Cow and Ewe Cheeses Made with Saffron: Characterization of Bioactive Compounds and Their Antiproliferative Effect in Cervical Adenocarcinoma (HeLa) and Breast Cancer (MDA-MB-231) Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Saffron Bioactive Molecules in Cheese
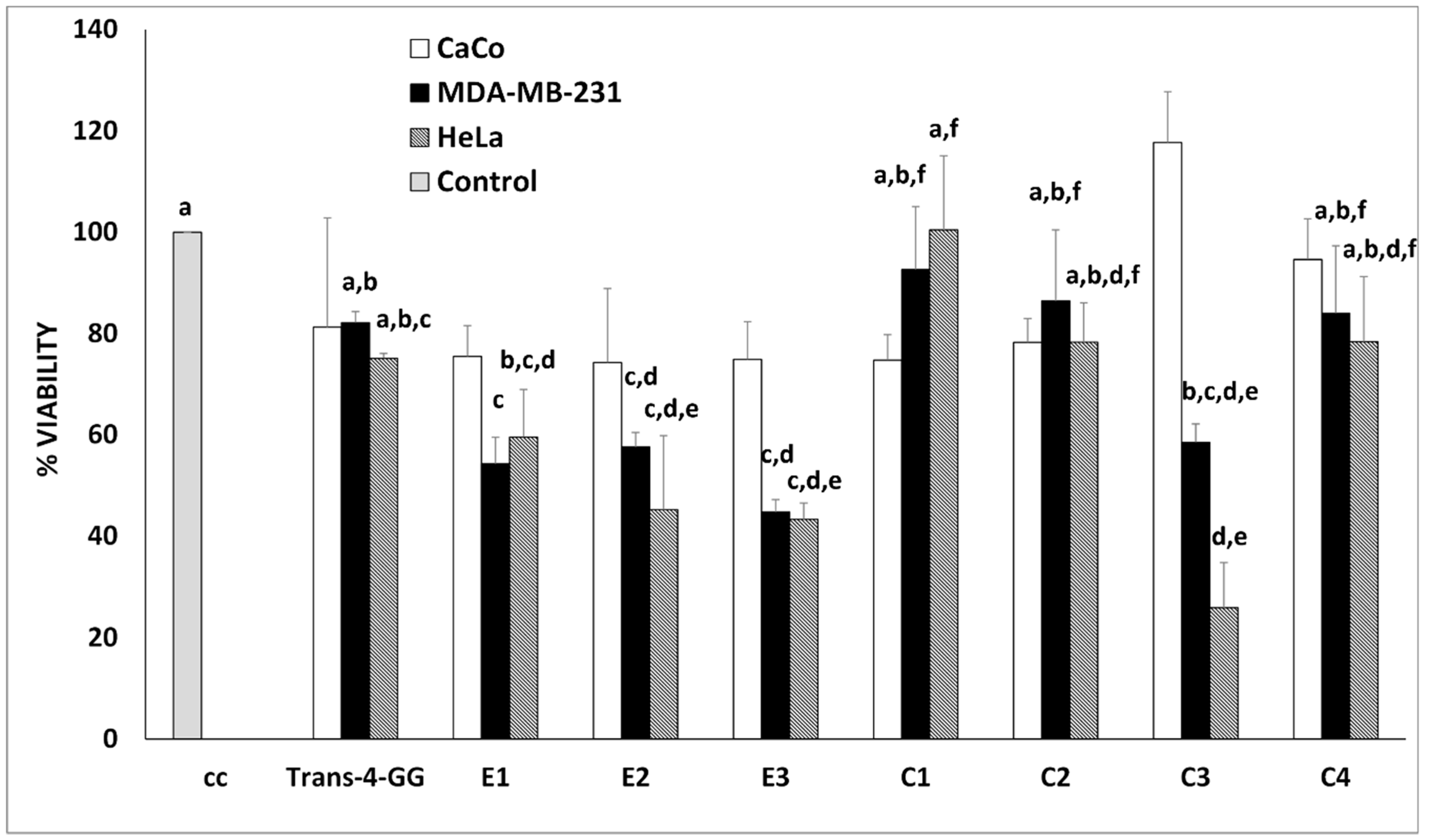
2.2. In Vitro Antiproliferative Assay of the Crocin-Rich Extracts
2.3. Characterization of the Crocin-Rich Extracts: Residual Protein Nitrogen and Fat
3. Materials and Methods
3.1. Samples and Chemicals
- three ewe milk cheeses (E1–E3) produced in three different farms and differing for ripening time and saffron addition.
- four cow milk cheeses (C1–C4), produced in the same farm and differing for indoor or local pasture feeding and for ripening time.
3.2. Saffron Bioactive Molecules in Cheese
3.3. Cell Lines and Treatment
3.4. In Vitro Viability Assay (MTT Assay)
3.5. Residual Protein Nitrogen and Fat in the Crocin-Rich Extracts
3.6. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Basker, D.; Negbi, M. Uses of saffron. Econ. Bot. 1983, 37, 228–236. [Google Scholar] [CrossRef]
- Negbi, M. Saffron cultivation: Past, present and future prospects. In Saffron; CRC Press: Boca Raton, FL, USA, 1999; pp. 14–28. [Google Scholar]
- Melnyk, J.P.; Wang, S.; Marcone, M.F. Chemical and biological properties of the world’s most expensive spice: Saffron. Food Res. Int. 2010, 43, 1981–1989. [Google Scholar] [CrossRef]
- Abu-Izneid, T.; Rauf, A.; Khalil, A.A.; Olatunde, A.; Khalid, A.; Alhumaydhi, F.A.; Aljohani, A.S.; Sahab Uddin, M.; Heydari, M.; Khayrullin, M. Nutritional and health beneficial properties of saffron (Crocus sativus L.): A comprehensive review. Crit. Rev. Food Sci. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Akowuah, G.A.; Htar, T.T. Therapeutic properties of saffron and its chemical constituents. J. Natl. Prod. 2014, 7, 5–13. [Google Scholar]
- Fernández, J.-A. Anticancer properties of saffron, Crocus sativus Linn. Adv. Phytomed. 2006, 2, 313–330. [Google Scholar]
- Hoshyar, R.; Mollaei, H. A comprehensive review on anticancer mechanisms of the main carotenoid of saffron, crocin. J. Pharm. Pharmacol. 2017, 69, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Lambrianidou, A.; Koutsougianni, F.; Papapostolou, I.; Dimas, K. Recent advances on the anticancer properties of saffron (Crocus sativus L.) and its major constituents. Molecules 2021, 26, 86. [Google Scholar] [CrossRef]
- Bathaie, S.Z.; Bolhasani, A.; Hoshyar, R.; Ranjbar, B.; Sabouni, F.; Moosavi-Movahedi, A.-A. Interaction of saffron carotenoids as anticancer compounds with ctDNA, Oligo (dG. dC) 15, and Oligo (dA. dT) 15. DNA Cell Biol. 2007, 26, 533–540. [Google Scholar] [CrossRef]
- Festuccia, C.; Mancini, A.; Gravina, G.L.; Scarsella, L.; Llorens, S.; Alonso, G.L.; Tatone, C.; Di Cesare, E.; Jannini, E.A.; Lenzi, A. Antitumor effects of saffron-derived carotenoids in prostate cancer cell models. BioMed Res. Int. 2014, 2014, 135048. [Google Scholar] [CrossRef]
- Aktypis, A.; Christodoulou, E.D.; Manolopoulou, E.; Georgala, A.; Daferera, D.; Polysiou, M. Fresh ovine cheese supplemented with saffron (Crocus sativus L.): Impact on microbiological, physicochemical, antioxidant, color and sensory characteristics during storage. Small Rumin. Res. 2018, 167, 32–38. [Google Scholar] [CrossRef]
- Carpino, S.; Rapisarda, T.; Belvedere, G.; Licitra, G. Volatile fingerprint of Piacentinu cheese produced with different tools and type of saffron. Small Rumin. Res. 2008, 79, 16–21. [Google Scholar] [CrossRef]
- Licón, C.; Carmona, M.; Molina, A.; Berruga, M. Chemical, microbiological, textural, color, and sensory characteristics of pressed ewe milk cheeses with saffron (Crocus sativus L.) during ripening. J. Dairy Sci. 2012, 95, 4263–4274. [Google Scholar] [CrossRef] [PubMed]
- Librán, C.M.; Licón, C.C.; Serrano-Díaz, J.; Carmona, M.; Berruga, M.I. Safranal transference from ewe’s milk to cheese and whey and antifungal properties of fortified whey. Dairy Sci. Technol. 2014, 94, 83–89. [Google Scholar] [CrossRef][Green Version]
- Licón, C.C.; Carmona, M.; Berruga, M.I. Volatile compounds in pressed ewes’ milk cheese with saffron spice (Crocus sativus L.). Int. J. Dairy Technol. 2015, 68, 399–408. [Google Scholar] [CrossRef]
- Ritota, M.; Mattera, M.; Costanzo, M.G.D.; Manzi, P. Evaluation of Crocins in Cheeses Made with Saffron by UHPLC. J. Braz. Chem. Soc. 2018, 29, 248–257. [Google Scholar] [CrossRef]
- Aung, H.; Wang, C.; Ni, M.; Fishbein, A.; Mehendale, S.; Xie, J.; Shoyama, A.; Yuan, C. Crocin from Crocus sativus possesses significant anti-proliferation effects on human colorectal cancer cells. Exp. Oncol. 2007, 29, 175. [Google Scholar] [PubMed]
- Chryssanthi, D.G.; Dedes, P.G.; Karamanos, N.K.; Cordopatis, P.; Lamari, F.N. Crocetin inhibits invasiveness of MDA-MB-231 breast cancer cells via downregulation of matrix metalloproteinases. Planta Med. 2011, 77, 146–151. [Google Scholar] [CrossRef]
- Dhar, A.; Mehta, S.; Dhar, G.; Dhar, K.; Banerjee, S.; Van Veldhuizen, P.; Campbell, D.R.; Banerjee, S.K. Crocetin inhibits pancreatic cancer cell proliferation and tumor progression in a xenograft mouse model. Mol. Cancer Ther. 2009, 8, 315–323. [Google Scholar] [CrossRef]
- Mousavi, S.H.; Moallem, S.A.; Mehri, S.; Shahsavand, S.; Nassirli, H.; Malaekeh-Nikouei, B. Improvement of cytotoxic and apoptogenic properties of crocin in cancer cell lines by its nanoliposomal form. Pharm. Biol. 2011, 49, 1039–1045. [Google Scholar] [CrossRef]
- Bajbouj, K.; Schulze-Luehrmann, J.; Diermeier, S.; Amin, A.; Schneider-Stock, R. The anticancer effect of saffron in two p53 isogenic colorectal cancer cell lines. BMC Complement. Altern. Med. 2012, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.M.; Mancini, A.; Lizzi, A.R.; De Simone, A.; Marroccella, C.E.; Gravina, G.L.; Tatone, C.; Festuccia, C. Crocus sativus stigma extract and its major constituent crocin possess significant antiproliferative properties against human prostate cancer. Nutr. Cancer 2013, 65, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Boskabady, M.H.; Davoodi, S. Use of in vitro assays to assess the potential antiproliferative and cytotoxic effects of saffron (Crocus sativus L.) in human lung cancer cell line. Pharm. Mag. 2010, 6, 309. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Ortega, H.; Pereda-Miranda, R.; Abdullaev, F.I. HPLC quantification of major active components from 11 different saffron (Crocus sativus L.) sources. Food Chem. 2007, 100, 1126–1131. [Google Scholar] [CrossRef]
- Masi, E.; Taiti, C.; Heimler, D.; Vignolini, P.; Romani, A.; Mancuso, S. PTR-TOF-MS and HPLC analysis in the characterization of saffron (Crocus sativus L.) from Italy and Iran. Food Chem. 2016, 192, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Tarantilis, P.A.; Polissiou, M.G. Chemical analysis and antitumor activity of natural and semi-natural carotenoids of saffron. In Proceedings of the I International Symposium on Saffron Biology and Biotechnology 650, Albacete, Spain, 22–25 October 2003; pp. 447–461. [Google Scholar]
- Chavez, K.J.; Garimella, S.V.; Lipkowitz, S. Triple negative breast cancer cell lines: One tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010, 32, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zang, C.; Fenner, M.; Possinger, K.; Elstner, E. PPARγ ligands and ATRA inhibit the invasion of human breast cancer cells in vitro. Breast Cancer Res. Treat. 2003, 79, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Hire, R.R.; Srivastava, S.; Davis, M.B.; Kumar Konreddy, A.; Panda, D. Antiproliferative activity of crocin involves targeting of microtubules in breast cancer cells. Sci. Rep. 2017, 7, 44984. [Google Scholar] [CrossRef]
- Calvert, T.; Aidoo, K.; Candlish, A.; Fuat, A.M. Comparison of in vitro cytotoxicity of Fusarium mycotoxins, deoxynivalenol, T-2 toxin and zearalenone on selected human epithelial cell lines. Mycopathologia 2005, 159, 413–419. [Google Scholar] [CrossRef] [PubMed]
- William-Faltaos, S.; Rouillard, D.; Lechat, P.; Bastian, G. Cell cycle arrest and apoptosis induced by oxaliplatin (L-OHP) on four human cancer cell lines. Anticancer Res. 2006, 26, 2093–2099. [Google Scholar] [PubMed]
- Zhou, C.; Tabb, M.M.; Sadatrafiei, A.; Grün, F.; Blumberg, B. Tocotrienols activate the steroid and xenobiotic receptor, SXR, and selectively regulate expression of its target genes. Drug Metabol. Dispos. 2004, 32, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Abdullaev Jafarova, F.; Caballero Ortega, H.; Riverón Negrete, L.; Pereda Miranda, R.; Rivera Luna, R.; Hernández, J.M.; Pérez López, I.; Espinosa Aguirre, J.J. Evaluación in vitro del potencial quimiopreventivo del azafrán. Rev. Investig. Clín. 2002, 54, 430–436. [Google Scholar]
- Gezici, S. Comparative anticancer activity analysis of saffron extracts and a principle component, crocetin for prevention and treatment of human malignancies. J. Food Sci. Technol. 2019, 56, 5435–5443. [Google Scholar] [CrossRef]
- Givens, D.I. Saturated fats, dairy foods and health: A curious paradox? Nutr. Bull. 2017, 42, 274–282. [Google Scholar] [CrossRef]
- Lecerf, J.-M.; Legrand, P. Les effets des nutriments dépendent-ils des aliments qui les portent? L’effet matrice. Cah. Nutr. Diététique 2015, 50, 158–164. [Google Scholar] [CrossRef]
- Zou, L.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Utilizing food matrix effects to enhance nutraceutical bioavailability: Increase of curcumin bioaccessibility using excipient emulsions. J. Agric. Food Chem. 2015, 63, 2052–2062. [Google Scholar] [CrossRef]
- Capuano, E.; Oliviero, T.; van Boekel, M.A. Modeling food matrix effects on chemical reactivity: Challenges and perspectives. Crit. Rev. Food Sci. Nutr. 2018, 58, 2814–2828. [Google Scholar] [CrossRef]
- Fardet, A. Food health potential is primarily due to its matrix structure, then nutrient composition: A new paradigm for food classification according to technological processes applied. J. Nutr. Health Food Eng. 2014, 1, 208–209. [Google Scholar] [CrossRef]
- Mohanty, D.; Mohapatra, S.; Misra, S.; Sahu, P. Milk derived bioactive peptides and their impact on human health–A review. Saudi J. Biol. Sci. 2016, 23, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Meisel, H.; FitzGerald, R.J. Biofunctional peptides from milk proteins: Mineral binding and cytomodulatory effects. Curr. Pharm. Design 2003, 9, 1289–1296. [Google Scholar]
- López-Expósito, I.; Miralles, B.; Amigo, L.; Hernández-Ledesma, B. Health effects of cheese components with a focus on bioactive peptides. In Fermented Foods in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2017; pp. 239–273. [Google Scholar]
- Yasuda, S.; Ohkura, N.; Suzuki, K.; Yamasaki, M.; Nishiyama, K.; Kobayashi, H.; Hoshi, Y.; Kadooka, Y.; Igoshi, K. Effects of highly ripened cheeses on HL-60 human leukemia cells: Antiproliferative activity and induction of apoptotic DNA damage. J. Dairy Sci. 2010, 93, 1393–1400. [Google Scholar] [CrossRef]
- Rafiq, S.; Gulzar, N.; Huma, N.; Hussain, I.; Murtaza, M.S. Evaluation of anti-proliferative activity of Cheddar cheeses using colon adenocarcinoma (HCT-116) cell line. Int. J. Dairy Technol. 2020, 73, 255–260. [Google Scholar] [CrossRef]
- Rafiq, S.; Huma, N.; Gulzar, N.; Murtaza, M.A.; Hussain, I. Effect of cheddar cheese peptide extracts on growth inhibition, cell cycle arrest and apoptosis induction in human lung cancer (H-1299) cell line. Int. J. Dairy Technol. 2018, 71, 975–980. [Google Scholar] [CrossRef]
- Rafiq, S.; Huma, N.; Rakariyatham, K.; Hussain, I.; Gulzar, N.; Hayat, I. Anti-inflammatory and anticancer activities of water-soluble peptide extracts of buffalo and cow milk Cheddar cheeses. Int. J. Dairy Technol. 2018, 71, 432–438. [Google Scholar] [CrossRef]
- De Simone, C.; Picariello, G.; Mamone, G.; Stiuso, P.; Dicitore, A.; Vanacore, D.; Chianese, L.; Addeo, F.; Ferranti, P. Characterisation and cytomodulatory properties of peptides from Mozzarella di Bufala Campana cheese whey. J. Pept. Sci. 2009, 15, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Alcalá, L.M.; Castro-Gómez, M.P.; Pimentel, L.L.; Fontecha, J. Milk fat components with potential anticancer activity—A review. Biosci. Rep. 2017, 37, BSR20170705. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Kuwata, H.; Kawamoto, K.; Shirakawa, J.; Atobe, S.; Hoshi, Y.; Yamasaki, M.; Nishiyama, K.; Tachibana, H.; Yamada, K. Effect of highly lipolyzed goat cheese on HL-60 human leukemia cells: Antiproliferative activity and induction of apoptotic DNA damage. J. Dairy Sci. 2012, 95, 2248–2260. [Google Scholar] [CrossRef]
- Snow, D.R.; Jimenez-Flores, R.; Ward, R.E.; Cambell, J.; Young, M.J.; Nemere, I.; Hintze, K.J. Dietary milk fat globule membrane reduces the incidence of aberrant crypt foci in Fischer-344 rats. J. Agric. Food Chem. 2010, 58, 2157–2163. [Google Scholar] [CrossRef] [PubMed]
- Zanabria, R.; Tellez, A.M.; Griffiths, M.; Corredig, M. Milk fat globule membrane isolate induces apoptosis in HT-29 human colon cancer cells. Food Funct. 2013, 4, 222–230. [Google Scholar] [CrossRef]
- Santiago-López, L.; Aguilar-Toalá, J.E.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Liceaga, A.M.; González-Córdova, A.F. Invited review: Bioactive compounds produced during cheese ripening and health effects associated with aged cheese consumption. J. Dairy Sci. 2018, 101, 3742–3757. [Google Scholar] [CrossRef]
- Bathaie, S.Z.; Mousavi, S.Z. New applications and mechanisms of action of saffron and its important ingredients. Crit. Rev. Food Sci. Nutr. 2010, 50, 761–786. [Google Scholar] [CrossRef]
- ISO 707/IDF 50; Milk and Milk Products—Guidance on Sampling. ISO: Genève, Switzerland, 2008.
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- GU (Gazzetta Ufficiale della Repubblica Italiana). Metodi Ufficiali di Analisi per i Formaggi; GU: Rome, Italy, 1986; Volume Serie generale n. 229, pp. 1–40. [Google Scholar]
- Hammer, Ø.; Harper, D.; Ryan, P. PAST, v. 2.17c. Palaeontol. Electron. 2001, 4, 1–229. [Google Scholar]

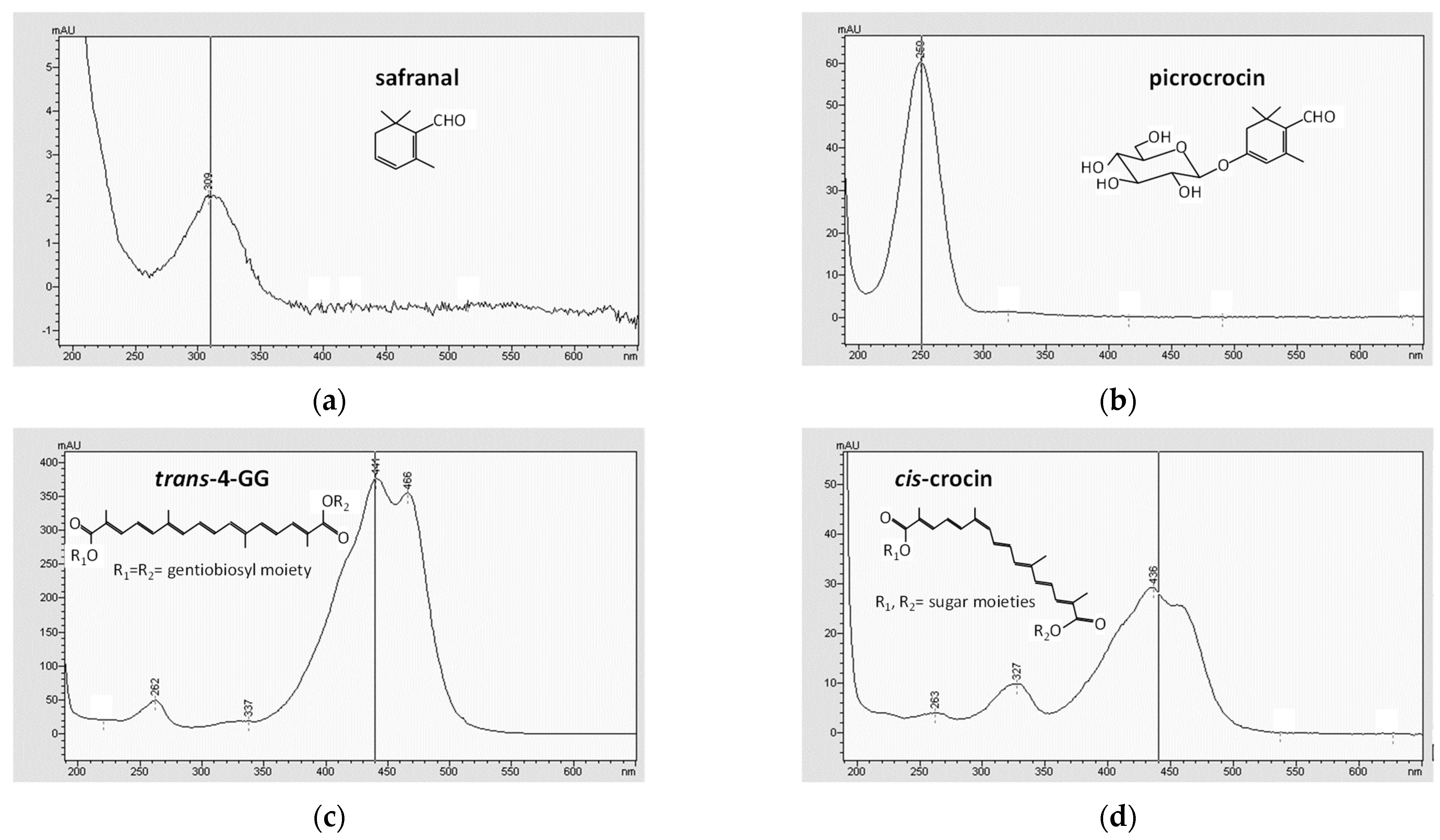
| Sample | Total Crocins (mg/100 g Cheese) | Safranal (μg/100 g Cheese) |
|---|---|---|
| E1 | 30.57 ± 0.49 a | 57 ± 1 |
| E2 | 2.87 ± 0.15 c | n.d. |
| E3 | 10.02 ± 0.12 b | n.d. |
| C1 | 0.83 ± 0.02 d | n.d. |
| C2 | 1.49 ± 0.01 e | n.d. |
| C3 | 0.54 ± 0.03 d | n.d. |
| C4 | 1.36 ± 0.09 e | n.d. |
| Sample | Total Crocins (μM) | Protein N Residue (mg/mL) | Fat Residue (mg/mL) | trans-3-Gg/trans-4-GG | trans-4-GG (% on Total Crocins) |
|---|---|---|---|---|---|
| E1 | 2.00 | 2.52 | 0.35 | 0.5 | 57.3 |
| E2 | 2.00 | 12.88 | 4.16 | 0.4 | 60.3 |
| E3 | 2.00 | 10.98 | 1.53 | 0.7 | 46.4 |
| C1 | 1.00 | 76.72 | 8.12 | 0.7 | 58.6 |
| C2 | 2.00 | 77.66 | 7.72 | 0.5 | 56.1 |
| C3 | 1.00 | 109.01 | 12.96 | 0.4 | 69.4 |
| C4 | 1.00 | 44.58 | 5.36 | 0.6 | 63.8 |
| Ewe Cheeses | ||||
|---|---|---|---|---|
| Protein N residue | Fat residue | HeLa Proliferation | MDA-MB-231 Proliferation | |
| Protein N residue | 1 | |||
| Fat residue | 0.844 | 1 | ||
| HeLa Proliferation | −0.961 | −0.663 | 1 | |
| MDA-MB-231 Proliferation | 0.098 | 0.450 | 0.370 | 1 |
| Cow Cheeses | ||||
| Protein N residue | Fat residue | HeLa Proliferation | MDA-MB-231 Proliferation | |
| Protein N residue | 1 | |||
| Fat residue | 0.970 | 1 | ||
| HeLa Proliferation | −0.675 | −0.796 | 1 | |
| MDA-MB-231 Proliferation | −0.688 | −0.822 | 0.993 | 1 |
| Sample | Cheese Variety | Milk | Ripening | Saffron Addition |
|---|---|---|---|---|
| E1 | Pecorino-type (ewe) | Pasteurized | 30–40 days | Powder added during cutting of the curd |
| E2 | Pecorino-type (ewe) | N.A. 1 | about 15 days | Stigmas added during cutting of the curd |
| E3 | Pecorino-type (ewe) | Raw | 10 months | Powder (previously suspended in milk) added after cutting of the curd |
| C1 | Cow hard cheese | Raw partially skimmed | 22 months | Powder added to milk, or to a mixture of whey and curd, before cutting of the curd |
| C2 | Cow hard cheese | Raw partially skimmed | 14 months | Powder added to milk, or to a mixture of whey and curd, before cutting of the curd |
| C3 | Cow hard cheese | Raw partially skimmed | 19 months | Powder added to milk, or to a mixture of whey and curd, before cutting of the curd |
| C4 | Cow hard cheese | Raw partially skimmed | 16 months | Powder added to milk, or to a mixture of whey and curd, before cutting of the curd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ritota, M.; Comitato, R.; Manzi, P. Cow and Ewe Cheeses Made with Saffron: Characterization of Bioactive Compounds and Their Antiproliferative Effect in Cervical Adenocarcinoma (HeLa) and Breast Cancer (MDA-MB-231) Cells. Molecules 2022, 27, 1995. https://doi.org/10.3390/molecules27061995
Ritota M, Comitato R, Manzi P. Cow and Ewe Cheeses Made with Saffron: Characterization of Bioactive Compounds and Their Antiproliferative Effect in Cervical Adenocarcinoma (HeLa) and Breast Cancer (MDA-MB-231) Cells. Molecules. 2022; 27(6):1995. https://doi.org/10.3390/molecules27061995
Chicago/Turabian StyleRitota, Mena, Raffaella Comitato, and Pamela Manzi. 2022. "Cow and Ewe Cheeses Made with Saffron: Characterization of Bioactive Compounds and Their Antiproliferative Effect in Cervical Adenocarcinoma (HeLa) and Breast Cancer (MDA-MB-231) Cells" Molecules 27, no. 6: 1995. https://doi.org/10.3390/molecules27061995
APA StyleRitota, M., Comitato, R., & Manzi, P. (2022). Cow and Ewe Cheeses Made with Saffron: Characterization of Bioactive Compounds and Their Antiproliferative Effect in Cervical Adenocarcinoma (HeLa) and Breast Cancer (MDA-MB-231) Cells. Molecules, 27(6), 1995. https://doi.org/10.3390/molecules27061995







