.; Abdallah, M.; Nader, S.; Amin, W.; Martinez, P.; Celemin, A.; Cerruti, M.; Tamimi, F. Hydrogen peroxide whitens teeth by oxidizing the organic structure. J. Dent. 2012, 40, e25–e33. [Google Scholar] [CrossRef] [PubMed]
- de Geus, J.; Wambier, L.; Kossatz, S.; Loguercio, A.; Reis, A. At-home vs in-office bleaching: A systematic review and meta-analysis. Oper. Dent. 2016, 41, 341–356. [Google Scholar] [CrossRef] [Green Version]
- Karaarslan, E.; Özmen, Z.; Aytac, F.; Bicakci, A.; Buldur, M.; Aydogan, L.; Hologlu, F.; Özkocak, B. Evaluation of biochemical changes in dental tissues after different office bleaching methods. Hum. Exp. Toxicol. 2019, 38, 389–397. [Google Scholar] [CrossRef]
- Toledano, M.; Yamauti, M.; Osorio, E.; Osorio, R. Bleaching agents increase metalloproteinases-mediated collagen degradation in dentin. J. Endod. 2011, 37, 1668–1672. [Google Scholar] [CrossRef]
- Breschi, L.; Martin, P.; Mazzoni, A.; Nato, F.; Carrilho, M.; Tjäderhane, L.; Visintini, E.; Cadenaro, M.; Tay, F.R.; Dorigo, E.D.S.; et al. Use of a specific MMP-inhibitor (galardin) for preservation of hybrid layer. Dent. Mater. 2010, 26, 571–578. [Google Scholar] [CrossRef] [Green Version]
- Mazzoni, A.; Apolonio, F.M.; Saboia, V.P.A.; Santi, S.; Angeloni, V.; Checchi, V.; Curci, R.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; et al. Carbodiimide inactivation of MMPs and effect on dentin bonding. J. Dent. Res. 2014, 93, 263–268. [Google Scholar] [CrossRef]
- Breschi, L.; Maravic, T.; Comba, A.; Cunha, S.R.; Loguercio, A.D.; Reis, A.; Hass, V.; Cadenaro, M.; Mancuso, E.; Mayer-Santos, E.; et al. Chlorhexidine preserves the hybrid layer in vitro after 10-years aging. Dent. Mater. 2020, 36, 672–680. [Google Scholar] [CrossRef]
- Maravic, T.; Mancuso, E.; Comba, A.; Checchi, V.; Generali, L.; Mazzitelli, C.; Josic, U.; Hass, V.; Reis, A.; Loguercio, A.D.; et al. Dentin cross-linking effect of carbodiimide after 5 years. J. Dent. Res. 2021, 100, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Mazzitelli, C.; Maravic, T.; Sebold, M.; Checchi, V.; Josic, U.; Breschi, L.; Mazzoni, A. Effect of shelf-life of a universal adhesive to dentin. Int. J. Adhes. Adhes. 2020, 102, 102673. [Google Scholar] [CrossRef]
- Maravic, T.; Breschi, L.; Paganelli, F.; Bonetti, G.A.; Martina, S.; Di Giorgio, G.; Bossù, M.; Polimeni, A.; Checchi, V.; Generali, L.; et al. Endogenous enzymatic activity of primary and permanent dentine. Materials 2021, 14, 4043. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Nascimento, F.; Carrilho, M.; Tersariol, I.; Papa, V.; Tjaderhane, L.; Di Lenarda, R.; Tay, F.; Pashley, D.; Breschi, L. MMP activity in the hybrid layer detected with in situ zymography. J. Dent. Res. 2012, 91, 467–472. [Google Scholar] [CrossRef] [Green Version]
- Mazzoni, A.; Breschi, L.; Carrilho, M.; Nascimento, F.D.; Orsini, G.; Ruggeri, A.; Gobbi, P.; Manzoli, L.; Tay, F.R.; Pashley, D.H.; et al. A review of the nature, role, and function of dentin non-collagenous proteins. Part II: Enzymes, serum proteins, and growth factors. Endod. Top. 2009, 21, 19–40. [Google Scholar] [CrossRef]
- Buzalaf, M.A.R.; Charone, S.; Tjäderhane, L. Role of host-derived proteinases in dentine caries and erosion. Caries Res. 2015, 49, 30–37. [Google Scholar] [CrossRef]
- Comba, A.; Maravic, T.; Valente, L.; Girlando, M.; Cunha, S.R.; Checchi, V.; Salgarello, S.; Tay, F.R.; Scotti, N.; Breschi, L.; et al. Effect of benzalkonium chloride on dentin bond strength and endogenous enzymatic activity. J. Dent. 2019, 85, 25–32. [Google Scholar] [CrossRef]
- Mazzitelli, C.; Maravic, T.; Mancuso, E.; Josic, U.; Generali, L.; Comba, A.; Mazzoni, A.; Breschi, L. Influence of the activation mode on long-term bond strength and endogenous enzymatic activity of dual-cure resin cements. Clin. Oral Investig. 2021, 26, 1683–1694. [Google Scholar] [CrossRef]
- Sato, C.; Rodrigues, F.A.; Garcia, D.M.; Vidal, C.M.P.; Pashley, D.H.; Tjäderhane, L.; Carrilho, M.R.; Nascimento, F.D.; Tersariol, I.L.S. Tooth bleaching increases dentinal protease activity. J. Dent. Res. 2013, 92, 187. [Google Scholar] [CrossRef] [Green Version]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [Green Version]
- Maravic, T.; Mazzoni, A.; Comba, A.; Scotti, N.; Checchi, V.; Breschi, L. How stable is dentin as a substrate for bonding? Curr. Oral Health Rep. 2017, 4, 248–257. [Google Scholar] [CrossRef]
- Breschi, L.; Maravic, T.; Cunha, S.R.; Comba, A.; Cadenaro, M.; Tjäderhane, L.; Pashley, D.H.; Tay, F.R.; Mazzoni, A. Dentin bonding systems: From dentin collagen structure to bond preservation and clinical applications. Dent. Mater. 2018, 34, 78–96. [Google Scholar] [CrossRef] [Green Version]
- Checchi, V.; Maravic, T.; Bellini, P.; Generali, L.; Consolo, U.; Breschi, L.; Mazzoni, A. The role of matrix metalloproteinases in periodontal disease. Int. J. Environ. Res. Public Health 2020, 17, 4923. [Google Scholar] [CrossRef]
- Mazzoni, A.; Tjäderhane, L.; Checchi, V.; Di Lenara, R.; Salo, T.; Tay, F.R.; Pashley, D.H.; Breschi, L. Role of dentin MMPs in caries progression and bond stability. J. Dent. Res. 2015, 94, 241–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulkala, M.; Tervahartiala, T.; Sorsa, T.; Larmas, M.; Salo, T.; Tjäderhane, L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch. Oral Biol. 2007, 52, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Ichinose, S.; Sadr, A.; Burrow, M.F.; Tagami, J. Localization of matrix metalloproteinases (MMPs-2, 8, 9 and 20) in normal and carious dentine. Aust. Dent. J. 2009, 54, 347–354. [Google Scholar] [CrossRef]
- Mazzoni, A.; Mannello, F.; Tay, F.R.; Tonti, G.A.M.; Papa, S.; Mazzotti, G.; Di Lenarda, R.; Pashley, D.H.; Breschi, L. Zymographic analysis and characterization of MMP-2 and -9 forms in human sound dentin. J. Dent. Res. 2007, 86, 436–440. [Google Scholar] [CrossRef]
- Mazzoni, A.; Papa, V.; Nato, F.; Carrilho, M.; Tjäderhane, L.; Ruggeri, A.; Gobbi, P.; Mazzotti, G.; Tay, F.R.; Pashley, D.H.; et al. Immunohistochemical and biochemical assay of MMP-3 in human dentine. J. Dent. 2011, 39, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Mazzoni, A.; Maravić, T.; Tezvergil-Mutluay, A.; Tjäderhane, L.; Scaffa, P.M.C.; Seseogullari-Dirihan, R.; Bavelloni, A.; Gobbi, P.; Pashley, D.H.; Tay, F.R.; et al. Biochemical and immunohistochemical identification of MMP-7 in human dentin. J. Dent. 2018, 79, 90–95. [Google Scholar] [CrossRef] [Green Version]
- Mazzoni, A.; Scaffa, P.; Carrilho, M.; Tjäderhane, L.; Di Lenarda, R.; Polimeni, A.; Tezvergil-Mutluay, A.; Tay, F.R.; Pashley, D.H.; Breschi, L. Effects of etch-and-rinse and self-etch adhesives on dentin MMP-2 and MMP-9. J. Dent. Res. 2013, 92, 82–86. [Google Scholar] [CrossRef] [Green Version]
- Cunha, S.R.; Maravic, T.; Comba, A.; Ramos, P.A.; Tay, F.R.; Pashley, D.H.; Rodrigues, E.; Cecília, A.; Mazzoni, A.; Breschi, L. In vivo and in vitro radiotherapy increased dentin enzymatic activity. J. Dent. 2020, 100, 103429. [Google Scholar] [CrossRef] [PubMed]
- Baena, E.; Cunha, S.R.; Maravić, T.; Comba, A.; Paganelli, F.; Alessandri-Bonetti, G.; Ceballos, L.; Tay, F.R.; Breschi, L.; Mazzoni, A. Effect of chitosan as a cross-linker on matrix metalloproteinase activity and bond stability with different adhesive systems. Mar. Drugs 2020, 18, 263. [Google Scholar] [CrossRef] [PubMed]
- Comba, A.; Maravić, T.; Villalta, V.; Tozzola, S.; Mazzitelli, C.; Checchi, V.; Mancuso, E.; Scotti, N.; Tay, F.R.; Breschi, L.; et al. Effect of an ethanol cross-linker on universal adhesive. Dent. Mater. 2020, 36, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Maravic, T.; Breschi, L.; Comba, A.; Cunha, S.R.; Angeloni, V.; Nucci, C.; Hebling, J.; Pashley, D.; Tay, F.; Mazzoni, A. Experimental use of an acrolein-based primer as collagen cross-linker for dentine bonding. J. Dent. 2017, 68, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Mancuso, E.; Comba, A.; Mazzitelli, C.; Maravic, T.; Josic, U.; Del Bianco, F.; Tay, F.R.; Breschi, L.; Mazzoni, A. Bonding to dentin using an experimental zirconium oxynitrate etchant. J. Dent. 2021, 108, 103641. [Google Scholar] [CrossRef]
- Maravić, T.; Baena, E.; Mazzitelli, C.; Josić, U.; Mancuso, E.; Checchi, V.; Generali, L.; Ceballos, L.; Breschi, L.; Mazzoni, A. Endogenous enzymatic activity in dentin treated with a chitosan primer. Int. J. Mol. Sci. 2021, 22, 8852. [Google Scholar] [CrossRef]
- Löffek, S.; Schilling, O.; Franzke, C.W. Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef] [Green Version]
- Klarić, E.; Marcius, M.; Ristic, M.; Sever, I.; Prskalo, K.; Tarle, Z. Surface changes of enamel and dentin after two different bleaching procedures. Acta Clin. Croat. 2013, 52, 419–429. [Google Scholar]
- Rodríguez-Martínez, J.; Valiente, M.; Sánchez-Martín, M. Tooth whitening: From the established treatments to novel approaches to prevent side effects. J. Esthet. Restor. Dent. 2019, 31, 431–440. [Google Scholar] [CrossRef]
- Turco, G.; Cadenaro, M.; Maravić, T.; Frassetto, A.; Marsich, E.; Mazzoni, A.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; Breschi, L. Release of ICTP and CTX telopeptides from demineralized dentin matrices: Effect of time, mass and surface area. Dent. Mater. 2018, 34, 452–459. [Google Scholar] [CrossRef]
- Turco, G.; Frassetto, A.; Fontanive, L.; Mazzoni, A.; Cadenaro, M.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; Breschi, L. Occlusal loading and cross-linking effects on dentin collagen degradation in physiological conditions. Dent. Mater. 2016, 32, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Uchida, T.; Yoshie, T.; Mizote, Y.; Ishikawa, F.; Katsuyama, M.; Shibanuma, M. A mitochondrial ROS pathway controls matrix metalloproteinase 9 levels and invasive properties in RAS-activated cancer cells. FEBS J. 2019, 286, 459–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viappiani, S.; Nicolescu, A.C.; Holt, A.; Sawicki, G.; Crawford, B.D.; León, H.; van Mulligen, T.; Schulz, R. Activation and modulation of 72 kDa matrix metalloproteinase-2 by peroxynitrite and glutathione. Biochem. Pharmacol. 2009, 77, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.; Pérez de la Lastra, J.; Plou, F.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Ezraty, B.; Gennaris, A.; Barras, F.; Collet, J.-F. Oxidative stress, protein damage and repair in bacteria. Nat. Rev. Microbiol. 2017, 15, 385–396. [Google Scholar] [CrossRef]
- Rotstein, I.; Lehr, Z.; Gedalia, I. Effect of bleaching agents on inorganic components of human dentin and cementum. J. Endod. 1992, 18, 290–293. [Google Scholar] [CrossRef]
- Tredwin, C.; Naik, S.; Lewis, N.; Scully, C. Hydrogen peroxide tooth-whitening (bleaching) products: Review of adverse effects and safety issues. Br. Dent. J. 2006, 200, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, R.M.; Tjäderhane, L.; Manso, A.P.; Carrilho, M.R.; Carvalho, C.A.R. Dentin as a bonding substrate. Endod. Top. 2012, 21, 62–88. [Google Scholar] [CrossRef]
- Tjäderhane, L.; Carrilho, M.R.; Breschi, L.; Tay, F.R.; Pashley, D.H. Dentin basic structure and composition-an overview. Endod. Top. 2009, 20, 3–29. [Google Scholar] [CrossRef]

| Method of Application | Number of Sessions | ||
|---|---|---|---|
| GROUP 1 | Hydrogen peroxide 35% | Application of 50 min per session (the gel was renewed every 15 min) | 4 * |
| GROUP 2 | Carbamide peroxide 10% | Application of 180 min per session | 21 * |
| GROUP 3 | Control group | No treatment | - |
| Products | Description |
|---|---|
| 35% hydrogen peroxide gel (Fórmula e Ação, São Paulo, Brazil) | 35% hydrogen peroxide, thickener, vegetable extracts, amide, sequestering agent, glycol, and water. pH = 4.5. |
| 10% carbamide peroxide gel (Fórmula e Ação) | 10% carbamide peroxide, 3% potassium nitrate, 0.24% blood fluoride, humectant, thickener, preservative, mint aroma, and purified water qsp. pH = 6.8. |
| Products | Description |
|---|---|
| 35% hydrogen peroxide liquid (Fórmula e Ação) | 35% hydrogen peroxide, vegetable extracts, sequestering agent, glycol, and water. pH = 4.5. |
| 10% carbamide peroxide liquid (Fórmula e Ação) | 10% carbamide peroxide, 3% potassium nitrate, 0.24% sodium fluoride, humectant, preservative, and purified water qsp. pH = 6.8. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayer-Santos, E.; Maravic, T.; Comba, A.; Freitas, P.M.; Marinho, G.B.; Mazzitelli, C.; Mancuso, E.; Scotti, N.; Florenzano, F.; Breschi, L.; et al. The Influence of Different Bleaching Protocols on Dentinal Enzymatic Activity: An In Vitro Study. Molecules 2022, 27, 1684. https://doi.org/10.3390/molecules27051684
Mayer-Santos E, Maravic T, Comba A, Freitas PM, Marinho GB, Mazzitelli C, Mancuso E, Scotti N, Florenzano F, Breschi L, et al. The Influence of Different Bleaching Protocols on Dentinal Enzymatic Activity: An In Vitro Study. Molecules. 2022; 27(5):1684. https://doi.org/10.3390/molecules27051684
Chicago/Turabian StyleMayer-Santos, Eric, Tatjana Maravic, Allegra Comba, Patricia Moreira Freitas, Giovanna Bueno Marinho, Claudia Mazzitelli, Edoardo Mancuso, Nicola Scotti, Federica Florenzano, Lorenzo Breschi, and et al. 2022. "The Influence of Different Bleaching Protocols on Dentinal Enzymatic Activity: An In Vitro Study" Molecules 27, no. 5: 1684. https://doi.org/10.3390/molecules27051684






