Microfluidic Microalgae System: A Review
Abstract
:1. Introduction
2. Cell Identification by Identification of Cell Property or Strain Selection or Species
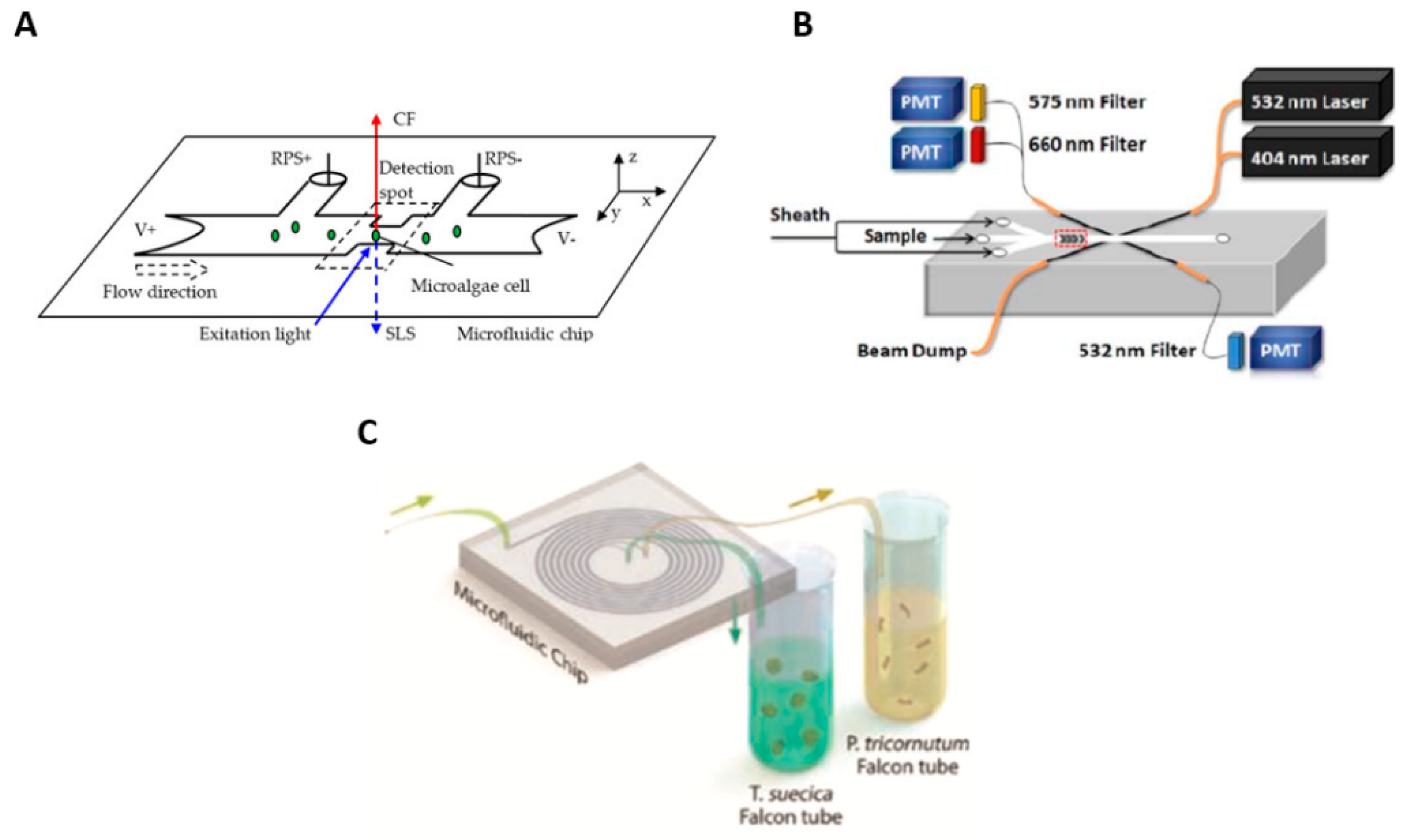
2.1. Microfluidic Flow Cytometry for Cell Characterization
2.2. Analysis of Cell Viability
3. Microfluidic-Based Microalgal Sorting
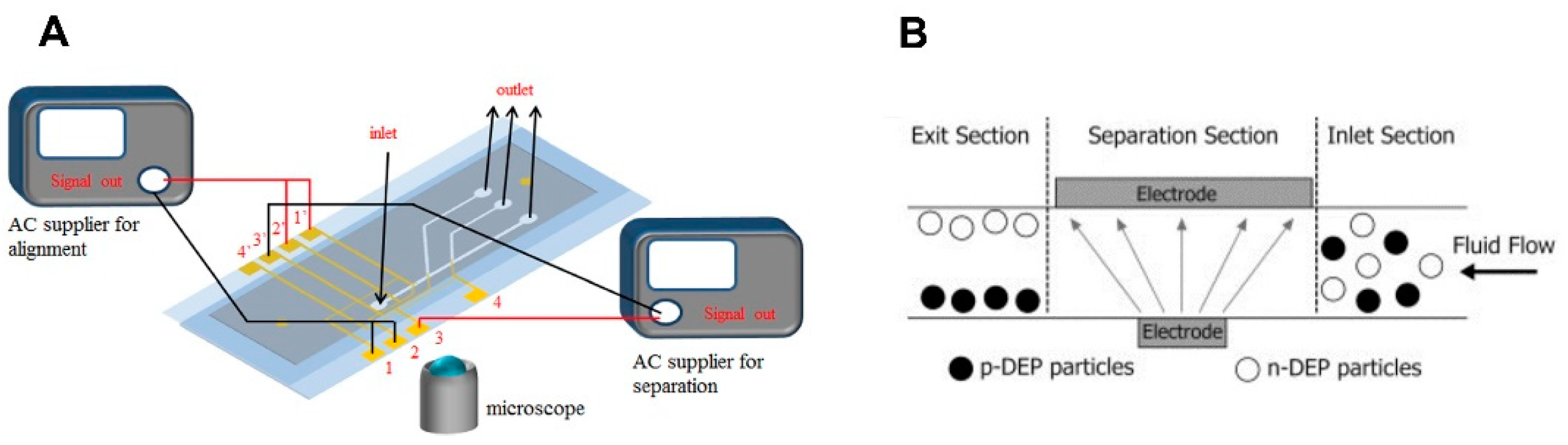
3.1. Electric Field-Based Sorting
3.2. Inertial Microfluidic-Based Sorting
4. Cell Transformation
4.1. Digital Microfluidic Platform
4.2. Droplet Microfluidics-Based Designs
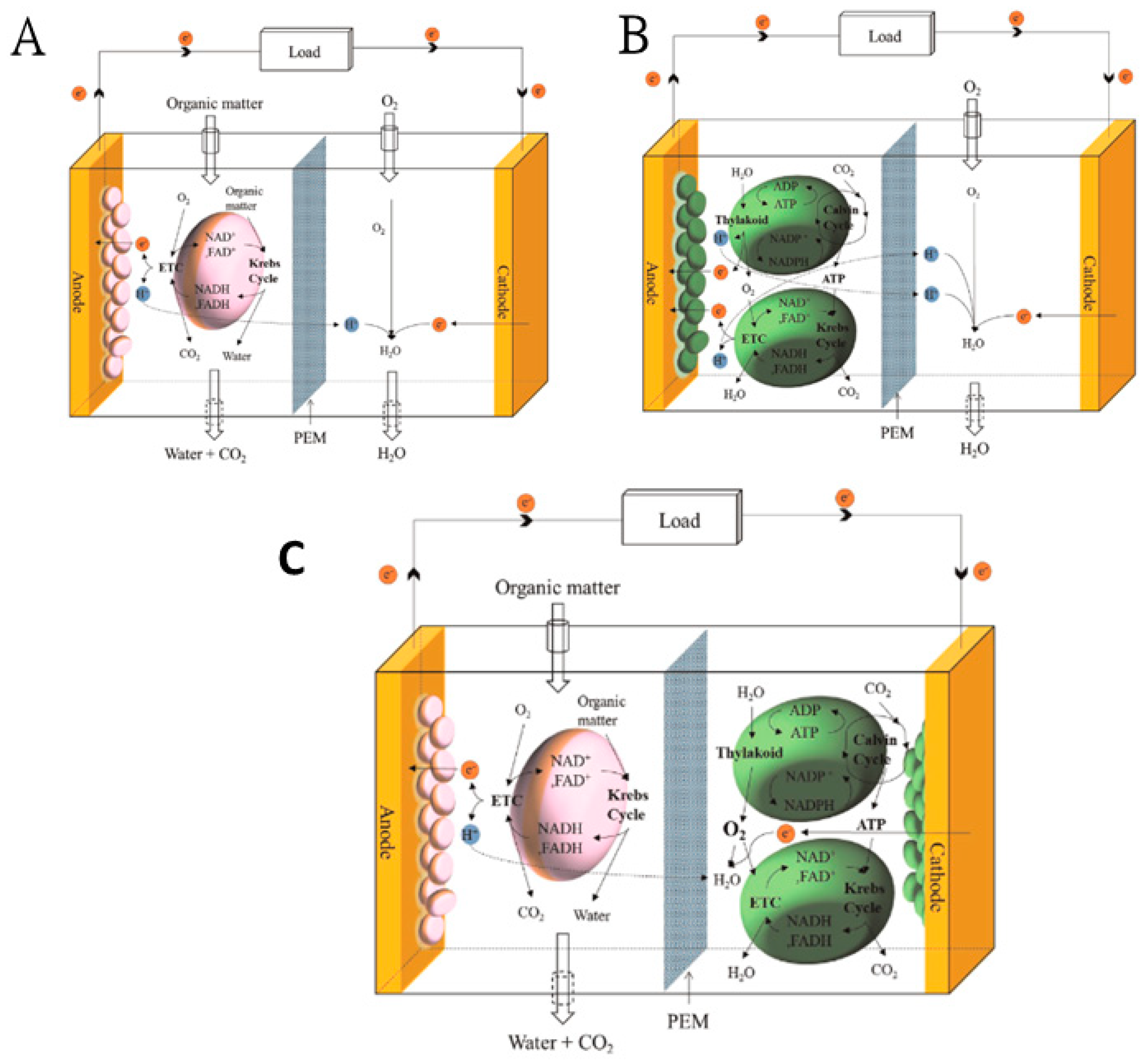
5. Fuel Cell
6. Other Applications of Microfluidic Technology
6.1. Metabolic Engineering
6.2. Light-Controllable Photobioreactor
6.3. Gradient Generators
7. Discussion
8. Conclusions and Future Scope
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microbial. Cell Fact. 2018, 17, 1–21. [Google Scholar] [CrossRef]
- Durrett, T.P.; Benning, C.; Ohlrogge, J. Plant triacylglycerols as feedstocks for the production of biofuels. Plant J. 2008, 54, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-F.; Jin, W.-B.; Tu, R.-J.; Wu, W. Biofuel production from microalgae as feedstock: Current status and potential. Crit. Rev. Biotechnol. 2013, 35, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Georgianna, D.R.; Mayfield, S.P. Exploiting diversity and synthetic biology for the production of algal biofuels. Nature 2012, 488, 329–335. [Google Scholar] [CrossRef]
- Stanier, R.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef]
- Guihéneuf, F.; Khan, A.; Tran, L.-S.P. Genetic engineering: A promising tool to engender physiological, biochemical, and molecular stress resilience in green microalgae. Front. Plant Sci. 2016, 7, 400. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.; Khanra, S.; Mondal, M.; Halder, G.; Tiwari, O.; Saini, S.; Bhowmick, T.K.; Gayen, K. Progress toward isolation of strains and genetically engineered strains of microalgae for production of biofuel and other value added chemicals: A review. Energy Convers. Manag. 2016, 113, 104–118. [Google Scholar] [CrossRef]
- Chisti, Y. Constraints to commercialization of algal fuels. J. Biotechnol. 2013, 167, 201–214. [Google Scholar] [CrossRef]
- Duong, V.T.; Li, Y.; Nowak, E.; Schenk, P.M. Microalgae isolation and selection for prospective biodiesel production. Energies 2012, 5, 1835–1849. [Google Scholar] [CrossRef]
- Alias, A.; Huang, H.-Y.; Yao, D.-J. A review on microfluidics: An aid to assisted reproductive technology. Molecules 2021, 26, 4354. [Google Scholar] [CrossRef] [PubMed]
- Alias, A.B.; Chiang, C.-E.; Huang, H.-Y.; Lin, K.-T.; Lu, P.-J.; Wang, Y.-W.; Wu, T.-H.; Jiang, P.-S.; Chen, C.-A.; Yao, D.-J. Extraction of cell-free DNA from an embryo-culture medium using micro-scale bio-reagents on ewod. Sci. Rep. 2020, 10, 9708. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-E.; Huang, H.-Y.; Lin, K.-T.; Alias, A.B.; Lu, P.-J.; Wang, Y.-W.; Wu, T.-H.; Jiang, P.-S.; Chen, C.-A.; Yao, D.-J. A medical innovation: A new and improved method of DNA extraction with electrowetting-on-dielectric of genetic testing in-vitro fertilization (IVF). Microfluid. Nanofluidics 2020, 24, 1–9. [Google Scholar] [CrossRef]
- Kaladharan, K.; Kumar, A.; Gupta, P.; Illath, K.; Santra, T.; Tseng, F.-G. Microfluidic based physical approaches towards single-cell intracellular delivery and analysis. Micromachines 2021, 12, 631. [Google Scholar] [CrossRef] [PubMed]
- Jesús-Pérez, N.M.; Lapizco-Encinas, B.H. Dielectrophoretic monitoring of microorganisms in environmental applications. Electrophoresis 2011, 32, 2331–2357. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Hou, H.; Li, L.; Kim, H.S.; de Figueiredo, P. Microfabricated devices in microbial bioenergy sciences. Trends Biotechnol. 2013, 31, 225–232. [Google Scholar] [CrossRef]
- Guzman, A.; Kim, H.S.; de Figueiredo, P.; Han, A. A three-dimensional electrode for highly efficient electrocoalescence-based droplet merging. Biomed. Microdevices 2015, 17, 1–9. [Google Scholar] [CrossRef]
- Neofotis, P.; Huang, A.; Sury, K.; Chang, W.; Joseph, F.; Gabr, A.; Twary, S.; Qiu, W.; Holguin, O.; Polle, J.E. Characterization and classification of highly productive microalgae strains discovered for biofuel and bioproduct generation. Algal Res. 2016, 15, 164–178. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhao, J.; Wang, Y.; Wang, W.; Gao, Y.; Xu, R.; Zhao, W. A new microfluidic device for classification of microalgae cells based on simultaneous analysis of chlorophyll fluorescence, side light scattering, resistance pulse sensing. Micromachines 2016, 7, 198. [Google Scholar] [CrossRef] [Green Version]
- Benazzi, G.; Holmes, D.; Sun, T.; Mowlem, M.; Morgan, H. Discrimination and analysis of phytoplankton using a microfluidic cytometer. IET Nanobiotechnol. 2007, 1, 94–101. [Google Scholar] [CrossRef]
- Hashemi, N.; Erickson, J.S.; Golden, J.P.; Jackson, K.M.; Ligler, F.S. Microflow Cytometer for optical analysis of phytoplankton. Biosens. Bioelectr. 2011, 26, 4263–4269. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, N.; Erickson, J.S.; Golden, J.P.; Ligler, F.S. Optofluidic characterization of marine algae using a microflow cytometer. Biomicrofluidics 2011, 5, 032009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaap, A.; Bellouard, Y.; Rohrlack, T. Optofluidic lab-on-a-chip for rapid algae population screening. Biomed. Opt. Express 2011, 2, 658–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaap, A.; Rohrlack, T.; Bellouard, Y. Optical classification of algae species with a glass lab-on-a-chip. Lab Chip 2012, 12, 1527–1532. [Google Scholar] [CrossRef]
- Polle, J.E. Microalgae strain isolation, screening, and identification for biofuels and high-value products. In Microalgal Production for Biomass and High-Value Products; CRC Press: Boca Raton, FL, USA, 2017; pp. 63–89. [Google Scholar]
- Guo, M.T.; Rotem, A.; Heyman, J.A.; Weitz, D.A. Droplet microfluidics for high-throughput biological assays. Lab Chip 2012, 12, 2146–2155. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Kim, H.S.; Guzman, A.R.; Shim, W.-B.; Han, A. A large-scale on-chip droplet incubation chamber enables equal microbial culture time. RSC Adv. 2016, 6, 20516–20519. [Google Scholar] [CrossRef]
- Lagus, T.P.; Edd, J.F. A review of the theory, methods and recent applications of high-throughput single-cell droplet microfluidics. J. Phys. D Appl. Phys. 2013, 46, 114005. [Google Scholar] [CrossRef]
- Schaap, A.; Rohrlack, T.; Bellouard, Y. Lab on a chip technologies for algae detection: A review. J. Biophotonics 2012, 5, 661–672. [Google Scholar] [CrossRef]
- Rusconi, R.; Garren, M.; Stocker, R. Microfluidics expanding the frontiers of microbial ecology. Annu. Rev. Biophys. 2014, 43, 65–91. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Sun, J.; Song, Y.; Xu, Y.; Pan, X.; Sun, Y.; Li, D. A label-free microfluidic biosensor for activity detection of single microalgae cells based on chlorophyll fluorescence. Sensors 2013, 13, 16075–16089. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.S.; Rafeie, M.; Vandamme, D.; Asadnia, M.; Henderson, R.; Taylor, R.A.; Warkiani, M.E. Selective separation of microalgae cells using inertial microfluidics. Bioresour. Technol. 2018, 252, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Li, M.; Yang, J.; Wang, J.; Pan, X.; Sun, Y.; Li, D. Capacitive detection of living microalgae in a microfluidic chip. Sensors Actuators B Chem. 2014, 194, 164–172. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Maw, M.M.; Song, Y.; Pan, X.; Sun, Y.; Li, D. Detection of size spectrum of microalgae cells in an integrated underwater microfluidic device. J. Exp. Mar. Biol. Ecol. 2015, 473, 129–137. [Google Scholar] [CrossRef]
- Song, Y.; Peng, R.; Wang, J.; Pan, X.; Sun, Y.; Li, D. Automatic particle detection and sorting in an electrokinetic microfluidic chip. Electrophoresis 2013, 34, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, F.; Juneau, P.; Izquierdo, R. Integration of fluorescence sensors using organic optoelectronic components for microfluidic platform. Sensors Actuators B Chem. 2015, 221, 1314–1320. [Google Scholar] [CrossRef]
- Antonacci, A.; Scognamiglio, V. Biotechnological advances in the design of algae-based biosensors. Trends Biotechnol. 2019, 38, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Attaallah, R.; Antonacci, A.; Mazzaracchio, V.; Moscone, D.; Palleschi, G.; Arduini, F.; Amine, A.; Scognamiglio, V. Carbon black nanoparticles to sense algae oxygen evolution for herbicides detection: Atrazine as a case study. Biosens. Bioelectron. 2020, 159, 112203. [Google Scholar] [CrossRef]
- Antonacci, A.; Attaallah, R.; Arduini, F.; Amine, A.; Giardi, M.T.; Scognamiglio, V. A dual electro-optical biosensor based on Chlamydomonas reinhardtii immobilised on paper-based nanomodified screen-printed electrodes for herbicide monitoring. J. Nanobiotechnol. 2021, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bayley, H.; Martin, C.R. Resistive-pulse sensing from microbes to molecules. Chem. Rev. 2000, 100, 2575–2594. [Google Scholar] [CrossRef]
- Müller, T.; Schnelle, T.; Fuhr, G. Dielectric single cell spectra in snow algae. Polar Biol. 1998, 20, 303–310. [Google Scholar] [CrossRef]
- Hübner, Y.; Hoettges, K.F.; Hughes, M.P. Water quality test based on dielectrophoretic measurements of fresh water algae Selenastrum capricornutum. J. Environ. Monit. 2003, 5, 861–864. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Sukhorukov, V.L.; Djuzenova, C.S.; Zimmermann, U.; Müller, T.; Fuhr, G. Electrorotational spectra of protoplasts generated from the giant marine alga Valonia utricularis. Protoplasma 1997, 196, 123–134. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, C.; Wang, L.; Miao, X.; Xing, W.; Cheng, J. Electrokinetic system to determine differences of electrorotation and traveling-wave electrophoresis between autotrophic and heterotrophic algal cells. Colloids Surfaces A Physicochem. Eng. Asp. 2005, 262, 57–64. [Google Scholar] [CrossRef]
- Ogata, S.; Yasukawa, T.; Matsue, T. Dielectrophoretic manipulation of a single chlorella cell with dual-microdisk electrode. Bioelectrochemistry 2001, 54, 33–37. [Google Scholar] [CrossRef]
- Marbà-Ardébol, A.-M.; Emmerich, J.; Neubauer, P.; Junne, S. Single-cell-based monitoring of fatty acid accumulation in Crypthecodinium cohnii with three-dimensional holographic and in situ microscopy. Process Biochem. 2016, 52, 223–232. [Google Scholar] [CrossRef]
- Dismukes, G.C.; Carrieri, D.; Bennette, N.; Ananyev, G.M.; Posewitz, M.C. Aquatic phototrophs: Efficient alternatives to land-based crops for biofuels. Curr. Opin. Biotechnol. 2008, 19, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Kindle, K.L. High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 1990, 87, 1228–1232. [Google Scholar] [CrossRef] [Green Version]
- Shimogawara, K.; Fujiwara, S.; Grossman, A.; Usuda, H. High-efficiency transformation of Chlamydomonas reinhardtii by electroporation. Genetics 1998, 148, 1821–1828. [Google Scholar] [CrossRef]
- Pethig, R. Dielectrophoresis: Status of the theory, technology, and applications. Biomicrofluidics 2010, 4, 022811. [Google Scholar] [CrossRef] [Green Version]
- Çetin, B.; Li, D. Dielectrophoresis in microfluidics technology. Electrophoresis 2011, 32, 2410–2427. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.-L.; Chang, J.-S.; Juang, Y.-J. Separation of microalgae with different lipid contents by dielectrophoresis. Bioresour. Technol. 2012, 135, 137–141. [Google Scholar] [CrossRef]
- Bae, S.; Park, S.; Kim, J.; Choi, J.S.; Kim, K.H.; Kwon, D.; Jin, E.; Park, I.; Kim, D.H.; Seo, T.S. Exogenous gene integration for microalgal cell transformation using a nanowire-incorporated microdevice. ACS Appl. Mater. Interfaces 2015, 7, 27554–27561. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.-L.; Kuo, M.-Y.; Juang, Y.-J. Development of flow through dielectrophoresis microfluidic chips for biofuel production: Sorting and detection of microalgae with different lipid contents. Biomicrofluidics 2014, 8, 064120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagnon, Z.R. Cellular dielectrophoresis: Applications to the characterization, manipulation, separation and patterning of cells. Electrophoresis 2011, 32, 2466–2487. [Google Scholar] [CrossRef]
- Michael, K.A.; Hiibel, S.R.; Geiger, E. Dependence of the dielectrophoretic upper crossover frequency on the lipid content of microalgal cells. Algal Res. 2014, 6, 17–21. [Google Scholar] [CrossRef]
- Song, H.; Rosano, J.M.; Wang, Y.; Garson, C.J.; Prabhakarpandian, B.; Pant, K.; Klarmann, G.J.; Perantoni, A.; Alvarez, L.M.; Lai, E. Continuous-flow sorting of stem cells and differentiation products based on dielectrophoresis. Lab Chip 2015, 15, 1320–1328. [Google Scholar] [CrossRef]
- Hadady, H.; Redelman, D.; Hiibel, S.R.; Geiger, E.J. Continuous-flow sorting of microalgae cells based on lipid content by high frequency dielectrophoresis. AIMS Biophys. 2016, 3, 398–414. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Gencoglu, A.; Minerick, A.R. DC insulator dielectrophoretic applications in microdevice technology: A review. Anal. Bioanal. Chem. 2010, 399, 301–321. [Google Scholar] [CrossRef] [PubMed]
- Gallo-Villanueva, R.C.; Jesús-Pérez, N.M.; Martínez-López, J.I.; Pacheco, A.; Lapizco-Encinas, B.H. Assessment of microalgae viability employing insulator-based dielectrophoresis. Microfluid. Nanofluidics 2011, 10, 1305–1315. [Google Scholar] [CrossRef]
- Song, Y.; Yang, J.; Shi, X.; Jiang, H.; Wu, Y.; Peng, R.; Wang, Q.; Gong, N.; Pan, X.-X.; Sun, Y.; et al. DC dielectrophoresis separation of marine algae and particles in a microfluidic chip. Sci. Chin. Chem. 2012, 55, 524–530. [Google Scholar] [CrossRef]
- Godino, N.; Jorde, F.; Lawlor, D.; Jaeger, M.; Duschl, C. Purification of microalgae from bacterial contamination using a disposable inertia-based microfluidic device. J. Micromech. Microeng. 2015, 25, 084002. [Google Scholar] [CrossRef]
- Di Carlo, D. Inertial microfluidics. Lab Chip 2009, 9, 3038–3046. [Google Scholar] [CrossRef] [PubMed]
- Amini, H.; Lee, W.; Di Carlo, D. Inertial microfluidic physics. Lab Chip 2014, 14, 2739–2761. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.M.; Toner, M. Inertial focusing in microfluidics. Annu. Rev. Biomed. Eng. 2014, 16, 371–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.S.; Devarenne, T.P.; Han, A. Microfluidic systems for microalgal biotechnology: A review. Algal Res. 2017, 30, 149–161. [Google Scholar] [CrossRef]
- Longsine-Parker, W.; Wang, H.; Koo, C.; Kim, J.; Kim, B.; Jayaraman, A.; Han, A. Microfluidic electro-sonoporation: A multi-modal cell poration methodology through simultaneous application of electric field and ultrasonic wave. Lab Chip 2013, 13, 2144–2152. [Google Scholar] [CrossRef]
- Im, D.J.; Jeong, S.-N.; Yoo, B.S.; Kim, B.; Kim, D.-P.; Jeong, W.-J.; Kang, I.S. Digital microfluidic approach for efficient electroporation with high productivity: Transgene expression of microalgae without cell wall removal. Anal. Chem. 2015, 87, 6592–6599. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Yuan, D.; Alici, G.; Nguyen, N.-T.; Warkiani, M.E.; Li, W. Fundamentals and applications of inertial microfluidics: A review. Lab Chip 2015, 16, 10–34. [Google Scholar] [CrossRef] [Green Version]
- Dewan, A.; Kim, J.; McLean, R.H.; Vanapalli, S.A.; Karim, M.N. Growth kinetics of microalgae in microfluidic static droplet arrays. Biotechnol. Bioeng. 2012, 109, 2987–2996. [Google Scholar] [CrossRef]
- Kim, H.S.; Guzman, A.R.; Thapa, H.R.; Devarenne, T.P.; Han, A. A droplet microfluidics platform for rapid microalgal growth and oil production analysis. Biotechnol. Bioeng. 2016, 113, 1691–1701. [Google Scholar] [CrossRef]
- Choi, S. Microscale microbial fuel cells: Advances and challenges. Biosens. Bioelectron. 2015, 69, 8–25. [Google Scholar] [CrossRef]
- Sivasankar, V.; Mylsamy, P.; Omine, K. Microbial Fuel Cell Technology for Bioelectricity; Springer: Berlin, Germany, 2018. [Google Scholar]
- Mishra, S.; Liu, Y.-J.; Chen, C.-S.; Yao, D.-J. An easily accessible microfluidic chip for high-throughput microalgae screening for biofuel production. Energies 2021, 14, 1817. [Google Scholar] [CrossRef]
- Juang, Y.-J.; Chang, J.-S. Applications of microfluidics in microalgae biotechnology: A review. Biotechnol. J. 2016, 11, 327–335. [Google Scholar] [CrossRef]
- Chiao, M.; Lam, K.B.; Lin, L. Micromachined microbial and photosynthetic fuel cells. J. Micromech. Microeng. 2006, 16, 2547–2553. [Google Scholar] [CrossRef]
- Velasquez-Orta, S.B.; Curtis, T.P.; Logan, B.E. Energy from algae using microbial fuel cells. Biotechnol. Bioeng. 2009, 103, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Shahparnia, M.; Packirisamy, M.; Juneau, P.; Zazubovich, V. Micro photosynthetic power cell for power generation from photosynthesis of algae. Technology 2015, 3, 119–126. [Google Scholar] [CrossRef]
- Bodénès, P.; Wang, H.-Y.; Lee, T.-H.; Chen, H.-Y.; Wang, C.-Y. Microfluidic techniques for enhancing biofuel and biorefinery industry based on microalgae. Biotechnol. Biofuels 2019, 12, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, R.; Kumar, S.J.; Mehendale, N.; Sevda, S.; Garlapati, V.K. Intervention of microfluidics in biofuel and bioenergy sectors: Technological considerations and future prospects. Renew. Sustain. Energy Rev. 2018, 101, 548–558. [Google Scholar] [CrossRef]
- Yang, Y.-T.; Wang, C.Y. Review of microfluidic photobioreactor technology for metabolic engineering and synthetic biology of cyanobacteria and microalgae. Micromachines 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, H.S.; Kim, J.Y.H.; Sim, S.J. A microscale approach for simple and rapid monitoring of cell growth and lipid accumulation in Neochloris oleoabundans. Bioprocess Biosyst. Eng. 2015, 38, 2035–2043. [Google Scholar] [CrossRef]
- Kwak, H.S.; Kim, J.Y.H.; Sim, S.J. A microreactor system for cultivation of Haematococcus pluvialis and astaxanthin production. J. Nanosci. Nanotechnol. 2015, 15, 1618–1623. [Google Scholar] [CrossRef]
- Schneider, F.; Draheim, J.; Kamberger, R.; Wallrabe, U. Process and material properties of polydimethylsiloxane (PDMS) for Optical MEMS. Sens. Actuators A Phys. 2009, 151, 95–99. [Google Scholar] [CrossRef]
- Perin, G.; Cimetta, E.; Monetti, F.; Morosinotto, T.; Bezzo, F. Novel micro-photobioreactor design and monitoring method for assessing microalgae response to light intensity. Algal Res. 2016, 19, 69–76. [Google Scholar] [CrossRef]
- Kim, H.S.; Devarenne, T.P.; Han, A. A high-throughput microfluidic single-cell screening platform capable of selective cell extraction. Lab Chip 2015, 15, 2467–2475. [Google Scholar] [CrossRef]
- Graham, P.J.; Riordon, J.; Sinton, D. Microalgae on display: A microfluidic pixel-based irradiance assay for photosynthetic growth. Lab Chip 2015, 15, 3116–3124. [Google Scholar] [CrossRef] [PubMed]
- Luke, C.S.; Selimkhanov, J.; Baumgart, L.; Cohen, S.E.; Golden, S.S.; Cookson, N.A.; Hasty, J. A microfluidic platform for long-term monitoring of algae in a dynamic environment. ACS Synth. Biol. 2015, 5, 8–14. [Google Scholar] [CrossRef]
- Eu, Y.-J.; Park, H.-S.; Kim, D.-P.; Hong, J.W. A microfluidic perfusion platform for cultivation and screening study of motile microalgal cells. Biomicrofluidics 2014, 8, 024113. [Google Scholar] [CrossRef] [Green Version]
- Holcomb, R.E.; Mason, L.J.; Reardon, K.F.; Cropek, N.M.; Henry, C.S. Culturing and investigation of stress-induced lipid accumulation in microalgae using a microfluidic device. Anal. Bioanal. Chem. 2011, 400, 245–253. [Google Scholar] [CrossRef]
- Bae, S.; Kim, C.W.; Choi, J.S.; Yang, J.-W.; Seo, T.S. An integrated microfluidic device for the high-throughput screening of microalgal cell culture conditions that induce high growth rate and lipid content. Anal. Bioanal. Chem. 2013, 405, 9365–9374. [Google Scholar] [CrossRef]
- Zheng, G.; Wang, Y.; Wang, Z.; Zhong, W.; Wang, H.; Li, Y. An integrated microfluidic device in marine microalgae culture for toxicity screening application. Mar. Pollut. Bull. 2013, 72, 231–243. [Google Scholar] [CrossRef]


| Microfluidic Application/ Operation | Parameter/Species | Operating Mechanism | Throughput | Reference |
|---|---|---|---|---|
| Heterotrophic MFC | Consortia containing Pseudomonas aeruginosa, Enterococcus faecium and Rhodoferax ferrireducens | Extracellular electron transfer (EET) | - | [71] |
| Photosynthetic MFC | cyanobacteria or algae | - | [71] | |
| Hybrid MFC | Heterotrophic and photosynthetic microorganism | - | [71] | |
| Identification of Cell size | Cyanothece aeruginosa, Scenedesmus acuminatus, Chlorella vulgaris, Microcystis viridis, Anabaenopsis sp., Navicula pelliculosa, Pseudokirchneriella subcapitata, Pseudana-baena sp., Monoraphidium griffithii | Optical sensing | - | [28] |
| Cell Separation | Chlamydomonas reinhardti | Dielectrophoresis | - | [55] |
| Cell Sorting | Chlamydomonas reinhardtii | Dielectrophoresis | 88.8% | [55] |
| Cell Purification | Coenochloris signiensis | Inertial focusing | 97.3% | [55] |
| Cell viability | Karenia mikimotoi Hansen, Chlorella vulgaris, N. closterium, Platymonas subcordiformis, P. delicatula, Dunaliella salina | Optical sensing | ~10 cells/min | [30] |
| Cell viability | Dunaliella salina | Capacitance-based sensing | ~40 cells/min | [32] |
| Screening/sorting by lipid content | Chlorella vulgaris | Dielectrophoresis | ~104 cells | [51] |
| Screening/sorting by lipid content | Chlorella vulgaris | Dielectrophoresis, hydrodynamic flow | ~100 cells/min | [53] |
| Size based sorting/screening | Chlorella vulgaris, Pseudokirchneriella subcapitata | Electro-osmotic field | 30–40 cells/min | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alias, A.B.; Mishra, S.; Pendharkar, G.; Chen, C.-S.; Liu, C.-H.; Liu, Y.-J.; Yao, D.-J. Microfluidic Microalgae System: A Review. Molecules 2022, 27, 1910. https://doi.org/10.3390/molecules27061910
Alias AB, Mishra S, Pendharkar G, Chen C-S, Liu C-H, Liu Y-J, Yao D-J. Microfluidic Microalgae System: A Review. Molecules. 2022; 27(6):1910. https://doi.org/10.3390/molecules27061910
Chicago/Turabian StyleAlias, Anand Baby, Shubhanvit Mishra, Gaurav Pendharkar, Chi-Shuo Chen, Cheng-Hsien Liu, Yi-Ju Liu, and Da-Jeng Yao. 2022. "Microfluidic Microalgae System: A Review" Molecules 27, no. 6: 1910. https://doi.org/10.3390/molecules27061910
APA StyleAlias, A. B., Mishra, S., Pendharkar, G., Chen, C.-S., Liu, C.-H., Liu, Y.-J., & Yao, D.-J. (2022). Microfluidic Microalgae System: A Review. Molecules, 27(6), 1910. https://doi.org/10.3390/molecules27061910








