Screening of Natural Products Inhibitors of SARS-CoV-2 Entry
Abstract
:1. Introduction
2. Results and Discussion
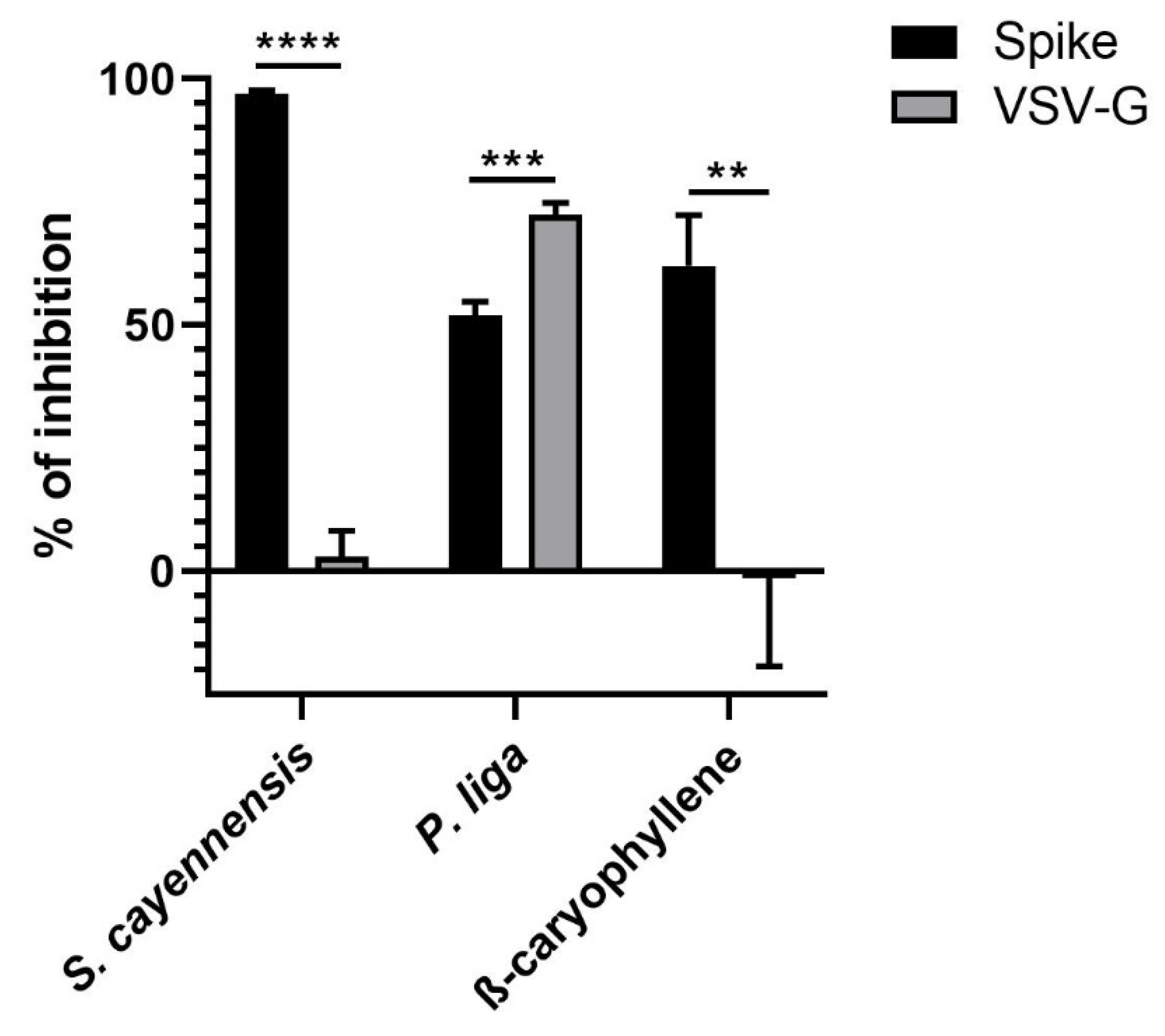
2.1. Antiviral Screening
2.2. Cytotoxicity, Inhibitory Concentration, and Selectivity Index
3. Materials and Methods
3.1. Cell Cultures and Plasmids
3.2. Natural Products
3.3. Cytotoxicity Assay
3.4. HIV-1-Based SARS-CoV-2 Pseudotyped Particles
3.5. Antiviral Activity
3.6. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Drosten, C.; Günther, S.; Preiser, W.; van der Werf, S.; Brodt, H.-R.; Becker, S.; Rabenau, H.; Panning, M.; Kolesnikova, L.; Fouchier, R.A.M.; et al. Identification of a Novel Coronavirus in Patients with Severe Acute Respiratory Syndrome. N. Engl. J. Med. 2003, 348, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Isolation of a Novel Coronavirus from a Man with Pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, N.C.; López-Cortés, A.; González, E.V.; Grimaldos, A.B.; Prado, E.O. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. NPJ Vaccines 2021, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.d.A.; Grosche, V.R.; Bergamini, F.R.G.; Sabino-Silva, R.; Jardim, A.C.G. Antivirals Against Coronaviruses: Candidate Drugs for SARS-CoV-2 Treatment? Front. Microbiol. 2020, 11, 1818. [Google Scholar] [CrossRef]
- Sheahan, T.P.; Sims, A.C.; Zhou, S.; Graham, R.L.; Hill, C.S.; Leist, S.R.; Schäfer, A.; Dinnon, K.H.; Montgomery, S.A.; Agostini, M.L.; et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 and multiple endemic, epidemic and bat coronavirus. bioRxiv 2020, 5883, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, R.L.; Kania, R.S.; Brothers, M.A.; Davies, J.F.; Ferre, R.A.; Gajiwala, K.S.; He, M.; Hogan, R.J.; Kozminski, K.; Li, L.Y.; et al. Discovery of Ketone-Based Covalent Inhibitors of Coronavirus 3CL Proteases for the Potential Therapeutic Treatment of COVID-19. J. Med. Chem. 2020, 63, 12725–12747. [Google Scholar] [CrossRef]
- Yan, R.; Yuanyuan, Z.; Yaning, L.; Lu, X.; Yingying, G.; Qiang, Z. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef] [Green Version]
- Mazzon, M.; Marsh, M. Targeting viral entry as a strategy for broad-spectrum antivirals. F1000Research 2019, 8, 1628. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.T.; Sarkar, C.; El-Kersh, D.M.; Jamaddar, S.; Uddin, S.J.; Shilpi, J.A.; Mubarak, M.S. Natural products and their derivatives against coronavirus: A review of the non-clinical and pre-clinical data. Phyther. Res. 2020, 34, 2471–2492. [Google Scholar] [CrossRef]
- Yang, Y.; Islam, M.S.; Wang, J.; Li, Y.; Chen, X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): A review and perspective. Int. J. Biol. Sci. 2020, 16, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Rolta, R.; Salaria, D.; Sharma, P.P.; Sharma, B.; Kumar, V.; Rathi, B.; Verma, M.; Sourirajan, A.; Baumler, D.J.; Dev, K. Phytocompounds of Rheum emodi, Thymus serpyllum, and Artemisia annua Inhibit Spike Protein of SARS-CoV-2 Binding to ACE2 Receptor: In Silico Approach. Curr. Pharmacol. Reports 2021, 7, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Sumaryada, T.; Pramudita, C.A. Molecular docking evaluation of some indonesian’s popular herbals for a possible covid-19 treatment. Biointerface Res. Appl. Chem. 2021, 11, 9827–9835. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Q.; Huang, W.; Li, X.; Wang, Y. Current status on the development of pseudoviruses for enveloped viruses. Rev. Med. Virol. 2018, 28, e1963. [Google Scholar] [CrossRef]
- Beltrán-Pavez, C.; Riquelme-Barrios, S.; Oyarzún-Arrau, A.; Gaete-Argel, A.; González-Stegmaier, R.; Cereceda-Solis, K.; Aguirre, A.; Travisany, D.; Palma-Vejares, R.; Barriga, G.P.; et al. Insights into neutralizing antibody responses in individuals exposed to SARS-CoV-2 in Chile. Sci. Adv. 2021, 7, eabe6855. [Google Scholar] [CrossRef]
- Yang, L.; Pei, R.J.; Li, H.; Ma, X.N.; Zhou, Y.; Zhu, F.H.; He, P.I.; Tang, W.; Zhang, Y.C.; Xiong, J.; et al. Identification of SARS-CoV-2 entry inhibitors among already approved drugs. Acta Pharmacol. Sin. 2021, 42, 1347–1353. [Google Scholar] [CrossRef]
- da Silva, J.K.R.; Figueiredo, P.L.B.; Byler, K.G.; Setzer, W.N. Essential oils as antiviral agents. Potential of essential oils to treat sars−cov−2 infection: An in−silico investigation. Int. J. Mol. Sci. 2020, 21, 3426. [Google Scholar] [CrossRef]
- Panikar, S.; Shoba, G.; Arun, M.; Sahayarayan, J.J.; Usha Raja Nanthini, A.; Chinnathambi, A.; Alharbi, S.A.; Nasif, O.; Kim, H.-J. Essential oils as an effective alternative for the treatment of COVID-19: Molecular interaction analysis of protease (Mpro) with pharmacokinetics and toxicological properties. J. Infect. Public Health 2021, 14, 601–610. [Google Scholar] [CrossRef]
- Schnitzler, P.; Astani, A.; Reichling, J. Screening for antiviral activities of isolated compounds from essential oils. Evid. Based Complement. Altern. Med. 2011, 2011, 56. [Google Scholar] [CrossRef] [Green Version]
- Hassanin, O.; Abdallah, F.; A.A.Galal, A. In vitro and in vivo experimental trials to assess the modulatory influence of β-caryophyllene on NDV replication and immunopathogenesis. Comp. Immunol. Microbiol. Infect. Dis. 2020, 73, 101547. [Google Scholar] [CrossRef]
- Drevinskas, T.; Maruška, A.; Telksnys, L.; Hjerten, S.; Stankevičius, M.; Lelešius, R.; Mickienė, R.; Karpovaitė, A.; Šalomskas, A.; Tiso, N.; et al. Chromatographic Data Segmentation Method: A Hybrid Analytical Approach for the Investigation of Antiviral Substances in Medicinal Plant Extracts. Anal. Chem. 2019, 91, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Chatow, L.; Nudel, A.; Nesher, I.; Hayo Hemo, D.; Rozenberg, P.; Voropaev, H.; Winkler, I.; Levy, R.; Kerem, Z.; Yaniv, Z.; et al. In Vitro Evaluation of the Activity of Terpenes and Cannabidiol against Human Coronavirus E229. Life 2021, 11, 290. [Google Scholar] [CrossRef]
- Jha, N.K.; Sharma, C.; Hashiesh, H.M.; Arunachalam, S.; Meeran, M.N.; Javed, H.; Patil, C.R.; Goyal, S.N.; Ojha, S. β-Caryophyllene, A Natural Dietary CB2 Receptor Selective Cannabinoid can be a Candidate to Target the Trinity of Infection, Immunity, and Inflammation in COVID-19. Front. Pharmacol. 2021, 12, 321. [Google Scholar] [CrossRef] [PubMed]
- Narkhede, R.R.; Pise, A.V.; Cheke, R.S.; Shinde, S.D. Recognition of Natural Products as Potential Inhibitors of COVID-19 Main Protease (Mpro): In-Silico Evidences. Nat. Products Bioprospect. 2020, 10, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Liew, P.M.; Yong, Y.K. Stachytarpheta jamaicensis (L.) Vahl: From Traditional Usage to Pharmacological Evidence. Evid. Based Complement. Altern. Med. 2016, 2016, 7842340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieroni, A.; Vandebroek, I.; Prakofjewa, J.; Bussmann, R.W.; Paniagua-Zambrana, N.Y.; Maroyi, A.; Torri, L.; Zocchi, D.M.; Dam, A.T.K.; Khan, S.M.; et al. Taming the pandemic? The importance of homemade plant-based foods and beverages as community responses to COVID-19. J. Ethnobiol. Ethnomed. 2020, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Olayode, O.A.; Daniyan, M.O.; Olayiwola, G. Biochemical, hematological and histopathological evaluation of the toxicity potential of the leaf extract of Stachytarpheta cayennensis in rats. J. Tradit. Complement. Med. 2020, 10, 544–554. [Google Scholar] [CrossRef]
- Okoye, T.C.; Akah, P.A.; Ezike, A.C.; Uzor, P.F.; Odoh, U.E.; Igboeme, S.O.; Onwuka, U.B.; Okafor, S.N. Immunomodulatory effects of Stachytarpheta cayennensis leaf extract and its synergistic effect with artesunate. BMC Complement. Altern. Med. 2014, 14, 376. [Google Scholar] [CrossRef] [Green Version]
- Sulaiman, M.R.; Zakaria, Z.A.; Chiong, H.S.; Lai, S.K.; Israf, D.A.; Azam Shah, T.M. Antinociceptive and anti-inflammatory effects of stachytarpheta jamaicensis (L.) Vahl (Verbenaceae) in experimental animal models. Med. Princ. Pract. 2009, 18, 272–279. [Google Scholar] [CrossRef]
- Dabbish, A.M.; Yonis, N.; Salama, M.; Essa, M.M.; Qoronfleh, M.W. Inflammatory pathways and potential therapies for COVID-19: A mini review. Eur. J. Inflamm. 2021, 19, 20587392211002986. [Google Scholar] [CrossRef]
- Chen, C.Z.; Xu, M.; Pradhan, M.; Gorshkov, K.; Petersen, J.D.; Straus, M.R.; Zhu, W.; Shinn, P.; Guo, H.; Shen, M.; et al. Identifying SARS-CoV-2 Entry Inhibitors through Drug Repurposing Screens of SARS-S and MERS-S Pseudotyped Particles. ACS Pharmacol. Transl. Sci. 2020, 3, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Ao, Z.; Chan, M.; Ouyang, M.J.; Olukitibi, T.A.; Mahmoudi, M.; Kobasa, D.; Yao, X. Identification and evaluation of the inhibitory effect of prunella vulgaris extract on sars-coronavirus 2 virus entry. PLoS ONE 2021, 16, e0251649. [Google Scholar] [CrossRef] [PubMed]
- Gabaglio, S.; Alvarenga, N.; Cantero-González, G.; Degen, R.; Ferro, E.A.; Langjahr, P.; Chnaiderman, J.; Sotelo, P.H. A quantitative PCR assay for antiviral activity screening of medicinal plants against Herpes simplex 1. Nat. Prod. Res. 2019, 6419, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.C.; Talarico, L.; Almeida, N.; Colombres, S.; Duschatzky, C.; Damonte, E.B. Virucidal activity of essential oils from aromatic plants of San Luis, Argentina. Phytother. Res. 2003, 17, 1073–1075. [Google Scholar] [CrossRef] [PubMed]
- Garber, A.; Barnard, L.; Pickrell, C. Review of Whole Plant Extracts with Activity Against Herpes Simplex Viruses In Vitro and In Vivo. J. Evid. Based Integr. Med. 2021, 26, 2515690X2097839. [Google Scholar] [CrossRef] [PubMed]
- Sakthiselvan, P.; Madhumathi, R.; Meenambiga, S.S. Moronic acid: An antiviral for herpes simplex virus. In A Centum of Valuable Plant Bioactives; Elsevier: Amsterdam, The Netherlands, 2021; pp. 143–158. [Google Scholar]
- Simões, C.M.O.; Falkenberg, M.; Mentz, L.A.; Schenkel, E.P.; Amoros, M.; Girre, L. Antiviral activity of South Brazilian medicinal plant extracts. Phytomedicine 1999, 6, 205–214. [Google Scholar] [CrossRef]
- Visintini Jaime, M.F.; Redko, F.; Muschietti, L.V.; Campos, R.H.; Martino, V.S.; Cavallaro, L. V In vitro antiviral activity of plant extracts from Asteraceae medicinal plants. Virol. J. 2013, 10, 245. [Google Scholar] [CrossRef] [Green Version]
- Kingston, R.E.; Chen, C.A.; Rose, J.K. Calcium Phosphate Transfection. Curr. Protoc. Mol. Biol. 2003, 63, 1–11. [Google Scholar] [CrossRef]



| Voucher | Species | MNTC 1 (µg/mL) | Antiviral Activity (% of Inhibition) |
|---|---|---|---|
| R. Degen 4164 (10.24) | Acacia caven (Molina) | 31.25 | 44.75 ± 4.32 |
| R. Degen 4061 (14.40) | Acanthospermum australe | 31.25 | −16.06 ± 14.66 |
| R. Degen 4042 (5.45) | Aloysia gratissima | 62.5 | 35.60 ± 11.72 |
| R. Degen 4224 (7.89) | Amphilophium paniculatum | 62.5 | −45.29 ± 14.43 |
| R. Degen 4064 (15.83) | Annona emarginata | 15 | 59.32 ± 8.20 |
| R. Degen 4079 (4.91) | Austroeupatorium inulifolium | 125 | −69.62 ± 30.92 |
| R. Degen 4272 (6.90) | Baccharis dracunculifolia | 62.5 | 50.46 ± 5.87 |
| R. Degen 4291 (5.25) | Calea uniflora | 62.5 | 25.93 ± 16.21 |
| R. Degen 4044 (5.23) | Centratherum punctatum | 7.8 | −75.99 ± 5.13 |
| R. Degen 4016 (6.30) | Chromolaena ivifolia | 31.25 | 2.75 ± 10.94 |
| R. Degen 4198 (4.89) | Croton paraguayensis | 31.25 | 54.14 ± 3.16 |
| R. Degen 4236 (4.51) | Lessingianthus niederleinii | 31.25 | 59.49 ± 2.50 |
| R. Degen 4257 (8.76) | Lippia origanoides | 125 | 1.77 ± 1.77 |
| R. Degen 4321 (10.05) | Phoradendron liga | 500 | 82.58 ± 4.75 |
| R. Degen 4065 (3.07) | Pluchea sagittalis | 15.62 | −6.31 ± 18.04 |
| R. Degen 4039 (4.07) | Pterocaulom angustifolium | 31.25 | 1.20 ± 12.44 |
| R. Degen 4065 (9.06) | Solanum sisymbriifolium | 62.5 | 29.72 ± 4.61 |
| R. Degen 4032 (6.01) | Solidago chilensis | 31.25 | 40.97 ± 1.28 |
| R. Degen 4038 (11.05) | Stachytarpheta cayennensis | 500 | 97.03 ± 0.64 |
| R. Degen 4127 (11.46) | Tessaria dodoneifolia | 31.25 | −49.87 ± 13.96 |
| R. Degen 4184 (EK 094 PA) | Zhathoxylum chilopexone | 31.25 | 25.39 ± 2.33 |
| Species | Vernacular Name | MNTC 1 (µg/mL) | Antiviral Activity (% of Inhibition) |
|---|---|---|---|
| Gonopterodendron sarmientoi | Palo Santo | 37.5 | −24.03 ± 10.37 |
| Citrus aurantium L. var. amara | Petit grain | 31.25 | −38.95 ± 9.50 |
| Myrocarpus frondosus | Cabreuva | 7.8 | 7.56 ± 2.87 |
| Anona emarginata | Aratiku’i | 18.25 | 1.37 ± 2.25 |
| Eucalyptus globulus | Eucalyptus | 250 | −131.35 ± 32.85 |
| Lippia alba | Salvia | 31.25 | 4.34 ± 3.69 |
| Cymbopogon citratusv | Cedron capi´i | 15.62 | −41.47 ± 6.84 |
| Compound | MNTC 1 (µg/mL) | Antiviral Activity (% of Inhibition) |
|---|---|---|
| β-Caryophyllene | 125 | 62.10 ± 10.31 |
| Caryophyllene Oxide | 31.25 | 43.82 ± 6.31 |
| Linalool | 125 | −9.42 ± 0.25 |
| Trans-anethole | 500 | 5.02 ± 0.71 |
| S-Limonene | 31.25 | −62.63 ± 22.17 |
| R-Limonene | 31.25 | −27.90 ± 16.41 |
| cis-Verbenol | 250 | −171.26 ± 32.54 |
| Guaiol | 33.3 | −60.20 ± 8.02 |
| Macrophominol | 36 | −91.39 ± 26.65 |
| Acetylphomolactone | 4.5 | −0.65 ± 6.16 |
| Botryodiplodin | 3 | −88.21 ± 12.51 |
| Asperline | 7.81 | −15.48 ± 8.34 |
| Isoasperline | 7.19 | −19.82 ± 10.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Maldonado, P.; Alvarenga, N.; Burgos-Edwards, A.; Flores-Giubi, M.E.; Barúa, J.E.; Romero-Rodríguez, M.C.; Soto-Rifo, R.; Valiente-Echeverría, F.; Langjahr, P.; Cantero-González, G.; et al. Screening of Natural Products Inhibitors of SARS-CoV-2 Entry. Molecules 2022, 27, 1743. https://doi.org/10.3390/molecules27051743
González-Maldonado P, Alvarenga N, Burgos-Edwards A, Flores-Giubi ME, Barúa JE, Romero-Rodríguez MC, Soto-Rifo R, Valiente-Echeverría F, Langjahr P, Cantero-González G, et al. Screening of Natural Products Inhibitors of SARS-CoV-2 Entry. Molecules. 2022; 27(5):1743. https://doi.org/10.3390/molecules27051743
Chicago/Turabian StyleGonzález-Maldonado, Pamela, Nelson Alvarenga, Alberto Burgos-Edwards, Ma. Eugenia Flores-Giubi, Javier E. Barúa, Ma. Cristina Romero-Rodríguez, Ricardo Soto-Rifo, Fernando Valiente-Echeverría, Patricia Langjahr, Guadalupe Cantero-González, and et al. 2022. "Screening of Natural Products Inhibitors of SARS-CoV-2 Entry" Molecules 27, no. 5: 1743. https://doi.org/10.3390/molecules27051743
APA StyleGonzález-Maldonado, P., Alvarenga, N., Burgos-Edwards, A., Flores-Giubi, M. E., Barúa, J. E., Romero-Rodríguez, M. C., Soto-Rifo, R., Valiente-Echeverría, F., Langjahr, P., Cantero-González, G., & Sotelo, P. H. (2022). Screening of Natural Products Inhibitors of SARS-CoV-2 Entry. Molecules, 27(5), 1743. https://doi.org/10.3390/molecules27051743






