Optimization of Ultrasound-Assisted Cellulase Extraction from Nymphaea hybrid Flower and Biological Activities: Antioxidant Activity, Protective Effect against ROS Oxidative Damage in HaCaT Cells and Inhibition of Melanin Production in B16 Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Single-Factor Experiments
2.2. Response Surface Design
2.3. AB-8 Macroporous Resin
2.3.1. Static Adsorption and Desorption
2.3.2. Dynamic Adsorption and Desorption
2.4. Activity Determination by Chemical Method
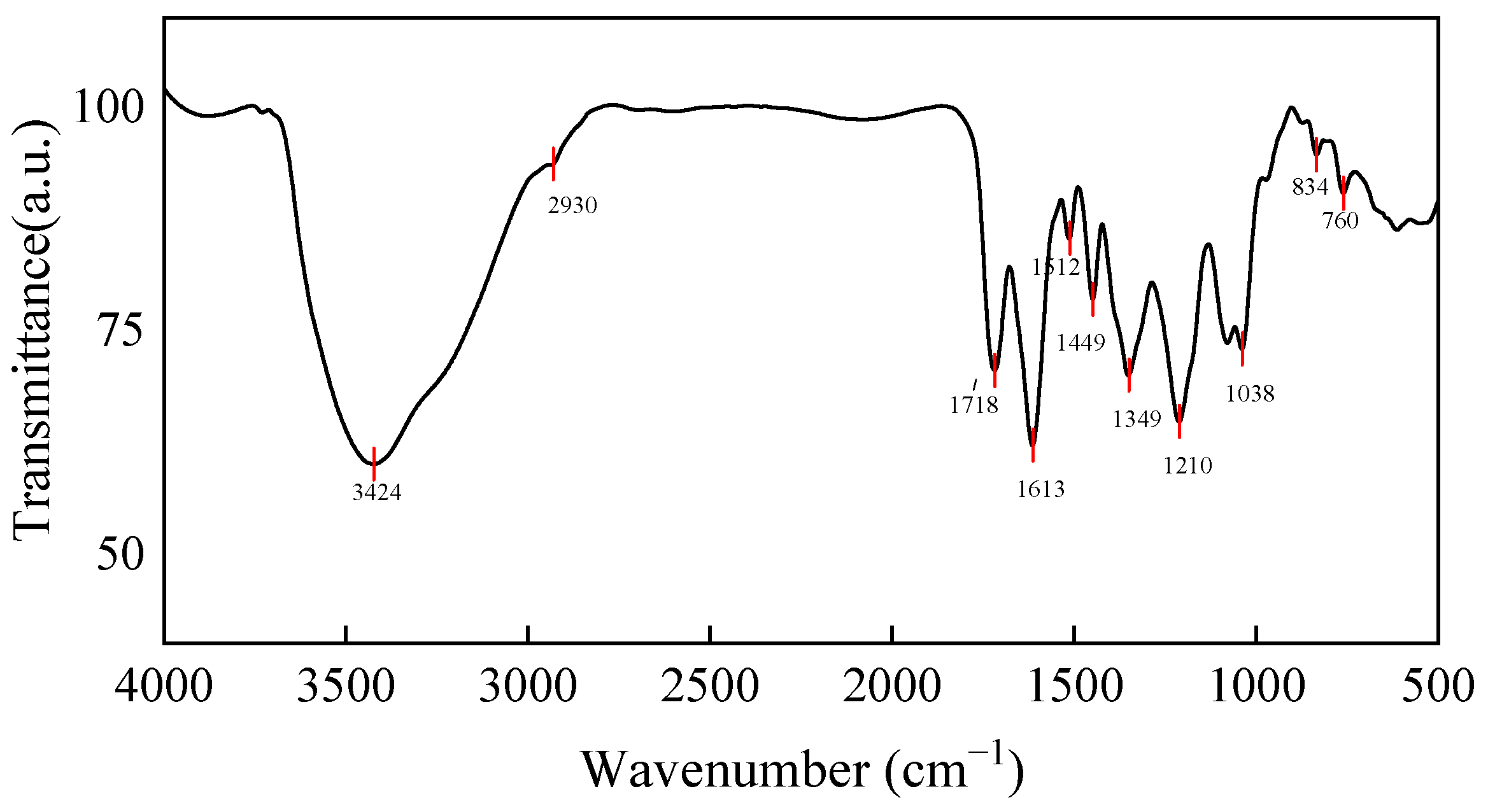
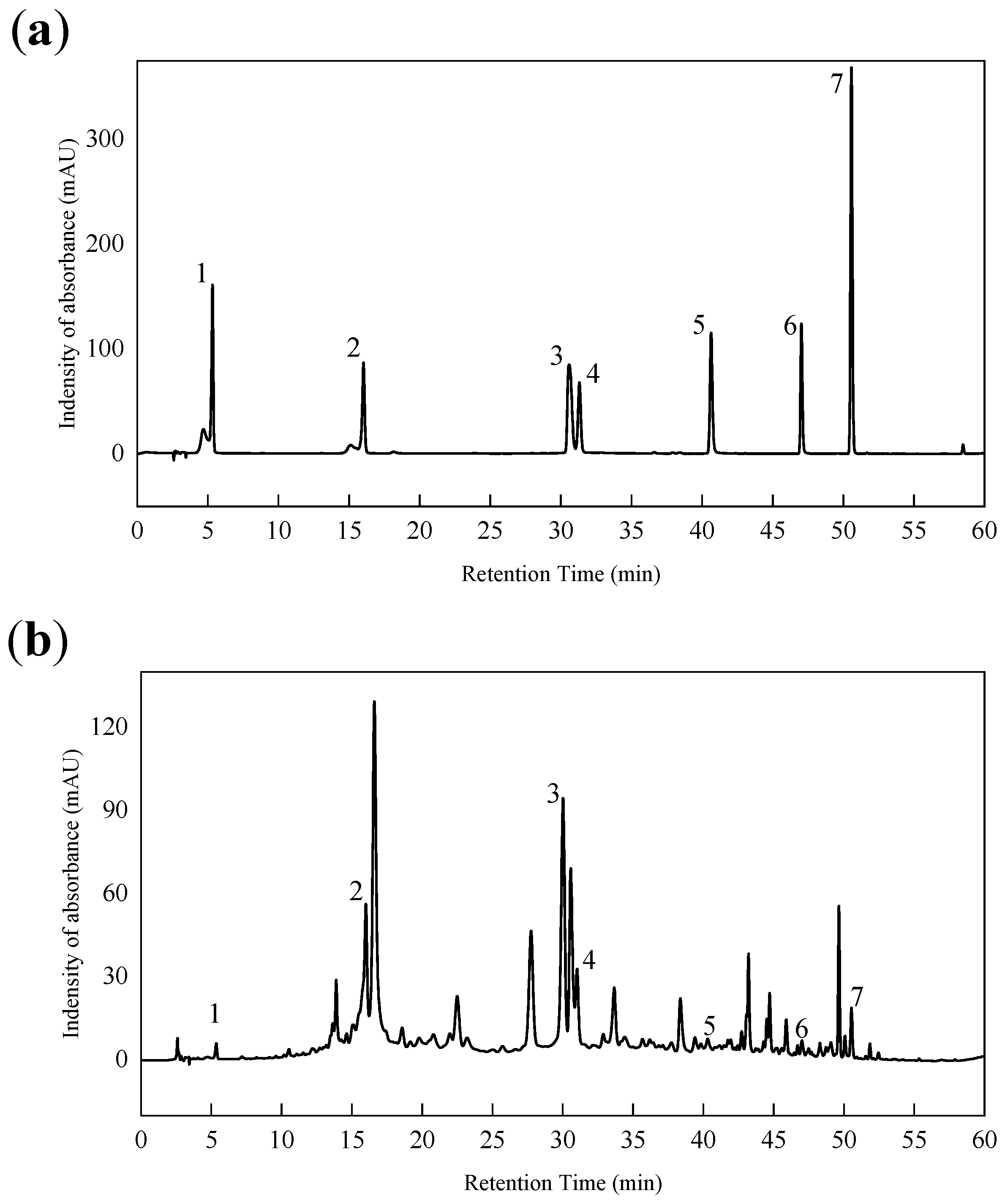
2.5. Characterization of FTIR and HPLC
2.6. HaCaT Cells Experiments
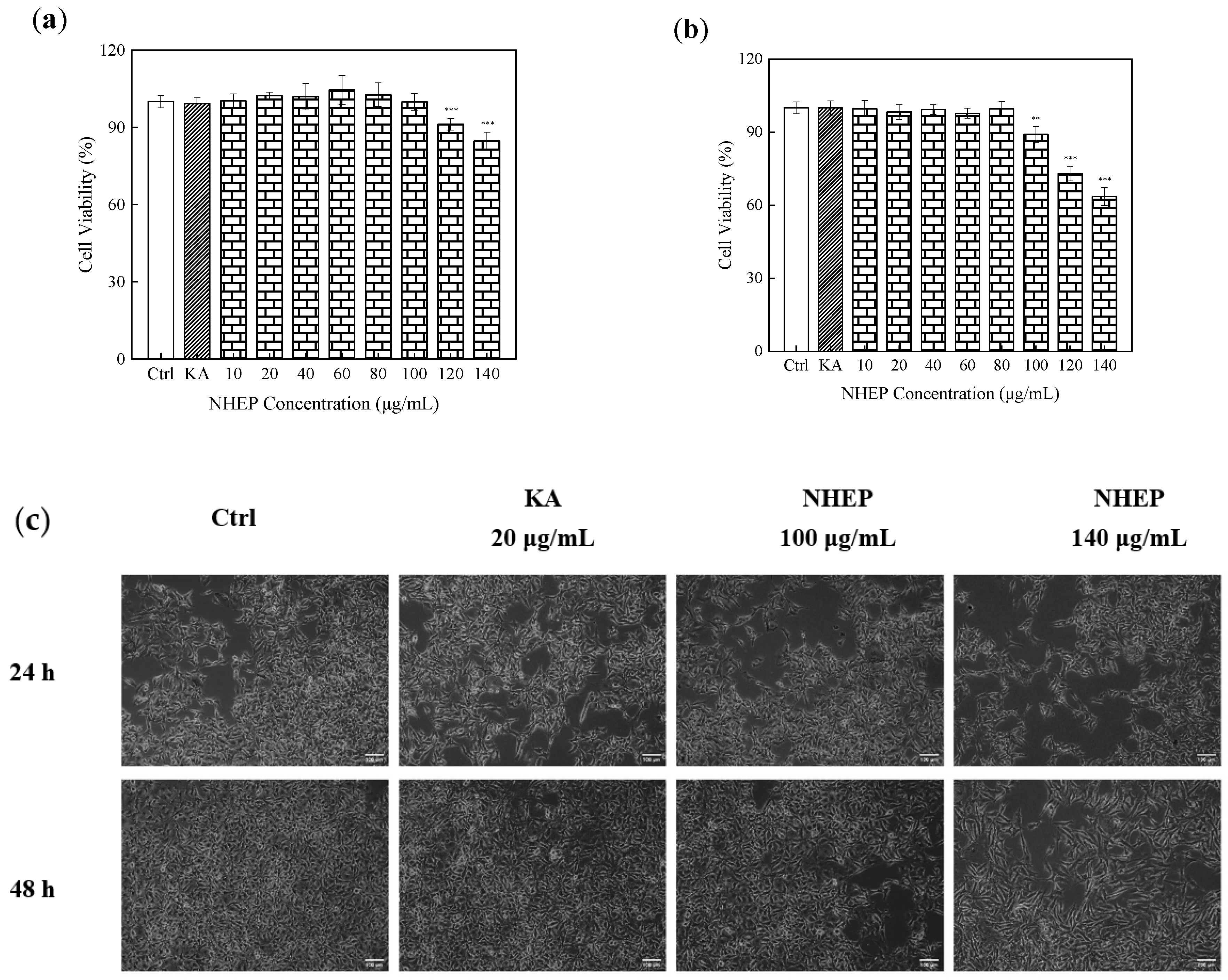
2.6.1. Effects of NHEP and H2O2 on Cell Viability of HaCaT Cells
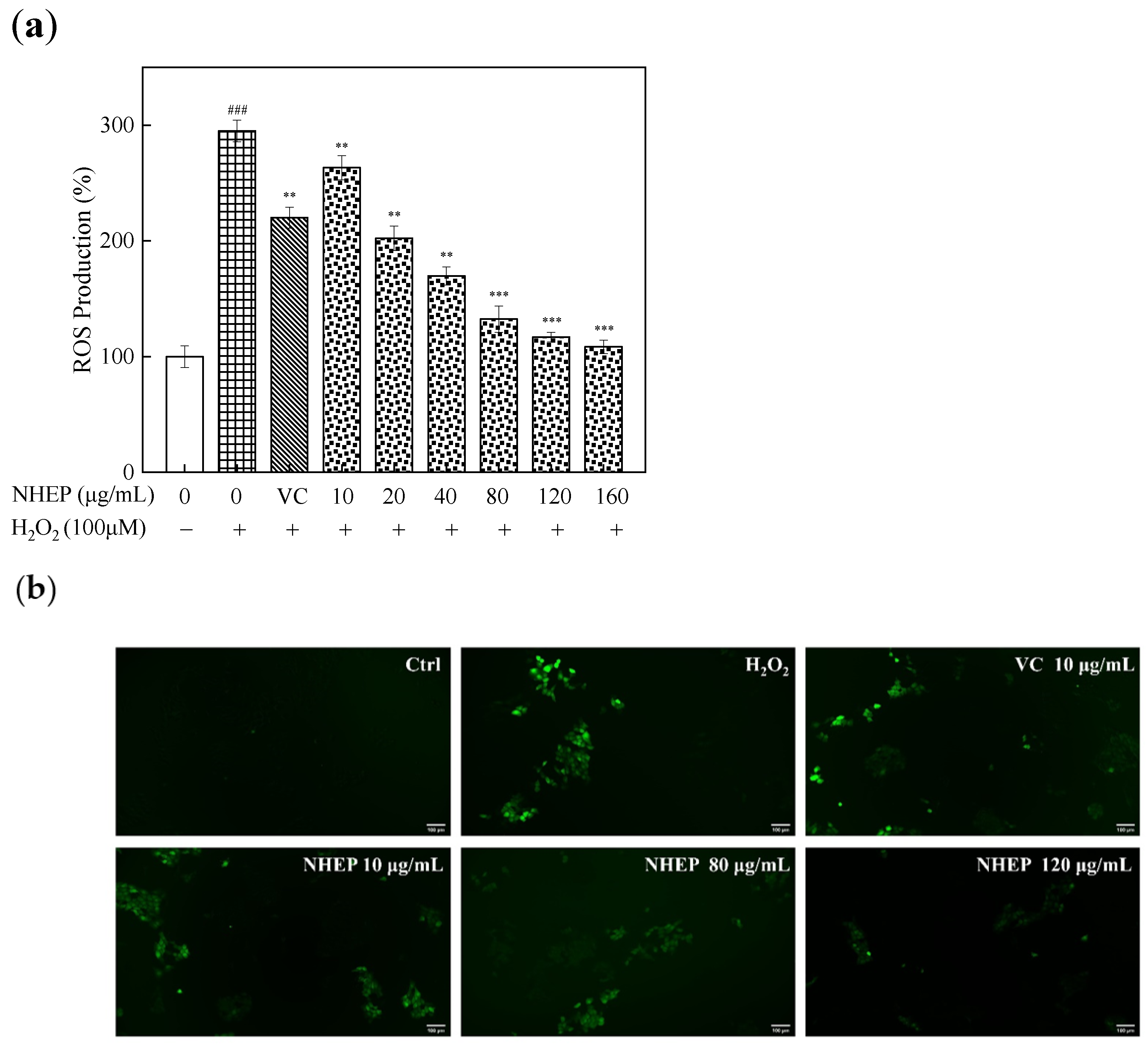
2.6.2. Effects of NHEP on the ROS Level of HaCaT Cells with Stimulation of H2O2
2.7. B16 Cell Experiments
2.7.1. Activity of B16 Cells
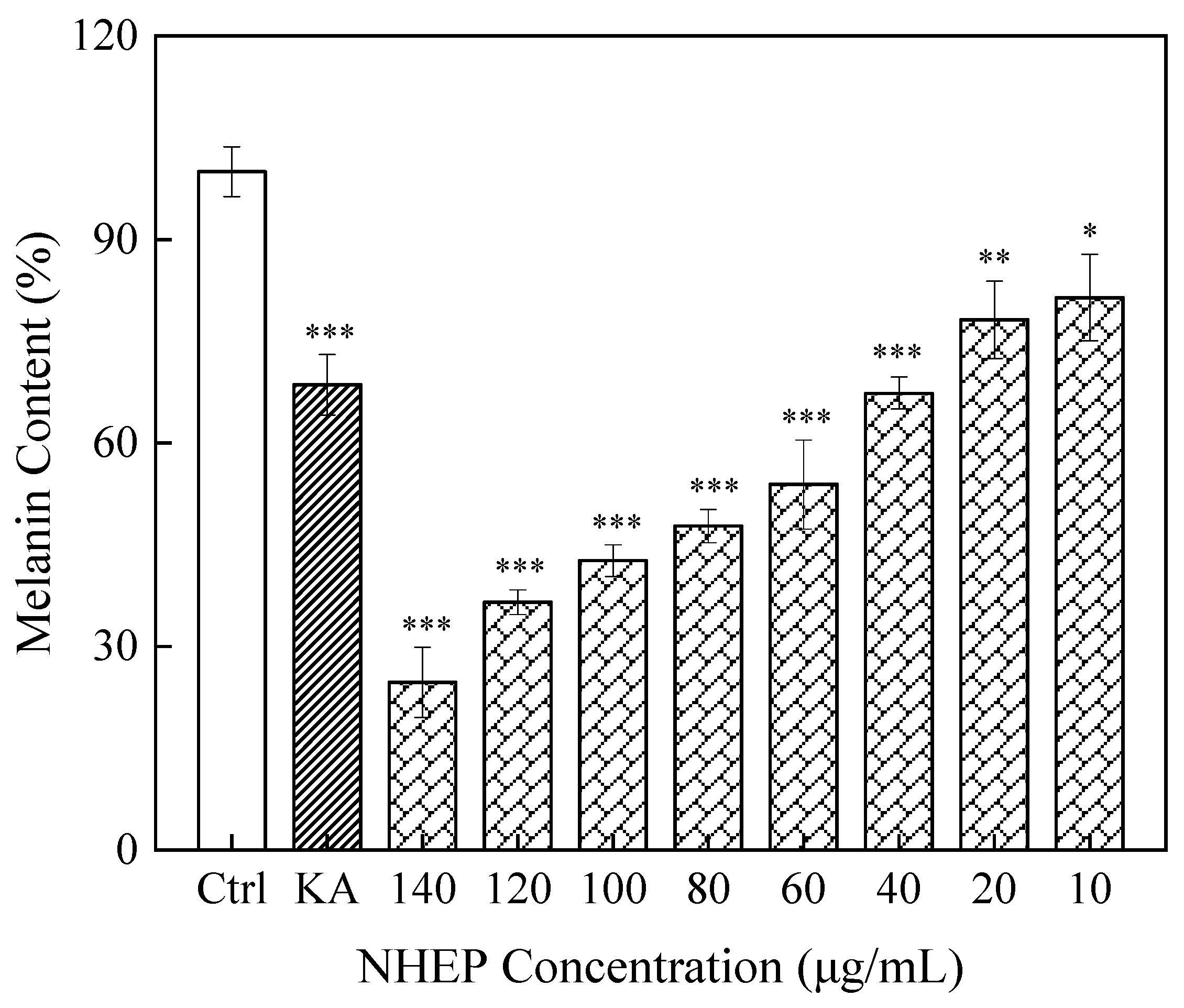
2.7.2. Effects of NHEP on B16 Melanin Production
3. Materials and Methods
3.1. Materials
3.2. Chemical Reagents
3.3. Preparation of NHE
3.4. Flavonoids and Polyphenol Determination
3.5. Response Surface Experiment
3.6. AB-8 Macroporous Resin Experiment
3.7. Antioxidant and Anti-Inflammatory Activity Analysis
3.8. Characterization of NHEP by HPLC and FTIR
3.9. HaCaT Cell Experiments
3.10. B16 Mouse Melanoma Cells Experiments
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhang, H.; Wu, H.; Zhou, Q.; Zhao, R.; Sheng, Q.; Zhu, Z. Flowering characteristics and reproductive biology of Nymphaea hybrid, a precious water lily. Sci. Hortic. 2021, 287, 110268. [Google Scholar] [CrossRef]
- Nierbauer, K.U.; Kanz, B.; Zizka, G. The widespread naturalisation of Nymphaea hybrids is masking the decline of wild-type Nymphaea alba in Hesse, Germany. Flora Morphol. Distrib. Funct. Ecol. Plants 2014, 209, 122–130. [Google Scholar] [CrossRef]
- Rasid, N.S.A.; Naim, M.N.; Man, H.C.; Bakar, N.F.A.; Mokhtar, M.N. Evaluation of surface water treated with lotus plant; Nelumbo nucifera. J. Environ. Chem. Eng. 2019, 7, 103048. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Drouet, S.; Kabra, A.; Hano, C. Enrichment in Antioxidant Flavonoids of Stamen Extracts from Nymphaea lotus L. Using Ultrasonic-Assisted Extraction and Macroporous Resin Adsorption. Antioxidants 2020, 9, 576. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.B.; Ahmed, A.; Motin, M.A.; Kim, S.; Lee, S.H. Attenuation of melanogenesis by Nymphaea nouchali (Burm. f) flower extract through the regulation of cAMP/CREB/MAPKs/MITF and proteasomal degradation of tyrosinase. Sci. Rep. 2018, 8, 13928. [Google Scholar] [CrossRef] [PubMed]
- Cudalbeanu, M.; Peitinho, D.; Silva, F.; Marques, R.; Pinheiro, T.; Ferreira, A.C.; Marques, F.; Paulo, A.; Soeiro, C.F.; Sousa, S.A.; et al. Sono-Biosynthesis and Characterization of AuNPs from Danube Delta Nymphaea alba Root Extracts and Their Biological Properties. Nanomaterials 2021, 11, 1562. [Google Scholar] [CrossRef] [PubMed]
- Jahan, I.; Mamun, M.; Hossen, M.A.; Sakir, J.; Haque, M.E. Antioxidant, Analgesic and Anti-Inflammatory Activities of Nymphaea Nouchali Flowers. Res. J. Pharmacol. 2012, 6, 62–70. [Google Scholar]
- Oyeyemi, I.T.; Akanni, O.O.; Adaramoye, O.A.; Bakare, A.A. Methanol extract of Nymphaea lotus ameliorates carbon tetrachloride-induced chronic liver injury in rats via inhibition of oxidative stress. J. Basic Clin. Physiol. Pharm. 2017, 28, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, S.; Sharma, V.; Dar, A.I.; Neetu Arya, R.C.S.; Chaurasia, I.D.; Shrivastava, R. In-vivo Antidiabetic activity of Alcoholic and Aqueous Extract of Nymphaea lotus in Rat Model. Inven. Rapid: Ethnopharmacol. 2011, 3, 2. [Google Scholar]
- Acharya, J.; Dutta, M.; Chaudhury, K.; De, B. Metabolomics and chemometric study for identification of acetylcholinesterase inhibitor(s) from the flower extracts of Nymphaea pubescens. J. Food Biochem. 2018, 42, e12575. [Google Scholar] [CrossRef]
- Ordonez, A.; Gomez, J.; Vattuone, M.; Lsla, M. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Mita, R.N.L.P.; Amir, H. Antioxidant activity of methanolic extract of Eucheuma spinosum extracted using a microwave. IOP Conf. Ser. Earth Environ. Sci. 2021, 763, 012028. [Google Scholar]
- El-Roby, A.M.; Hammad, K.S.M.; Galal, S.M. Enhancing oxidative stability of sunflower oil with sesame (Sesamum indicum) coat ultrasonic extract rich in polyphenols. J. Food Processing Preserv. 2020, 44, 8. [Google Scholar] [CrossRef]
- Salehi, H.; Karimi, M.; Raofie, F. Micronization and coating of bioflavonoids extracted from Citrus sinensis L. peels to preparation of sustained release pellets using supercritical technique. J. Iran. Chem. Soc. 2021, 18, 1–14. [Google Scholar] [CrossRef]
- Ines, I.M.; Arturo, R.J.; Sandra, D.M.; Montserrat, C.; Guadalupe, A.M. Enzymatic hydrolysis of lignocellulosic biomass using native cellulase produced by Aspergillus niger ITV02 under liquid state fermentation. Biotechnol. Appl. Biochem. 2021, 69, 198–208. [Google Scholar]
- Drevelegka, I.; Goula, A.M. Recovery of grape pomace phenolic compounds through optimized extraction and adsorption processes. Chem. Eng. Processing Process Intensif. 2020, 149, 107845. [Google Scholar] [CrossRef]
- Hu, B.; Li, Y.; Song, J.; Li, H.; Zhou, Q.; Li, C.; Zhang, Z.; Liu, Y.; Liu, A.; Zhang, Q.; et al. Oil extraction from tiger nut (Cyperus esculentus L.) using the combination of microwave-ultrasonic assisted aqueous enzymatic method-design, optimization and quality evaluation. J. Chromatogr. A 2020, 1627, 461380. [Google Scholar] [CrossRef]
- Tran, D.D.; Hoang, L.D.; Tai, N.; Thi, P.M.T.; My, P.L.T.; Thanh, V.H.; Viet, N.D.; Thuy, L.N.T.; Nam, H.M.; Phong, M.T.; et al. Utilization of Response Surface Methodology in Optimization of Polysaccharides Extraction from Vietnamese Red by Ultrasound-Assisted Enzymatic Method and Examination of Bioactivities of the Extract. Sci. World J. 2021, 11, 7594092. [Google Scholar]
- Costa-Junior, R.B.; Brandao-Costa, R.M.P.; Albuquerque, W.W.C.; Batista, J.M.S.; Pedrosa, R.B.; Porto, A.L.F. Ultrasound-Assisted Enzyme-Catalyzed Hydrolysis of Collagen to Produce Peptides with Biomedical Potential: Collagenase From Aspergillus terreus UCP1276. Bioelectromagnetics 2020, 41, 113–120. [Google Scholar] [CrossRef]
- Xu, X.Y.; Meng, J.M.; Mao, Q.Q.; Shang, A.; Li, B.Y.; Zhao, C.N.; Tang, G.Y.; Cao, S.Y.; Wei, X.L.; Gan, R.Y.; et al. Effects of Tannase and Ultrasound Treatment on the Bioactive Compounds and Antioxidant Activity of Green Tea Extract. Antioxidants 2019, 8, 362. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Li, X. Ultrasonic-assisted enzymatic extraction of silymarin from the Silybum marianum seed shell and evaluation of its antioxidant activity in vitro. EXCLI J. 2015, 14, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, H.; He, L.; Li, Y.; Li, C.; Zhang, Z.; Liu, Y.; Zhou, K.; Zhang, Q.; Liu, A.; et al. A method for extracting oil from cherry seed by ultrasonic-microwave assisted aqueous enzymatic process and evaluation of its quality. J. Chromatogr. A 2019, 1587, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Tan, J.; Li, Q.; Tang, J.; Cai, X. Ultrasound-Assisted Enzymatic Extraction of Anthocyanins from Raspberry Wine Residues: Process Optimization, Isolation, Purification, and Bioactivity Determination. Food Anal. Methods 2021, 14, 1369–1386. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Yang, Y.; Song, X.; Yu, Z. Optimization of ultrasound-assisted compound enzymatic extraction and characterization of polysaccharides from blackcurrant. Carbohydr. Polym. 2015, 117, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.-Q. Optimization of Ultrasonic Extraction of Polysaccharides from Lentinus Edodes Based on Enzymatic Treatment. J. Food Processing Preserv. 2015, 39, 254–259. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, G.; Zhang, J.; Jia, S.; Li, F.; Wang, Y.; Wu, S. Response surface optimization of ultrasound-assisted enzymatic extraction polysaccharides from Lycium barbarum. Carbohydr. Polym. 2014, 110, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Yoon, C.H.; Row, K.H. Ultrasonic-Assisted Enzymatic Ionic Liquid-Based Extraction and Separation of Flavonoids from Chamaecyparis Obtusa. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 2029–2043. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, M.N. Ultrasonic pre-irradiation effect upon aqueous enzymatic oil extraction from almond and apricot seeds. Ultrason. Sonochem. 2006, 13, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Cudalbeanu, M.; Ghinea, I.O.; Furdui, B.; Dah-Nouvlessounon, D.; Raclea, R.; Costache, T.; Cucolea, I.E.; Urlan, F.; Dinica, R.M. Exploring New Antioxidant and Mineral Compounds from Nymphaea alba Wild-Grown in Danube Delta Biosphere. Molecules 2018, 23, 1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.Z.; Wu, W.; Jiao, L.L.; Yang, P.F.; Guo, M.Q. Analysis of Flavonoids in Lotus (Nelumbo nucifera) Leaves and Their Antioxidant Activity Using Macroporous Resin Chromatography Coupled with LC-MS/MS and Antioxidant Biochemical Assays. Molecules 2015, 20, 10553–10565. [Google Scholar] [CrossRef] [Green Version]
- Mariotti-Celis, M.S.; Martínez-Cifuentes, M.; Huamán-Castilla, N.; Pedreschi, F.; Iglesias-Rebolledo, N.; Pérez-Correa, J.R. Impact of an integrated process of hot pressurised liquid extraction-macroporous resin purification over the polyphenols, hydroxymethylfurfural and reducing sugars content of Vitis vinifera ‘Carménère’ pomace extracts. Int. J. Food Sci. Technol. 2018, 53, 1072–1078. [Google Scholar] [CrossRef]
- Limwachiranon, J.; Huang, H.; Li, L.; Duan, Z.; Luo, Z. Recovery of lotus (Nelumbo nucifera Gaertn.) seedpod flavonoids using polar macroporous resins: The updated understanding on adsorption/desorption mechanisms and the involved intermolecular attractions and bonding. Food Chem. 2019, 299, 125108. [Google Scholar] [CrossRef] [PubMed]
- Xiaoyong, W.; Yuan, T.; Yanxia, S.; Dabing, X.; Yan, W.; Qi, W.; Gang, Z. Retracted Article: Extraction of flavonoids and kinetics of purification by macroporous resins from quinoa. J. Nanoparticle Res. 2020, 22, 181. [Google Scholar] [CrossRef]
- Yang, X.; Bai, Z.F.; Zhang, D.W.; Zhang, Y.; Cui, H.; Zhou, H.L. Enrichment of flavonoid-rich extract from Bidens bipinnata L. by macroporous resin using response surface methodology, UHPLC-Q-TOF MS/MS-assisted characterization and comprehensive evaluation of its bioactivities by analytical hierarchy process. Biomed. Chromatogr. 2020, 34, e4933. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jiang, Y.; Zhang, L.; Zhou, J.; Yu, Y.; Zhang, S.; Zhou, Y. Green and efficient extraction of total glucosides from Paeonia lactiflora Pall. ‘Zhongjiang’ by subcritical water extraction combined with macroporous resin enrichment. Ind. Crops Prod. 2019, 141, 111699. [Google Scholar] [CrossRef]
- Sklirou, A.D.; Angelopoulou, M.T.; Argyropoulou, A.; Chaita, E.; Boka, V.I.; Cheimonidi, C.; Niforou, K.; Mavrogonatou, E.; Pratsinis, H.; Kalpoutzakis, E.; et al. Phytochemical Study and In Vitro Screening Focusing on the Anti-Aging Features of Various Plants of the Greek Flora. Antioxidants 2021, 10, 1206. [Google Scholar] [CrossRef]
- Kunnaja, P.; Chansakaow, S.; Wittayapraparat, A.; Yusuk, P.; Sireeratawong, S. In Vitro Antioxidant Activity of Litsea martabanica Root Extract and Its Hepatoprotective Effect on Chlorpyrifos-Induced Toxicity in Rats. Molecules 2021, 26, 1906. [Google Scholar] [CrossRef]
- Cosin-Tomas, M.; Senserrich, J.; Arumi-Planas, M.; Alquezar, C.; Pallas, M.; Martin-Requero, A.; Sunol, C.; Kaliman, P.; Sanfeliu, C. Role of Resveratrol and Selenium on Oxidative Stress and Expression of Antioxidant and Anti-Aging Genes in Immortalized Lymphocytes from Alzheimer’s Disease Patients. Nutrients 2019, 11, 1764. [Google Scholar] [CrossRef] [Green Version]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Sarteshnizi, R.A.; Udenigwe, C.C.; Aluko, R.E. A Concise Review of Current In Vitro Chemical and Cell-Based Antioxidant Assay Methods. Molecules 2021, 26, 4865. [Google Scholar] [CrossRef]
- Li, Y.L.; Chen, X.; Niu, S.Q.; Zhou, H.Y.; Li, Q.S. Protective Antioxidant Effects of Amentoflavone and Total Flavonoids from Hedyotis diffusa on H2O2-Induced HL-O2 Cells through ASK1/p38 MAPK Pathway. Chem. Biodivers. 2020, 17, e2000251. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Gao, T.; Yang, H.; Du, Y.; Li, C.; Wei, L.; Zhou, T.; Lu, J.; Bi, H. Simultaneous microwave/ultrasonic-assisted enzymatic extraction of antioxidant ingredients from Nitraria tangutorun Bobr. juice by-products. Ind. Crops Prod. 2015, 66, 229–238. [Google Scholar] [CrossRef]
- Huang, D.; Zhou, X.; Si, J.; Gong, X.; Wang, S. Studies on cellulase-ultrasonic assisted extraction technology for flavonoids from Illicium verum residues. Chem. Cent. J. 2016, 10, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laffort, P. A Revisited Definition of the Three Solute Descriptors Related to the Van der Waals Forces in Solutions. Open J. Phys. Chem. 2016, 6, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Xi, L.; Mu, T.; Sun, H. Preparative purification of polyphenols from sweet potato (Ipomoea batatas L.) leaves by AB-8 macroporous resins. Food Chem. 2015, 172, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Fangma, Y.; Jin, W.; Jin, Z.; Li, X.; He, Y. Response surface optimization of the water immersion extraction and macroporous resin purification processes of anhydrosafflor yellow B from Carthamus tinctorius L. J. Food Sci. 2020, 85, 3191–3201. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Lu, X. Enrichment and purification of madecassoside and asiaticoside from Centella asiatica extracts with macroporous resins. J. Chromatogr. A 2008, 1193, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Gomathi, D.; Ravikumar, G.; Kalaiselvi, M.; Vidya, B.; Uma, C. In Vitro Free Radical Scavenging Activity of Ethanolic Extract of the Whole Plant of Evolvulus alsinoides (L.) L. Chin. J. Integr. Med. 2015, 21, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Pereira, J.; Ferreira, N.; Paiva, N.; Ferra, J.; Magalhães, F.D.; Martins, J.M.; Dulyanska, Y.; Carvalho, L.H. Valorisation of non-timber by-products from maritime pine (Pinus pinaster, Ait) for particleboard production. Ind. Crops Prod. 2021, 168. [Google Scholar] [CrossRef]
- García, D.E.; Delgado, N.; Aranda, F.L.; Toledo, M.A.; Cabrera-Barjas, G.; Sintjago, E.M.; Escobar-Avello, D.; Paczkowski, S. Synthesis of maleilated polyflavonoids and lignin as functional bio-based building-blocks. Ind. Crops Prod. 2018, 123, 154–163. [Google Scholar] [CrossRef]
- Escobar-Avello, D.; Avendano-Godoy, J.; Santos, J.; Lozano-Castellon, J.; Mardones, C.; von Baer, D.; Luengo, J.; Lamuela-Raventos, R.M.; Vallverdu-Queralt, A.; Gomez-Gaete, C. Encapsulation of Phenolic Compounds from a Grape Cane Pilot-Plant Extract in Hydroxypropyl Beta-Cyclodextrin and Maltodextrin by Spray Drying. Antioxidants 2021, 10, 1130. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.; Sharma, P.; Kumar, V. Structural profiling of wax biopolymer from Pinus roxburghii Sarg. needles using spectroscopic methods. Int. J. Biol. Macromol. 2017, 104, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Felhi, S.; Hajlaoui, H.; Ncir, M.; Bakari, S.; Ktari, N.; Saoudi, M.; Gharsallah, N.; Kadri, A. Nutritional, phytochemical and antioxidant evaluation and FT-IR analysis of freeze dried extracts of Ecballium elaterium fruit juice from three localities. Food Sci. Technol. 2016, 36, 646–655. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.N.; Samad, M.A.; Zubair, M.A.; Haque, M.Z.; Mitra, K.; Khan, T.; Hossain, M.; Syed, A.; Afroze, A. Potential bioactive phytochemicals, antioxidant properties and anticancer pathways of Nymphaea nouchali. Asian Pac. J. Trop. Bio. 2020, 10, 555–562. [Google Scholar] [CrossRef]
- Gunhyuk, P.; Yeomoon, S.; Wonil, L.; Hyun, S.S.; Sook, O.M. Protection on Skin Aging Mediated by Antiapoptosis Effects of the Water Lily (Nymphaea Tetragona Georgi) via Reactive Oxygen Species Scavenging in Human Epidermal Keratinocytes. Pharmacology 2016, 97, 282–293. [Google Scholar]
- Medrano-Padial, C.; Puerto, M.; Moreno, F.J.; Richard, T.; Cantos-Villar, E.; Pichardo, S. In Vitro Toxicity Assessment of Stilbene Extract for Its Potential Use as Antioxidant in the Wine Industry. Antioxidants 2019, 8, 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Wang, W.; Mao, X. Chitopentaose protects HaCaT cells against H2O2-induced oxidative damage through modulating MAPKs and Nrf2/ARE signaling pathways. J. Funct. Foods 2020, 72, 104086. [Google Scholar] [CrossRef]
- Chang, T.M.; Tsen, J.H.; Yen, H.; Yang, T.Y.; Huang, H.C. Extract from Periostracum cicadae Inhibits Oxidative Stress and Inflammation Induced by Ultraviolet B Irradiation on HaCaT Keratinocytes. Evid. Based Complementary Altern. Med. 2017, 2017, 8325049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinsons Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [Green Version]
- Hou, D.-X. Potential Mechanisms of Cancer Chemoprevention by Anthocyanins. Curr. Mol. Med. 2003, 3, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Diaconeasa, Z.; Leopold, L.; Rugina, D.; Ayvaz, H.; Socaciu, C. Antiproliferative and antioxidant properties of anthocyanin rich extracts from blueberry and blackcurrant juice. Int. J. Mol. Sci. 2015, 16, 2352–2365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, H.; Fan, X.; Lv, Q.; Zhang, Q.; Xiao, J.; Qian, Y.; Zheng, B.; Gao, H.; Gao, S.; Chen, L. Folium nelumbinis (Lotus leaf) volatile-rich fraction and its mechanisms of action against melanogenesis in B16 cells. Food Chem. 2020, 330, 127030. [Google Scholar] [CrossRef] [PubMed]
- Aimvijarn, P.; Rodboon, T.; Payuhakrit, W.; Suwannalert, P. Nymphaea pubescens Induces Apoptosis, Suppresses Cellular Oxidants-Related Cell Invasion in B16 Melanoma Cells. Pharm. Sci. 2018, 24, 199–206. [Google Scholar] [CrossRef]
- Parimala, M.; Shoba, F.G. Phytochemical analysis and In vitro antioxidant acitivity of hydroalcoholic seed extract of Nymphaea nouchali Burm. f. Asian Pac. J. Trop. Biomed. 2013, 3, 887–895. [Google Scholar] [CrossRef] [Green Version]
- Shaddel, R.; Maskooki, A.; Haddad-Khodaparast, M.H.; Azadmard-Damirchi, S.; Mohamadi, M.; Fathi-Achachlouei, B. Optimization of extraction process of bioactive compounds from Bene hull using subcritical water. Food Sci. Biotechnol. 2014, 23, 1459–1468. [Google Scholar] [CrossRef]
- Mancarz, G.F.F.; Laba, L.C.; Morais Silva, T.A.; Pazzim, M.D.S.; de Souza, D.; Prado, M.R.M.; de Souza, L.M.; Nakashima, T.; Mello, R.G. Chemical composition and biological activity of Liquidambar styraciflua L. leaf essential oil. Ind. Crops Prod. 2019, 138, 111446. [Google Scholar] [CrossRef]
- Jesse, A.O. Fourier Transform Infrared Spectroscopy Analysis of Allium sativum L. and Nymphaea lotus L. Asian J. Appl. Chem. Res. 2020, 6, 7–24. [Google Scholar] [CrossRef]
- Liu, H.M.; Xu, P.F.; Cheng, M.Y.; Lei, S.N.; Liu, Q.L.; Wang, W. Optimization of Fermentation Process of Pomegranate Peel and Schisandra Chinensis and the Biological Activities of Fermentation Broth: Antioxidant Activity and Protective Effect Against H2O2-induced Oxidative Damage in HaCaT Cells. Molecules 2021, 26, 3432. [Google Scholar] [CrossRef]
- Song, I.B.; Gu, H.; Han, H.J.; Lee, N.Y.; Cha, J.Y.; Son, Y.K.; Kwon, J. Effects of 7-MEGA(TM) 500 on Oxidative Stress, Inflammation, and Skin Regeneration in H2O2-Treated Skin Cells. Toxicol. Res. 2018, 34, 103–110. [Google Scholar] [CrossRef]
- Zanoni, T.B.; Hudari, F.; Munnia, A.; Peluso, M.; Godschalk, R.W.; Zanoni, M.V.; den Hartog, G.J.; Bast, A.; Barros, S.B.; Maria-Engler, S.S.; et al. The oxidation of p-phenylenediamine, an ingredient used for permanent hair dyeing purposes, leads to the formation of hydroxyl radicals: Oxidative stress and DNA damage in human immortalized keratinocytes. Toxicol. Lett. 2015, 239, 194–204. [Google Scholar] [CrossRef]
- Lee, H.; Yeom, M.; Shin, S.; Jeon, K.; Park, D.; Jung, E. Protective Effects of Aqueous Extract of Mentha suaveolens against Oxidative Stress-Induced Damages in Human Keratinocyte HaCaT Cells. Evid. Based Complementary Altern. Med. 2019, 2019, 5045491. [Google Scholar] [CrossRef] [PubMed]
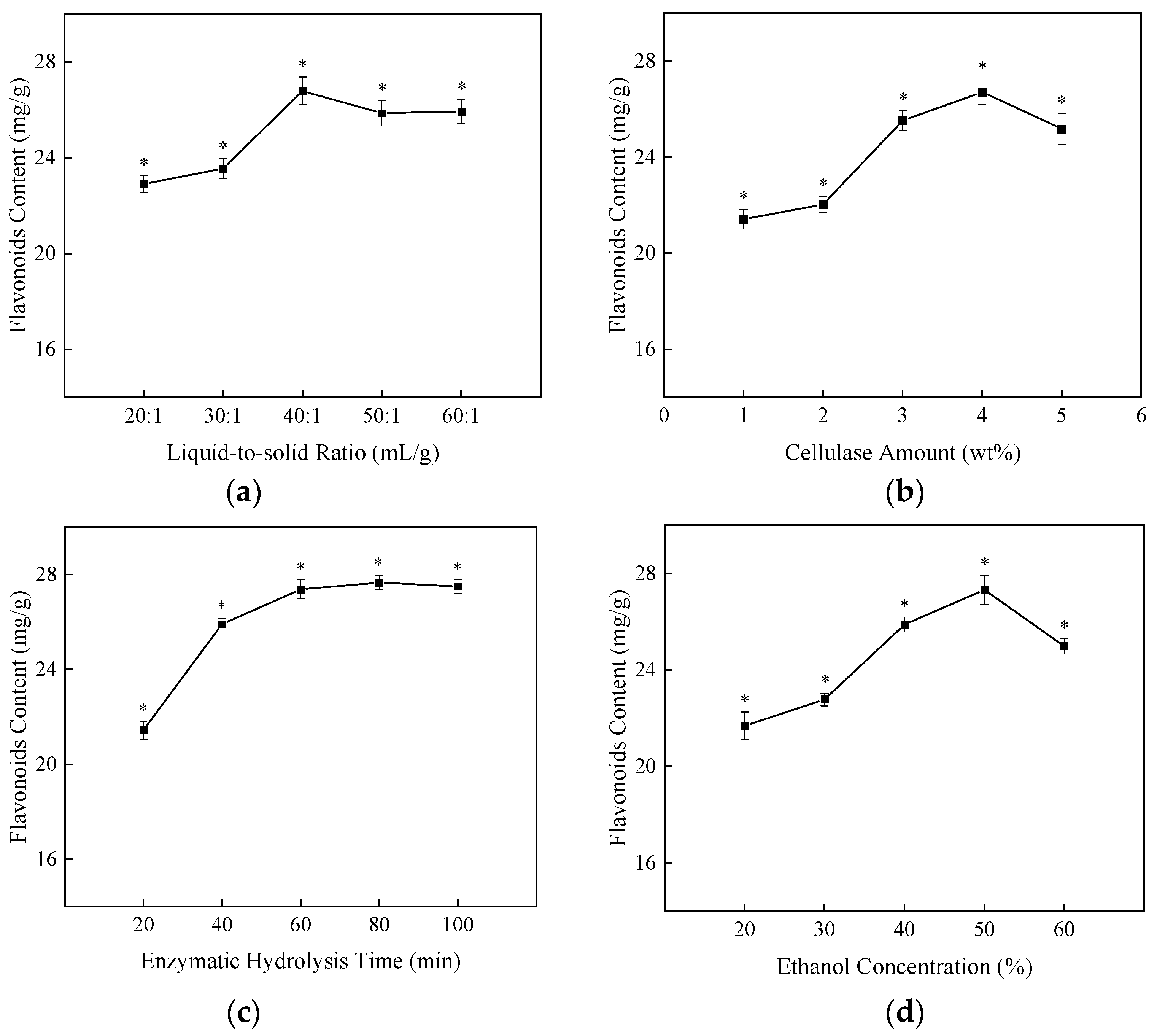
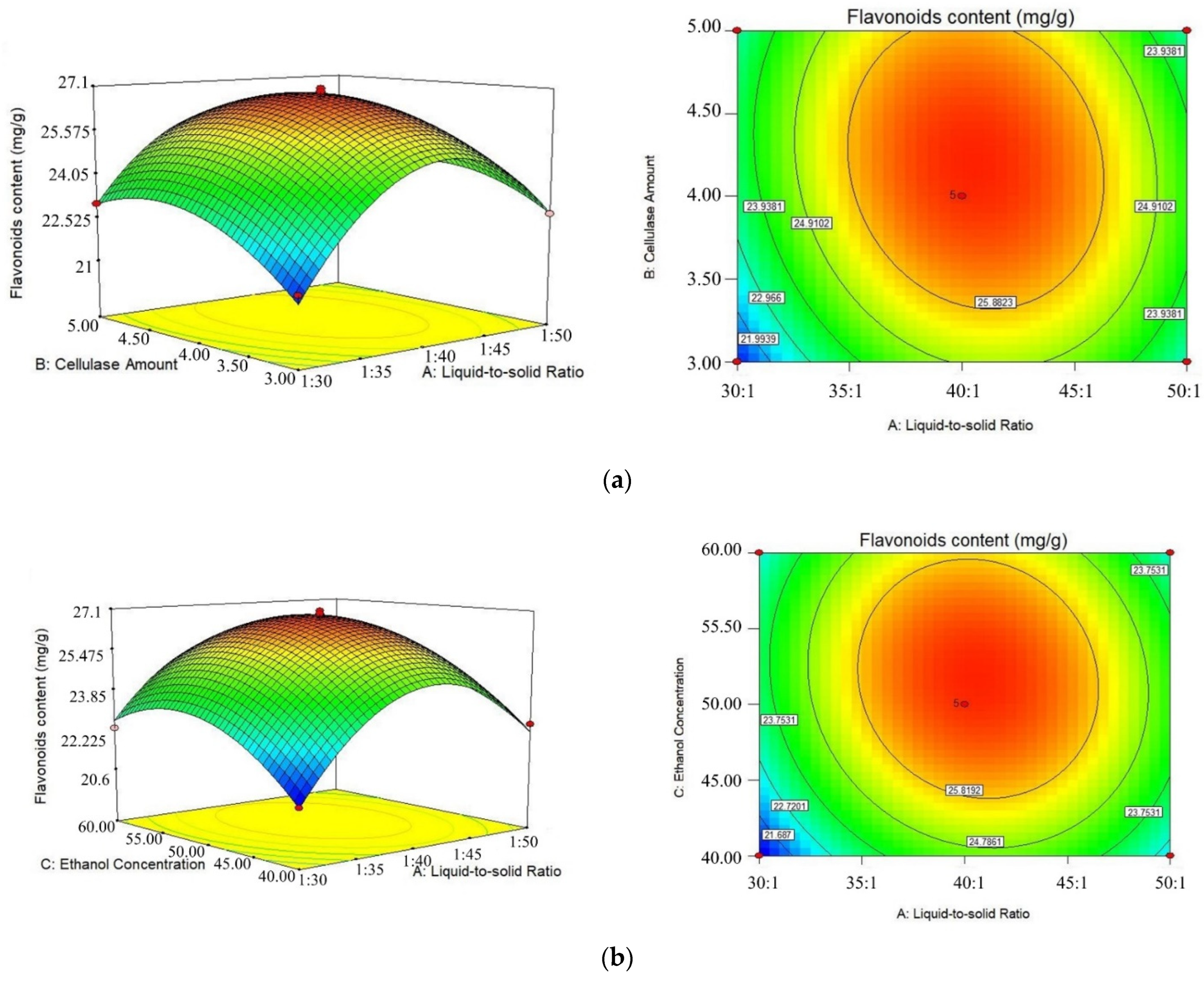
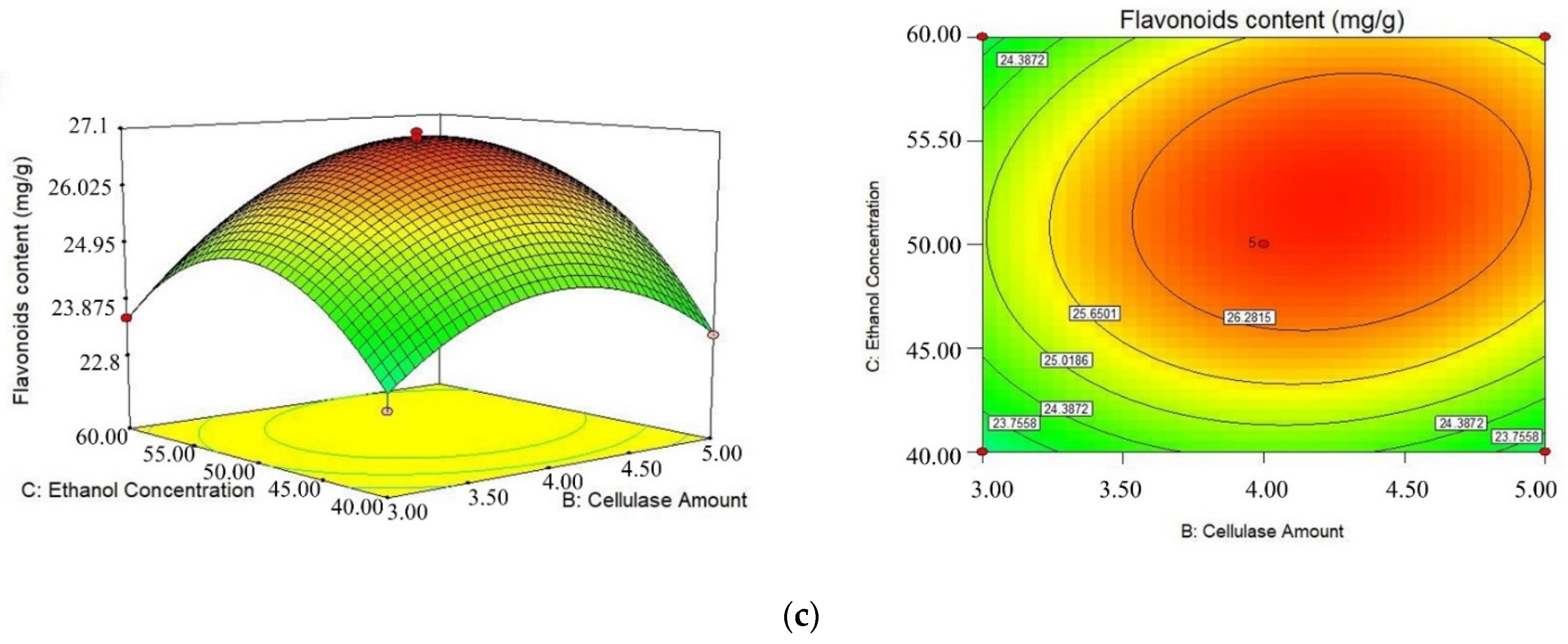


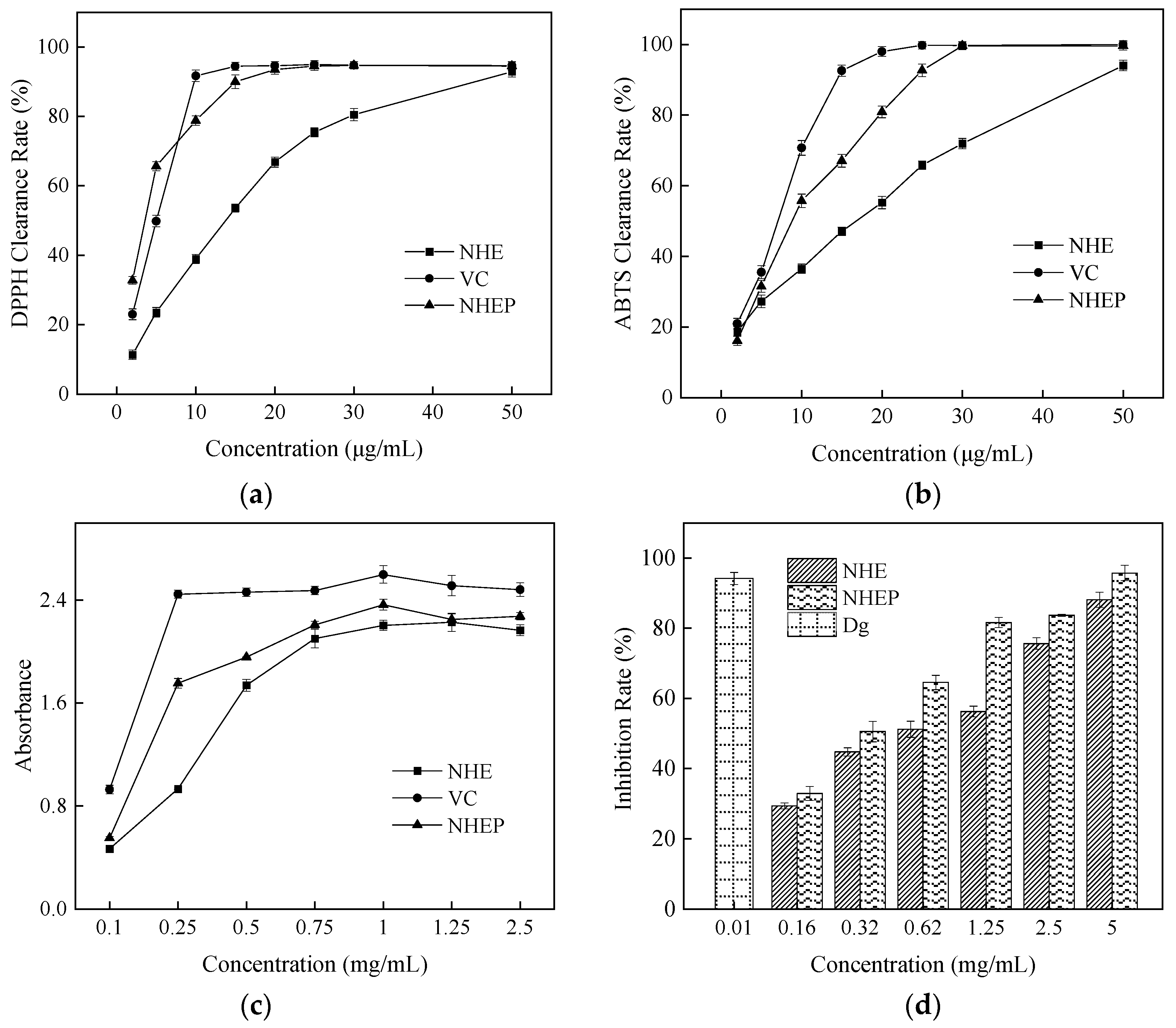


| NO. | A/(mL/g) | B/(%) | C/(%) | Flavonoids Content (mg/g) |
|---|---|---|---|---|
| 1 | 40:1 | 4 | 50 | 26.62 |
| 2 | 40:1 | 5 | 60 | 25.66 |
| 3 | 30:1 | 3 | 50 | 21.30 |
| 4 | 40:1 | 5 | 40 | 23.34 |
| 5 | 40:1 | 3 | 40 | 22.83 |
| 6 | 50:1 | 4 | 60 | 22.73 |
| 7 | 40:1 | 4 | 50 | 26.40 |
| 8 | 40:1 | 4 | 50 | 27.06 |
| 9 | 30:1 | 4 | 60 | 22.30 |
| 10 | 50:1 | 4 | 40 | 22.65 |
| 11 | 30:1 | 4 | 40 | 20.67 |
| 12 | 40:1 | 4 | 50 | 26.92 |
| 13 | 30:1 | 5 | 50 | 23.02 |
| 14 | 50:1 | 3 | 50 | 22.85 |
| 15 | 40:1 | 4 | 50 | 26.95 |
| 16 | 50:1 | 5 | 50 | 22.72 |
| 17 | 40:1 | 3 | 60 | 23.52 |
| Source | Sum of Squares | df | Mean Squares | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 71.7596 | 9 | 7.9733 | 67.6019 | <0.0001 *** |
| A | 1.6801 | 1 | 1.6801 | 14.2445 | 0.0069 ** |
| B | 2.2521 | 1 | 2.2521 | 19.0949 | 0.0033 ** |
| C | 2.7817 | 1 | 2.7817 | 23.5852 | 0.0018 ** |
| AB | 0.8540 | 1 | 0.8540 | 7.2411 | 0.0310 * |
| AC | 0.5987 | 1 | 0.5987 | 5.0763 | 0.0589 |
| BC | 0.6680 | 1 | 0.6680 | 5.6636 | 0.0489 * |
| A2 | 38.6989 | 1 | 38.6989 | 328.1105 | <0.0001 *** |
| B2 | 6.9468 | 1 | 6.9468 | 58.8986 | 0.0001 *** |
| C2 | 11.7205 | 1 | 11.7205 | 99.3726 | <0.0001 *** |
| Residual | 0.8256 | 7 | 0.1179 | ||
| Lack of Fit | 0.5251 | 3 | 0.1750 | 2.3293 | 0.2159 |
| Pure Error | 0.3006 | 4 | 0.0751 | ||
| Cor Total | 72.5852 | 16 | |||
| R2 | 0.9886 | ||||
| Sample | Compound | |
|---|---|---|
| Flavonoids Content (mg/g) | Polyphenols Content (mg/g) | |
| NHE | 55.60 ± 0.38 | 414.97 ± 2.95 |
| NHEP | 157.39 ± 1.18 * | 888.63 ± 4.41 * |
| Standard | Retention Time (min) | Regression Equation | R2 | NHEP Compound (mg/g) |
|---|---|---|---|---|
| Gallic acid | 5.33 | y = 28.696x – 6.5794 | 0.9997 | 1.60 ± 0.36 |
| Corilagin | 16.21 | y = 14.902x – 40.435 | 0.9992 | 65.60 ± 3.36 |
| Ellagic acid | 30.58 | y = 10.3x – 11.978 | 0.9999 | 97.72 ± 0.92 |
| Rutin | 31.32 | y = 10.71x – 9.2865 | 0.9999 | 34.41 ± 4.24 |
| Myricetin | 40.86 | y = 12.76x + 4.3092 | 0.9996 | 3.13 ± 0.87 |
| Quercetin | 47.15 | y = 11.001x + 2.6758 | 0.9999 | 4.64 ± 0.15 |
| Naringin | 50.69 | y = 34.715x + 24.424 | 0.9999 | 4.03 ± 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.-M.; Lei, S.-N.; Tang, W.; Xun, M.-H.; Zhao, Z.-W.; Cheng, M.-Y.; Zhang, X.-D.; Wang, W. Optimization of Ultrasound-Assisted Cellulase Extraction from Nymphaea hybrid Flower and Biological Activities: Antioxidant Activity, Protective Effect against ROS Oxidative Damage in HaCaT Cells and Inhibition of Melanin Production in B16 Cells. Molecules 2022, 27, 1914. https://doi.org/10.3390/molecules27061914
Liu H-M, Lei S-N, Tang W, Xun M-H, Zhao Z-W, Cheng M-Y, Zhang X-D, Wang W. Optimization of Ultrasound-Assisted Cellulase Extraction from Nymphaea hybrid Flower and Biological Activities: Antioxidant Activity, Protective Effect against ROS Oxidative Damage in HaCaT Cells and Inhibition of Melanin Production in B16 Cells. Molecules. 2022; 27(6):1914. https://doi.org/10.3390/molecules27061914
Chicago/Turabian StyleLiu, Hui-Min, Sheng-Nan Lei, Wei Tang, Meng-Han Xun, Zhi-Wei Zhao, Ming-Yan Cheng, Xiao-Dan Zhang, and Wei Wang. 2022. "Optimization of Ultrasound-Assisted Cellulase Extraction from Nymphaea hybrid Flower and Biological Activities: Antioxidant Activity, Protective Effect against ROS Oxidative Damage in HaCaT Cells and Inhibition of Melanin Production in B16 Cells" Molecules 27, no. 6: 1914. https://doi.org/10.3390/molecules27061914
APA StyleLiu, H.-M., Lei, S.-N., Tang, W., Xun, M.-H., Zhao, Z.-W., Cheng, M.-Y., Zhang, X.-D., & Wang, W. (2022). Optimization of Ultrasound-Assisted Cellulase Extraction from Nymphaea hybrid Flower and Biological Activities: Antioxidant Activity, Protective Effect against ROS Oxidative Damage in HaCaT Cells and Inhibition of Melanin Production in B16 Cells. Molecules, 27(6), 1914. https://doi.org/10.3390/molecules27061914






