Adsorption Behavior and Dynamic Interactions of Anionic Acid Blue 25 on Agricultural Waste
Abstract
:1. Introduction
2. Results and Discussion
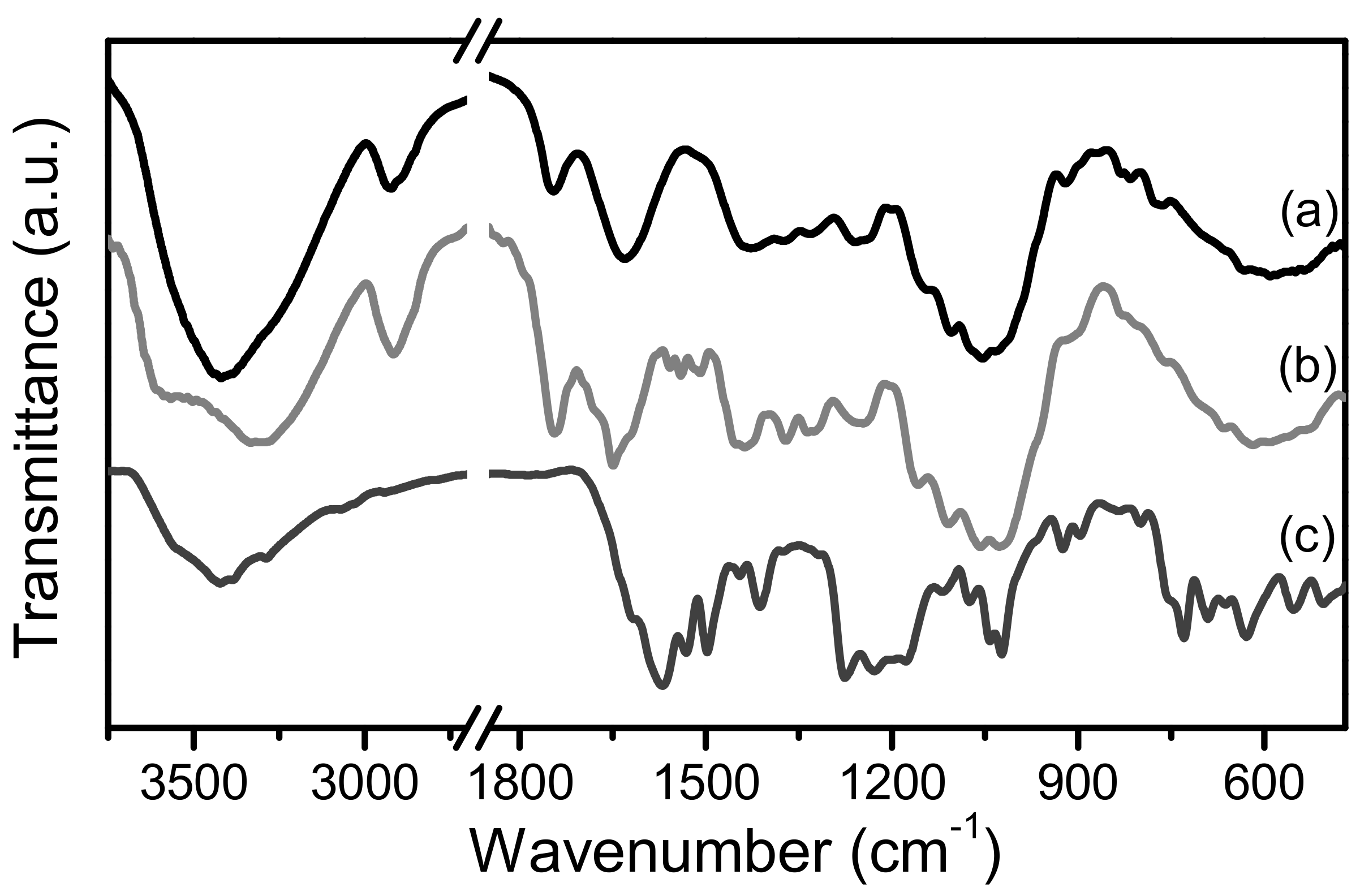
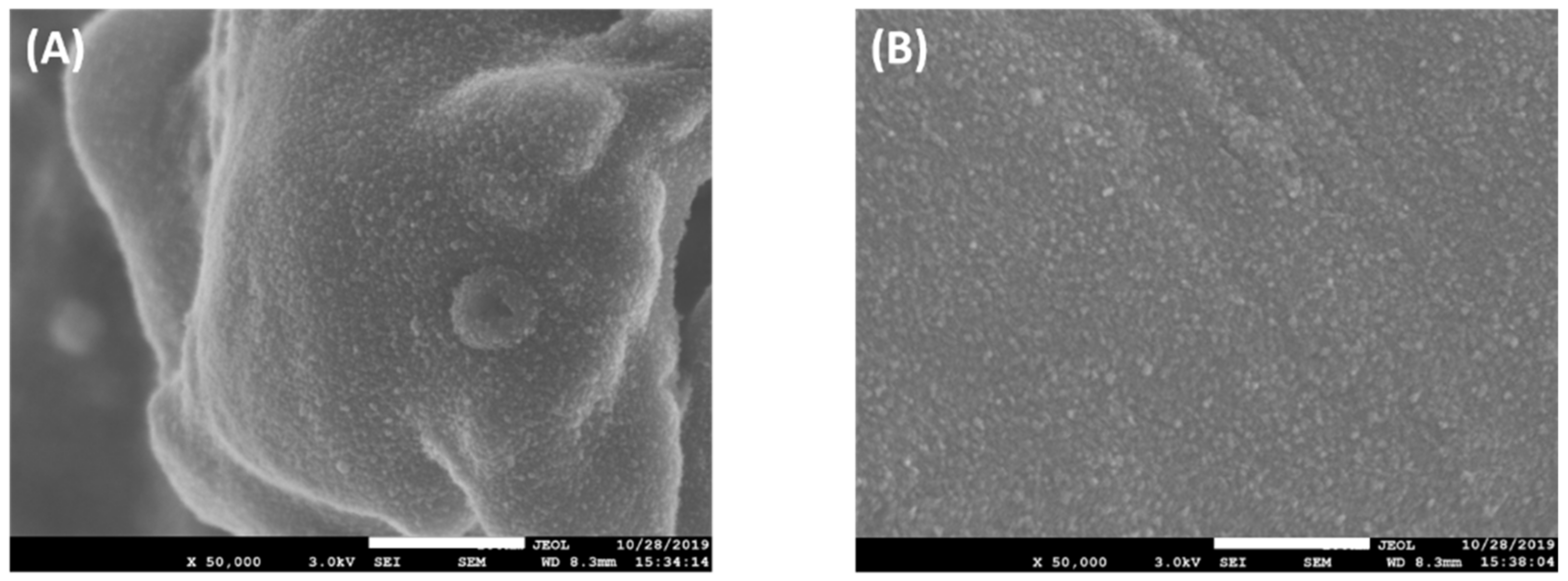
2.1. The Functional Groups and Surface Morphology of PP
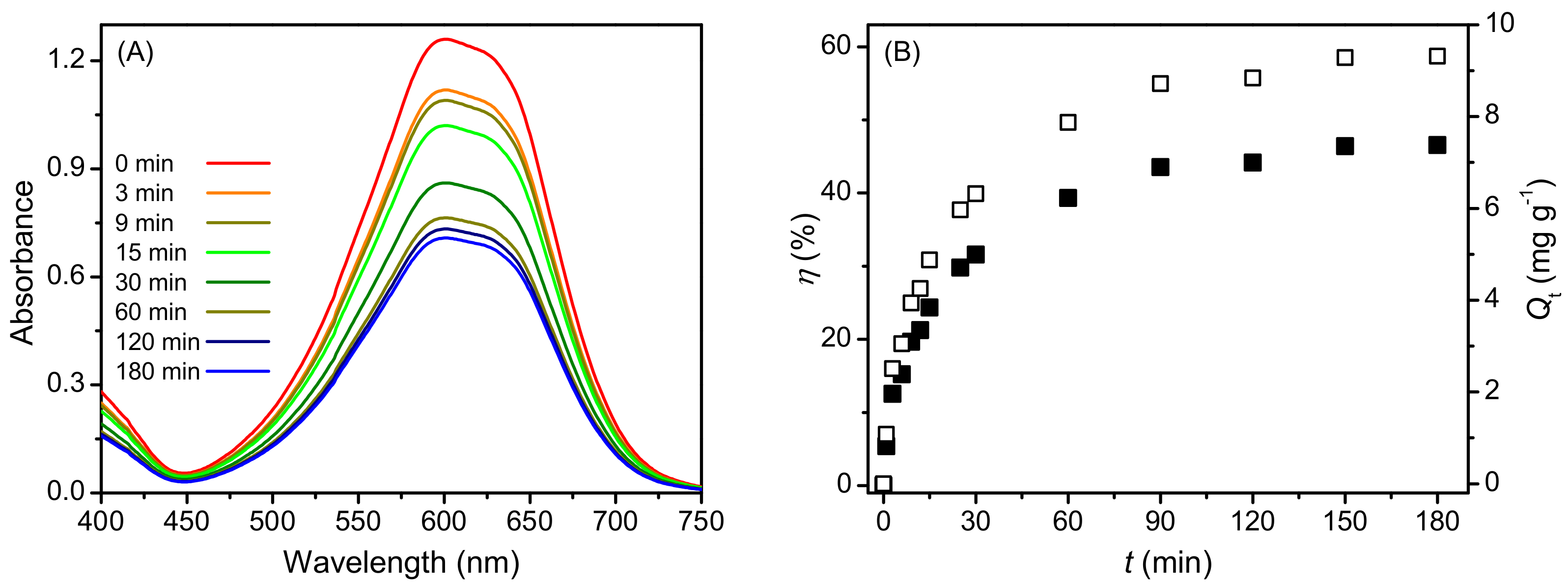
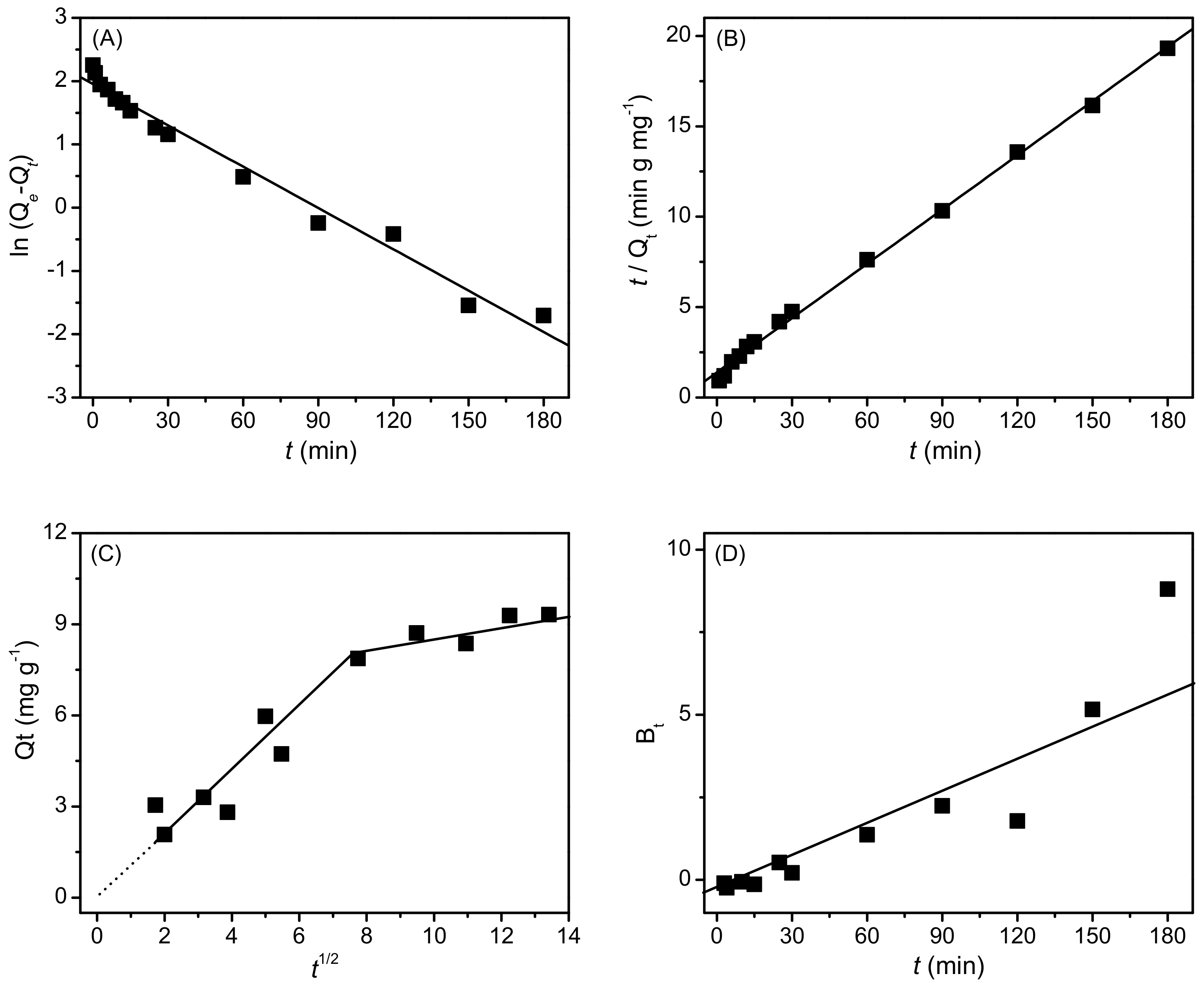
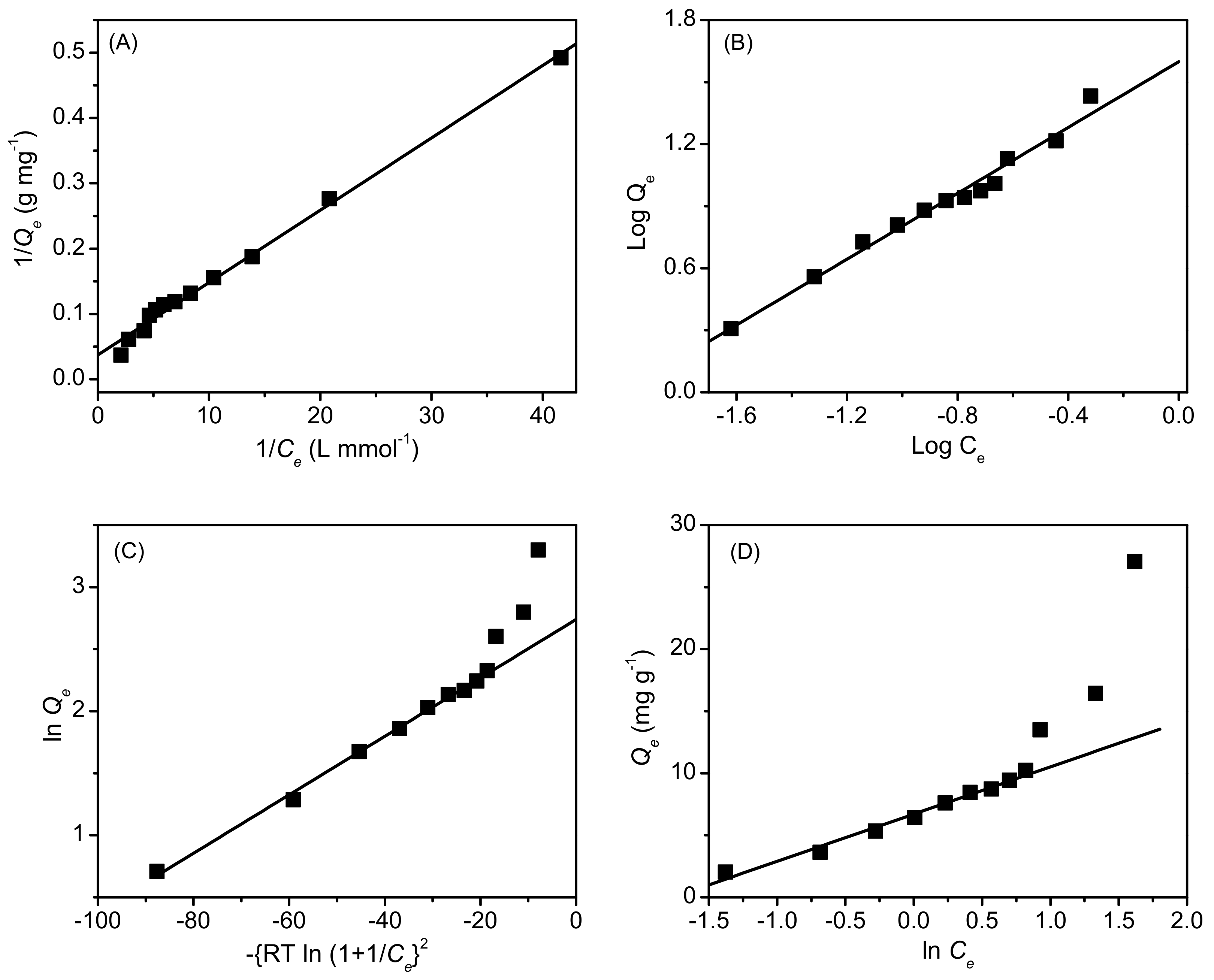
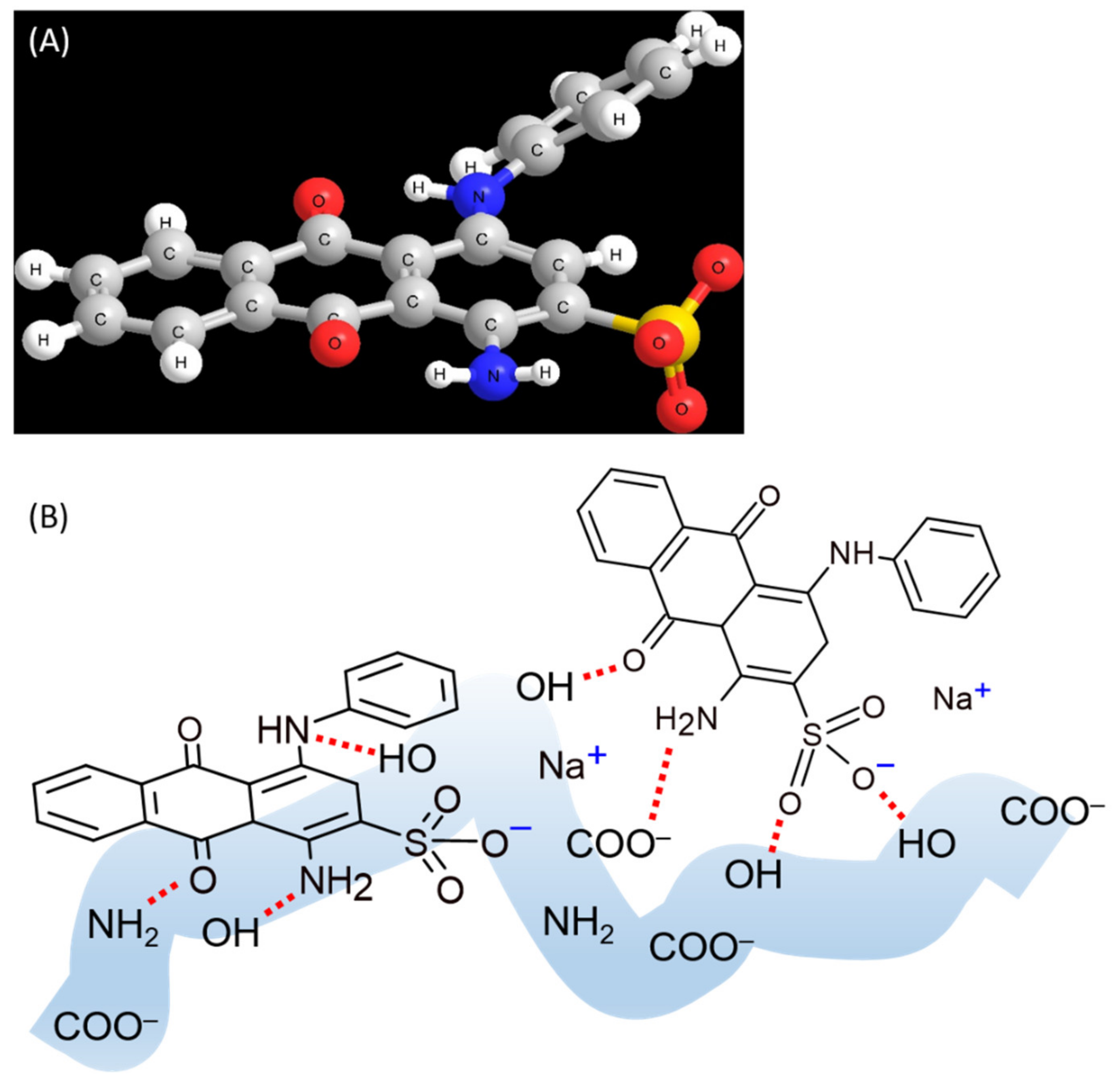
2.2. Adsorption Characteristics of AB25 on PP
2.3. Comparison of the of AB25
2.4. Thermodynamic Parameters of AB25 Adsorption
3. Materials and Methods
3.1. Chemicals
3.2. Characterizations
3.3. Adsorption of AB25 of PP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ardila-Leal, L.D.; Poutou-Piñales, R.A.; Pedroza-Rodríguez, A.M.; Quevedo-Hidalgo, B.E. A brief history of colour, the environmental impact of synthetic dyes and removal by using laccases. Molecules 2021, 26, 3813. [Google Scholar] [CrossRef] [PubMed]
- Palani, G.; Arputhalatha, A.; Kannan, K.; Lakkaboyana, S.K.; Hanafiah, M.M.; Kumar, V.; Marella, R.K. Current trends in the application of nanomaterials for the removal of pollutants from industrial wastewater treatment–A review. Molecules 2021, 26, 2799. [Google Scholar] [CrossRef] [PubMed]
- Zazouli, M.A.; Kalankesh, L.R. Removal of precursors and disinfection by-products (DBPs) by membrane fltration from water: A review. J. Environ. Health Sci. Eng. 2017, 15, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadekar, M.R.; Ahammed, M.M. Use of water treatment residuals for colour removal from real textile dye wastewater. Appl. Water Sci. 2020, 10, 160. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.J. Advanced oxidation processes for wastewater treatment: Formation of hydroxyl radical and application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Suhaimi, N.A.A.; Shahri, N.N.M.; Samat, J.H.; Kusrini, E.; Lim, J.-W.; Hobley, J.; Usman, A. Domination of methylene blue over rhodamine B during simultaneous photocatalytic degradation by TiO2 nanoparticles in an aqueous binary solution under UV irradiation. React. Kinet. Mech. Cat. 2022, 135, 511–527. [Google Scholar] [CrossRef]
- Bagheri, F.; Chaibakhsh, N. Efficient visible-light photocatalytic ozonation for dye degradation using Fe2O3/MoS2 nanocomposite. Sep. Sci. Technol. 2021, 56, 3022–3032. [Google Scholar] [CrossRef]
- Hynes, N.R.J.; Kumar, J.S.; Kamyab, H.; Sujana, J.A.J.; Al-Khashman, O.A.; Kuslu, Y.; Ene, A.; Kumar, B.S. Modern enabling techniques and adsorbents based dye removal with sustainability concerns in textile industrial sector—A comprehensive review. J. Clean. Prod. 2020, 272, 122636. [Google Scholar] [CrossRef]
- Zamri, N.I.I.; Zulmajdi, S.L.N.; Daud, N.Z.A.; Mahadi, A.H.; Kusrini, E.; Usman, A. Insight into the adsorption kinetics, mechanism, and thermodynamics of methylene blue from aqueous solution onto pectin-alginate-titania composite microparticles. SN Appl. Sci. 2021, 3, 222. [Google Scholar] [CrossRef]
- Asbollah, M.A.; Mahadi, A.H.; Kusrini, E.; Usman, A. Synergistic effect in concurrent removal of toxic methylene blue and acid red-1 dyes from aqueous solution by durian rind: Kinetics, isotherm, thermodynamics, and mechanism. Int. J. Phytoremediat. 2021, 23, 1432–1443. [Google Scholar] [CrossRef]
- Samat, J.H.; Shahri, N.N.M.; Abdullah, M.A.; Suhaimi, N.A.A.; Padmosoedarso, K.M.; Kusrini, E.; Mahadi, A.H.; Hobley, J.; Usman, A. Adsorption of Acid Blue 25 on agricultural wastes: Efficiency, kinetics, mechanism, and regeneration. Air Soil Water Res. 2021, 14, 1–12. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M.; Hubicki, Z. Anion Exchange Resins as Efective Sorbents for Removal of Acid, Reactive, and Direct Dyes from Textile Wastewaters. In Ion Exchange-Studies and Applications; Kilislioglu, A., Ed.; IntechOpen: Rijeka, Croatia, 2015. [Google Scholar]
- Zhang, M.-H.; Dong, H.; Zhao, L.; Wang, D.-X.; Meng, D. A review on Fenton process for organic wastewater treatment based onoptimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef]
- Kousha, M.; Daneshvar, E.; Esmaeili, A.R.; Zilouei, H.; Karimi, K. Biosorption of toxic acidic dye–acid blue 25 by aquatic plants. Desalin. Water Treat. 2014, 52, 6756–6769. [Google Scholar] [CrossRef]
- Ferrero, F. Dye removal by low cost adsorbents: Hazelnut shells in comparison with wood sawdust. J. Hazard. Mater. 2007, 142, 144–152. [Google Scholar] [CrossRef]
- Zaidi, N.A.H.M.; Lim, L.B.L.; Priyantha, N.; Usman, A. Artocarpus odoratissimus leaves as an eco-friendly adsorbent for the removal of toxic rhodamine B dye in aqueous solution: Equilibrium isotherm, kinetics, thermodynamics and regeneration studies. Arab. J. Sci. Eng. 2018, 43, 6011–6020. [Google Scholar] [CrossRef]
- Kusrini, E.; Usman, A.; Sani, F.A.; Wilson, L.D.; Abdullah, M.A.A. Simultaneous adsorption of lanthanum and yttrium from aqueous solution by durian rind biosorbent. Environ. Monit. Assess. 2019, 191, 488. [Google Scholar] [CrossRef]
- Dinh, V.-P.; Huynh, T.D.; Le, H.M.; Nguyen, V.-D.; Dao, V.-A.; Hung, N.Q.; Tuyen, L.A.; Lee, S.; Yi, J.; Nguyen, T.D.; et al. Insight into the adsorption mechanisms of methylene blue and chromium(III) from aqueous solution onto pomelo fruit peel. RSC Adv. 2019, 9, 25847–25860. [Google Scholar] [CrossRef] [Green Version]
- Lewis, D.M. The Coloration of Wool. In Advances in Wool Technology; Johnson, N.A.G., Russell, I.M., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2009; pp. 183–213. [Google Scholar]
- El-Hamshary, H.; Elsherbiny, A.S.; El-Newehy, M.H.; El-Hefnawy, M.E. Polyaspartate-Ionene/Na+-Montmorillonite nanocomposites as novel adsorbent for anionic dye; effect of ionene structure. Polymers 2020, 12, 2843. [Google Scholar] [CrossRef]
- Shahrin, E.W.E.S.; Narudin, N.A.H.; Padmosoedarso, K.M.; Kusrini, E.; Mahadi, A.H.; Shahri, N.N.M.; Usman, A. Pectin derived from pomelo pith as a superior adsorbent to remove toxic Acid Blue 25 from aqueous solution. Carbohydr. Polym. Technol. Appl. 2021, 2, 100116. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Preparation of waste tea activated carbon using potassium acetate as an activating agent for adsorption of Acid Blue 25 dye. Chem. Eng. J. 2011, 171, 502–509. [Google Scholar] [CrossRef]
- Tovar-Gómez, R.; Rivera-Ramírez, D.A.; Hernández-Montoya, V.; Bonilla-Petriciolet, A.; Durán-Valle, C.J.; Montes-Morán, M.A. Synergic adsorption in the simultaneous removal of Acid Blue 25 and heavy metals from water using a Ca(PO3)2-modified carbon. J. Hazard. Mater. 2012, 199–200, 290–300. [Google Scholar] [CrossRef]
- Hanafiah, M.A.K.M.; Ngah, W.S.W.; Zolkafly, S.H.; Teong, L.C.; Majid, Z.A.A. Acid blue 25 adsorption on base treated Shorea dasyphylla sawdust: Kinetic, isotherm, thermodynamic and spectroscopic analysis. J. Environ. Sci. 2012, 24, 261–268. [Google Scholar] [CrossRef]
- Dahri, M.K.; Lim, L.B.L.; Priyantha, N.; Chan, C.M. Removal of Acid Blue 25 using cempedak durian peel from aqueous medium: Isotherm, kinetics and thermodynamics studies. Int. Food Res. J. 2016, 23, 1154–1163. [Google Scholar]
- Lakkaboyana, S.K.; Khantong, S.; Kabir, M.A.; Ali, Y.; Yaacob, W.Z.W. Removal of Acid Blue 25 dye from wastewater using rambutan (Nephelium lappaceum Linn.) seed as an efficient natural biosorbent. Indian J. Adv. Chem. Sci. 2018, 6, 111–117. [Google Scholar]
- Kousha, M.; Tavakoli, S.; Daneshvar, E.; Vazirzadeh, A.; Bhatnagar, A. Central composite design optimization of Acid Blue 25 dye biosorption using shrimp shell biomass. J. Mol. Liq. 2015, 207, 266–273. [Google Scholar] [CrossRef]
- Kul, A.R.; Aldemir, A.; Elik, H. Adsorption of Acid Blue 25 on peach seed powder: Isotherm, kinetic and thermodynamic studies. Environ. Res. Technol. 2019, 2, 233–242. [Google Scholar] [CrossRef]
- Khalid, K.; Ngah, W.S.W.; Hanafiah, M.A.K.M.; Malek, N.S.A.; Khazaai, S.N.M. Acid Blue 25 adsorption onto phosphoric acid treated rubber leaf powder. Am. J. Environ. Eng. 2015, 5, 19–25. [Google Scholar]
- Jain, S.N.; Gogate, P.R. NaOH-treated dead leaves of Ficus racemosa as an efficient biosorbent for Acid Blue 25 removal. Int. J. Environ. Sci. Technol. 2017, 14, 531–542. [Google Scholar] [CrossRef]
- Kooh, M.R.R.; Dahri, M.K.; Lim, L.B.L.; Lim, L.H. Batch adsorption studies on the removal of acid blue 25 from aqueous solution using Azolla pinnata and soya bean waste. Arab. J. Sci. Eng. 2016, 41, 2453–2464. [Google Scholar] [CrossRef]
- Jawad, A.H.; Rashid, R.A.; Ishak, M.A.M.; Ismail, K. Adsorptive removal of methylene blue by chemically treated cellulosic waste banana (Musa sapientum) peels. J. Taibah Univ. Sci. 2018, 12, 809–819. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, N.A.H.M.; Lim, L.B.L.; Usman, A. Enhancing adsorption of malachite green dye using base-modified Artocarpus odoratissimus leaves as adsorbents. Environ. Technol. Innov. 2019, 13, 211–223. [Google Scholar] [CrossRef]
- Pirali-Hamedani, M.; Mehdipour-Ataei, S. Effect of sulfonation degree on molecular weight, thermal stability, and proton conductivity of poly(arylene ether sulfone)s membrane. Des. Monomers Polym. 2017, 20, 54–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dotto, G.L.; Pinto, L.A.A. Analysis of mass transfer kinetics in the biosorption of synthetic dyes onto Spirulina platensis nanoparticles. Biochem. Eng. J. 2012, 68, 85–90. [Google Scholar] [CrossRef]
- Dotto, G.L.; McKay, G. Current scenario and challenges in adsorption for water treatment. J. Environ. Chem. Eng. 2020, 8, 103988. [Google Scholar] [CrossRef]
- Asbollah, M.A.; Sahid, M.S.M.; Padmosoedarso, K.M.; Mahadi, A.H.; Kusrini, E.; Hobley, J.; Usman, A. Individual and competitive adsorption of negatively charged Acid Blue 25 and Acid Red 1 onto Raw Indonesian kaolin clay. Arab. J. Sci. Eng. 2022, 1–14. [Google Scholar] [CrossRef]
- Yang, Y.; Jin, D.; Wang, G.; Wang, S.; Jia, X.; Zhao, Y. Competitive biosorption of Acid Blue 25 and Acid Red 337 onto unmodified and CDAB-modified biomass of Aspergillus oryzae. Bioresour. Technol. 2011, 102, 7429–7436. [Google Scholar] [CrossRef]
- Saheed, I.O.; Oh, W.-D.; Suah, F.B.M. Enhanced adsorption of acid Blue-25 dye onto chitosan/porous carbon composite modified in 1-allyl-3-methyl imidazolium bromide ionic liquid. Int. J. Biol. Macromol. 2021, 183, 1026–1033. [Google Scholar] [CrossRef]
- Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Fruit peel waste: Characterization and its potential uses. Curr. Sci. 2017, 113, 444–454. [Google Scholar] [CrossRef]
- Zeng, Y.; Chen, X.; Zhao, D.; Li, H.; Zhang, Y.; Xiao, X. Estimation of pKa values for carboxylic acids, alcohols, phenols and amines using changes in the relative Gibbs free energy. Fluid Phase Equilibria 2012, 313, 148–155. [Google Scholar] [CrossRef]
- Goswami, S.; Ghosh, U.C. Studies on adsorption behaviour of Cr(VI) onto synthetic hydrous stannic oxide. Water SA 2005, 31, 597–602. [Google Scholar]
- Jiménez-Ángeles, F.; Khoshnood, A.; Firoozabadi, A. Molecular dynamics simulation of the adsorption and aggregation of ionic surfactants at liquid-solid interfaces. J. Phys. Chem. C 2017, 121, 25908–25920. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Ho, Y.S.; Ng, J.C.Y.; McKay, G. Kinetics of pollutant sorption by biosorbents: Review. Sep. Purif. Methods 2000, 29, 189–232. [Google Scholar] [CrossRef]
- Viegas, R.M.C.; Campinas, M.; Costa, H.; Rosa, M.J. How do the HSDM and Boyd’s model compare for estimating intraparticle diffusion coefficients in adsorption processes. Adsorption 2014, 20, 737–746. [Google Scholar]
- Sağ, Y.; Aktay, Y. Mass transfer and equilibrium studies for the sorption of chromium ions onto chitin. Process Biochem. 2000, 36, 157–173. [Google Scholar] [CrossRef]
- Ermolenko, A.; Shevelev, A.; Vikulova, M.; Blagova, T.; Altukhov, S.; Gorokhovsky, A.; Godymchuk, A.; Burmistrov, I.; Offor, P.O. Wastewater treatment from lead and strontium by potassium polytitanates: Kinetic analysis and adsorption mechanism. Processes 2020, 8, 217. [Google Scholar] [CrossRef] [Green Version]
- Ghaffari, H.R.; Pasalari, H.; Tajvar, A.H.; Dindarloo, K.; Goudarzi, B.; Alipour, V.; Ghanbarneajd, A. Linear and nonlinear two-parameter adsorption isotherm modeling: A case-study. Int. J. Eng. Sci. 2017, 6, 1–11. [Google Scholar]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Moreno-Piraján, J.C.; Anastopoulos, I. A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J. Mol. Liq. 2019, 273, 425–434. [Google Scholar] [CrossRef]
| Isotherm Model | Parameters | Quantity |
|---|---|---|
| Langmuir | ||
| (mg g−1) | 26.9 | |
| (dm−3 mmol−1) | 3.36 | |
| R2 | 0.993 | |
| Freundlich | ||
| 39.7 | ||
| 1.26 | ||
| R2 | 0.998 | |
| Dubinin–Radushkevich | ||
| (mg g−1) | 20.3 | |
| 28.6 | ||
| E (kJ mol−1) | 0.132 | |
| R2 | 0.914 | |
| Temkin | ||
| (dm−3 mmol−1) | 2.95 | |
| (kJ mol−1) | 0.354 | |
| R2 | 0.758 |
| Adsorbent | pHpzc | pH | Qm (mg g−1) | Reference |
|---|---|---|---|---|
| Raw kaolin | 5.70 | 7.3 | 16.5 | [37] |
| Shorea dasyphylla sawdust | 8.25 | 2.0 | 24.4 | [24] |
| Pine sawdust | 2.3–3.8 | 2.5–4.2 | 26.2 | [15] |
| Cempedak durian peel | 4.01 | 2 | 26.6 | [25] |
| Pomelo pith | 4.23 | 6.7 | 26.9 | This study |
| Oak sawdust | 2.3–3.8 | 2.5–4.2 | 27.9 | [15] |
| Rubber leaves powder | NR * | 2 | 28.1 | [29] |
| Nephelium lappaceum Linn. seed | 6.2 | 2 | 35.6 | [26] |
| Walnut sawdust | 2.3–3.8 | 2.5–4.2 | 37.0 | [15] |
| Soybean waste | NR * | 2 | 38.3 | [31] |
| Azolla pinnata | NR * | 2 | 50.5 | [31] |
| Hazelnut shell | 2.3–3.8 | 2.5–4.2 | 60.2 | [15] |
| Banana peel | 6.32 | 6.7 | 70.0 | [11] |
| Ficus rasemosa leaves powder | NR * | 2 | 83.3 | [30] |
| Durian peel | 6.18 | 6.7 | 89.7 | [11] |
| Peach seed powder | NR * | 2 | 95.2 | [28] |
| Aspergillus oryzae | NR * | 2 | 105.3 | [38] |
| Egg shell modified activated carbon | 5.7 | 5.2–5.7 | 109.8 | [23] |
| Ceratophylum demersum | NR * | 2 | 129.7 | [14] |
| Potamogeton pusillus | NR * | 2 | 183.5 | [14] |
| Waste tea activated carbon | 7.2 | 7–11 | 203.3 | [22] |
| Pectin derived from pomelo peel | 2.63 | 6.7 | 739.0 | [21] |
| Chitosan-activated carbon composite | NR * | 4 | 909.1 | [39] |
| Adsorbent | T (K) | KL | ΔGads | ΔH | ΔS | |
|---|---|---|---|---|---|---|
| (kJ mol−1) | (kJ mol−1) | (kJ mol−1) | (J mol−1 K−1) | |||
| 293 | 3.23 | 7420 | −4.77 | |||
| 298 | 3.36 | 7728 | −4.95 | |||
| PP | 303 | 3.95 | 9078 | −5.43 | 10.4 | 52 |
| 308 | 3.96 | 9106 | −5.53 | |||
| 313 | 4.22 | 9683 | −5.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahrin, E.W.E.S.; Narudin, N.A.H.; Shahri, N.N.M.; Verinda, S.B.; Nur, M.; Hobley, J.; Usman, A. Adsorption Behavior and Dynamic Interactions of Anionic Acid Blue 25 on Agricultural Waste. Molecules 2022, 27, 1718. https://doi.org/10.3390/molecules27051718
Shahrin EWES, Narudin NAH, Shahri NNM, Verinda SB, Nur M, Hobley J, Usman A. Adsorption Behavior and Dynamic Interactions of Anionic Acid Blue 25 on Agricultural Waste. Molecules. 2022; 27(5):1718. https://doi.org/10.3390/molecules27051718
Chicago/Turabian StyleShahrin, Ensan Waatriah E. S., Nur Alimatul Hakimah Narudin, Nurulizzatul Ningsheh M. Shahri, Sera Budi Verinda, Muhammad Nur, Jonathan Hobley, and Anwar Usman. 2022. "Adsorption Behavior and Dynamic Interactions of Anionic Acid Blue 25 on Agricultural Waste" Molecules 27, no. 5: 1718. https://doi.org/10.3390/molecules27051718
APA StyleShahrin, E. W. E. S., Narudin, N. A. H., Shahri, N. N. M., Verinda, S. B., Nur, M., Hobley, J., & Usman, A. (2022). Adsorption Behavior and Dynamic Interactions of Anionic Acid Blue 25 on Agricultural Waste. Molecules, 27(5), 1718. https://doi.org/10.3390/molecules27051718









