Anthraquinones from the Aerial Parts of Rubia cordifolia with Their NO Inhibitory and Antibacterial Activities
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. General Experimental Procedures
3.2. Plant Materials
3.3. Extraction and Isolation
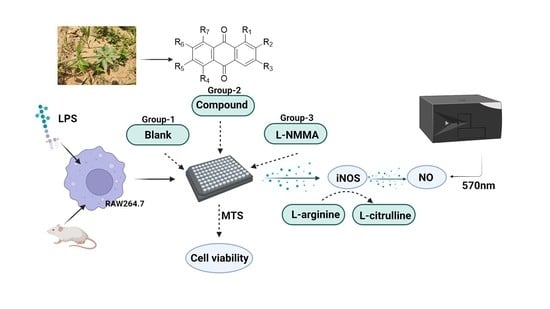
3.4. NO Inhibitory Assay
3.5. Antibacterial Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| ID | infectious diarrhea |
| NO | nitric oxide |
| iNOS | inducible nitric oxide synthase |
| TCM | Traditional Chinese Medicine |
| HR-ESIMS | high resolution electrospray ionization mass spectroscopy |
| UV | ultraviolet-visible |
| NMR | nuclear magnetic resonance |
| HMBC | heteronuclear multiple bond correlation |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromid |
| IR | infrared ra |
| Q-TOF | quadrupole-time of flight |
| HPLC | high performance liquid chromatography |
| TLC | thin-layer chromatography |
| Si CC | silica gel column chromatography |
| EtOH | ethyl alcohol |
| EtOAc | ethyl acetate |
| MeOH | Methanol |
| USA | The United States of America |
References
- Snyder, J.D.; Merson, M. The magnitude of the global problem of acute diarrhoeal disease: A review of active surveillance data. Bull. WHO 1982, 60, 605–613. [Google Scholar]
- Liu, L.; Johnson, H.L.; Cousens, S.; Perin, J.; Scott, S.; Lawn, J.E.; Rudan, I.; Campbell, H.; Cibulskis, R.; Li, M.; et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet 2012, 379, 2151–2161. [Google Scholar] [CrossRef]
- Stephen, J. Pathogenesis of infectious diarrhea. Can. J. Gastroenterol. 2001, 15, 669–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993, 2, 2002–2012. [Google Scholar]
- Ignarro, L.J.; Buga, G.M.; Wood, K.S.; Byrns, R.E.; Chaudhuri, G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. USA 1987, 84, 9265–9269. [Google Scholar] [CrossRef] [Green Version]
- Wink, D.A.; Mitchell, J.B. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic. Biol. Med. 1998, 25, 434–456. [Google Scholar] [CrossRef]
- Grisham, M.B.; Jourd’Heuil, D.; Wink, D.A. Nitric oxide—I. Physiological chemistry of nitric oxide and its metabolites: Implications in inflammation. Am. J. Physiol. 1999, 276, 315–321. [Google Scholar] [CrossRef]
- Umer, S.; Tekewe, A.; Kebede, N. Antidiarrhoeal and antimicrobial activity of Calpurnia aurea leaf extract. BMC Complementary Altern. Med. 2013, 13, 21. [Google Scholar] [CrossRef] [Green Version]
- Orsi, P.R.; Bonamin, F.; Severi, J.A.; Santos, R.C.; Vilegas, W.; Hiruma-Lima, C.A.; Di Stasi, L.C. Hymenaea stigonocarpa Mart. ex Hayne: A Brazilian medicinal plant with gastric and duodenal anti-ulcer and antidiarrheal effects in experimental rodent models. J. Ethnopharmacol. 2012, 143, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Ali, N.; Alam, H.; Khan, A.; Ahmed, G.; Shah, W.A.; Nabi, M.; Junaid, M. Antispasmodic and antidiarrhoeal activity of the fruit of Rosa moschata. BMC Complementary Altern. Med. 2014, 14, 485. [Google Scholar] [CrossRef]
- Atta, A.H.; Mouneir, S.M. Antidiarrhoeal activity of some Egyptian medicinal plant extracts. J. Ethnopharmacol. 2004, 92, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Mbagwu, H.O.C.; Adeyemi, O.O. Anti-diarrhoeal activity of the aqueous extract of Mezoneuron benthamianum Baill (Caesalpiniaceae). J. Ethnopharmacol. 2008, 116, 16–20. [Google Scholar] [CrossRef]
- Praxedes de Sales, I.R.; Frota Machado, F.D.; Marinho, A.F.; Suassuna Carneiro Lucio, A.S.; Barbosa Filho, J.M.; Batista, L.M. Cissampelos sympodialis Eichl. (Menispermaceae), a medicinal plant, presents antimotility and antidiarrheal activity in vivo. BMC Complement. Altern. Med. 2015, 15, 253. [Google Scholar]
- Chinese Academy of Sciences Flora of China Commission. Flora of China; Science Press: Beijing, China, 1999; Volume 71, pp. 287–315. [Google Scholar]
- Itokawa, H.; Takeya, K.; Mihara, K.; Mori, N.; Hamanaka, T.; Sonobe, T.; Iitaka, Y. Studies on the antitumor cyclic hexapeptides obtained from Rubiae radix. Chem. Pharm. Bull. 1983, 31, 1424–1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, X.P.; Sun, Y.Y.; Chen, W.; Guo, X.; Guan, J.K.; Li, D.Y.; Du, G. Anti-diarrheal and anti-inflammatory activities of aqueous extract of the aerial part of Rubia cordifolia. BMC Complementary Altern. Med. 2017, 17, 20. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Gao, L.; Zhang, Q.; Zhang, Y.; Yao, W.F.; Zhang, M.; Tang, Y.P.; Ding, A.W.; Zhang, L. Revealing the mechanisms and the material basis of Rubia cordifolia L. on abnormal uterine bleeding with uniting simultaneous determination of four components and systematic pharmacology approach-experimental validation. J. Pharm. Biomed. Anal. 2020, 189, 113475. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020; Volume 1, p. 245. [Google Scholar]
- Itokawa, H.; Mihara, K.; Takeya, K. Studies on a novel anthraquinone and its glycosides isolated from Rubia cordifolia and R. akane. Chem. Pharm. Bull. 1983, 31, 2353–2358. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.Y.; Zhang, X.J.; Zhang, Z.H.; Wang, Z.; Tan, N.H. Qualitative and quantitative analyses of quinones in multi-origin Rubia species by ultra-performance liquid chromatography-tandem mass spectrometry combined with chemometrics. J. Pharm. Biomed. Anal. 2020, 189, 113471. [Google Scholar] [CrossRef]
- Arisawa, M.; Ueno, H.; Nimura, M.; Hayashi, T.; Morita, N. Rubiatriol, a new triterpenoid from the Chinese drug Qian Cao Gen, Rubia cordifolia. J. Nat. Prod. 1986, 49, 1114–1116. [Google Scholar] [CrossRef]
- Chen, X.Q.; Zhao, S.M.; Wang, Z.; Zeng, G.Z.; Huang, M.B.; Tan, N.H. Rubicordins A-C, new cyclopeptides from Rubia cordifolia with cytotoxicity and inhibiting NF-kappa B signaling pathway. Tetrahedron 2015, 71, 9673–9678. [Google Scholar] [CrossRef]
- Zhang, M.T.; Yang, L.; Hu, J.M.; Zhang, H.; Shi, X.Q.; Liu, S.J. Study on lignan constituents from aerial part of Rubia cordifolia. Chin. Tradit. Herb. Drugs. 2017, 48, 4856–4859. [Google Scholar]
- Qiao, Y.F.; Wang, S.K.; Wu, L.J.; Li, X.; Zhu, T.R. Antibacterial constituents from the roots of Rubia cordifolia L. Acta. Pharm. Sin. 1990, 25, 834–839. [Google Scholar]
- Tao, J.; Morikawa, T.; Ando, S.; Matsuda, H.; Yoshikawa, M. Bioactive constituents from chinese natural medicines. XI. Inhibitors on NO production and degranulation in RBL-2H3 from Rubia yunnanensis: Structures of rubianosides II, III, and IV, rubianol-g, and rubianthraquinone. Chem. Pharm. Bull. 2003, 51, 654–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, J.K.; Jung, S.J.; Jung, J.H.; Fang, Z.; Lee, C.S.; Seo, C.S.; Moon, D.C.; Min, B.S.; Kim, M.R.; Woo, M.H. Anticancer constituents from the roots of Rubia cordifolia L. Chem. Pharm. Bull. 2008, 56, 213–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunez Montoya, S.C.; Agnese, A.M.; Cabrera, J.L. Anthraquinone Derivatives from Heterophyllaea pustulata. J. Nat. Prod. 2006, 69, 801–803. [Google Scholar] [CrossRef] [PubMed]
- El-Lakany, A.M.; Aboul-Ela, M.A.; Abdel-Kader, M.S.; Badr, J.M.; Sabri, N.N.; Goher, Y. Anthraquinones with antibacterial activities from Crucianella maritima L. growing in Egypt. Nat. Prod. Sci. 2004, 10, 63–68. [Google Scholar]
- Ismail, N.H.; Ali, A.M.; Aimi, N.; Kitajima, M.; Takayama, H.; Lajis, N.H. Anthraquinones from Morinda elliptica. Phytochemistry 1997, 45, 1723–1725. [Google Scholar] [CrossRef] [Green Version]
- Koyama, J.; Ogura, T.; Tagahara, K. Anthraquinones of Galium spurium. Phytochemistry 1993, 33, 1540–1542. [Google Scholar] [CrossRef]
- Kang, X.D.; Li, X.; Zhao, C.C.; Mao, Y. Two new anthraquinones from Hedyotis diffusa W. J. Asian. Nat. Prod. Res. 2008, 10, 193–197. [Google Scholar] [CrossRef]
- Itokawa, H.; Ibraheim, Z.Z.; Qiao, Y.F.; Takeya, K. Anthraquinones, naphthohydroquinones and naphthohydroquinone dimers from Rubia cordifolia and their cytotoxic activity. Chem. Pharm. Bull. 1993, 41, 1869–1872. [Google Scholar] [CrossRef] [Green Version]
- Longue Ekon, J.P.; Zra, T.; Lobe Songue, J.; Wondja Ngoko, M.L.; Ngassoum, M.B.; Talla, E.; Kamdem Waffo, A.F.; Sewald, N.; Wansi, J.D. New anthraquinone derivatives from the stem barks of Morinda lucida Benth. Phytochem. Lett. 2020, 39, 94–98. [Google Scholar] [CrossRef]
- El-Gamal, A.A.; Takeya, K.; Itokawa, H.; Halim, A.F.; Amer, M.M.; Saad, H.E.A.; Awad, S.A. Anthraquinones from the polar fractions of Galium sinaicum. Phytochemistry 1996, 42, 1149–1155. [Google Scholar] [CrossRef]
- Banthorpe, D.V.; White, J.J. Novel anthraquinones from undifferentiated cell cultures of Galium verum. Phytochemistry 1995, 38, 107–111. [Google Scholar] [CrossRef]
- Wisetsai, A.; Lekphrom, R.; Schevenels, F.T. New anthracene and anthraquinone metabolites from Prismatomeris filamentosa and their antibacterial activities. Nat. Prod. Res. 2021, 35, 1582–1589. [Google Scholar] [CrossRef]
- Ling, S.K.; Komorita, A.; Tanaka, T.; Fujioka, T.; Mihashi, K.; Kouno, I. Iridoids and anthraquinones from the Malaysian medicinal plant, Saprosma scortechinii (Rubiaceae). Chem. Pharm. Bull. 2002, 50, 1035–1040. [Google Scholar] [CrossRef] [Green Version]
- Halim, A.F.; Abd El-Fattah, H.; El-Gamal, A.A.; Thomson, R.H. Anthraquinones from Galium sinaicum. Phytochemistry 1991, 31, 355–356. [Google Scholar] [CrossRef]
- Leistner, E. Second pathway leading to anthraquinones in higher plants. Phytochemistry 1971, 10, 3015–3020. [Google Scholar] [CrossRef]
- Sieweke, H.J.; Leistner, E. o-Succinylbenzoate:coenzyme A ligase from anthraquinone producing cell suspension cultures of Galium mollugo. Phytochemistry 1992, 31, 2329–2335. [Google Scholar] [CrossRef]
- Lv, X.; Wang, F.; Zhou, P.; Ye, L.; Xie, W.; Xu, H.; Yu, H. Dual regulation of cytoplasmic and mitochondrial acetyl-CoA utilization for improved isoprene production in Saccharomyces cerevisiae. Nat. Commun. 2016, 7, 12851. [Google Scholar] [CrossRef]
- Han, Y.S.; van der Heijden, R.; Lefeber, A.W.M.; Erkelens, C.; Verpoorte, R. Biosynthesis of anthraquinones in cell cultures of Cinchona ‘Robusta’ proceeds via the methylerythritol 4-phosphate pathway. Phytochemistry 2002, 59, 45–55. [Google Scholar] [CrossRef]
- Ignarro, L.J. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu. Rev. Pharmacol. Toxicol. 1990, 30, 535–560. [Google Scholar] [CrossRef] [PubMed]
- Reif, D.W.; McCreedy, S.A. N-Nitro-L-arginine and N-monomethyl-L-arginine exhibit a different pattern of inactivation toward the three nitric oxide synthases. Arch. Biochem. Biophys. 1995, 320, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.D. Standards for antimicrobial susceptibility testing. Am. J. Vet. Res. 1999, 60, 64–65. [Google Scholar]



| No | 1 a | 2 a | 3 b | 4 a | ||||
|---|---|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | δH | δC | |
| 1 | 8.01, d (1.8) | 127.2 | 158.6 | 8.12, d (1.7) | 123.5 | 8.06, d (1.7) | 124.0 | |
| 2 | 145.5 | 138.3 | 149.8 | 149.5 | ||||
| 3 | 7.71, dd (7.9, 1.8) | 135.4 | 7.63, d (7.8) | 133.1 | 7.76, dd (7.9, 1.7) | 131.6 | 7.78, dd (7.9, 1.7) | 131.7 |
| 4 | 8.13, d (7.9) | 127.8 | 7.79, d (7.8) | 118.8 | 8.06, d (7.9) | 126.8 | 8.13, d (7.9) | 126.3 |
| 4 a | 133.5 | 131.8 | 131.7 | 132.1 | ||||
| 5 | 148.4 | 7.54, s | 113.4 | 7.19, s | 109.9 | 157.0 | ||
| 6 | 158.1 | 155.1 | 157.0 | 139.8 | ||||
| 7 | 7.38, d (8.5) | 121.6 | 153.5 | 139.6 | 160.3 | |||
| 8 | 8.04, d (8.5) | 125.8 | 7.44, s | 108.8 | 159.2 | 7.16, s | 110.5 | |
| 8 a | 127.9 | 125.2 | 109.6 | 129.3 | ||||
| 9 | 182.6 | 187.8 | 186.1 | 181.9 | ||||
| 9 a | 133.7 | 115.2 | 133.2 | 132.9 | ||||
| 10 | 182.7 | 181.8 | 181.4 | 185.5 | ||||
| 10 a | 127.5 | 129.2 | 129.1 | 109.0 | ||||
| 2-Me | 2.53, s | 21.6 | ||||||
| 2-CH2OH | 4.62, s | 57.9 | 4.67, s | 62.2 | 4.66, s | 62.2 | ||
| 5-OMe | 3.95, s | 61.7 | ||||||
| 6-OMe | 3.82, s | 59.8 | ||||||
| 7-OMe | 3.95, s | 56.3 | 3.83, s | 59.9 | ||||
 | |||||||
|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | R5 | R6 | R7 | |
| 1 | H | Me | H | OMe | OH | H | H |
| 2 | OH | CH2OH | H | H | OH | OMe | H |
| 3 | H | CH2OH | H | H | OH | OMe | OH |
| 4 | H | CH2OH | H | OH | OMe | OH | H |
| 5 | H | Me | H | H | OMe | OH | H |
| 6 | H | Me | H | OH | OMe | OH | H |
| 7 | OH | Me | H | H | OH | H | H |
| 8 | OH | Me | H | OMe | OH | H | H |
| 9 | OH | Me | H | OH | OMe | H | H |
| 10 | OH | Me | H | OH | OMe | OH | H |
| 11 | OH | OMe | OH | H | H | H | H |
| 12 | OH | OMe | OH | H | OH | H | H |
| 13 | H | CH2OH | H | H | OMe | OH | H |
| 14 | OH | CH2OH | H | H | H | H | H |
| 15 | OH | CH2OH | H | OMe | OH | H | H |
| 16 | OH | CH2OH | H | OMe | OH | OMe | H |
| 17 | OH | CH2OH | OH | H | OMe | H | H |
| 18 | OH | CH2OH | OH | OMe | OMe | H | H |
| 19 | OMe | CH2OH | OH | H | OH | H | H |
| 20 | OMe | OH | H | H | H | H | H |
| Compounds | NO Inhibitory Effects (IC50/μmol·L−1) | RAW 264.7 Cell Viability a (%) |
|---|---|---|
| 1 | 14.05 ± 0.48 | 105.69 ± 0.25 |
| 3 | 23.48 ± 1.05 | 97.67 ± 1.21 |
| 10 | 29.23 ±0.34 | 101.80 ± 1.10 |
| L-NMMA b | 42.36 ± 2.47 | 98.72 ± 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, H.; Qin, W.; Zhang, H.; Ren, F.-C.; Fang, W.-T.; Kong, Q.-H.; Yang, L.; Zhang, J.-M.; Fang, C.-W.; Hu, J.-M.; et al. Anthraquinones from the Aerial Parts of Rubia cordifolia with Their NO Inhibitory and Antibacterial Activities. Molecules 2022, 27, 1730. https://doi.org/10.3390/molecules27051730
Luo H, Qin W, Zhang H, Ren F-C, Fang W-T, Kong Q-H, Yang L, Zhang J-M, Fang C-W, Hu J-M, et al. Anthraquinones from the Aerial Parts of Rubia cordifolia with Their NO Inhibitory and Antibacterial Activities. Molecules. 2022; 27(5):1730. https://doi.org/10.3390/molecules27051730
Chicago/Turabian StyleLuo, Han, Wei Qin, Hong Zhang, Fu-Cai Ren, Wen-Tao Fang, Qing-Hua Kong, Liu Yang, Jian-Mei Zhang, Cheng-Wu Fang, Jiang-Miao Hu, and et al. 2022. "Anthraquinones from the Aerial Parts of Rubia cordifolia with Their NO Inhibitory and Antibacterial Activities" Molecules 27, no. 5: 1730. https://doi.org/10.3390/molecules27051730
APA StyleLuo, H., Qin, W., Zhang, H., Ren, F.-C., Fang, W.-T., Kong, Q.-H., Yang, L., Zhang, J.-M., Fang, C.-W., Hu, J.-M., & Liu, S.-J. (2022). Anthraquinones from the Aerial Parts of Rubia cordifolia with Their NO Inhibitory and Antibacterial Activities. Molecules, 27(5), 1730. https://doi.org/10.3390/molecules27051730