Effect of High Hydrostatic Pressure in the Storage of Spanish-Style Table Olive Fermented with Olive Leaf Extract and Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Results and Discussion
2.1. Evolution of the Microbiota during Storage
2.2. Evolution of Physical-Chemical Parameters during the Storage Period
2.3. Profile of Phenolic Compounds
2.4. Sensory Analysis
3. Materials and Methods
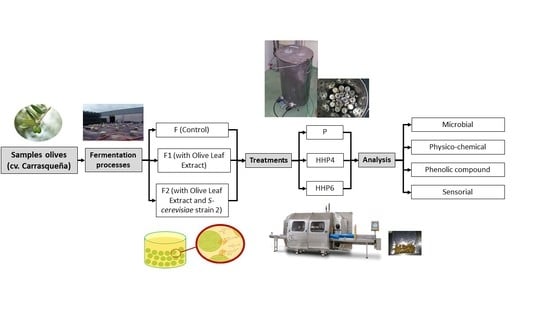
3.1. Samples
- -
- F: spontaneously fermented olives.
- -
- F1: olives fermented spontaneously and with olive leaf extract (OLE).
- -
- F2: olives fermented with a starter culture of a strain of Saccharomyces cerevisiae (6 log10 cfu/mL) and with OLE.
3.2. Microbiological Analysis
3.3. Physicochemical Analysis
3.4. Determination of the Phenolic Profile
3.5. Sensory Analysis
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministry of Agriculture Fisheries and Food. Areas and Annual Crop Production 2020 (Advancement). Spanish Government. Available online: https://www.mapa.gob.es/en/ministerio/ministerio-exterior/brexit/Papel-MAPA.aspx (accessed on 10 October 2021).
- International Olive Council (IOC). World Table Olive and Olive Oil Production; International Olive: Madrid, Spain, 2020; Available online: http://www.internationaloliveoil.org (accessed on 20 December 2020).
- Lavermicocca, P.; Valerio, F.; Lonigro, S.L.; De Angelis, M.; Morelli, L.; Callegari, M.L.; Rizzello, C.G.; Visconti, A. Study of Adhesion and Survival of Lactobacilli and Bifidobacteria on Table Olives with the Aim of Formulating a New Probiotic Food. Appl. Environ. Microbiol. 2005, 71, 4233–4240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanza, B. Abnormal fermentations in table-olive processing: Microbial origin and sensory evaluation. Front. Microbiol. 2013, 4, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Angelis, M.; Campanella, D.; Cosmai, L.; Summo, C.; Rizzello, C.G.; Caponio, F. Microbiota and metabolome of un-started and started Greek-type fermentation of Bella di Cerignola table olives. Food Microbiol. 2015, 52, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Blana, V.A.; Grounta, A.; Tassou, C.; Nychas, G.-J.; Panagou, E. Inoculated fermentation of green olives with potential probiotic Lactobacillus pentosus and Lactobacillus plantarum starter cultures isolated from industrially fermented olives. Food Microbiol. 2014, 38, 208–218. [Google Scholar] [CrossRef]
- Talhaoui, N.; Gómez-Caravaca, A.M.; León, L.; De la Rosa, R.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Determination of phenolic compounds of ‘Sikitita’olive leaves by HPLC-DAD-TOF-MS. Comparison with its parents ‘Arbequina’ and ‘Picual’ olive leaves. LWT-Food. Sci. Technol. 2014, 58, 28–34. [Google Scholar] [CrossRef]
- Rahmanian, N.; Jafari, S.M.; Wani, T.A. Bioactive profile, dehydration, extraction and application of the bioactive components of olive leaves. Trends Food Sci. Technol. 2015, 42, 150–172. [Google Scholar] [CrossRef]
- Quirantes-Piné, R.; Lozano-Sánchez, J.; Herrero, M.; Ibáñez, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC–ESI–QTOF–MS as a powerful analytical tool for characterizing phenolic compounds in olive-leaf extracts. Phytochem. Anal. 2013, 24, 213–223. [Google Scholar] [CrossRef]
- González, E.; Gómez-Caravaca, A.M.; Giménez, B.; Cebrian, R.; Maqueda, M.; Martinez-Ferez, A.; Segura-Carretero, A.; Robert, P. Evolution of the phenolic compounds profile of olive leaf extract encapsulated by spray-drying during in vitro gastrointestinal digestion. Food Chem. 2018, 279, 40–48. [Google Scholar] [CrossRef]
- Delgado-Adámez, J.; Baltasar, M.N.F.; Yuste, M.C.A.; Martín-Vertedor, D. Oxidative stability, phenolic compounds and antioxidant potential of a virgin olive oil enriched with natural bioactive compounds. J. Oleo Sci. 2014, 63, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Martín-Vertedor, D.; Garrido, M.; Pariente, J.A.; Espino, J.; Delgado-Adámez, J. Bioavailability of Bioactive Molecules from Olive Leaf Extracts and its Functional Value. Phytother. Res. 2016, 30, 1172–1179. [Google Scholar] [CrossRef]
- Difonzo, G.; Russo, A.; Trani, A.; Paradiso, V.M.; Ranieri, M.; Pasqualone, A.; Summo, C.; Tamma, G.; Silletti, R.; Caponio, F. Green extracts from Coratina olive cultivar leaves: Antioxidant characterization and biological activity. J. Funct. Foods 2017, 31, 63–70. [Google Scholar] [CrossRef]
- Caponio, F.; Difonzo, G.; Calasso, M.; Cosmai, L.; De Angelis, M. Effects of olive leaf extract addition on fermentative and oxidative processes of table olives and their nutritional properties. Food Res. Int. 2018, 116, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- Schaide, T.; Cabrera-Beñegal, M.; Pérez-Nevado, F.; Esperilla, A.; Martín-Vertedor, D. Effect of olive leaf extract combined with Saccharomyces cerevisiae in the fermentation process of table olives. J. Food Sci. Technol. 2019, 56, 3001–3013. [Google Scholar] [CrossRef]
- Rodgers, S. Minimally Processed Functional Foods: Technological and Operational Pathways. J. Food Sci. 2016, 81, R2309–R2319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdramidis, V.; Koutsoumanis, K.P. Challenges and perspectives of advanced technologies in processing, distribution and storage for improving food safety. Curr. Opin. Food Sci. 2016, 12, 63–69. [Google Scholar] [CrossRef]
- Abriouel, H.; Benomar, N.; Gálvez, A.; Pulido, R.P. Preservation of Manzanilla Aloreña cracked green table olives by high hydrostatic pressure treatments singly or in combination with natural antimicrobials. LWT 2014, 56, 427–431. [Google Scholar] [CrossRef]
- Tokuşoğlu, Ö.; Alpas, H.; Bozoğlu, F. High hydrostatic pressure effects on mold flora, citrinin mycotoxin, hydroxytyrosol, oleuropein phenolics and antioxidant activity of black table olives. Innov. Food Sci. Emerg. Technol. 2010, 11, 250–258. [Google Scholar] [CrossRef]
- Pradas, I.; del Pino, B.; Peña, F.; Ortiz, V.; Moreno-Rojas, J.; Fernández-Hernández, A.; García-Mesa, J. The use of high hydrostatic pressure (HHP) treatments for table olives preservation. Innov. Food Sci. Emerg. Technol. 2012, 13, 64–68. [Google Scholar] [CrossRef]
- Fernández, A.; Talaverano, M.I.; Pérez-Nevado, F.; Boselli, E.; Cordeiro, A.M.; Martillanes, S.; Martín-Vertedor, D. Evaluation of phenolics and acrylamide and their bioavailability in high hydrostatic pressure treated and fried table olives. J. Food Process. Preserv. 2020, 44, e14384. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, F.; Su, X. Direct extraction of lipids from wet microalgae slurries by super-high hydrostatic pressure. Algal Res. 2021, 58, 102412. [Google Scholar] [CrossRef]
- De Lamo-Castellví, S.; Capellas, M.; López-Pedemonte, T.; Hernández-Herrero, M.M.; Guamis, B.; Sagués, A.X.R. Behavior of Yersinia enterocolitica Strains Inoculated in Model Cheese Treated with High Hydrostatic Pressure. J. Food Prot. 2005, 68, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Tewari, G.; Jayas, D.S.; Holley, R.A. High pressure processing of foods: An overview. Emerg. Technol. Food Processing 1999, 19, 619–661. [Google Scholar]
- Patterson, M.F.; Margey, D.M.; Mills, G.; Simpson, R.; Glimour, A. The Effect of High Pressure Treatment on Microorganisms in Foods. In High Pressure Research, in the Bioscincesand Biothecnology; Heremans, K., Ed.; Leuven University Press: Leuven, Belgium, 1997; pp. 269–272. [Google Scholar]
- Codex Stan 118-1979. Codex Standard for Foods; Codex Alimentarius Commission: Geneva, Switzerland, 2015. [Google Scholar]
- Rastogi, N.K.; Raghavarao, K.S.M.S.; Balasubramaniam, V.; Niranjan, K.; Knorr, D. Opportunities and Challenges in High Pressure Processing of Foods. Crit. Rev. Food Sci. Nutr. 2007, 47, 69–112. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.; Delgado-Adamez, J.; Franco, M.N.; De Miguel, C.; Ramirez, M.R.; Martín-Vertedor, M. Comparative effect of high pressure processing and traditional thermal treatment on the physicochemical, microbiology, and sensory analysis of olive jam. Grasas Aceites 2013, 64, 432–441. [Google Scholar] [CrossRef] [Green Version]
- Parra, J.G.; González-Cebrino, F.; Delgado-Adamez, J.; Lozano, M.; Hernández, T.; Ramírez, R. Effect of Thermal and High-Pressure Processing on the Nutritional Value and Quality Attributes of a Nectarine Purée with Industrial Origin during the Refrigerated Storage. J. Food Sci. 2011, 76, C618–C625. [Google Scholar] [CrossRef]
- Sánchez, J.; De Miguel, C.; Ramírez, M.R.; Delgado, J.; Franco, M.N.; Martín-Vertedor, D. Efecto de las altas presiones hidrostáticas respecto a la pasteurización térmica en los aspectos microbiológicos, sensoriales y estabilidad oxidativa de un paté de aceituna. Grasas Aceites 2012, 63, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Mújica-Paz, H.; Valdez-Fragoso, A.; Samson, C.T.; Welti-Chanes, J.; Torres, J.A. High-Pressure Processing Technologies for the Pasteurization and Sterilization of Foods. Food Bioprocess Technol. 2011, 4, 969–985. [Google Scholar] [CrossRef]
- IOC. Method for the Sensory Analysis of Table Olives COI/OT/MO/Doc. No. 1/Rev. 2; International Olive Oil Council: Madrid, Spain, 2011. [Google Scholar]
- Rodríguez-Gómez, F.; López-López, A.; Romero-Gil, V.; Arroyo-López, F.; Moreno-Baquero, J.; Garrido-Fernández, A.; García-García, P. Effect of post-fermentation storage on Spanish-style green Manzanilla olives. LWT 2014, 57, 789–793. [Google Scholar] [CrossRef]
- Güngör, F.; Ocak, O.; Ünal, M.K. Effects of different preservation methods and storage on Spanish-style domat olives fermented with different chloride salts. J. Food Process. Preserv. 2021, e15236. [Google Scholar] [CrossRef]
- Casado, F.J.; Sánchez, A.H.; Rejano, L.; Montaño, A. Study of new procedures of elaboration of alkali treated green table olives, not fermented, preserved by heat treatments. Grasas Aceites 2007, 58, 275–282. [Google Scholar]
- Clydesdale, F.M.; Francis, F.J. Chlorophylls. Princ. Food Sci. 1976, 1, 386. [Google Scholar]
- Ramírez, E.; Gandul-Rojas, B.; Romero, C.; Brenes, M.; Gallardo-Guerrero, L. Composition of pigments and color changes in green table olives related to processing type. Food Chem. 2015, 166, 115–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aparicio-Ruiz, R.; Gandul-Rojas, B. Decoloration kinetics of chlorophylly and catotenoids in virgin olive oil by autoxidation. Food Res. Int. 2014, 65, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Geraldi, M.V.; Cazarin, C.B.B.; Dias-Audibert, F.L.; Pereira, G.A.; Carvalho, G.G.; Kabuki, D.Y.; Catharino, R.R.; Pastore, G.M.; Behrens, J.H.; Cristianini, M.; et al. Influence of high isostatic pressure and thermal pasteurization on chemical composition, color, antioxidant properties and sensory evaluation of jabuticaba juice. LWT 2020, 139, 110548. [Google Scholar] [CrossRef]
- Fiori, F.; Di Lecce, G.; Boselli, E.; Pieralisi, G.; Frega, N.G. Effects of olive paste fast preheating on the quality of extra virgin olive oil during storage. LWT 2014, 58, 511–518. [Google Scholar] [CrossRef]
- Lalas, S.; Athanasiadis, V.; Gortzi, O.; Bounitsi, M.; Giovanoudis, I.; Tsaknis, J.; Bogiatzis, F. Enrichment of table olives with polyphenols extracted from olive leaves. Food Chem. 2011, 127, 1521–1525. [Google Scholar] [CrossRef]
- Ferrari, G.; Maresca, P.; Ciccarone, R. The application of high hydrostatic pressure for the stabilization of functional foods: Pomegranate juice. J. Food Eng. 2010, 100, 245–253. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, Y.; Zhang, F.; Wang, Y.; Yi, J.; Liao, X. Effects of high hydrostatic pressure on enzymes, phenolic compounds, anthocyanins, polymeric color and color of strawberry pulps. J. Sci. Food Agric. 2011, 91, 877–885. [Google Scholar] [CrossRef]
- Huang, W.; Bi, X.; Zhang, X.; Liao, X.; Hu, X.; Wu, J. Comparative study of enzymes, phenolics, carotenoids and color of apricot nectars treated by high hydrostatic pressure and high temperature short time. Innov. Food Sci. Emerg. Technol. 2013, 18, 74–82. [Google Scholar] [CrossRef]
- Kaşikçi, M.B.; Bağdatlioğlu, N. High hydrostatic pressure treatment of fruit, fruit products and fruit juices: A review on phenolic compounds. Food Health 2016, 2, 27–39. [Google Scholar]
- Aponte, M.; Ventorino, V.; Blaiotta, G.; Volpe, G.; Farina, V.; Avellone, G.; Lanza, C.M.; Moschetti, G. Study of green Sicilian table olive fermentations through microbiological, chemical and sensory analyses. Food Microbiol. 2010, 27, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Fernández, A.; Fernández- Díez, M.J.; Adams, R.M. Table Olives: Production and Processing; Chapman, H., Ed.; Springer Science & Business Media: London, UK, 1997; pp. 134–197. [Google Scholar]
- Cabrera-Bañegil, M.; Schaide, T.; Manzano, R.; Delgado-Adámez, J.; Durán-Merás, I.; Martín-Vertedor, D. Optimization and validation of a rapid liquid chromatography method for determination of the main polyphenolic compounds in table olives and in olive paste. Food Chem. 2017, 233, 164–173. [Google Scholar] [CrossRef] [PubMed]
- González, M.; Navarro, T.; Gómez, G.; Pérez, R.A.; De Lorenzo, C. Análisis sensorial de aceituna de mesa: II. Aplicabilidad práctica y correlación con el análisis instrumenta. Grasas Aceites 2007, 58, 231–236. [Google Scholar]





| Process | Time (Days) | Treatment | pH | Free Acidity (% Lactic Acid) | Total Chlorides (% NaCl) |
|---|---|---|---|---|---|
| F | 0 (*) | Untreated | 3.4 ± 0.0 nsA | 0.3 ± 0.0 nsB | 4.2 ± 0.1 nsB |
| Untreated | 3.4 ± 0.0 nsB | 0.3 ± 0.0 nsA | 4.2 ± 0.1 nsA | ||
| Untreated | 3.4 ± 0.0 nsB | 0.3 ± 0.0 nsNS | 4.2 ± 0.1 nsNS | ||
| 117 | FP | 3.5 ± 0.0 bB | 0.3 ± 0.1 nsA | 4.6 ± 0.1 bC | |
| FHHP4 | 3.3 ± 0.0 aB | 0.3 ± 0.1 nsA | 4.5 ± 0.2 bB | ||
| FHHP6 | 3.3 ± 0.0 aB | 0.3 ± 0.2 nsNS | 4.4 ± 0.1 aNS | ||
| 300 | FP | 3.4 ± 0.0 bB | 0.3 ± 0.1 nsB | 4.1 ± 0.3 nsA | |
| FHHP4 | 3.2 ± 0.0 aA | 0.4 ± 0.0 nsB | 4.3 ± 0.1 nsA | ||
| FHHP6 | 3.2 ± 0.0 aA | 0.3 ± 0.1 nsNS | 4.4 ± 0.1 nsNS | ||
| F1 | 0 (*) | Untreated | 3.6 ± 0.5 nsC | 0.3 ± 0.3 nsA | 4.2 ± 0.4 nsA |
| Untreated | 3.6 ± 0.5 nsC | 0.3 ± 0.3 nsA | 4.2 ± 0.4 nsA | ||
| Untreated | 3.6 ± 0.5 nsC | 0.3 ± 0.3 nsA | 4.2 ± 0.4 nsA | ||
| 117 | F1P | 3.5 ± 0.0 bC | 0.3 ± 0.2 nsC | 4.5 ± 0.1 nsB | |
| F1HHP4 | 3.4 ± 0.0 aB | 0.3 ± 0.2 nsB | 4.4 ± 0.1 nsB | ||
| F1HHP6 | 3.4 ± 0.0 aB | 0.3 ± 0.3 nsB | 4.4 ± 0.1 nsC | ||
| 300 | F1P | 3.4 ± 0.0 bB | 0.3 ± 0.0 aB | 4.2 ± 0.0 aA | |
| F1HHP4 | 3.3 ± 0.0 aA | 0.3 ± 0.0 aB | 4.4 ± 0.1 bB | ||
| F1HHP6 | 3.3 ± 0.0 aA | 0.4 ± 0.0 bC | 4.2 ± 0.1 aB | ||
| F2 | 0 (*) | Untreated | 3.4 ± 0.4 nsA | 0.3 ± 0.0 nsA | 4.1± 0.7 nsA |
| Untreated | 3.4 ± 0.4 nsB | 0.3 ± 0.0 nsA | 4.1± 0.7 nsA | ||
| Untreated | 3.4 ± 0.4 nsB | 0.3 ± 0.0 nsA | 4.1± 0.7 nsA | ||
| 117 | F2P | 3.5 ± 0.0 bA | 0.3 ± 0.2 nsB | 4.5 ± 0.1 bB | |
| F2HHP4 | 3.3 ± 0.0 aA | 0.3 ± 0.2 nsB | 4.0 ± 0.2 aA | ||
| F2HHP6 | 3.4 ± 0.0 bA | 0.3 ± 0.1 nsB | 4.4 ± 0.2 bB | ||
| 300 | F2P | 3.6 ± 0.0 bB | 0.4 ± 0.0 bC | 4.7 ± 0.1 bC | |
| F2HHP4 | 3.3 ± 0.0 aA | 0.4 ± 0.0 aC | 4.3 ± 0.1 aB | ||
| F2HHP6 | 3.3 ± 0.1 aA | 0.4 ± 0.0 aC | 4.3 ± 0.1 aB |
| Process | Time (Days) | Treatment | L* | a* | b* |
|---|---|---|---|---|---|
| F | 0 (*) | Untreated | 57.0 ± 2.9 nsB | −6.5 ± 3 nsA | 40.5 ± 4.8 nsB |
| Untreated | 57.0 ± 2.9 nsB | −6.5 ± 3 nsA | 40.5 ± 4.8 nsB | ||
| Untreated | 57.0 ± 2.9 nsB | −6.5 ± 3 nsNS | 40.5 ± 4.8 nsB | ||
| 117 | FP | 56.1 ± 3.2 bB | −5.0 ± 1.5 aAB | 39.3 ± 3.9 cB | |
| FHHP4 | 50.7 ± 3.6 aA | −1.6 ± 1.4 bC | 31.4 ± 4.3 bB | ||
| FHHP6 | 49.4 ± 3.6 aA | −0.4 ± 2.0 cNS | 27.5 ± 5.9 aA | ||
| 300 | FP | 52.0 ± 0.4 nsA | −3.4 ± 1.9 aB | 34.9 ± 3.3 bA | |
| FHHP4 | 54.8 ± 0.2 nsB | −3.3 ± 2.1 aB | 35.3 ± 3.0 bA | ||
| FHHP6 | 54.4 ± 0.3 nsB | −0.8 ± 2.3 bNS | 29.2 ± 3.1 aA | ||
| F1 | 0 (*) | Untreated | 61.1 ± 3.1 nsB | −8.6 ± 1.3 nsA | 44.1 ± 3.7 nsB |
| Untreated | 61.1 ± 3.1 nsB | −8.6 ± 1.3 nsA | 44.1 ± 3.7 nsB | ||
| Untreated | 61.1 ± 3.1 nsB | −8.6 ± 1.3 nsA | 44.1 ± 3.7 nsB | ||
| 117 | F1P | 54.3 ± 2.5 bA | −6.7 ± 2.3 aB | 37.9 ± 3.0 bA | |
| F1HHP4 | 47.9 ± 3.0 aA | −0.8 ± 1.5 bB | 29.9 ± 4.0 aA | ||
| F1HHP6 | 46.8 ± 3.8 aA | −0.6 ± 1.6 bB | 28.3 ± 4.9 aA | ||
| 300 | F1P | 56.4 ± 0.6 cA | −5.3 ± 1.6 aB | 41.8 ± 2.7 bA | |
| F1HHP4 | 44.8 ± 0.3 bA | −0.3 ± 1.2 bB | 27.6 ± 4.1 aA | ||
| F1 HHP6 | 42.5 ± 0.2 aA | −0.4 ± 1.1 bB | 28.8 ± 3.5 aA | ||
| F2 | 0 (*) | Untreated | 59.2 ± 5.0 nsB | −8.6 ± 1.5 nsNS | 42.3 ± 3.8 nsB |
| Untreated | 59.2 ± 5.0 nsB | −8.6 ± 1.5 nsA | 42.3 ± 3.8 nsC | ||
| Untreated | 59.2 ± 5.0 nsB | −8.6 ± 1.558 nsA | 42.3 ± 3.8 nsB | ||
| 117 | F2P | 56.4 ± 3.3 bB | −7.1 ± 2.0 aNS | 40.9 ± 3.6 bB | |
| F2HHP4 | 50.7 ± 3.1 aB | −1.8 ± 1.3 bB | 33.6 ± 4.4 aB | ||
| F2HHP6 | 51.6 ± 3.0 aB | −1.5 ± 1.5 bB | 34.0 ± 4.1 aA | ||
| 300 | F2P | 45.3 ± 0.2 bA | −7.0 ± 0.5 aNS | 29.1 ± 2.1 nsA | |
| F2HHP4 | 42.2 ± 0.1 aA | −0.6 ± 1.4 bB | 27.3 ± 2.7 nsA | ||
| F2HHP6 | 45.7 ± 0.5 bA | −0.4 ± 0.1 nsB | 30.0 ± 2.7 nsA |
| Process | Time (Days) | Treatment | Hydroxytyrosol | Tyrosol | PB1 | Vanillic Acid | Oleuropein | Luteolin | Luteolin-7-O-g | Verbascoside | p-Coumaric Acid |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | 0 (*) | Untreated | 876 ± 82 nsC | 64 ± 10 nsC | 15 ± 2 nsC | 15 ± 5 nsC | 3 ± 1 ns | 11 ± 1 nsB | 14 ± 2 nsC | 32 ± 32 nsC | 20 ± 3 nsC |
| Untreated | 876 ± 82 nsC | 64 ± 10 nsC | 15 ± 2 nsC | 15 ± 5 nsC | 3 ± 1 nsNS | 11 ± 1 nsNS | 14 ± 2 nsC | 32 ± 32 nsC | 20 ± 3 nsB | ||
| Untreated | 876 ± 82 nsC | 64 ± 10 nsB | 15 ± 2 nsC | 15 ± 5 nsC | 3 ± 1 nsNS | 11 ± 1 ns | 14 ± 2 nsC | 32 ± 32 nsC | 20 ± 3 nsC | ||
| 117 | FP | 606 ± 22 aB | 43 ± 5 aB | 8 ± 2 aB | 10 ± 5 nsB | n.d. | 5 ± 1 aA | 8 ± 1 aB | 21 ± 12 aB | 12 ± 2 aB | |
| FHHP4 | 813 ± 21 bB | 71 ± 6 bB | 13 ± 2 bB | 12 ± 3 nsB | 3 ± 1 nsNS | 10 ± 1 bNS | 12 ± 1 bB | 31 ± 12 bB | 18 ± 3 cB | ||
| FHHP6 | 805 ± 22 bB | 65 ± 5 bB | 13 ± 2 bB | 12 ± 3 nsB | 3 ± 1 nsNS | 9 ± 1 b | 12 ± 2 bB | 26 ± 15 bB | 15 ± 2 bB | ||
| 300 | FP | 509 ± 20 aA | 33 ± 4 aA | 5 ± 1 aA | 6 ± 2 nsA | n.d. | n.d. | 3 ± 1 aA | 11 ± 10 aA | 6 ± 1 aA | |
| FHHP4 | 753 ± 19 cA | 62 ± 5 cA | 7 ± 2 bA | 6 ± 2 nsA | n.d. | 5 ± 1 b | 6 ± 1 cA | 16 ± 6 cA | 13 ± 1 cA | ||
| FHHP6 | 721 ± 18 bA | 55 ± 3 bA | 8 ± 1 bA | 5 ± 2 nsA | n.d. | 4 ± 1 a | 5 ± 1 bA | 13 ± 10 bA | 11 ± 1 bA | ||
| F1 | 0 (*) | Untreated | 1801 ± 247 nsC | 102 ± 10 nsC | 46 ± 1 nsC | 33 ± 4 nsC | 30 ± 5 nsC | 18 ± 1 nsB | 49 ± 10 nsC | 32 ± 2 nsB | 30 ± 5 nsC |
| Untreated | 1801 ± 247 nsC | 102 ± 10 nsC | 46 ± 1 nsC | 33 ± 4 nsC | 30 ± 5 nsC | 18 ± 1 nsC | 49 ± 10 nsC | 32 ± 2 nsC | 30 ± 5 nsB | ||
| Untreated | 1801 ± 112 nsC | 102 ± 10 nsC | 46 ± 1 nsC | 33 ± 4 nsC | 30 ± 5 nsC | 18 ± 1 nsC | 49 ± 10 nsC | 32 ± 2 nsC | 30 ± 5 nsB | ||
| 117 | F1P | 1121 ± 20 aB | 74 ± 2 aB | 20 ± 2 aB | 11 ± 1 aB | 15 ± 5 aB | 7 ± 1 aA | 25 ± 2 aB | 9 ± 1 aA | 12 ± 1 aB | |
| F1HHP4 | 1725 ± 12 cB | 90 ± 1 bB | 40 ± 2 cB | 27 ± 2 cB | 25 ± 4 bB | 15 ± 2 bB | 37 ± 3 cB | 26 ± 2 bB | 29 ± 1 cB | ||
| F1HHP6 | 1675 ± 11 bB | 86 ± 3 bB | 30 ± 3 bB | 24 ± 2 bB | 26 ± 5 bB | 15 ± 2 bB | 32 ± 2 bB | 24 ± 1 bB | 25 ± 2 bB | ||
| 300 | F1P | 995 ± 11 aA | 55 ± 1 aA | 16 ± 1 aA | 6 ± 1 aA | 10 ± 4 aA | 6 ± 1 aA | 15 ± 2 aA | 7 ± 1 aA | 9 ± 1 aA | |
| F1HHP4 | 1621 ± 10 bA | 75 ± 1 cA | 27 ± 2 bA | 20 ± 1 bA | 15 ± 3 cA | 12 ± 1 bA | 28 ± 2 bA | 19 ± 1 cA | 19 ± 1 bA | ||
| F1HHP6 | 1611 ± 10 bA | 60 ± 2 bA | 25 ± 1 bA | 19 ± 1 bA | 13 ± 6 bA | 12 ± 1 bA | 26 ± 1 bA | 16 ± 1 bA | 19 ± 1 bA | ||
| F2 | 0 (*) | Untreated | 1540 ± 114 nsC | 90 ± 10 nsC | 35 ± 6 nsB | 25 ± 5 nsB | 26 ± 3 nsB | 14 ± 2 nsB | 40 ± 5 nsC | 31 ± 38 ns | 30 ± 5 nsC |
| Untreated | 1540 ± 114 nsC | 90 ± 10 nsC | 35 ± 6 nsC | 25 ± 5 nsC | 26 ± 3 nsB | 14 ± 2 nsNS | 40 ± 5 nsC | 31 ± 38 ns | 30 ± 5 nsC | ||
| Untreated | 1540 ± 114 nsC | 90 ± 10 nsB | 35 ± 6 nsC | 25 ± 5 nsC | 26 ± 3 nsB | 14 ± 2 nsNS | 40 ± 5 nsC | 31 ± 38 ns | 30 ± 5 nsC | ||
| 117 | F2P | 902 ± 21 aB | 52 ± 3 aB | 12 ± 1 aA | 8 ± 1 aA | 10 ± 1 aA | 6 ± 1 aA | 21 ± 2.1 aB | 14 ± 1 aB | 10 ± 1 aB | |
| F2HHP4 | 1452 ± 15 cB | 82 ± 2 cB | 28 ± 1 bB | 22 ± 1 cB | 27 ± 1 bB | 15 ± 1 cNS | 35 ± 2 cB | 29 ± 1 cB | 28 ± 2 cB | ||
| F2HHP6 | 1395 ± 12 bB | 75 ± 3 bB | 26 ± 3 bB | 17 ± 1 bB | 25 ± 2 bB | 13 ± 2 bNS | 31 ± 3 bB | 25 ± 3 bB | 23 ± 2 bB | ||
| 300 | F2P | 811 ± 12 aA | 41 ± 2 aA | 10 ± 1 aA | 6 ± 1 aA | 8 ± 1 aA | 5 ± 1 aA | 16 ± 2 aA | 11 ± 1 aA | 5 ± 1 aA | |
| F2HHP4 | 1312 ± 11 cA | 61 ± 1 cA | 22 ± 1 bA | 17 ± 1 cA | 22 ± 1 bA | 13 ± 1 bNS | 29 ± 2 cA | 21 ± 1 bA | 21 ± 1 cA | ||
| F2HHP6 | 1222 ± 11 bA | 56 ± 3 bA | 19 ± 1 bA | 15 ± 1 bA | 18 ± 1 bA | 12 ± 1 bNS | 25 ± 1 bA | 18 ± 1 bA | 17 ± 1 bA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Vertedor, D.; Schaide, T.; Boselli, E.; Martínez, M.; García-Parra, J.; Pérez-Nevado, F. Effect of High Hydrostatic Pressure in the Storage of Spanish-Style Table Olive Fermented with Olive Leaf Extract and Saccharomyces cerevisiae. Molecules 2022, 27, 2028. https://doi.org/10.3390/molecules27062028
Martín-Vertedor D, Schaide T, Boselli E, Martínez M, García-Parra J, Pérez-Nevado F. Effect of High Hydrostatic Pressure in the Storage of Spanish-Style Table Olive Fermented with Olive Leaf Extract and Saccharomyces cerevisiae. Molecules. 2022; 27(6):2028. https://doi.org/10.3390/molecules27062028
Chicago/Turabian StyleMartín-Vertedor, Daniel, Thais Schaide, Emanuele Boselli, Manuel Martínez, Jesús García-Parra, and Francisco Pérez-Nevado. 2022. "Effect of High Hydrostatic Pressure in the Storage of Spanish-Style Table Olive Fermented with Olive Leaf Extract and Saccharomyces cerevisiae" Molecules 27, no. 6: 2028. https://doi.org/10.3390/molecules27062028
APA StyleMartín-Vertedor, D., Schaide, T., Boselli, E., Martínez, M., García-Parra, J., & Pérez-Nevado, F. (2022). Effect of High Hydrostatic Pressure in the Storage of Spanish-Style Table Olive Fermented with Olive Leaf Extract and Saccharomyces cerevisiae. Molecules, 27(6), 2028. https://doi.org/10.3390/molecules27062028