Toxicity of Selected Monoterpenes and Essential Oils Rich in These Compounds
Abstract
:1. Introduction
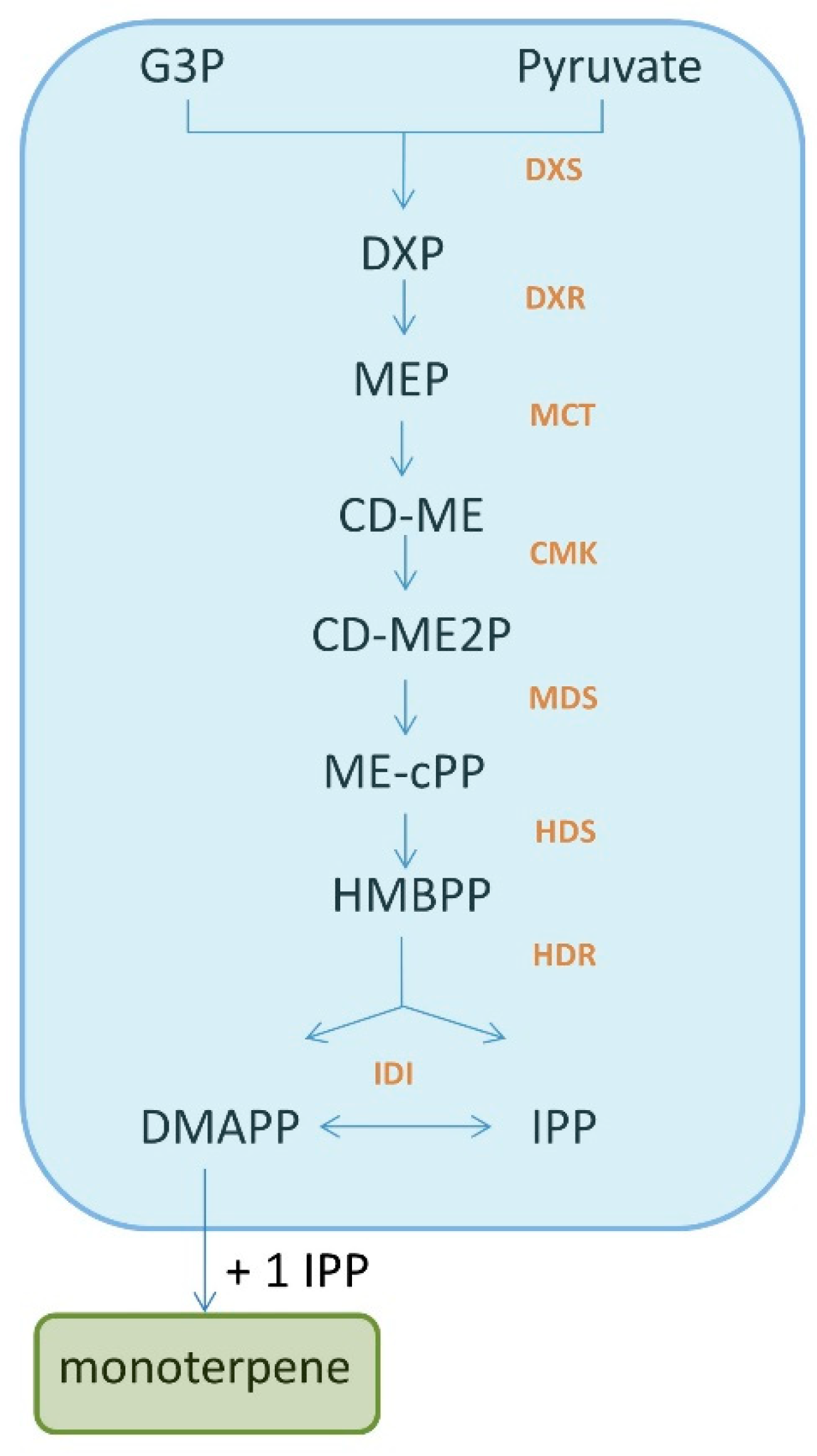
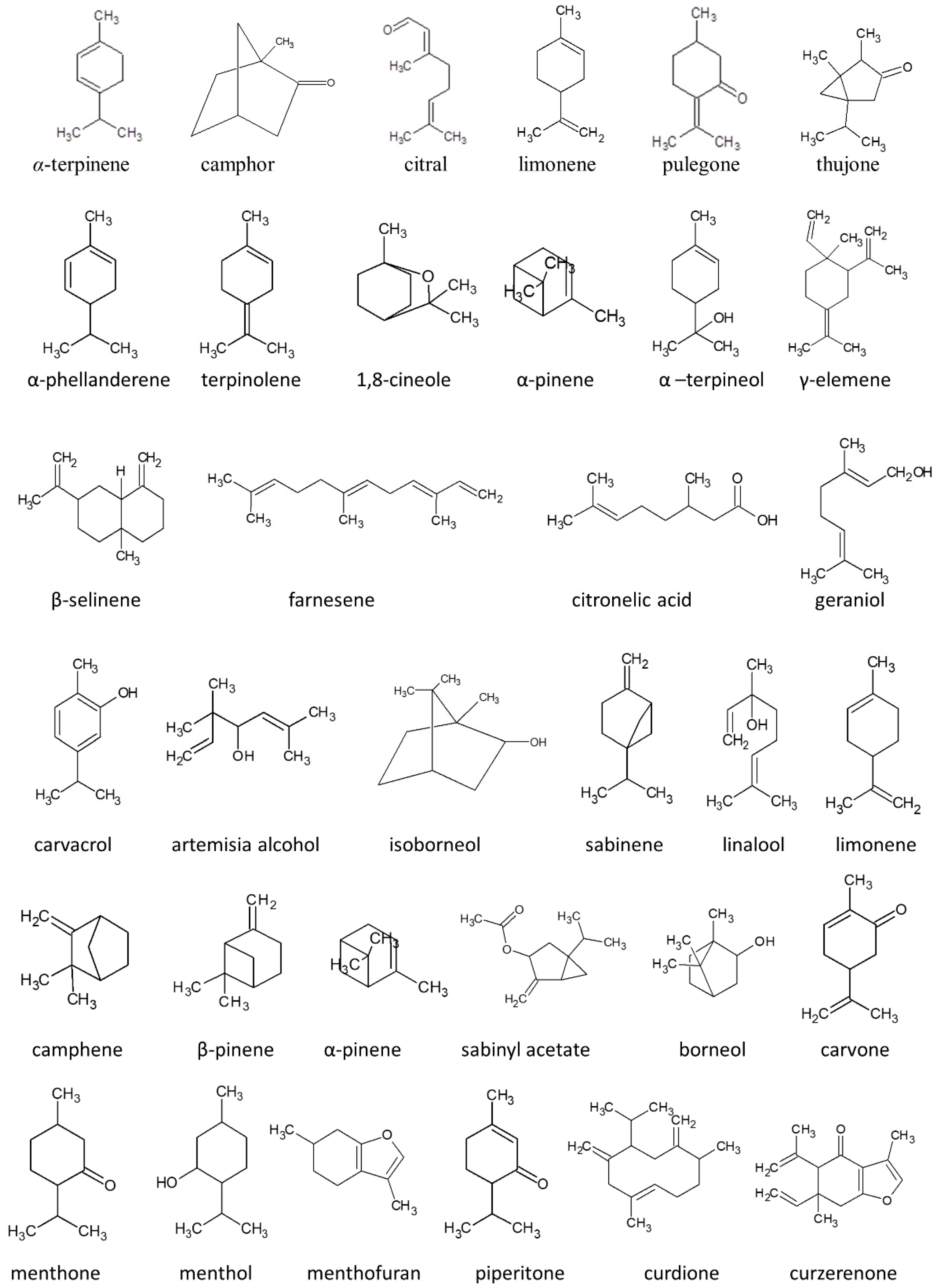
2. Monoterpenes: A Group of Isoprene Derivatives
3. Toxicity of Selected Monoterpenes
3.1. α-Terpinene
3.2. Camphor
3.3. Citral
3.4. Limonene
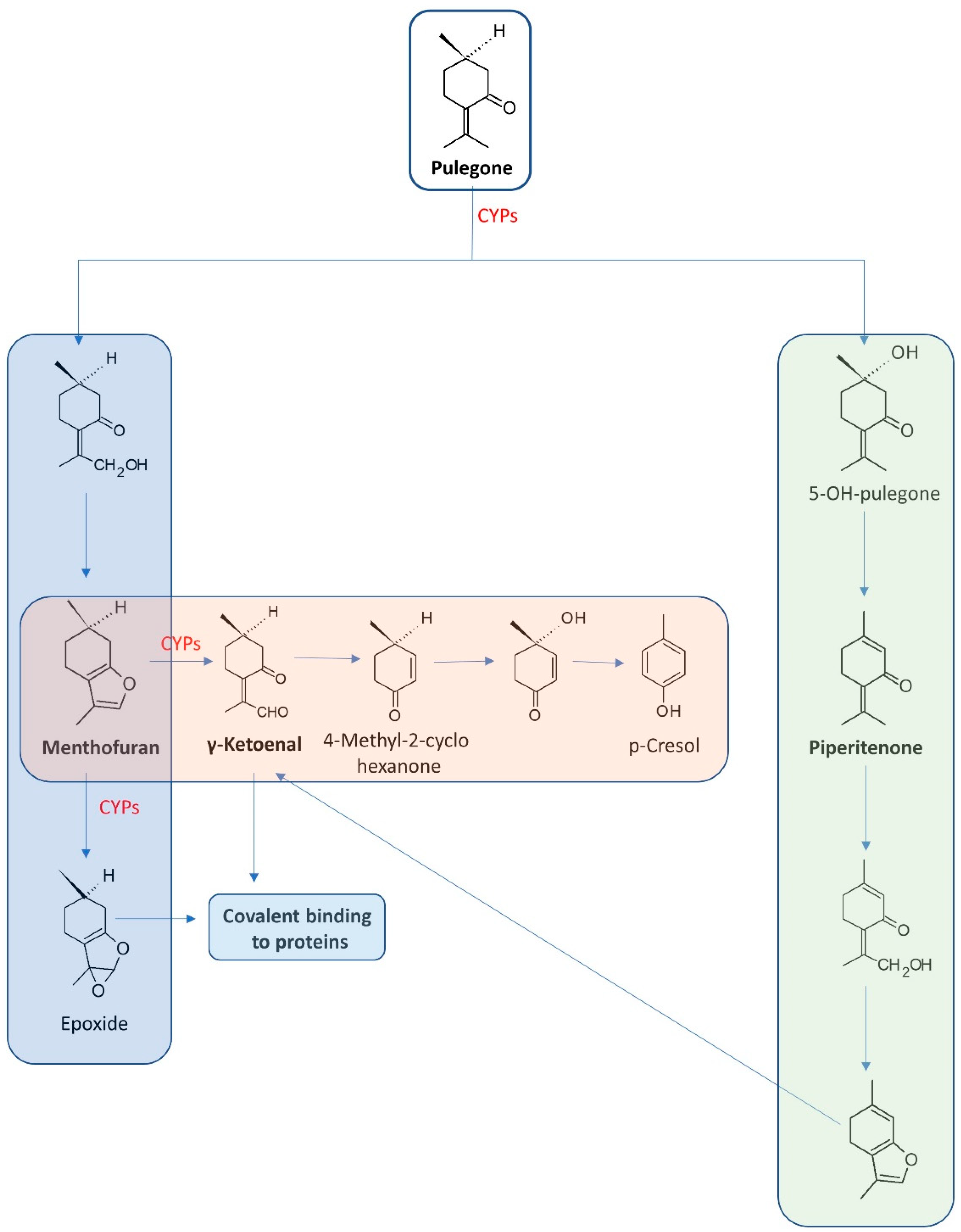
3.5. Pulegone
3.6. Thujone
4. Plants and Their Essential Oils Rich in Monoterpenes
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Singh, H.; Bhardwaj, N.; Bhardwaj, S.K.; Khatri, M.; Kim, K.-H.; Peng, W. An exploration on the toxicity mechanisms of phytotoxins and their potential utilities. Crit. Rev. Environ. Sci. Technol. 2020, 52, 395–435. [Google Scholar] [CrossRef]
- Wojtunik, K.A.; Ciesla, L.M.; Waksmundzka-Hajnos, M. Model Studies on the Antioxidant Activity of Common Terpenoid Constituents of Essential Oils by Means of the 2,2-Diphenyl-1-picrylhydrazyl Method. J. Agric. Food Chem. 2014, 62, 9088–9094. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Błajet, M.; Feder-Kubis, M. Monoterpenes and Their Derivatives—Recent Development in Biological and Medical Applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Sharma, V.; Thakur, A. PhytotoxinsA mini review. J. Pharmacogn Phytochem. 2018, 7, 2705–2708. [Google Scholar]
- Khodaei, N.; Nguyen, M.M.; Mdimagh, A.; Bayen, S.; Karboune, S. Compositional diversity and antioxidant properties of essential oils: Predictive models. LWT 2021, 138, 8110684. [Google Scholar] [CrossRef]
- Srivastava, S.; Lal, R.; Maurya, R.; Mishra, A.; Yadav, A.K.; Pandey, G.; Rout, P.; Chanotiya, C. Chemical diversity of essential oil among basil genotypes (Ocimum viride Willd.) across the years. Ind. Crops Prod. 2021, 173, 114153. [Google Scholar] [CrossRef]
- Maggio, A.; Rosselli, S.; Bruno, M. Essential Oils and Pure Volatile Compounds as Potential Drugs in Alzheimer’s Disease Therapy: An Updated Review of the Literature. Curr. Pharm. Des. 2016, 22, 4011–4027. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Rudkowska, M.; Kasprzak-Drozd, K.; Oniszczuk, A.; Borowicz-Reutt, K. Activity of Selected Group of Monoterpenes in Alzheimer’s Disease Symptoms in Experimental Model Studies—A Non-Systematic Review. Int. J. Mol. Sci. 2021, 22, 7366. [Google Scholar] [CrossRef]
- Wang, Q.; Quan, S.; Xiao, H. Towards efficient terpenoid biosynthesis: Manipulating IPP and DMAPP supply. Bioresour. Bioprocess. 2019, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.-D.; Jiang, L.-L.; Li, H.-Y.; Yan, P.-F.; Zhang, Y.-L. Chemical Components and Pharmacological Activities of Terpene Natural Products from the Genus Paeonia. Molecules 2016, 21, 1362. [Google Scholar] [CrossRef]
- Tetali, S.D. Terpenes and isoprenoids: A wealth of compounds for global use. Planta 2019, 249, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.A.; Kasprzak, K.; Oniszczuk, T.; Oniszczuk, A. Natural Monoterpenes: Much More than Only a Scent. Chem. Biodivers. 2019, 16, e1900434. [Google Scholar] [CrossRef] [PubMed]
- Hausch, B.J.; Lorjaroenphon, Y.; Cadwallader, K.R. Flavor Chemistry of Lemon-Lime Carbonated Beverages. J. Agric. Food Chem. 2015, 63, 112–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Ma, X.-W.; Zhan, R.-L.; Wu, H.-X.; Yao, Q.-S.; Xu, W.-T.; Luo, C.; Zhou, Y.-G.; Liang, Q.-Z.; Wang, S.-B. Profiling of volatile fragrant components in a mini-core collection of mango germplasms from seven countries. PLoS ONE 2017, 12, e0187487. [Google Scholar] [CrossRef]
- Brahim, M.A.S.; Fadli, M.; Hassani, L.; Boualy, B.; Markouk, M.; Bekkouche, K.; Abbad, A.; Ali, M.A.; Larhsini, M. Chenopodium ambrosioides var. ambrosioides used in Moroccan traditional medicine can enhance the antimicrobial activity of conventional antibiotics. Ind. Crops Prod. 2015, 71, 37–43. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.D.F.; Mourão, R.H.V.; Da Silva, L.V.F.; Monteiro, S.G. Trypanocidal action of Lippia alba and Lippia origanoides essential oils against Trypanosoma evansi in vitro and in vivo used mice as experimental model. J. Parasit Dis. 2017, 41, 345–351. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A.; Targowska-Duda, K.; Klimek, K.; Ginalska, G.; Jóźwiak, K.; Waksmundzka-Hajnos, M.; Cieśla, Ł. Volatile terpenoids as potential drug leads in Alzheimer’s disease. Open Chem. 2017, 15, 332–343. [Google Scholar] [CrossRef] [Green Version]
- Araujo, I.; Souza, C.; De-Carvalho, R.; Kuriyama, S.; Rodrigues, R.; Vollmer, R.; Alves, E.; Paumgartten, F.J.R. Study of the embryofoetotoxicity of α-terpinene in the rat. Food Chem. Toxicol. 1996, 34, 477–482. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Grando, T.H.; Sagrillo, M.; De Brum, G.F.; Nascimento, K.; Peres, D.S.; Maciel, M.F.; Silveira, S.O.; Da Luz, S.C.; et al. Memory deficit, toxic effects and activity of Na+, K+-ATPase and NTPDase in brain of Wistar rats submitted to orally treatment with alpha-terpinene. Environ. Toxicol. Pharmacol. 2016, 46, 1–8. [Google Scholar] [CrossRef]
- Wyse, A.T.; Streck, E.L.; Barros, S.V.; Brusque, A.M.; Zugno, A.I.; Wajner, M. Methylmalonate administration decreases Na+,K+-ATPase activity in cerebral cortex of rats. Neuroreport 2000, 11, 2331–2334. [Google Scholar] [CrossRef]
- Moseley, A.E.; Williams, M.; Schaefer, T.L.; Bohanan, C.S.; Neumann, J.C.; Behbehani, M.M.; Vorhees, C.; Lingrel, J.B. Deficiency in Na,K-ATPase Isoform Genes Alters Spatial Learning, Motor Activity, and Anxiety in Mice. J. Neurosci. 2007, 27, 616–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldissera, M.D.; Souza, C.F.; Dolci, G.S.; Grando, T.H.; Sagrillo, M.; Vaucher, R.A.; Da Luz, S.C.; Silveira, S.O.; Duarte, M.M.; Duarte, T.; et al. Monoterpene alpha-terpinene induced hepatic oxidative, cytotoxic and genotoxic damage is associated to caspase activation in rats. J. Appl. Biomed. 2017, 15, 187–195. [Google Scholar] [CrossRef]
- Herraiz-Peñalver, D.; Usano-Alemany, J.; Cuadrado, J.; Jordan, M.J.; Lax, V.; Sotomayor, J.A.; Palá-Paúl, J. Essential oil composition of wild populations of Salvia lavandulifolia Vahl. from Castilla-La Mancha (Spain). Biochem. Syst. Ecol. 2010, 38, 1224–1230. [Google Scholar] [CrossRef]
- Santos, C.D.; Cabot, J.C. Persistent effects after camphor ingestion: A case report and literature review. J. Emerg. Med. 2015, 48, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Manoguerra, A.S.; Erdman, A.R.; Wax, P.M.; Nelson, L.; Caravati, E.M.; Cobaugh, D.J.; Chyka, P.A.; Olson, K.R.; Booze, L.L.; Woolf, A.D.; et al. Camphor Poisoning: An evidence-based practice guideline for out-of-hospital management. Clin. Toxicol 2006, 44, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Khine, H.; Weiss, D.; Graber, N.; Hoffman, R.S.; Esteban-Cruciani, N.; Avner, J.R. A cluster of children with seizures caused by camphor poisoning. Pediatrics 2009, 123, 1269–1272. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.F.; Brown, A.L.; Arnold, W.C.; Byrne, W.J. Chronic camphor ingestion mimicking Reye’s syndrome. Gastroenterology 1983, 84, 394–398. [Google Scholar] [CrossRef]
- Völkel, W.; Colnot, T.; Schauer, U.M.; Broschard, T.H.; Dekant, W. Toxicokinetics and biotransformation of 3-(4-methylbenzylidene)camphor in rats after oral administration. Toxicol. Appl. Pharmacol. 2006, 216, 331–338. [Google Scholar] [CrossRef]
- Park, T.; Seo, H.-K.; Kang, B.-J.; Kim, K.-T. Noncompetitive inhibition by camphor of nicotinic acetylcholine receptors. Biochem. Pharmacol. 2001, 61, 787–793. [Google Scholar] [CrossRef]
- Oladeji, O.; Adelowo, F.; Ayodele, D.; Kehinde, A.; Abraham, O. Phytochemistry and pharmacological activities of Cymbopogon citratus: A review. Sci. Afr. 2019, 6, e00137. [Google Scholar] [CrossRef]
- Saddiq, A.; Khayyat, S. Chemical and antimicrobial studies of monoterpene: Citral. Pestic Biochem Physiol. 2010, 98, 89. [Google Scholar] [CrossRef]
- Nogueira, A.C.M.; Carvalho, R.R.; Souza, C.A.; Chahoud, I.; Paumgartten, F.J. Study on the embryofeto-toxicity of citral in the rat. Toxicology 1995, 96, 105–113. [Google Scholar] [CrossRef]
- Duerksen-Hughes, P.J.; Yang, J.; Ozcan, O. p53 induction as a genotoxic test for twenty-five chemicals undergoing in vivo carcinogenicity testing. Environ. Health Perspect. 1999, 107, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.C.S.; Silva, L.K.; Queiroz, T.B.; Marques, E.S.; Hiruma-Lima, C.; Gaivao, I.; Maistro, E.L. Citral presents cytotoxic and genotoxic effects in human cultured cells. Drug Chem. Toxicol. 2020, 43, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Jothiramajayam, M.; Ghosh, M.; Mukherjee, A. Evaluation of toxicity of essential oils palmarosa, citronella, lemongrass and vetiver in human lymphocytes. Food Chem Toxicol. 2014, 68, 71–77. [Google Scholar] [CrossRef]
- Porto, M.D.P.; Da Silva, G.N.; Luperini, B.C.O.; Bachiega, T.F.; Marcondes, J.P.D.C.; Sforcin, J.M.; Salvadori, D.M.F. Citral and eugenol modulate DNA damage and pro-inflammatory mediator genes in murine peritoneal macrophages. Mol. Biol. Rep. 2014, 41, 7043–7051. [Google Scholar] [CrossRef]
- European Chemicals Agency. Substance Infocard. Citral. Available online: https://echa.europa.eu/pl/substance-information/-/substanceinfo/100.023.994 (accessed on 22 February 2022).
- Api, A.; Belsito, D.; Biserta, S.; Botelho, D.; Bruze, M.; Burton, G.; Buschmann, J.; Cancellieri, M.; Dagli, M.; Date, M.; et al. RIFM fragrance ingredient safety assessment, citral, CAS Registry Number 5392-40-5. Food Chem. Toxicol. 2020, 141, 111339. [Google Scholar] [CrossRef]
- Sun, J. D-Limonene: Safety and clinical applications. Altern. Med. Rev. 2007, 12, 259–264. [Google Scholar]
- Bacanlı, M.; Başaran, A.A.; Başaran, N. Chapter 32—Effects and Usage of a Citrus Compound, Limonene. In Polyphenols: Prevention and Treatment of Human Disease (Second Edition); Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 419–424. [Google Scholar] [CrossRef]
- Rolseth, V.; Djurhuus, R.; Svardal, A.M. Additive toxicity of limonene and 50% oxygen and the role of glutathione in detoxification in human lung cells. Toxicology 2002, 170, 75–88. [Google Scholar] [CrossRef]
- Nikfar, S.; Behboudi, A.F. Limonene. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 78–82. [Google Scholar] [CrossRef]
- Whysner, J.; Williams, G.M. d-limonene mechanistic data and risk assessment: Absolute species-specific cytotoxicity, enhanced cell proliferation, and tumor promotion. Pharmacol. Ther. 1996, 71, 127–136. [Google Scholar] [CrossRef]
- Crowell, P.L. Prevention and therapy of cancer by dietary monoterpenes. J. Nutr. 1999, 129, 775S–778S. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency. Substance Infocard. (R)-p-mentha-1,8-diene. Available online: https://echa.europa.eu/pl/substance-information/-/substanceinfo/100.025.284 (accessed on 22 February 2022).
- Raza, A.; Muhammad, F.; De Sousa, D.P.; Khaliq, T.; Aslam, B.; Andrade, L.; Bashir, S.; Anwar, M.I.; Shahid, M.; Qamar, M. In vitro and in vivo toxicological evaluations of methyl ferulate, methyl p-coumarate, and pulegone 1,2-epoxide. Pharm. Biol. 2016, 54, 523–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, A.; Park, H.-J.; Abdul, Q.A.; Jung, H.A.; Choi, J.S. Pulegone Exhibits Anti-inflammatory Activities through the Regulation of NF-κB and Nrf-2 Signaling Pathways in LPS-stimulated RAW 264.7 cells. Nat. Prod. Sci. 2018, 24, 28–35. [Google Scholar] [CrossRef]
- Hilfiger, L.; Triaux, Z.; Marcic, C.; Héberlé, E.; Emhemmed, F.; Darbon, P.; Marchioni, E.; Petitjean, H.; Charlet, A. Anti-hyperalgesic Properties of Menthol and Pulegone. Front. Pharmacol. 2021, 12, 03413892. [Google Scholar] [CrossRef]
- Gordon, P.; Khojasteh, S.C. A decades-long investigation of acute metabolism-based hepatotoxicity by herbal constituents: A case study of pennyroyal oil. Drug Metab. Rev. 2015, 47, 12–20. [Google Scholar] [CrossRef]
- Chen, L.-J.; Lebetkin, E.H.; Burka, L.T. Metabolism of (R)-(+)-menthofuran in Fischer-344 rats: Identification of sulfonic acid metabolites. Drug Metab. Dispos. 2003, 31, 1208–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.-W.; Serag, E.S.; Sneed, K.B.; Zhou, S.-F. Herbal bioactivation, molecular targets and the toxicity relevance. Chem. Biol. Interact. 2011, 192, 161–176. [Google Scholar] [CrossRef]
- Dosoky, N.; Setzer, W. Maternal Reproductive Toxicity of Some Essential Oils and Their Constituents. Int. J. Mol. Sci. 2021, 22, 2380. [Google Scholar] [CrossRef]
- Bakerink, J.A.; Gospe, S.M.; Dimand, R.J.; Eldridge, M.W. Multiple organ failure after ingestion of pennyroyal oil from herbal tea in two infants. Pediatrics 1996, 98, 944–947. [Google Scholar] [CrossRef]
- Andersen, A. Final report on the safety assessment of sodium p -chloro- m -cresol, p -chloro- m -cresol, chlorothymol, mixed cresols, m -cresol, o -cresol, p -cresol, isopropyl cresols, thymol, o -cymen-5-ol, and carvacrol1. Int. J. Toxicol. 2006, 25, 29–127. [Google Scholar] [CrossRef]
- Zárybnický, T.; Matoušková, P.; Lancošová, B.; Šubrt, Z.; Skálová, L.; Boušová, I. Inter-Individual Variability in Acute Toxicity of R-Pulegone and R-Menthofuran in Human Liver Slices and Their Influence on miRNA Expression Changes in Comparison to Acetaminophen. Int. J. Mol. Sci. 2018, 19, 1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Medicines Agency. Public Statement on the Use of Herbal Medicinal Products Containing Pulegone and Menthofuran; 12 EMA/HMPC/138386/2005 Rev. 1.; European Medicines Agency: Amsterdam, The Netherlands, 2016.
- El Euch, S.K.; Hassine, D.; Cazaux, S.; Bouzouita, N.; Bouajila, J. Salvia officinalis essential oil: Chemical analysis and evaluation of anti-enzymatic and antioxidant bioactivities. S. Afr. J. Bot. 2019, 120, 253–260. [Google Scholar] [CrossRef]
- Moacă, E.-A.; Pavel, I.Z.; Danciu, C.; Crăiniceanu, Z.; Minda, D.; Ardelean, F.; Antal, D.S.; Ghiulai, R.; Cioca, A.; Derban, M.; et al. Romanian Wormwood (Artemisia absinthium L.): Physicochemical and Nutraceutical Screening. Molecules 2019, 24, 3087. [Google Scholar] [CrossRef] [Green Version]
- Lachenmeier, D.W.; Walch, S.G.; Padosch, S.A.; Kröner, L.U. Absinthe—A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 365–377. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Uebelacker, M. Risk assessment of thujone in foods and medicines containing sage and wormwood—Evidence for a need of regulatory changes? Regul. Toxicol. Pharmacol. 2010, 58, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Committee on Herbal Medicinal Products (HMPC). European Union Herbal Monograph on Artemisia Absinthium L., Herba; European Medicines Agency: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Pelkonen, O.; Abass, K.; Wiesner, J. Thujone and thujone-containing herbal medicinal and botanical products: Toxicological assessment. Regul. Toxicol. Pharmacol. 2013, 65, 100–107. [Google Scholar] [CrossRef]
- Németh, Z.; Nguyen, H.T. Thujone, a widely debated volatile compound: What do we know about it? Phytochem. Rev. 2020, 19, 405–423. [Google Scholar] [CrossRef]
- Siveen, K.; Kuttan, G. Augmentation of humoral and cell mediated immune responses by Thujone. Int. Immunopharmacol. 2011, 11, 1967–1975. [Google Scholar] [CrossRef]
- Waidyanatha, S.; Johnson, J.D.; Hong, S.P.; Robinson, V.G.; Gibbs, S.; Graves, S.W.; Hooth, M.J.; Smith, C.S. Toxicokinetics of α-thujone following intravenous and gavage administration of α-thujone or α- and β-thujone mixture in male and female F344/N rats and B6C3F1 mice. Toxicol. Appl. Pharmacol. 2013, 271, 216–228. [Google Scholar] [CrossRef]
- Nikolić, B.; Vasilijević, B.; Mitić-Ćulafić, D.; Vuković-Gačić, B.; Knežević-Vukćević, J. Comparative study of genotoxic, antigenotoxic and cytotoxic activities of monoterpenes camphor, eucalyptol and thujone in bacteria and mammalian cells. Chem. Biol. Interact. 2015, 242, 263–271. [Google Scholar] [CrossRef]
- European Medicines Agency. Public Statement on the Use of Herbal Medicinal Products Containing Thujone; EMA/HMPC/732886/2010; European Medicines Agency: Amsterdam, The Netherlands, 2011.
- Surendran, S.; Qassadi, F.; Surendran, G.; Lilley, D.; Heinrich, M. Myrcene—What Are the Potential Health Benefits of This Flavouring and Aroma Agent? Front. Nutr. 2021, 8, 699666. [Google Scholar] [CrossRef] [PubMed]
- Tisserand, R. Essential Oil Safety: A Guide for Health Care Professionals, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Ahangarpour, A.; Najimi, S.A.; Farbood, Y. Effects of Vitex agnus-castus fruit on sex hormones and antioxidant indices in a d-galactose-induced aging female mouse model. J. Chin. Med. Assoc. 2016, 79, 589–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Medicines Agency. Assessment Report on Vitex Agnus-Castus L., Fructus; EMA/HMPC/606741/2018; European Medicines Agency: Amsterdam, The Netherlands, 2018.
- Horváth, G.; Szabó, L.G.; Héthelyi, É.; Lemberkovics, E. Essential Oil Composition of Three Cultivated Thymus Chemotypes from Hungary. J. Essent. Oil Res. 2006, 18, 315–317. [Google Scholar] [CrossRef]
- Southwell, I.A.; Russell, M.; Smith, R.L.; Archer, D.W. Backhousia citriodora F. Muell. (Myrtaceae), A Superior Source of Citral. J. Essent Oil Res. 2000, 12, 735–741. [Google Scholar] [CrossRef]
- Southwell, I. Backhousia citriodora F. Muell. (Lemon Myrtle), an Unrivalled Source of Citral. Foods 2021, 10, 1596. [Google Scholar] [CrossRef]
- Lachenmeier, D.W. Wormwood (Artemisia absinthium L.)—A curious plant with both neurotoxic and neuroprotective properties? J. Ethnopharmacol. 2010, 131, 224–227. [Google Scholar] [CrossRef] [Green Version]
- Usano-Alemany, J.; Herraiz-Peñalver, D.; Cuadrado, J.; Díaz, S.; Santa-Cruz, M.; Palá-Paúl, J. Seasonal Variation of the Essential Oils of Salvia lavandulifolia: Antibacterial Activity. J. Essent. Oil Bear. Plants 2012, 15, 195–203. [Google Scholar] [CrossRef]
- Porres-Martínez, M.; Accame, M.E.C.; Cuadrado, M.P.G.-S. Pharmacological activity of Salvia lavandulifolia and chemical components of its essential oil. A Review. Lazaroa 2013, 34, 237–254. [Google Scholar] [CrossRef] [Green Version]
- Amri, I.; DE Martino, L.; Marandino, A.; Lamia, H.; Mohsen, H.; Scandolera, E.; De Feo, V.; Mancini, E. Chemical Composition and Biological Activities of the Essential Oil from Artemisia herba-alba Growing Wild in Tunisia. Nat. Prod. Commun. 2013, 8, 407–410. [Google Scholar] [CrossRef] [Green Version]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. Genus: A Review of Bioactive Essential Oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef] [Green Version]
- Malekmohammad, K.; Rafieian-Kopaei, M.; Sardari, S.; Sewell, R.D.E. Toxicological effects of Mentha × piperita (peppermint): A review. Toxin Rev. 2021, 40, 445–459. [Google Scholar] [CrossRef]
- European Medicines Agency. Assessment Report on Mentha × Piperita L., Folium and Aetheroleum; EMA/HMPC/522409/2013; European Medicines Agency: Amsterdam, The Netherlands, 2020.
- Güney, M.; Oral, B.; Karahanli, N.; Mungan, T.; Akdogan, M. The effect of Mentha spicata Labiatae on uterine tissue in rats. Toxicol. Ind. Health. 2006, 22, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.S.; Prakash, O.; Padalia, R.C.; Vivekanand; Pant, A.K.; Mathela, C.S. Chemical Diversity in Mentha spicata: Antioxidant and Potato Sprout Inhibition Activity of its Essential Oils. Nat. Prod. Commun. 2011, 6, 1934578X1100600938. [Google Scholar] [CrossRef] [Green Version]
- Angel, G.; Menon, N.; Vimala, B.; Nambisan, B. Essential oil composition of eight starchy Curcuma species. Ind. Crops Prod. 2014, 60, 233–238. [Google Scholar] [CrossRef]
| Monoterpene | Toxicity | Type of Study | References |
|---|---|---|---|
| α-Terpinene | Embryo foetotoxicity | In vivo—rats model | [18] |
| neurotoxic | In vivo—rats model | [19] | |
| memory and learning dysfunction | In vivo—mice model | [21] | |
| hepatic oxidative cytotoxic genotoxic | In vivo—rats model | [22] | |
| lipid peroxidation | In vivo—rats model | [22] | |
| Camphor | neurotoxic | In vivo—human case report | [24] |
| hepatotoxicity granulomatous hepatitis | In vivo—human case report | [25] | |
| Citral | Embryo foetotoxicity maternal toxicity | In vivo—rats model | [32] |
| genotoxic/DNA damage | Cultured cells | [33] | |
| genotoxic/HepG2/leukocytes | Cultured cells | [34] | |
| DNA damage | human lymphocytes | [35] | |
| Limonene | cancerogenic allergenic sensitising | In vivo—mice and rats model | [41] |
| toxic for human lung cells | Human lung cell culture | [41] | |
| impact on uridine diphosphoglucuronosyl transferase | Human cell culture | [44] | |
| Pulegone and its metabolites p-cresol and menthofuran | hepatotoxin nasal epithelia atrophy of female reproductive organs | In vivo—mice models | [51] |
| hepatotoxic change in expression of miRNAs | Human liver | [55] | |
| Thujone | neurotoxic/modulation of GABA-gated chloride channels | In vivo—mice and rats models In vivo—human liver preparations | [62,63] |
| decrease in organ weight behaviour changes disturbances in hepatic and renal functions | In vivo—mice models | [64] | |
| genotoxicity carcinogenicity | Bacteria and mammalian cells | [64] |
| Plant/Essential Oil | Oil Composition * | Hazards | Commentary | References |
|---|---|---|---|---|
| Eucalyptus staigeriana F. v. Muell. ex F. M. Bailey | d-limonene+ α-phellandrene (30.5%), geranial (9.9%), neral (7.7%), and terpinolene (6.6%) | Teratogenicity (malformation and abnormal eye development) | Maximum oral dose in pregnancy: 238 mg/day | [69] |
| Vitex agnus-castus | Leaf EO: 1,8-cineole (15.6–35.2%), sabinene (6.9–17.1%), α-pinene (1.0–13.9%), α-terpineol (1.4–9.2%), γ-elemene (0–9.1%), β-selinene (0–9.0%), β-caryophyllene (2.3–8.9%), (Z)-β-farnesene (0–8.6%), citronellyl acetate (0.3–7.8%), and citronellic acid (0–6.6%), may contain methyleugenol | Reproductive hormone modulation (lower prolactin levels and prolongation of menstrual phase) | The component that is probably responsible for the side effect is methyleugenol. Study results support the use of 20 mg VAC dry extract, ethanol 60% m/m, daily for treatment of PMS | [70,71] |
| Thymus citriodorus (Pers.) Schreb. (Synonyms: Thymus lanuginosus Mill. var. citriodorum Pers., Thymus serpyllum var. citriodorus (Hort.), Thymus serpyllum var. vulgaris Benth.); a cross between Thymus vulgaris and Thymus pulegioides. | geraniol (39.2%), carvacrol (15.4%), geranial (9.2%) and neral (7.1%) | Teratogenicity (changes based on oxidation processes and effects on proliferation level) | Maximum oral dose in pregnancy: 258 mg/day. | [69,72] |
| Backhousia citriodora F. Muell. | geranial (46.1–60.7%) and neral (32.0–40.9%) | Teratogenicity (dose-dependent malformations in chicken embryos; fetal cranial development) | Maximum oral dose in pregnancy: 46 mg/day. | [73,74] |
| Artemisia vulgaris | camphor (20.8%), artemisia alcohol (15.3%), α-thujone (11.4%), β-caryophyllene (10.6%), isoborneol (9.3%), 1,8-cineole (9.0%), and sabinene (6.1%) | Neurotoxic (due to -aminobutyric acid type A (GABAA) receptor modulation of thujone) | Thujone was revealed as having the highest neurotoxic activity within the plant and is considered as being responsible for its neurotoxic character. | [75] |
| Salvia lavandulifolia Vahl | 1,8-cineole (12.0–40.3%), camphor (12.9–36.1%), α-terpinyl acetate (0.5–15.5%), linalool (0.2–11.2%), α-pinene (4.7–10.9%), camphene (4.6–10.6%), β-pinene (3.3–7.3%), (Z)-sabinyl acetate (0.5–9.0%), borneol (1.5–6.4%), linalyl acetate (0.1–5.8%), and limonene (2.4–5.0%) | abortifacient (reduction of maternal weight leading to abortifacient effect), teratogenicity | Studies performed on mice have demonstrated teratogenicity and dose-dependent abortifacient effects of sabinyl acetate. Sage is contra-indicated during pregnancy and lactation. | [76,77] |
| Artemisia herba-alba Asso | camphor (34.0–55.0%), α-thujone (25.7–36.8%), β-thujone (2.0–9.0%), camphene (0.5–9.0%), and 1,8-cineole (1.5–8.0%) | Neurotoxic (due to -aminobutyric acid type A (GABAA) receptor modulation of thujone) | The essential oil is recognised as neurotoxic due to contained high levels of thujone. | [78,79] |
| Mentha piperita | menthol (29–48%), menthone (20–31%), menthofuran (6–8%), pulegone, menthyl acetate (3–10%), limonene, pinene and piperitone | Nephropathy (hyaline droplet formation), carcinogenic effect (hepatocellular carcinoma at higher doses) | This side effect was observed in rats after subchronic administration of peppermint oil (100 mg/kg/day). The essential oil is able to exchange human lymphocytes and induce chromosomal aberrations. It is to ensure that the sum of pulegone and menthofuran within the daily dose is < 37.5 mf for adults. To reach the limits, peppermint oil with adequate quality (specification of adequate limits of pulegone and menthofuran) is required. | [80,81] |
| Mentha spicata | piperetenone oxide (49.4%), carvone (15.3%), 1,8-cineole (5.8%) and limonene (2.7%) | Uterine damage (caused by apoptosis and diffuse eosinophil leucocyte infiltration in surface and stromal glandular epithelium in both endometrium and endocervix strictly connected with lipid peroxidation) | Histopathological changes such as apoptosis and diffuse eosinophil leucocyte infiltration were observed in rats administered M. spicata tea. To reach the limits, peppermint oil with adequate quality (specification of adequate limits of pulegone and menthofuran) is required. As A general precaution, it is not recommended to use M. spicata during pregnancy and lactation, unless medical advice proposed benefit is higher than the potential risk. | [82,83] |
| Curcuma zedoaria Roscoe | epicurzerene (19.0–46.6%), curzerene (10.4%), curdione (7.0–19.6%), curzerenone (22.3–31.6%), debromofiliforminol (31.5%), 1,8-cineole (18.5–40.8%), β-sesquiphellandrene (21.5%), p-cymene (18.4%), curcumenene (18.7%), and α-phellandrene (14.9%) | antigestational embryotoxicity, antifertility; and abortifacient (strictly connected with decreased body weight of maternal; embryonic angiogenesis inhibition was observed) | The component responsible for the side effects has not been identified. Reproductive toxicity observed in mice treated with up to 10 g/kg/day water extraction of the plant. | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtunik-Kulesza, K.A. Toxicity of Selected Monoterpenes and Essential Oils Rich in These Compounds. Molecules 2022, 27, 1716. https://doi.org/10.3390/molecules27051716
Wojtunik-Kulesza KA. Toxicity of Selected Monoterpenes and Essential Oils Rich in These Compounds. Molecules. 2022; 27(5):1716. https://doi.org/10.3390/molecules27051716
Chicago/Turabian StyleWojtunik-Kulesza, Karolina A. 2022. "Toxicity of Selected Monoterpenes and Essential Oils Rich in These Compounds" Molecules 27, no. 5: 1716. https://doi.org/10.3390/molecules27051716






