New Insights on Acanthus ebracteatus Vahl: UPLC-ESI-QTOF-MS Profile, Antioxidant, Antimicrobial and Anticancer Activities
Abstract
:1. Introduction
2. Results
2.1. Evaluation of the Antioxidant Activity
2.2. Total Phenolic and Flavonoid Content
2.3. Evaluation of Antimicrobial Activity
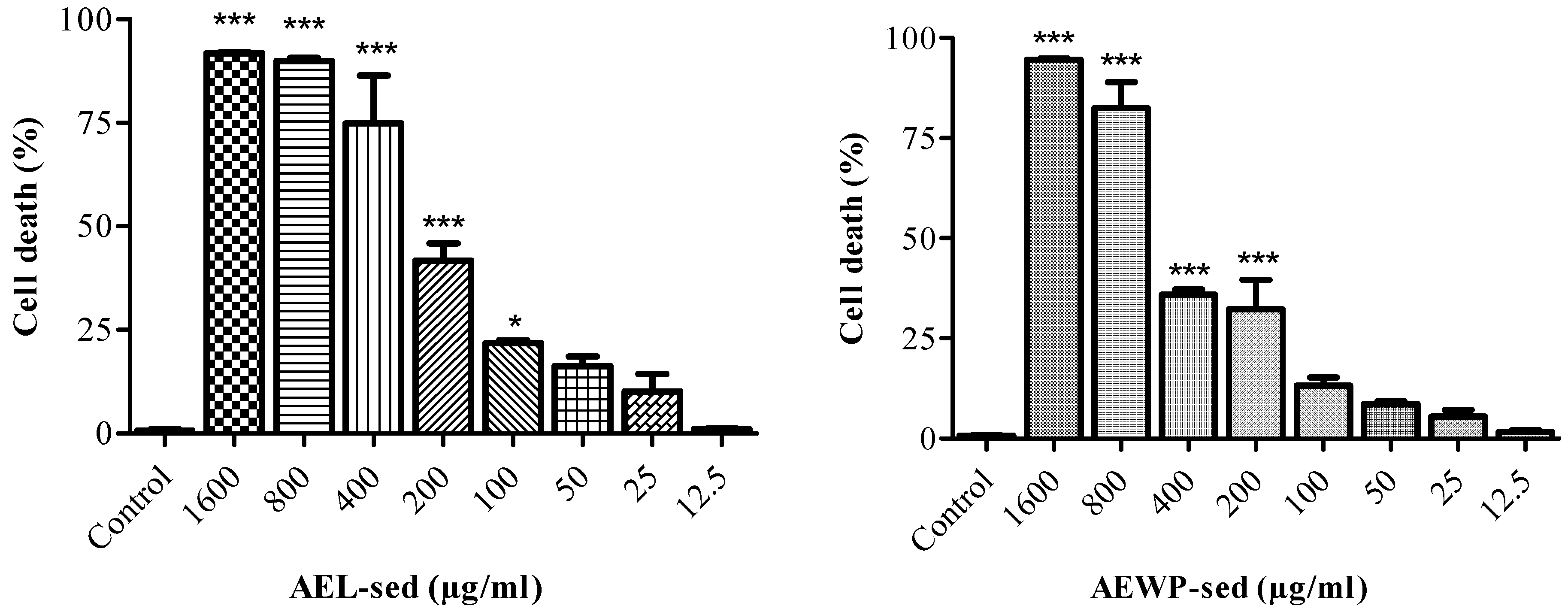
2.4. Evaluation of Anticancer Activity
2.5. Evaluation of the Anti-Proliferative Effect
2.6. Identification of Compounds in AEL-sed and AEPW-sed
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material
4.3. Preparation of A. ebracteatus Extracts
4.3.1. Classical Ethanol Extraction
4.3.2. Extraction Using the Sedimentation Method
4.4. Total Phenolic and Flavonoid Content
4.5. Antioxidant Activity
4.6. Antibacterial Activity
4.7. Anticancer Efficacy Compounds
4.8. Anti-Proliferative Effect of Extract
4.9. UHPLC-ESI-QTOF-MS Profiling of the Extracts
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Makinde, E.A.; Radenahmad, N.; Adekoya, A.E.; Olatunji, O.J. Tiliacora triandra extract possesses antidiabetic effects in high fat diet/streptozotocin-induced diabetes in rats. J. Food Biochem. 2020, 44, e13239. [Google Scholar] [CrossRef] [PubMed]
- Sinan, K.I.; Cádiz-Gurrea, M.D.L.L.; Leyva-Jiménez, F.J.; Fernández-Ochoa, Á.; Segura-Carretero, A.; Glamocilija, J.; Sokovic, M.; Nenadić, M.; Aktumsek, A.; Dall’Acqua, S.; et al. New insights on Phyllanthus reticulatus Poir. leaves and stem bark extracts: UPLC-ESI-TOF-MS profiles, and biopharmaceutical and in silico analysis. New J. Chem. 2021, 45, 21049–21065. [Google Scholar] [CrossRef]
- Salehi, B.; Azzini, E.; Zucca, P.; Maria Varoni, E.; Anil Kumar, N.V.; Dini, L.; Panzarini, E.; Rajkovic, J.; Valere Tsouh Fokou, P.; Peluso, I.; et al. Plant-derived bioactives and oxidative stress-related disorders: A key trend towards healthy aging and longevity promotion. Appl. Sci. 2020, 10, 947. [Google Scholar] [CrossRef] [Green Version]
- Forni, C.; Facchiano, F.; Bartoli, M.; Pieretti, S.; Facchiano, A.; D’Arcangelo, D.; Norelli, S.; Valle, G.; Nisini, R.; Beninati, S.; et al. Beneficial Role of Phytochemicals on Oxidative Stress and Age-Related Diseases. BioMed Res. Int. 2019, 2019, 8748253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somchaichana, J.; Bunaprasert, T.; Patumraj, S. Acanthus ebracteatusVahl. Ethanol Extract Enhancement of the Efficacy of the Collagen Scaffold in Wound Closure: A Study in a Full-Thickness-Wound Mouse Model. J. Biomed. Biotechnol. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasansuklab, A.; Tencomnao, T. Acanthus ebracteatus leaf extract provides neuronal cell protection against oxidative stress injury induced by glutamate. BMC Complement. Altern. Med. 2018, 18, 278. [Google Scholar] [CrossRef]
- Li, M.-Y.; Xiao, Q.; Pan, J.-Y.; Wu, J. Natural products from semi-mangrove flora: Source, chemistry and bioactivities. Nat. Prod. Rep. 2009, 26, 281–298. [Google Scholar] [CrossRef]
- Wisuitiprot, V.; Ingkaninan, K.; Chakkavittumrong, P.; Wisuitiprot, W.; Neungchamnong, N.; Chantakul, R.; Waranuch, N. Effects of Acanthus ebracteatus Vahl. extract and verbascoside on human dermal papilla and murine macrophage. Sci. Rep. 2022, 12, 1–13. [Google Scholar] [CrossRef]
- Pratoomsoot, C.; Wongkattiya, N.; Sanguansermsri, D. Synergistic Antimicrobial and Antioxidant Properties of Coccinia grandis (L.) Voigt, Clerodendrum inerme (L.) Gaertn. and Acanthus ebracteatus Vahl. Extracts and Their Potential as a Treatment for Xerosis Cutis. Complement. Med. Res. 2020, 27, 410–420. [Google Scholar] [CrossRef]
- Awadelkareem, A.M.; Al-Shammari, E.; Elkhalifa, A.E.O.; Adnan, M.; Siddiqui, A.J.; Snoussi, M.; Khan, M.I.; Azad, Z.R.A.A.; Patel, M.; Ashraf, S.A. Phytochemical and In Silico ADME/Tox Analysis of Eruca sativa Extract with Antioxidant, Antibacterial and Anticancer Potential against Caco-2 and HCT-116 Colorectal Carcinoma Cell Lines. Molecules 2022, 27, 1409. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physio-logical functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Eze, F.N.; Jayeoye, T.J. Chromolaena odorata (Siam weed): A natural reservoir of bioactive compounds with potent an-ti-fibrillogenic, antioxidative, and cytocompatible properties. Biomed. Pharmacother. 2021, 141, 111811. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Sun, C.; Li, J.; Long, T.; Yan, Y.; Qin, H.; Makinde, E.A.; Famurewa, A.C.; Jaisi, A.; Nie, Y.; et al. Tiliacora triandra extract and its major constituent attenuates diabetic kidney and testicular impairment by modulating redox imbalance and pro-inflammatory responses in rats. J. Sci. Food Agric. 2021, 101, 1598–1608. [Google Scholar] [CrossRef] [PubMed]
- Zafirah, A.; Shiou, A.; Lee, Y.; Mohamed, R. Pharmacological properties of agarwood tea derived from Aquilaria (Thymelaeaceae) leaves: An emerging contemporary herbal drink. J. Herb. Med. 2017, 10, 37–44. [Google Scholar]
- Wang, C.; Liao, Y.; Wang, S.; Wang, D.; Wu, N.; Xu, Q.; Jiang, W.; Qiu, M.; Liu, C. Cytoprotective effects of diosmetin against hydrogen peroxide-induced L02 cell oxidative damage via activation of the Nrf2-ARE signaling pathway. Mol. Med. Rep. 2018, 17, 7331–7338. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yu, J.-Y.; Sun, Y.; Wang, H.; Shan, H.; Wang, S. Baicalin protects LPS-induced blood–brain barrier damage and activates Nrf2-mediated antioxidant stress pathway. Int. Immunopharmacol. 2021, 96, 107725. [Google Scholar] [CrossRef]
- Ilori, N.T.O.; Liew, C.X.; Fang, C.M. The anti-inflammatory properties of Acanthus ebracteatus, Barleria lupulina and Clinacanthus nutans: A systematic review. Mol. Biol. Rep. 2020, 47, 9883–9894. [Google Scholar] [CrossRef]
- Limsuwan, S.; Jarukitsakul, S.; Issuriya, A.; Chusri, S.; Joycharat, N.; Jaisamut, P.; Saising, J.; Jetwanna, K.W.-N.; Voravuthikunchai, S.P. Thai herbal formulation ‘Ya-Pit-Samut-Noi’: Its antibacterial activities, effects on bacterial virulence factors and in vivo acute toxicity. J. Ethnopharmacol. 2020, 259, 112975. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Adnan, M.; Siddiqui, A.J.; Hamadou, W.S.; Patel, M.; Ashraf, S.A.; Jamal, A.; Awadelkareem, A.M.; Sachidanandan, M.; Snoussi, M.; De Feo, V. Phytochemistry, Bioactivities, Pharmacokinetics and Toxicity Prediction of Selaginella repanda with Its Anticancer Potential against Human Lung, Breast and Colorectal Carcinoma Cell Lines. Molecules 2021, 26, 768. [Google Scholar] [CrossRef]
- Williams, H.K. Molecular pathogenesis of oral squamous carcinoma. Mol. Pathol. 2000, 53, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Thirayan, V.; Jameson, M.B.; Gregor, R.T. Prophylactic versus reactive percutaneous endoscopic gastrostomy in oro-pharyngeal squamous cell carcinoma patients undergoing radical radiotherapy. N. Z. J. Surg. 2021, 91, 2720–2725. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, S.; Tufail, S.; Islam, N. Phenethyl isothiocyanate induces apoptosis through ROS generation and caspase-3 activation in cervical cancer cells. Front. Pharmacol. 2021, 12, 1651. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, P.S.; Ramos, P.S.; Ferreira, C.; Silva, J.L.; El-Bacha, T.; Fialho, E. Pro-Oxidant Effect of Resveratrol on Human Breast Cancer MCF-7 Cells is Associated with CK2 Inhibition. Nutr. Cancer 2021, 1–10. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, X.; Lin, J.; Song, F.; Shao, Y. Low curcumin concentration enhances the anticancer effect of 5-fluorouracil against colorectal cancer. Phytomedicine 2021, 85, 153547. [Google Scholar] [CrossRef]
- Ning, R.; Chen, G.; Fang, R.; Zhang, Y.; Zhao, W.; Qian, F. Diosmetin inhibits cell proliferation and promotes apoptosis through STAT3/c-Myc signaling pathway in human osteosarcoma cells. Biol. Res. 2021, 54, 40. [Google Scholar] [CrossRef]
- Duan, J.; Shi, J.; Ma, X.; Xuan, Y.; Li, P.; Wang, H.; Fan, Y.; Gong, H.; Wang, L.; Pang, Y.; et al. Esculetin inhibits proliferation, migration, and invasion of clear cell renal cell carcinoma cells. Biomed. Pharmacother. 2020, 125, 110031. [Google Scholar] [CrossRef]
- Wu, S.-T.; Liu, B.; Ai, Z.-Z.; Hong, Z.-C.; You, P.-T.; Wu, H.-Z.; Yang, Y.-F. Esculetin Inhibits Cancer Cell Glycolysis by Binding Tumor PGK2, GPD2, and GPI. Front. Pharmacol. 2020, 11, 379. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Guo, F.; Peng, Q.; Liu, Y.; Yang, B. Suppression of in vitro and in vivo human ovarian cancer growth by isoac-teoside is mediated via sub-G1 cell cycle arrest, ROS generation, and modulation of AKT/PI3K/m-TOR signalling pathway. J. BUON. 2019, 24, 285–290. [Google Scholar]
- Zhao, F.; Zhao, Z.; Han, Y.; Li, S.; Liu, C.; Jia, K. Baicalin suppresses lung cancer growth phenotypes via miR-340-5p/NET1 axis. Bioengineering 2021, 12, 1699–1707. [Google Scholar] [CrossRef]
- Katagi, A.; Sui, L.; Kamitori, K.; Suzuki, T.; Katayama, T.; Hossain, A.; Noguchi, C.; Dong, Y.; Yamaguchi, F.; Tokuda, M. Inhibitory effect of isoamericanol A from Jatropha curcas seeds on the growth of MCF-7 human breast cancer cell line by G2/M cell cycle arrest. Heliyon 2016, 2, e00055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Aslam Gondal, T.; Atif, M.; Shahbaz, M.; Batool Qaisarani, T.; Hanif Mughal, M.; Salehi, B.; Martorell, M.; Sharifi-Rad, J. Apigenin as an anticancer agent. Phytother. Res. 2020, 34, 1812–1828. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, Z.-J.; He, X.; Zhang, R.-Q.; Zhang, C.-F.; Li, F.; Wang, C.-Z.; Yuan, C.-S. Antitumor and immunomodulatory activity of genkwanin on colorectal cancer in the APC Min/+ mice. Int. Immunopharmacol. 2015, 29, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, C.; Song, X.; An, M.; Liu, M.; Yao, L.; Famurewa, A.C.; Olatunji, O.J. Antioxidant and Anti-inflammatory Properties Mediate the Neuroprotective Effects of Hydro-ethanolic Extract of Tiliacora triandra Against Cisplatin-induced Neurotoxicity. J. Inflamm. Res. 2021, 14, 6735–6748. [Google Scholar] [CrossRef] [PubMed]
- Olatunde, O.O.; Della Tan, S.L.; Shiekh, K.A.; Benjakul, S.; Nirmal, N.P. Ethanolic guava leaf extracts with different chlorophyll removal processes: Anti-melanosis, antibacterial properties and the impact on qualities of Pacific white shrimp during refrigerated storage. Food Chem. 2021, 341, 128251. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K.; Amnuaikit, T. Liposomal Encapsulated Ethanolic Coconut Husk Extract: Antioxidant and Antibacterial Properties. J. Food Sci. 2019, 84, 3664–3673. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Antioxidant and antibacterial properties of guava leaf extracts as affected by solvents used for prior dechlorophyllization. J. Food Biochem. 2018, 42, e12600. [Google Scholar] [CrossRef]
- Odedina, G.F.; Vongkamjan, K.; Voravuthikunchai, S.P. Potential Bio-Control Agent from Rhodomyrtus tomentosa against Listeria monocytogenes. Nutrients 2015, 7, 7451–7468. [Google Scholar] [CrossRef] [Green Version]
- Utaipan, T.; Boonyanuphong, P.; Chuprajob, T.; Suksamrarn, A.; Chunglok, W. A trienone analog of curcumin, 1,7-bis(3-hydroxyphenyl)-1,4,6-heptatrien-3-one, possesses ROS- and caspase-mediated apoptosis in human oral squa-mous cell carcinoma cells in vitro. Appl. Biol. Chem. 2020, 63, 7. [Google Scholar] [CrossRef]
- Eze, F.N.; Tola, A.J. Protein glycation and oxidation inhibitory activity of Centella asiatica phenolics (CAP) in glucose-mediated bovine serum albumin glycoxidation. Food Chem. 2020, 332, 127302. [Google Scholar] [CrossRef] [PubMed]


| Sample/Assay | AEWP-nor | AEWP-sed | AEL-nor | AEL-sed |
|---|---|---|---|---|
| TPC (mg GAE/g dry extract) | 30.49 ± 0.10 e | 36.88 ± 0.10 d | 138.20 ± 0.10 b | 140.50 ± 0.10 a |
| TFC (mg CE/g dry extract) | 20.24 ± 0.20 e | 28.82 ± 0.10 d | 107.60 ± 0.02 b | 110.40 ± 0.50 a |
| DPPH-RSA (µmol TE/g dry extract) | 91.90 ± 0.40 f | 104.90 ± 0.08 e | 448.10 ± 1.20 b | 498.80 ± 0.40 a |
| ABTS-RSA (µmol TE/g dry extract) | 44.01 ± 0.10 d | 48.59 ± 0.08 c | 57.05 ±0.10 b | 67.73 ± 0.50 a |
| FRAP (µmol TE/g dry extract) | 182.80 ± 0.40 c | 223.01 ± 0.40 b | 1098.20 ± 7.1 a | 1113.20 ± 4.20 a |
| MCA (µmol EDTA/g dry extract) | 36.32 ± 0.10 e | 37.29 ± 0.05 d | 46.87 ± 0.20 b | 47.83 ± 0.01 a |
| ORAC (µmol TE/g dry extract) | 3.22 ± 0.20 b | 3.32 ± 0.60 b | 11.51 ± 0.50 a | 11.52 ± 0.30 a |
| Samples | MIC | MBC | ||
|---|---|---|---|---|
| EC | LM | EC | LM | |
| AEWP-nor | 1.00 a | 1.00 a | 2.00 a | 2.00 a |
| AEWP-sed | 1.00 a | 1.00 a | 2.00 a | 2.00 a |
| AEL-nor | 0.25 c | 0.50 b | 0.50 c | 1.00 b |
| AEL-sed | 0.25 c | 0.50 b | 0.50 c | 1.00 b |
| No | Rt (min) | Accurate Mass (m/z) | Calculated Mass (Da) | Score (DB) | Predicted Formula | Compound Identity |
|---|---|---|---|---|---|---|
| 1 | 2.997 | 337.0775 | 338.0848 | 98.14 | C12H18O11 | L-Ascorbic acid-2-glucoside |
| 2 | 3.311 | 225.0015 | 226.009 | 63.57 | C8H7ClN4S | 6-(2-Chloroallylthio)purine |
| 3 | 4.076 | 134.0471 | 135.0544 | 87.88 | C5H5N5 | Adenine |
| 4 | 4.604 | 330.119 | 331.1262 | 98.71 | C14H21NO8 | 5′-O-beta-D-Glucosylpyridoxine |
| 5 | 4.729 | 128.0351 | 129.0426 | 95.05 | C5H7NO3 | (R)-(+)-2-Pyrrolidone-5-carboxylic acid |
| 6 | 4.767 | 243.0624 | 244.0697 | 99.79 | C9H12N2O6 | Pseudouridine |
| 7 | 4.805 | 174.077 | 175.0843 | 99.74 | C7H13NO4 | Calystegine B5 |
| 8 | 4.854 | 180.0664 | 181.0736 | 99.62 | C9H11NO3 | 3-Amino-3-(4-hydroxyphenyl)propanoate |
| 9 | 5.005 | 282.0842 | 283.0915 | 98.4 | C10H13N5O5 | Guanosine |
| 10 | 5.055 | 150.042 | 151.0492 | 98.18 | C5H5N5O | 8-Hydroxyadenine |
| 11 | 5.256 | 405.1395 | 406.1468 | 98.28 | C17 H26O11 | Morroniside |
| 12 | 5.269 | 477.1608 | 478.1681 | 97.79 | C20H30O13 | Kelampayoside A |
| 13 | 5.356 | 108.0456 | 109.0529 | 98.09 | C6H7NO | 3-Hydroxy-2-Methylpyridine |
| 14 | 5.52 | 371.0979 | 372.1052 | 98.94 | C16H20O10 | Dihydroferulic acid 4-O-glucuronide |
| 15 | 5.532 | 331.0668 | 332.0741 | 99.44 | C13H16O10 | 4-Glucogallic acid |
| 16 | 5.959 | 355.1044 | 356.1116 | 95.68 | C16H20O9 | 1-O-2′-Hydroxy-4′-methoxycinnamoyl-b-D-glucose |
| 17 | 6.16 | 461.1665 | 462.1738 | 99.05 | C20H30O12 | Verbasoside |
| 18 | 6.26 | 403.1245 | 404.1318 | 96.86 | C17H24O11 | Oleoside 11-methyl ester |
| 19 | 6.336 | 341.0875 | 342.0947 | 83.78 | C15H18O9 | Glucocaffeic acid |
| 20 | 6.36 | 179.0349 | 180.0423 | 96.04 | C9H8O4 | Caffeic Acid |
| 21 | 6.637 | 339.0721 | 340.0794 | 99.12 | C15H16O9 | Aesculin |
| 22 | 7.038 | 353.088 | 354.0953 | 99.19 | C16H18O9 | Chlorogenic acid |
| 23 | 7.114 | 593.151 | 594.1583 | 98.71 | C27H30O15 | Saponarin |
| 24 | 7.164 | 387.1657 | 388.1729 | 97.14 | C18H28O9 | 2-[4-(3-Hydroxypropyl)-2-methoxyphenoxy]-1,3-propanediol-1-xyloside |
| 25 | 7.39 | 639.1929 | 640.2024 | 57.65 | C29H36O16 | beta-Hydroxyacteoside |
| 26 | 7.415 | 637.1046 | 638.1119 | 99.11 | C27H26O18 | Scutellarein-7-glucuronosyl-(1->2)-glucuronide |
| 27 | 7.54 | 563.1406 | 564.1476 | 97.71 | C26H28O14 | Luteolin 3′-methyl ether 7,4′-dixyloside |
| 28 | 7.565 | 415.1608 | 416.1682 | 95.16 | C19H28O10 | Phenylethyl primeveroside |
| 29 | 7.817 | 177.0195 | 178.0267 | 86.68 | C9H6O4 | Esculetin |
| 30 | 7.842 | 399.166 | 400.1734 | 95.33 | C19H28O9 | Corchoionoside B |
| 31 | 7.917 | 621.11 | 622.117 | 98.15 | C27H26O17 | Genistein 4′,7-O-diglucuronide |
| 32 | 7.942 | 383.0622 | 384.0693 | 97.99 | C16H16O11 | 2-O-Feruloylhydroxycitric acid |
| 33 | 7.967 | 353.0517 | 354.0588 | 98.05 | C15H14O10 | 2-O-p-Coumaroylhydroxycitric acid |
| 34 | 8.018 | 337.0932 | 338.1004 | 98.79 | C16H18O8 | Hydrojuglone glucoside |
| 35 | 8.369 | 461.0725 | 462.0797 | 98.96 | C21H18O12 | 3-Methylellagic acid 8-rhamnoside |
| 36 | 8.432 | 623.1996 | 624.2065 | 96.46 | C29H36O15 | Isoacteoside |
| 37 | 8.62 | 637.2143 | 638.2212 | 98.89 | C30H38O15 | 4′-Hydroxy-5,7,2′-trimethoxyflavanone-4′-rhamnosyl-(1->6)-glucoside |
| 38 | 8.745 | 429.1764 | 430.1837 | 97.61 | C20H30O10 | Phenethyl rutinoside |
| 39 | 8.871 | 579.1728 | 580.1799 | 97.92 | C27H32O14 | Cascaroside F |
| 40 | 8.884 | 445.0774 | 446.0847 | 97.07 | C21H18O11 | Baicalin |
| 41 | 8.971 | 475.088 | 476.0953 | 98.09 | C22H20O12 | Diosmetin 7-O-beta-D-glucuronopyranoside |
| 42 | 9.147 | 665.2081 | 666.2159 | 88.16 | C31H38O16 | Tetramethylquercetin 3-rutinoside |
| 43 | 9.273 | 651.2289 | 652.2359 | 97.31 | C31H40O15 | (-)-Matairesinol-4′-[apiosyl-(1->2)-glucoside] |
| 44 | 9.373 | 413.2173 | 414.2247 | 95.11 | C21H34O8 | (4R,5S,7R,11S)-11,12-Dihydroxy-1(10)-spirovetiven-2-one 11-glucoside |
| 45 | 9.386 | 433.1498 | 434.1571 | 96.09 | C22H26O9 | Vestitone 7-glucoside |
| 46 | 9.624 | 431.1345 | 432.1419 | 95.75 | C22H24O9 | 4′-O-Methylglucoliquiritigenin |
| 47 | 9.8 | 591.2074 | 592.2147 | 96.83 | C29H36O13 | Osmanthuside B |
| 48 | 10.151 | 503.1552 | 504.1625 | 72.14 | C25H28O11 | Sergeolide |
| 49 | 10.277 | 473.1438 | 474.1511 | 92.54 | C24H26O10 | Luteolin 7,3′-dimethyl ether 5-glucoside |
| 50 | 10.327 | 275.092 | 276.0993 | 98.16 | C15H16O5 | 5-De-O-methyltoddanol |
| 51 | 10.478 | 285.0405 | 286.0478 | 98.52 | C15H10O6 | Luteolin |
| 52 | 10.955 | 329.1037 | 330.1107 | 85.17 | C18H18O6 | Isoamericanol A |
| 53 | 11.03 | 207.0666 | 208.0738 | 97.72 | C11H12O4 | 5-(3′,5′-Dihydroxyphenyl)-gamma valerolactone |
| 54 | 11.18 | 220.0613 | 221.0685 | 99.48 | C11H11NO4 | Methyl dioxindole-3-acetate |
| 55 | 11.193 | 329.2332 | 330.2404 | 98.67 | C18 H34O5 | 9S,10S,11R-trihydroxy-12Z-octadecenoic acid |
| 56 | 11.281 | 269.0455 | 270.0528 | 98.69 | C15H10O5 | Apigenin |
| 57 | 11.432 | 299.0563 | 300.0635 | 98.53 | C16 H12O6 | Diosmetin |
| 58 | 11.482 | 619.1446 | 620.1518 | 96.93 | C32H28O13 | Apigenin-7-(3″-acetyl-6″-E-p-coumaroylglucoside) |
| 59 | 11.934 | 268.0611 | 269.0682 | 97.25 | C15H11NO4 | Evoxanthidine |
| 60 | 13.088 | 307.1907 | 308.198 | 81.32 | C18H28O4 | Dihydrocapsiate |
| 61 | 13.289 | 675.358 | 676.3652 | 94.84 | C33H56O14 | Gingerglycolipid A |
| 62 | 13.44 | 293.1752 | 294.1825 | 98.16 | C17H26O4 | Myrsinone |
| 63 | 14.067 | 273.0765 | 274.0837 | 98.17 | C15 H14O5 | 2,3,4-Trihydroxy-4′-ethoxybenzophenone |
| 64 | 14.545 | 241.0866 | 242.0938 | 98.73 | C15H14O3 | Resveratrol 4′-methyl Ether |
| 65 | 15.8 | 291.0425 | 292.0498 | 98.05 | C15H13ClO4 | Chlorosesamone |
| No | Rt (min) | Accurate Mass (m/z) | Calculated Mass (Da) | Score (DB) | Predicted Formula | Compound Identity |
|---|---|---|---|---|---|---|
| 1 | 2.251 | 629.1697 | 630.1768 | 77.85 | C27H34O17 | Leucodelphinidin-3-O-(beta-D-glucopyranosyl-(1->4)-alpha-L-rhamnopyranoside) |
| 2 | 2.302 | 191.0562 | 192.0635 | 99.67 | C7H12O6 | Quinic acid |
| 3 | 2.427 | 827.2658 | 828.2731 | 97.02 | C30H52O26 | Verbascose |
| 4 | 2.528 | 503.1612 | 504.1685 | 98.71 | C18H32 O16 | Nephritogenoside |
| 5 | 2.653 | 683.225 | 684.2323 | 80.52 | C37H36N2O11 | Citbismine C |
| 6 | 2.654 | 341.1091 | 342.1163 | 98.89 | C12 H22 O11 | 2-O-a-D-Galactopyranuronosyl-L-rhamnose |
| 7 | 3.156 | 290.0878 | 291.095 | 99.06 | C11H17NO8 | Sarmentosin epoxide |
| 8 | 3.193 | 665.2136 | 666.2208 | 97.88 | C24H42O21 | Fagopyritol A3 |
| 9 | 3.331 | 225.0016 | 226.009 | 65.47 | C8H7ClN4S | 6-(2-Chloroallylthio)purine |
| 10 | 3.381 | 203.0196 | 204.0269 | 99.89 | C7H8O7 | Daucic acid |
| 11 | 4.762 | 243.0623 | 244.0697 | 99.5 | C9H12N2O6 | Pseudouridine |
| 12 | 4.837 | 174.077 | 175.0843 | 99.62 | C7H13NO4 | Calystegine B5 |
| 13 | 5.038 | 282.0839 | 283.0913 | 98.71 | C10H13N5O5 | Guanosine |
| 14 | 5.264 | 477.1609 | 478.1681 | 98.24 | C20H30O13 | Kelampayoside A |
| 15 | 5.904 | 329.0876 | 330.0948 | 84.7 | C14H18O9 | 3′-Glucosyl-2′,4′,6′-trihydroxyacetophenone |
| 16 | 6.093 | 359.0981 | 360.1055 | 97.46 | C15H20O10 | 6′-Methoxypolygoacetophenoside |
| 17 | 6.168 | 461.1666 | 462.1738 | 99.21 | C20H30O12 | Verbasoside |
| 18 | 6.243 | 167.0348 | 168.0421 | 99.62 | C8H8O4 | Dihydroxyphenylacetic acid |
| 19 | 6.268 | 343.1027 | 344.1102 | 97.17 | C15H20O9 | 4′,6′-Dihydroxy-2′-methoxyacetophenone 6′-glucoside |
| 20 | 6.344 | 341.0875 | 342.0947 | 82.32 | C15H18O9 | Glucocaffeic acid |
| 21 | 6.368 | 403.1241 | 404.1315 | 97.32 | C17H24O11 | Oleoside 11-methyl ester |
| 22 | 6.469 | 179.035 | 180.0416 | 60.28 | C9H8O4 | Caffeic Acid |
| 23 | 6.871 | 513.2184 | 514.2256 | 98.64 | C21H38O14 | 2-O-(beta-D-galactopyranosyl-(1->6)-beta-D-galactopyranosyl) 2S,3R-dihydroxynonanoic acid |
| 24 | 6.921 | 431.1556 | 432.1629 | 99.13 | C19H28O11 | Benzyl gentiobioside |
| 25 | 6.971 | 387.1293 | 388.1365 | 98.02 | C17H24O10 | Geniposide |
| 26 | 6.984 | 326.0886 | 327.0955 | 94.43 | C14H17NO8 | Blepharin |
| 27 | 7.047 | 457.1356 | 458.1427 | 98.51 | C20H26O12 | 7-Hydroxy-4-methylphthalide O-[arabinosyl-(1->6)-glucoside] |
| 28 | 7.122 | 593.1515 | 594.1587 | 98.85 | C27H30O15 | Saponarin |
| 29 | 7.222 | 293.124 | 294.1313 | 99.71 | C12H22O8 | Ethyl 3-O-beta-D-glucopyranosyl-butanoate |
| 30 | 7.272 | 785.2499 | 786.257 | 97.39 | C35H46O20 | Echinacoside |
| 31 | 7.461 | 639.193 | 640.2006 | 93.47 | C29H36O16 | beta-Hydroxyacteoside |
| 32 | 7.511 | 563.1404 | 564.1476 | 97.59 | C26H28O14 | Luteolin 3′-methyl ether 7,4′-dixyloside |
| 33 | 7.524 | 137.0247 | 138.0319 | 99.35 | C7H6O3 | 2,5-Dihydroxybenzaldehyde |
| 34 | 7.649 | 327.1085 | 328.1158 | 99.26 | C15H20O8 | Dihydromelilotoside |
| 35 | 7.787 | 581.224 | 582.2314 | 97.41 | C28H38O13 | (+)-Lyoniresinol 9-glucoside |
| 36 | 7.925 | 383.0619 | 384.0691 | 99.1 | C16H16O11 | 2-O-Feruloylhydroxycitric acid |
| 37 | 7.95 | 621.1096 | 622.1166 | 98.47 | C27H26O17 | Genistein 4′,7-O-diglucuronide |
| 38 | 7.975 | 353.0514 | 354.0586 | 99.36 | C15H14O10 | 2-O-p-Coumaroylhydroxycitric acid |
| 39 | 8.051 | 337.0925 | 338.0999 | 99.16 | C16H18O8 | Hydrojuglone glucoside |
| 40 | 8.377 | 551.2125 | 552.2198 | 97.21 | C27 H36O12 | Prupaside |
| 41 | 8.427 | 623.1992 | 624.2061 | 98.06 | C29H36O15 | Isoacteoside |
| 42 | 8.628 | 637.2133 | 638.2204 | 97.93 | C30H38O15 | 4′-Hydroxy-5,7,2′-trimethoxyflavanone 4′-rhamnosyl-(1->6)-glucoside |
| 43 | 9.055 | 563.1037 | 564.1109 | 98.35 | C25H24O15 | Larycitrin 3-(4″-malonylrhamnoside) |
| 44 | 9.18 | 533.093 | 534.1001 | 95.67 | C24H22O14 | 2′-Hydroxygenistein 7-(6″-malonylglucoside) |
| 45 | 9.256 | 665.2085 | 666.2156 | 98.23 | C31H38O16 | Tetramethylquercetin 3-rutinoside |
| 46 | 9.381 | 503.0817 | 504.089 | 92.63 | C23H20O13 | Gomphrenol 3-methylether 4′-glucuronide |
| 47 | 9.557 | 393.1545 | 394.1624 | 88.04 | C20H26O8 | Gibberellin A43 |
| 48 | 9.633 | 431.134 | 432.1416 | 86.12 | C22H24O9 | 4′-O-Methylglucoliquiritigenin |
| 49 | 9.833 | 144.0455 | 145.0528 | 87.81 | C9H7NO | 4-formyl Indole |
| 50 | 9.934 | 291.0871 | 292.0943 | 85.16 | C15H16O6 | trans-Grandmarin |
| 51 | 10.197 | 395.2066 | 396.214 | 94.7 | C21H32O7 | Isopetasoside |
| 52 | 10.335 | 275.0919 | 276.0993 | 97.91 | C15H16O5 | 5-De-O-methyltoddanol |
| 53 | 10.36 | 213.0917 | 214.099 | 98.96 | C14H14O2 | Ethyl 1-naphthylacetic acid |
| 54 | 10.436 | 285.0403 | 286.0476 | 99.79 | C15H10O6 | Luteolin |
| 55 | 10.586 | 721.2332 | 722.2405 | 43.96 | C34 H42O17 | Amorphigenol O-vicianoside |
| 56 | 10.687 | 135.0815 | 136.0889 | 94.23 | C9H12O | 2-(1-Pentenyl)furan |
| 57 | 11.038 | 207.0662 | 208.0734 | 99.66 | C11H12O4 | 5-(3′,5′-Dihydroxyphenyl)-gamma-valerolactone |
| 58 | 11.314 | 269.0454 | 270.0527 | 98.57 | C15H10O5 | Apigenin |
| 59 | 11.402 | 299.0558 | 300.0631 | 97.59 | C16H12O6 | Diosmetin |
| 60 | 11.515 | 619.1449 | 620.1521 | 96.73 | C32H28O13 | Apigenin 7-(3″-acetyl-6″-E-p-coumaroylglucoside) |
| 61 | 12.569 | 375.1447 | 376.1519 | 83.09 | C20H24O7 | alpha-Peroxyachifolide |
| 62 | 12.946 | 223.1338 | 224.1411 | 99.62 | C13H20O3 | Methyl jasmonate |
| 63 | 13.096 | 381.0973 | 382.1046 | 97.02 | C21H18O7 | Mollicellin B |
| 64 | 13.247 | 227.0709 | 228.0782 | 98.97 | C14H12O3 | 3,4′,5-Trihydroxystilbene |
| 65 | 13.373 | 283.0606 | 284.0679 | 84.78 | C16 H12O5 | Genkwanin |
| 66 | 13.398 | 305.1752 | 306.1825 | 84.31 | C18H26O4 | Capsiate |
| 67 | 13.448 | 309.2069 | 310.2141 | 84.83 | C18H30O4 | Auxin b |
| 68 | 13.448 | 293.1755 | 294.1828 | 95.67 | C17H26O4 | Myrsinone |
| 69 | 14.076 | 273.0764 | 274.0838 | 96.81 | C15H14O5 | 6′-Hydroxy-O-desmethylangolensin |
| 70 | 14.565 | 307.1912 | 308.1984 | 84.43 | C18H28O4 | Dihydrocapsiate |
| 71 | 15.08 | 487.3423 | 488.3494 | 95.88 | C30H48O5 | 21beta-Hydroxyhederagenin |
| 72 | 15.959 | 423.1805 | 424.1877 | 97.88 | C25H28O6 | 1,7-Dihydroxy-3,6-dimethoxy-2,8-diprenylxanthone |
| 73 | 16.737 | 407.1858 | 408.193 | 98.62 | C25H28O5 | 1-Hydroxy-3,5-dimethoxy-2,4-diprenylxanthone |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olatunji, O.J.; Olatunde, O.O.; Jayeoye, T.J.; Singh, S.; Nalinbenjapun, S.; Sripetthong, S.; Chunglok, W.; Ovatlarnporn, C. New Insights on Acanthus ebracteatus Vahl: UPLC-ESI-QTOF-MS Profile, Antioxidant, Antimicrobial and Anticancer Activities. Molecules 2022, 27, 1981. https://doi.org/10.3390/molecules27061981
Olatunji OJ, Olatunde OO, Jayeoye TJ, Singh S, Nalinbenjapun S, Sripetthong S, Chunglok W, Ovatlarnporn C. New Insights on Acanthus ebracteatus Vahl: UPLC-ESI-QTOF-MS Profile, Antioxidant, Antimicrobial and Anticancer Activities. Molecules. 2022; 27(6):1981. https://doi.org/10.3390/molecules27061981
Chicago/Turabian StyleOlatunji, Opeyemi Joshua, Oladipupo Odunayo Olatunde, Titilope John Jayeoye, Sudarshan Singh, Sirinporn Nalinbenjapun, Sasikarn Sripetthong, Warangkana Chunglok, and Chitchamai Ovatlarnporn. 2022. "New Insights on Acanthus ebracteatus Vahl: UPLC-ESI-QTOF-MS Profile, Antioxidant, Antimicrobial and Anticancer Activities" Molecules 27, no. 6: 1981. https://doi.org/10.3390/molecules27061981
APA StyleOlatunji, O. J., Olatunde, O. O., Jayeoye, T. J., Singh, S., Nalinbenjapun, S., Sripetthong, S., Chunglok, W., & Ovatlarnporn, C. (2022). New Insights on Acanthus ebracteatus Vahl: UPLC-ESI-QTOF-MS Profile, Antioxidant, Antimicrobial and Anticancer Activities. Molecules, 27(6), 1981. https://doi.org/10.3390/molecules27061981








