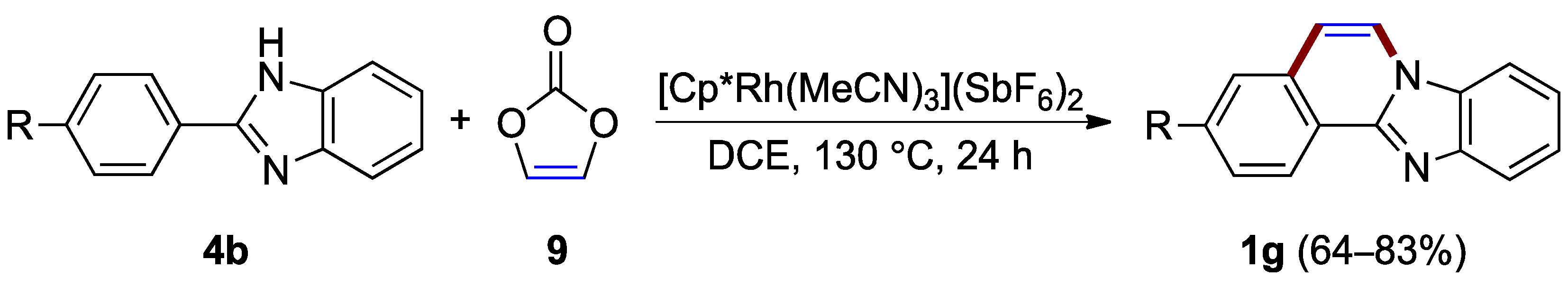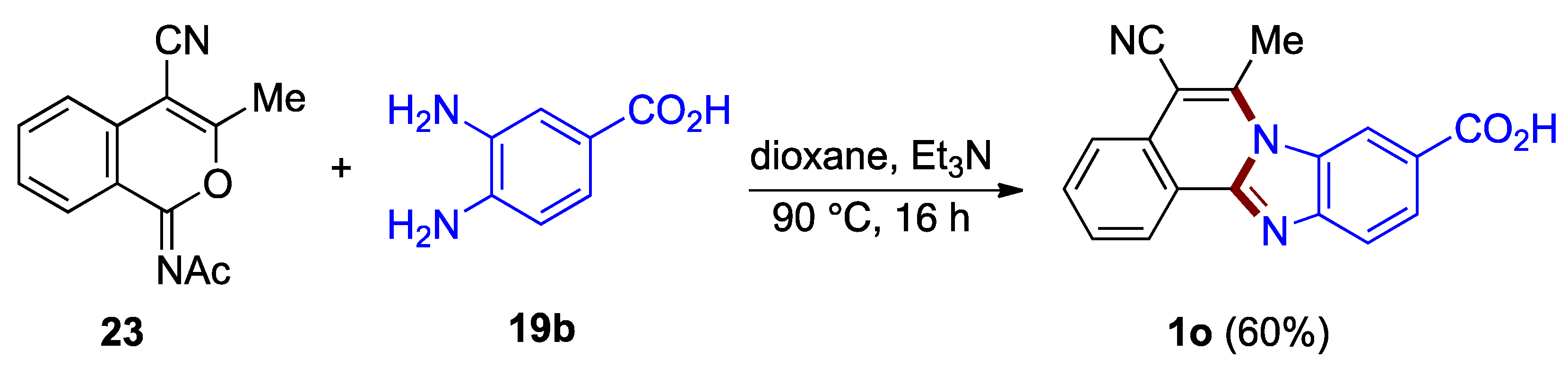Abstract
This review includes recent developments in the synthesis of benzo[4,5]imidazo[2,1-a]isoquinolines with particular attention given to categorizing protocols based on the structural features of the ring architecture and crystallographically characterized reaction products.
1. Introduction
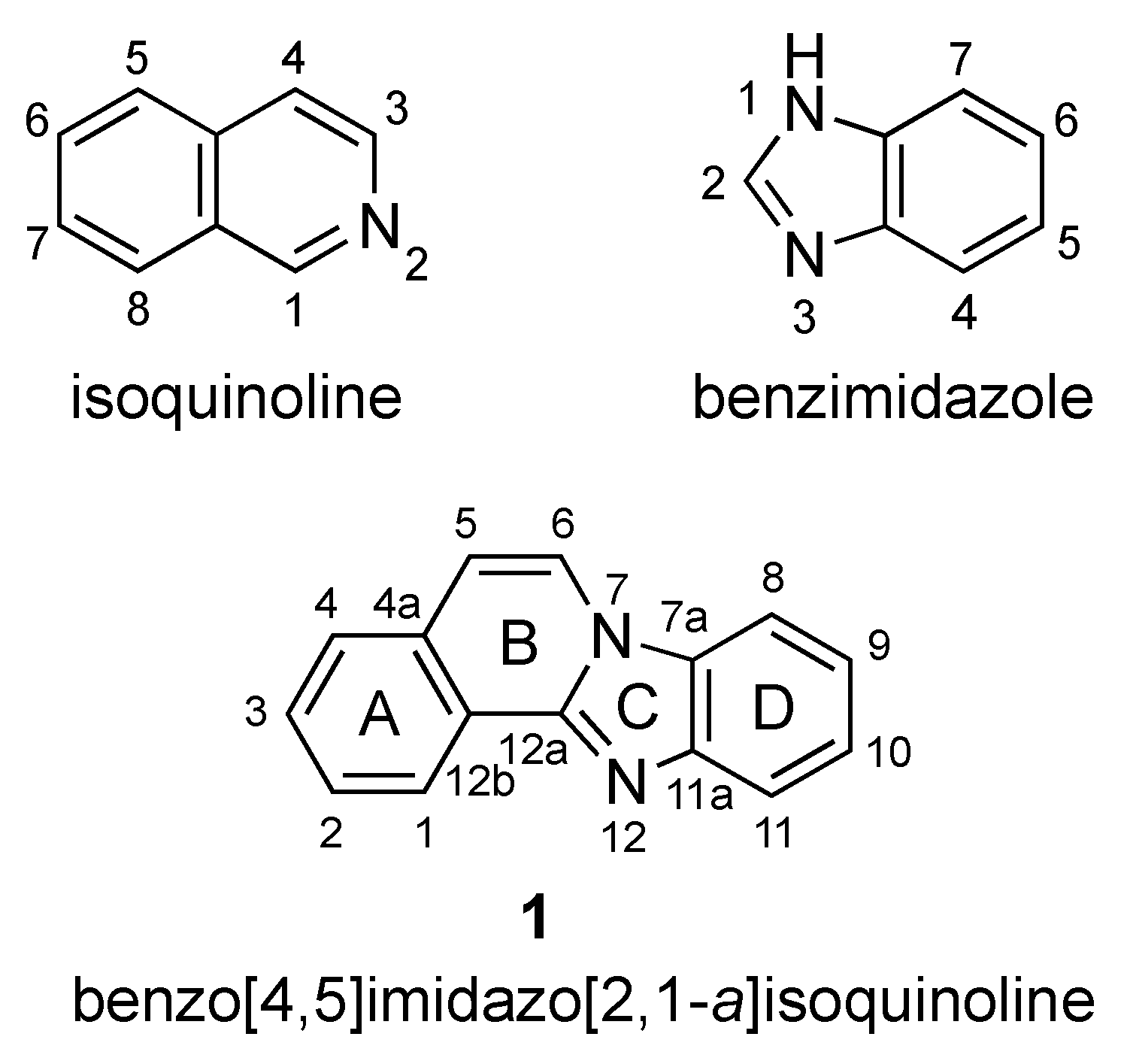
Benzo[4,5]imidazo[2,1-a]isoquinoline 1 is a heterotetracyclic compound that combines fused benzimidazole and isoquinoline moieties (Figure 1), with each structural feature exhibiting broad and multifaceted biological activity. The association of these two ring systems has so far resulted in studies directed at the inhibition of topoisomerase I [1] and the cyclic AMP-dependent protein kinase (PKA) catalytic subunit [2], as well as anticancer activities [3].
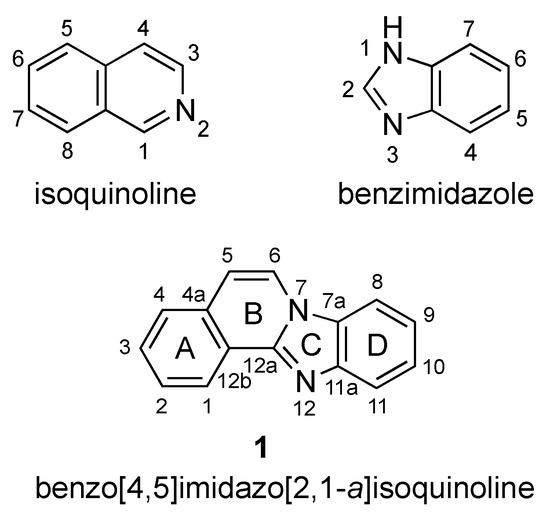
Figure 1.
Isoquinoline, benzimidazole, and benzimidazo[2,1-a]isoquinoline 1.
Synthetic protocols for this class of compounds often include the use of metal reagents/catalysts. Separation of the targeted organic product(s) from these metal-containing catalysts or reagents is required to minimize sources of auxiliary toxicity and meet the standards of biological testing or specific pharmaceutical thresholds [4]. This review highlights the development of methods that reduce cost, improve product selectivity or purity, and offer more effective routes for the synthesis of benzimidazo[2,1-a]isoquinolines. Moreover, emphasis is placed on green methods that support contemporary environmental and safety improvements.
The seminal work of Sun and LaVoie (1996) provided one of the earliest comprehensive synthetic routes to benzimidazo[2,1-a]isoquinolines [1]. Since then, especially within the years of 2016–2021, researchers have developed and refined a variety of alternative synthetic protocols for these molecules. Figure 1 provides a labeling scheme for the rings of benzimidazo[2,1-a]isoquinolines to facilitate the sorting of synthetic methods as it relates to specific ring formation. Within each group of ring(s) formation, the reactions based on traditional halogen-containing components are presented first, followed by C–H activation-based protocols. Beyond the four-ring system, selected references that describe homologs containing extended fused aromatic systems are occasionally included.
2. Ring B Formation
Aryl-substituted benzimidazoles are versatile starting materials that necessitate the construction of a C–5/C–6 vinylene bridge between the ortho position of an aryl group (C–4a in ring A) and the benzimidazole unit (N–7 in ring C, Figure 1). The source of the -CH=CH- moiety may vary. The functionalization of an aryl ring via a halogen atom provides a convenient anchor for such a synthetic approach.
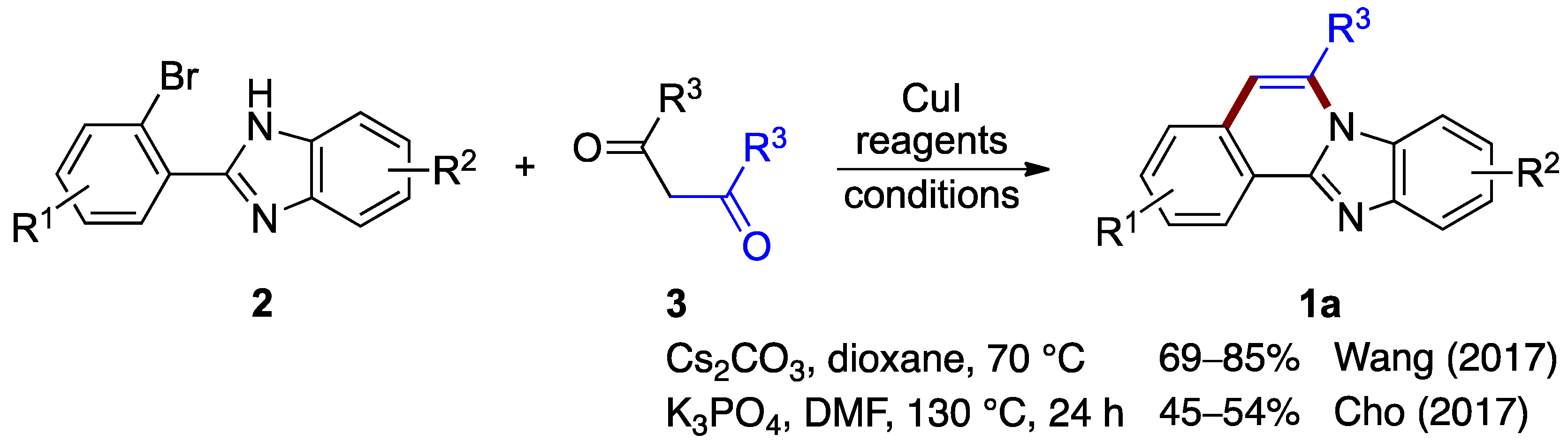
Wang et al. [5] and Cho et al. [6] independently reported protocols for forming benzimidazo[2,1-a]isoquinolines by reacting o-bromophenyl-substituted benzimidazoles 2 with substituted 1,3-diketones (3; Figure 2). Both procedures proceed by copper-catalyzed coupling followed by a deacylation (debenzoylation) step. Wang et al. optimized reaction yields for the 2-(2-bromo-phenyl)-1H-benzo[d]imidazole 2 and the diphenyl 1,3-diketone (dibenzoylmethane) 3 using copper(I) iodide as the catalyst in the presence of cesium carbonate (69–85% yield) [5]. Cho et al. optimized a similar copper-catalyzed reaction using potassium phosphate as a base (45–54% yield) [6]. These protocols show the utility of various diphenyl 1,3-diketones for the production of benzimidazo[2,1-a]isoquinolines 1a (Figure 2).
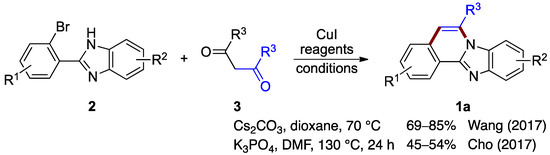
Figure 2.
Copper(I)-catalyzed coupling protocols with o-bromophenyl benzimidazoles 2 and 1,3-diketones 3 [5,6].
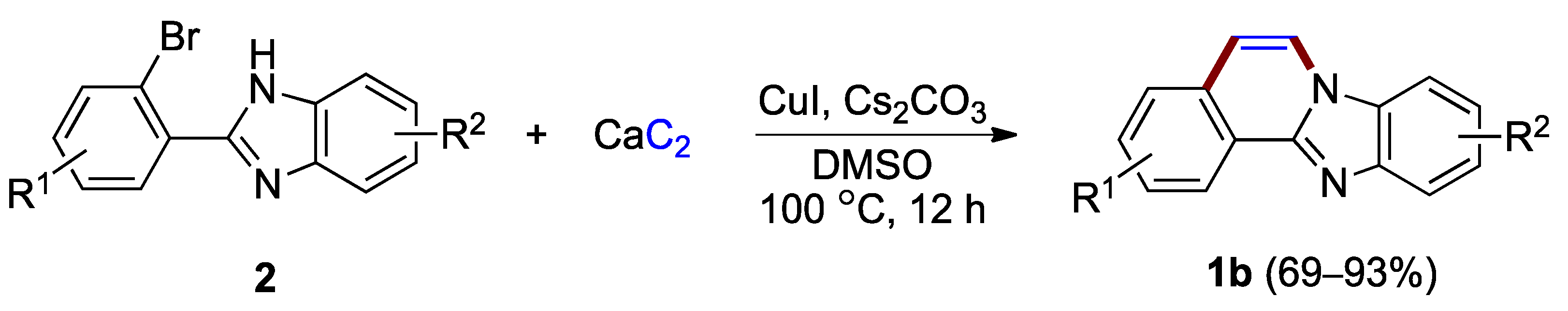
More recently, Liu and Li reported the application of calcium acetylide (calcium carbide), a low-cost industrial commodity, for the synthesis of benzimidazo[2,1-a]isoquinolines from o-haloaryl-substituted benzimidazoles 2 (Figure 3) [7]. The protocol was used to prepare a variety of benzimidazo[2,1-a]isoquinoline derivatives 1b with no substituents at the C–5 and C–6 positions (69–93%), while tolerating halogens such as chlorine and fluorine attached to the phenyl ring.
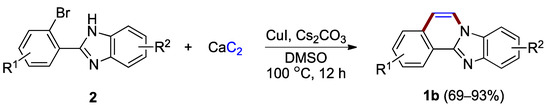
Figure 3.
Copper(I)-catalyzed coupling protocol using o-bromophenyl benzimidazoles 2 and calcium carbide as reactants.
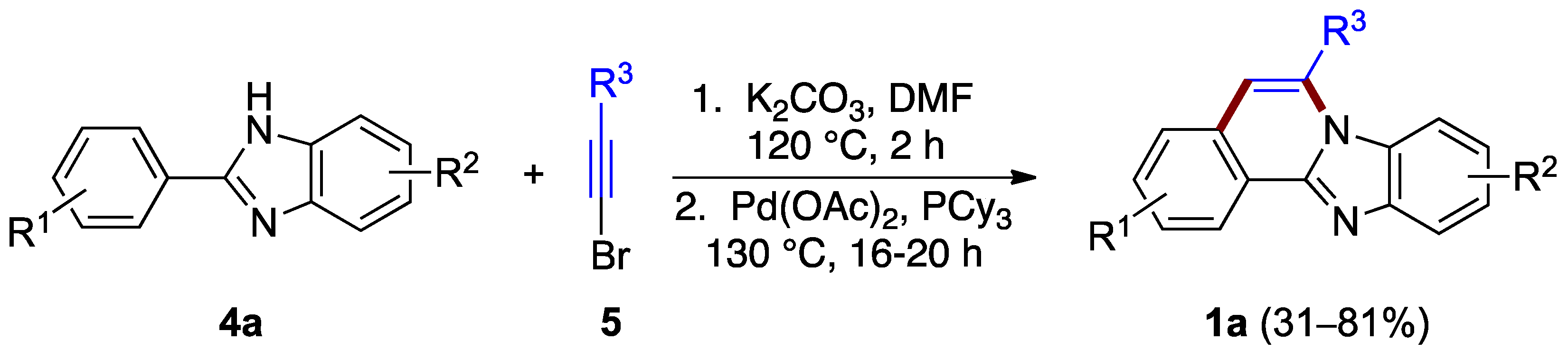
The ring B formation can be achieved with the halogen located on the alkyne reagent as well. Li et al. reported a two-step synthesis that uses phenyl benzimidazole 4a and bromoacetylene 5 derivatives (Figure 4) [8]. The first step is thought to involve nucleophilic addition (hydroamination) of the benzimidazole to bromoalkyne 5 followed by palladium-catalyzed C–H vinylation. This process effectively generates a bond between the benzimidazole phenyl substituent leading to ring B formation and the benzimidazo[2,1-a]isoquinoline 1a product (31–81% yield). The ortho regioselectivity results from the length of already anchored bromovinyl intermediate (Figure 4).
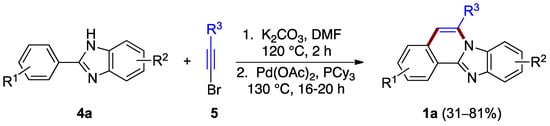
Figure 4.
Hydroamination of bromoacetylene 5 by benzimidazole 4a.
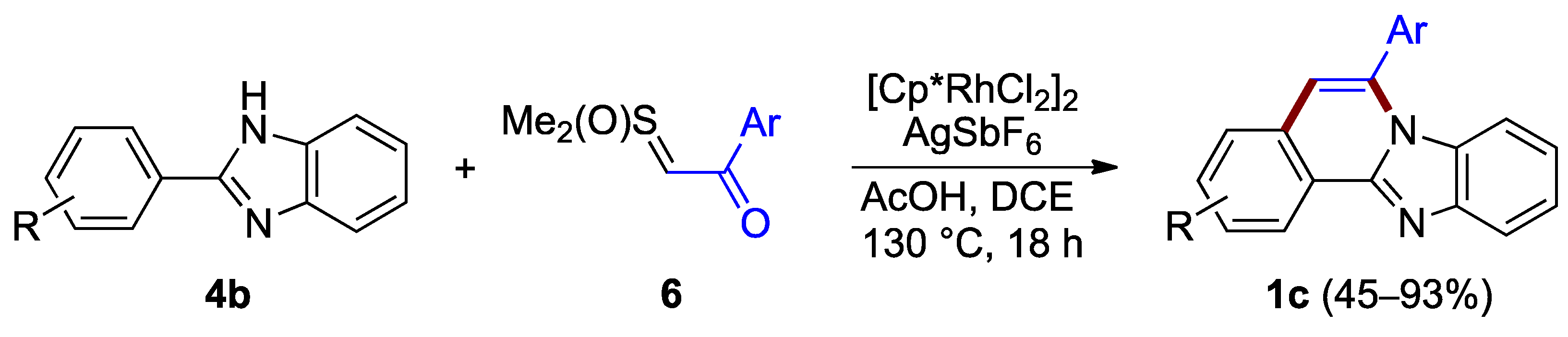
Ring B can also be formed in the absence of a halogen anchor. Cheng et al. reported the synthesis of benzimidazo[2,1-a]isoquinolines 1c through the annulation of non-halogenated 2-arylimidazoles 4b and the acetophenone-derived sulfoxonium ylide 6, under aerobic conditions (45–93% yield; Figure 5) [9]. The procedure relies on a rhodium species generated in situ, facilitating a C–C coupling and forming a C–N bond. The dichloro(pentamethylcyclopentadienyl)rhodium(III) precatalyst plays an important role in the reaction process where it is activated by acetic acid combined with a catalytic amount of silver hexafluoroantimonate.
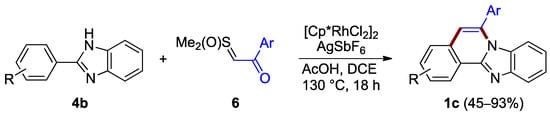
Figure 5.
Annulation of benzimidazole 4b with sulfoxonium ylides 6.
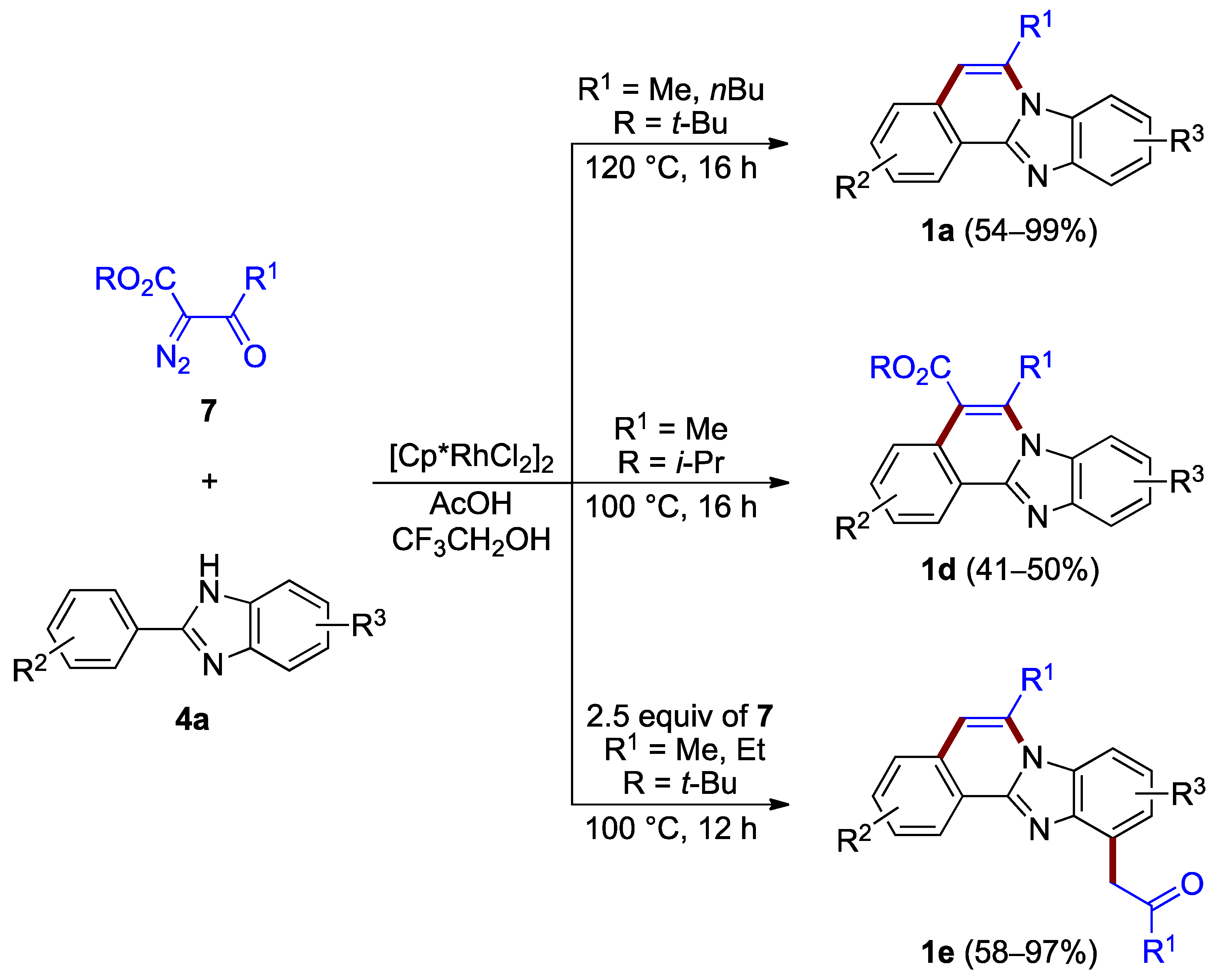
During the same time period, Song et al. reported the synthesis of benzimidazo[2,1-a]isoquinolines 1a (54–99%) and 1d (41–50%) via the rhodium(III)-catalyzed [4 + 2] annulation of α-diazoketoesters 7 and 2-arylbenzimidazoles 4a. The choice of appropriate ester groups (–COO-t-Bu or –COO-i-Pr) or additive (AcOH) controls the reaction outcome with up to two C–C bond-breaking steps (retro-Claisen and decarboxylation) during the C–H functionalization/condensation process (Figure 6 top and center) [10]. The related reaction also produced 5-hydroxy C5–C6 saturated derivatives (not illustrated).
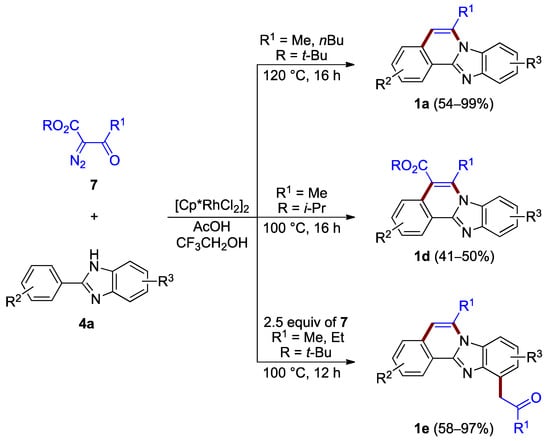
Figure 6.
Synthesis of functionalized benzimidazo[2,1-a]isoquinolines 1a,d,e via rhodium(III)-catalyzed annulation of α-diazoketoesters 7 and 2-arylbenzimidazoles 4a.
The presence of excess of diazo compounds 7, allows for double C–H activation and the addition of a methylene ketone unit, and leads to more functionalized derivatives 1e (58–97%; Figure 6 bottom) [11].
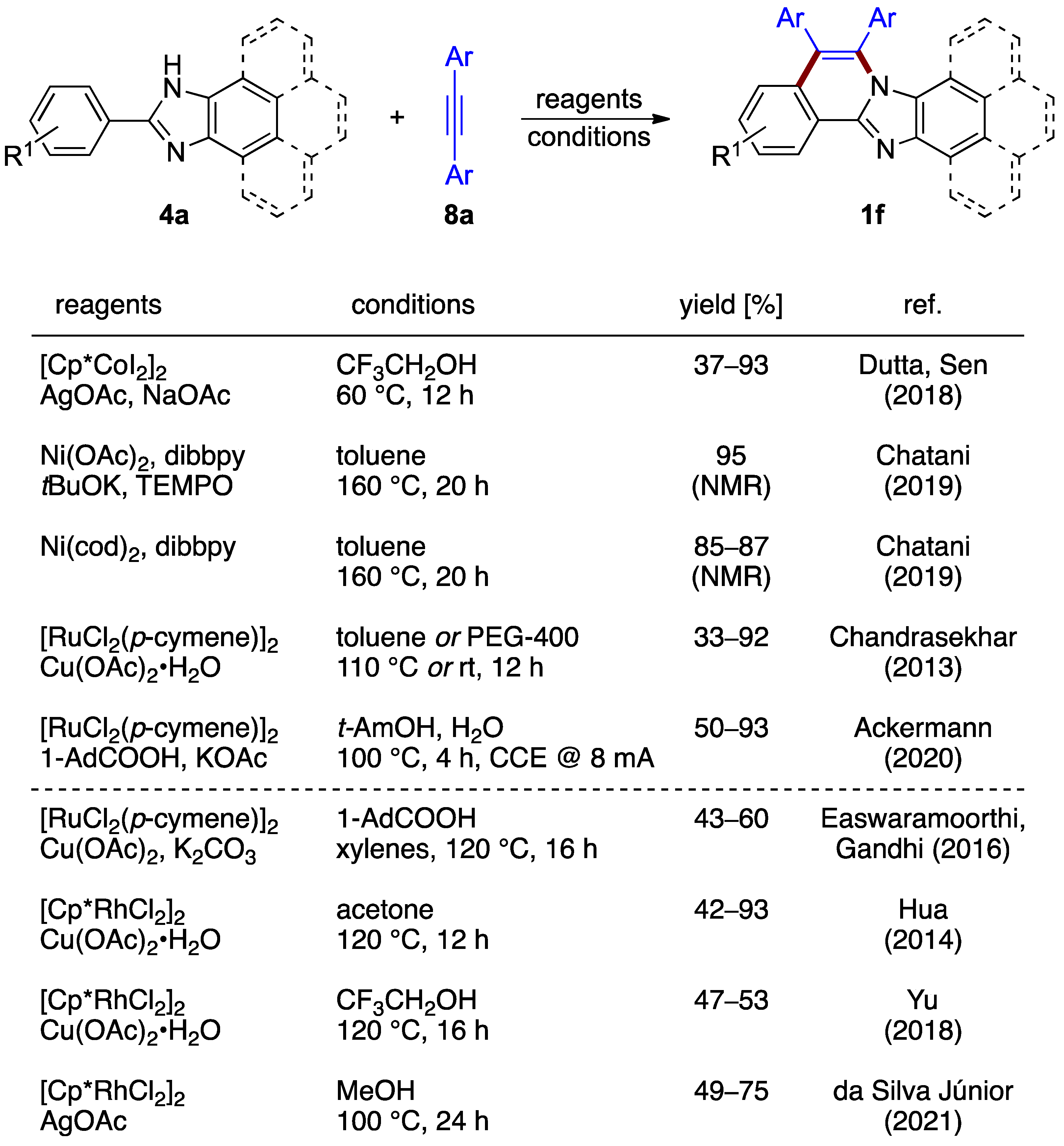
The oxidative annulation procedures rely on the formation of a vinylene unit between C–H and N–H moieties. The non-halogenated 2-arylbenzimidazoles 4a and a variety of diarylacetylenes 8a react in the presence of a catalytic amount of cobalt(III) catalyst and silver acetate as an oxidant, as reported by Dutta and Sen, yielding structures 1f (37–93%; Figure 7) [12]. Nickel complexes also have been shown by Chatani et al. to be active for an analogous reaction with diphenyl acetylene 8a (Ar = Ph) [13]. Various protocols featuring the presence or absence of base were elaborated (Figure 7). A similar synthesis catalyzed by [RuCl2(p-cymene)]2 was also reported (Chandrasekhar et al.; 43–60% yield) [14]. Another ruthenium-catalyzed process, elaborated by Ackermann et al., integrated an innovative electrochemical oxidation approach [15]. Molecular hydrogen is the sole byproduct, with the reaction proceeding with 50–93% yields (Figure 7). The key catalytic intermediate, aza-ruthena(II)-bicyclo-[3.2.0]-heptadiene, was isolated and characterized, increasing the mechanistic understanding of the process.
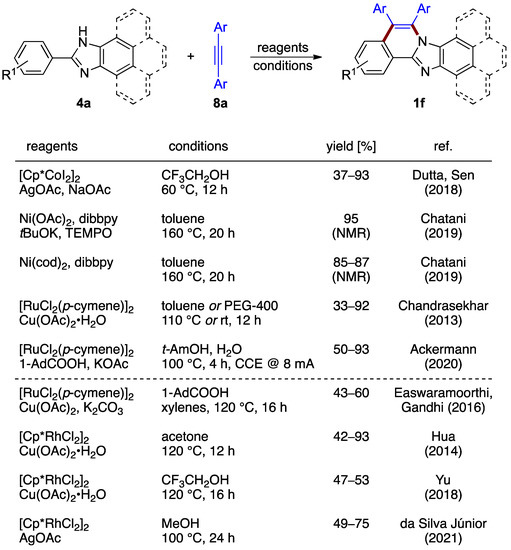
Figure 7.
Oxidative annulation of 2-phenylbenzimidazoles 4a and their fused homologs with internal alkynes 8 by different protocols [12,13,14,15,16,17,18,19].
The use of alkynes in the reaction design strategy was applied to the synthesis of related phenantroimidazolo- and pyrenoimidazolo-derived compounds. In these cases, the reaction outcomes were achieved by [RuCl2(p-cymene)]2 (Easwaramoorthi and Gandhi et al.; 43–60% yield) [16] or [Cp*RhCl2]2 (Hua et al.; 42–93% yield) [17], catalysts aided by copper(II) acetate as the oxidant. A related multicomponent protocol using a [Cp*RhCl2]2 catalyst for the synthesis of phenantroimidazolo structures was described by Yu et al. [18]. The use of silver acetate oxidant combined with a [Cp*RhCl2]2 catalyst was reported as well (da Silva Júnior et al.; 49–75% yield) [19].
Without a demand for C–5 and C–6 substituents, vinylene carbonate 9 offers an effective source of the C–4a to N–7 bridging unit in the rhodium(III)-catalyzed annulation of 2-arylbenzimidazoles 4b, as reported by Nishii, Miura et al. (Figure 8) [20]. This protocol eliminated the need for an additive since vinylene carbonate acts as an internal oxidant to regenerate the rhodium(III) species while producing benzimidazo[2,1-a]isoquinolines 1g (64–83% yield).
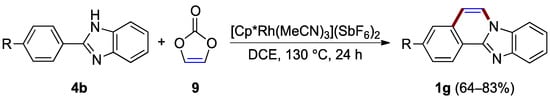
Figure 8.
Application of vinylene carbonate 9 in rhodium-catalyzed oxidative annulation of 2-arylbenzimidazoles 1g.
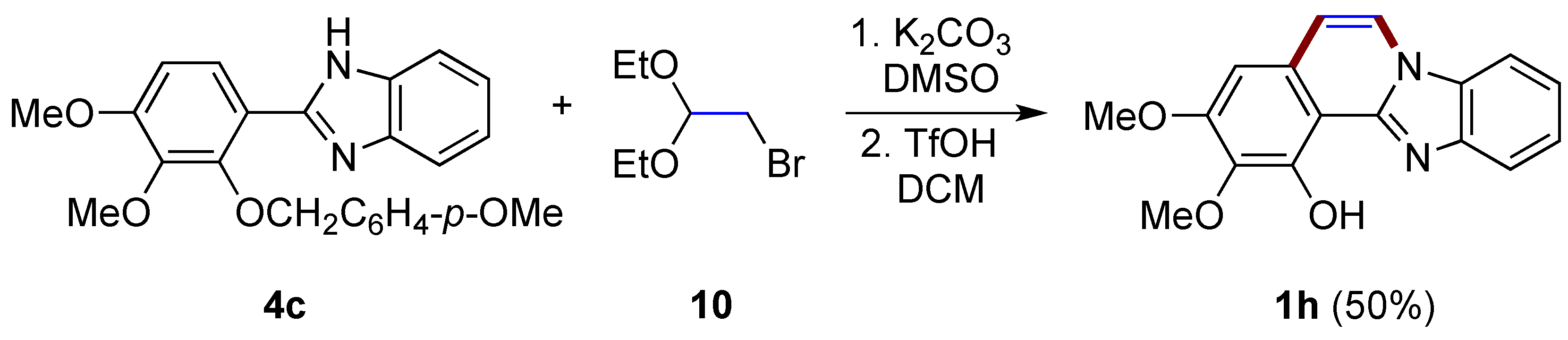
A two-step synthesis first involving the N-alkylation of 2-arylbenzimidazole 4c with bromoacetaldehyde diethyl acetal 10 and then the triflic acid-catalyzed intramolecular cyclization/deprotection of the phenolic hydroxy group allows the formation of benzimidazo[2,1-a]isoquinoline 1h in a moderate yield (50%; Figure 9), as described by Takagi et al. [21]. As clarified by the authors, this same reaction cannot be achieved using concentrated HCl.
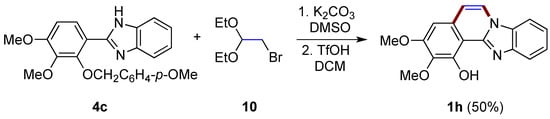
Figure 9.
Synthesis of diversely functionalized benzimidazo[2,1-a]isoquinoline 1h with the use of bromoacetaldehyde derivative 10.
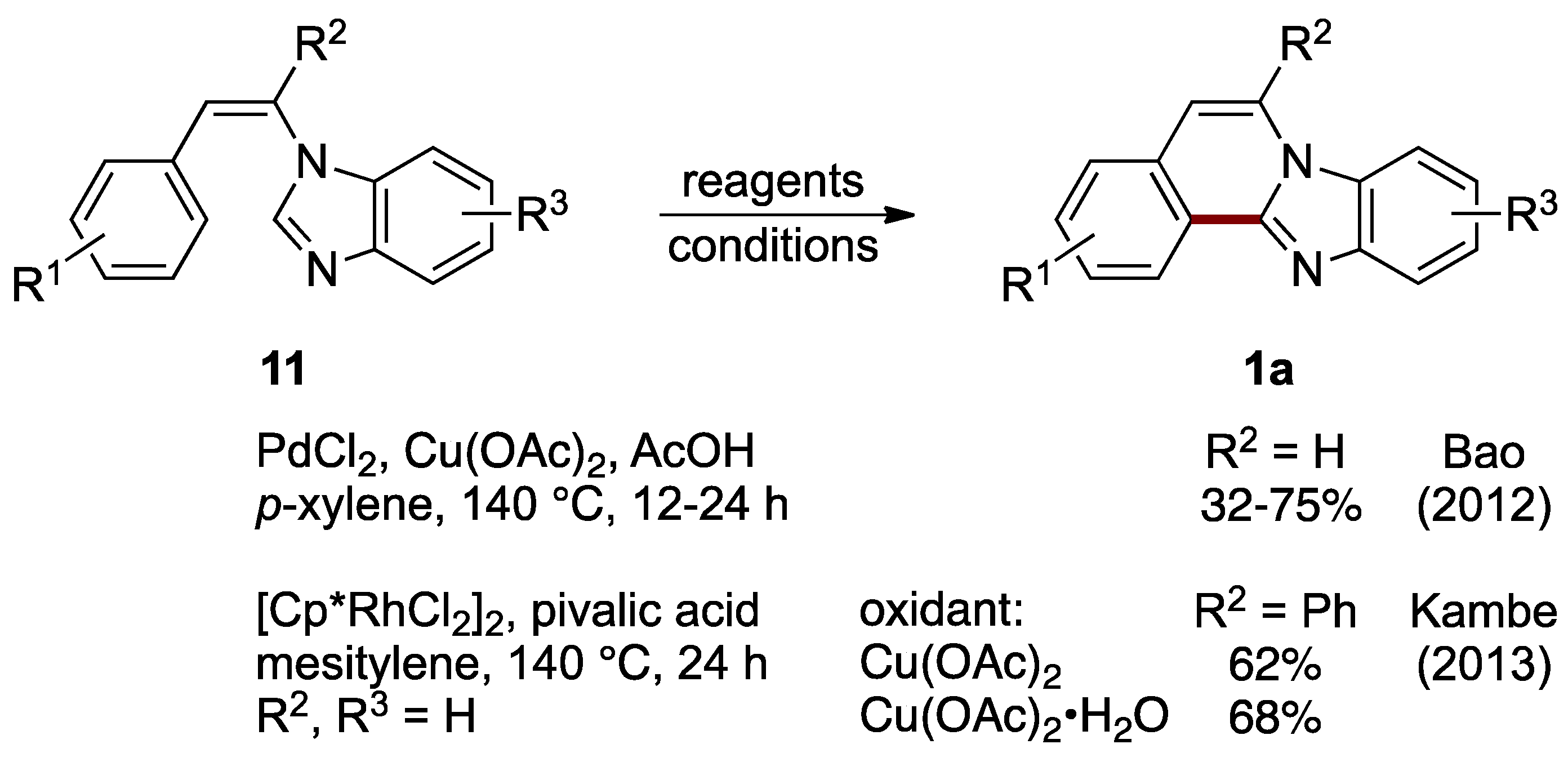
Formation of ring B can be accomplished by connecting C–12a and C–12b atoms (see Figure 1 for the numbering of structure 1). Bao et al. achieved oxidative intramolecular C–C bond formation via double sp2 C–H activation between the 2-position of an imidazole ring and a benzene ring of N-styrylbenzimidazoles 11 catalyzed by palladium(II) chloride, with copper(II) acetate as an oxidant and acetic acid as an additive. This route effectively leads to C–5 and C–6 unsubstituted benzimidazo[2,1-a]isoquinolines 1a (R2 = H, 32–75%; Figure 10) [22]. Soon after that report, Kambe et al. described a related oxidative cross-coupling with the aid of a dichloro(pentamethylcyclopentadienyl)rhodium(III) catalyst [23]. The protocol uses copper(II) acetate as an oxidant and pivalic acid as an additive to yield 6-phenylbenzimidazo[2,1-a]isoquinolines 1a (62–68%). The optimized conditions were applied to a phenyl-substituted N-styrylbenzimidazole 11 (R2 = Ph) to compare anhydrous and monohydrate copper(II) acetate oxidation reagents (Figure 10).
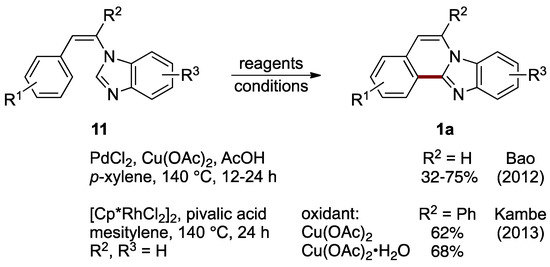
Figure 10.
Palladium- and rhodium-catalyzed intramolecular cross-coupling of N-styrylbenzimidazoles 11 [22,23].
3. Ring C Formation
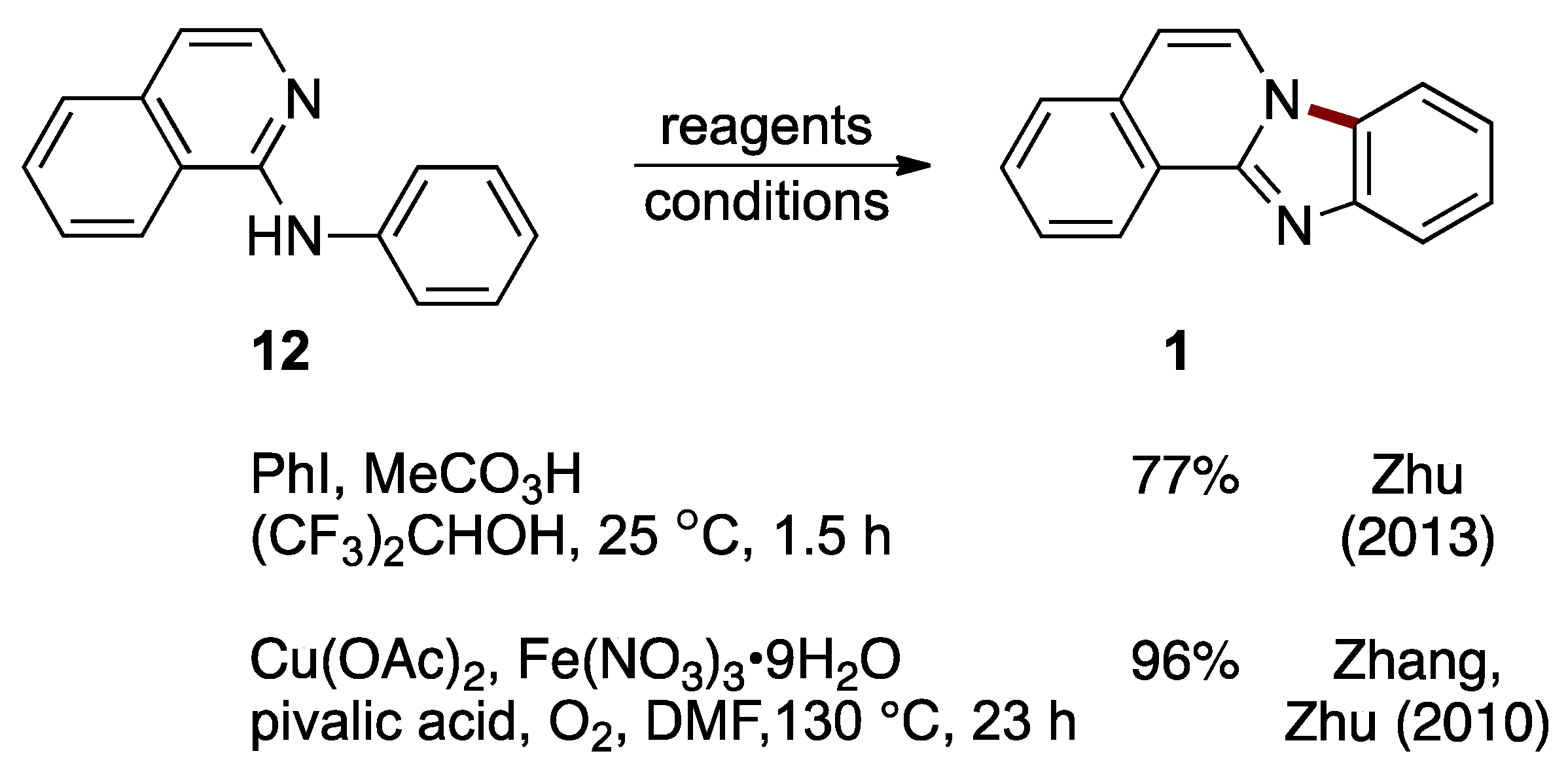
A metal-free synthesis of benzimidazo[2,1-a]isoquinoline 1 from N-phenyl-1-aminoisoquinoline 12 was developed by Zhu et al. This C–H cycloamination reaction is catalyzed by hypervalent iodine(III) generated in situ from iodobenzene (catalytic) and peracetic acid (stoichiometric). The reaction proceeded under ambient conditions with a 77% yield (Figure 11) [24]. Earlier work by Zhang, Zhu et al. established the preparation of benzimidazo[2,1-a]isoquinoline 1 (96%) using direct intramolecular aromatic C–H amination of N-phenyl-1-aminoisoquinoline 12, catalyzed by copper(II) acetate and iron(III) nitrate nonahydrate (Figure 11). In this process, the pyridinyl nitrogen in the starting material 12 acts as both a directing group and nucleophile [25].
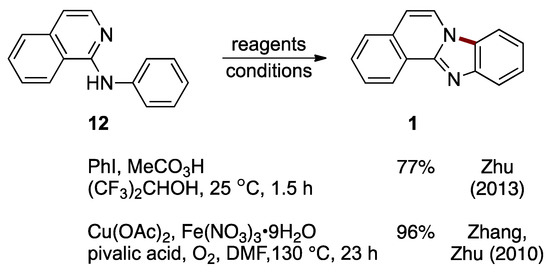
Figure 11.
C–H cycloamination of N-phenyl-2-aminoisoquinoline 12 catalyzed by an in situ-generated hypervalent iodine reagent or copper/iron bimetallic system [24,25].
4. Ring B and C Formation
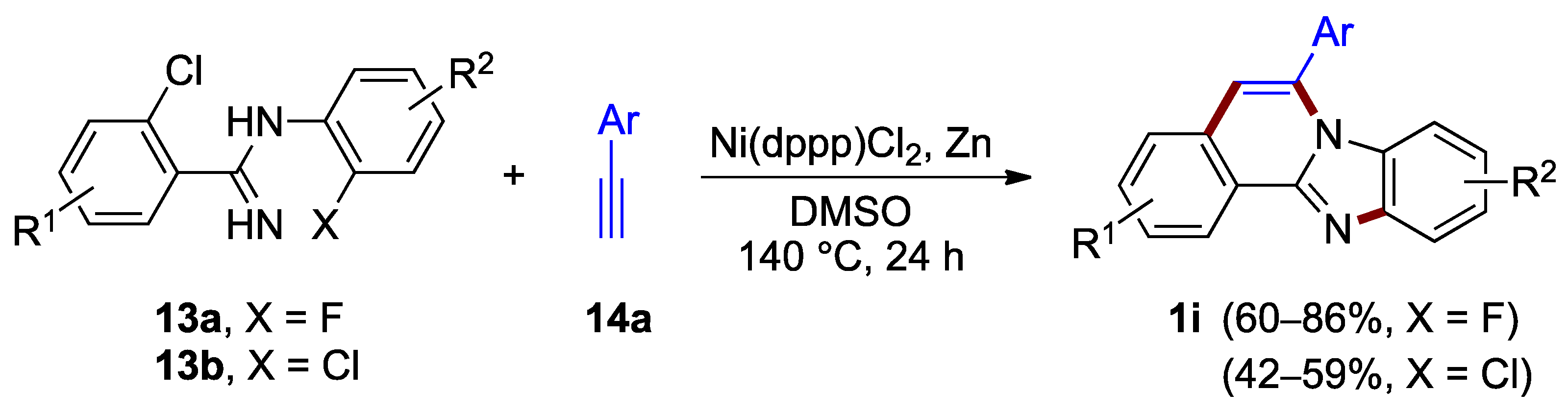
A method that facilitates the construction of both the imidazole ring C and the pyridine ring B utilizes two halogen anchors. Nickel complexes offer an effective tool for activating the reaction of these halogens and terminal alkynes. Xiao, Deng et al. reported the formation of benzimidazo[2,1-a]isoquinoline 1i as a major product in the nickel-catalyzed annulation of 2-chloro-N-(2-halophenyl)benzimidamides 13 with terminal alkynes such as 14a, under anhydrous conditions and elevated temperature (140 °C; Figure 12) [26]. The yield of the product is dependent on halogen in the starting material. The inspection of the yields for benzimidazo[2,1-a]isoquinolines 1i revealed the increased reactivity of the fluoroaryl reactant 13a (X = F; 60–86% yield) as compared to the chloroaryl reactant 13b (X = Cl; 42–59% yield).
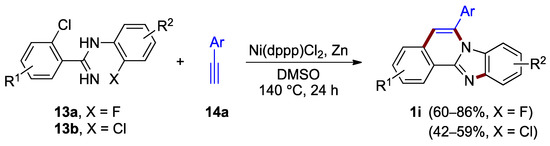
Figure 12.
Nickel-catalyzed annulation of chlorohalobenzimidamides 13 with terminal alkynes 14a.
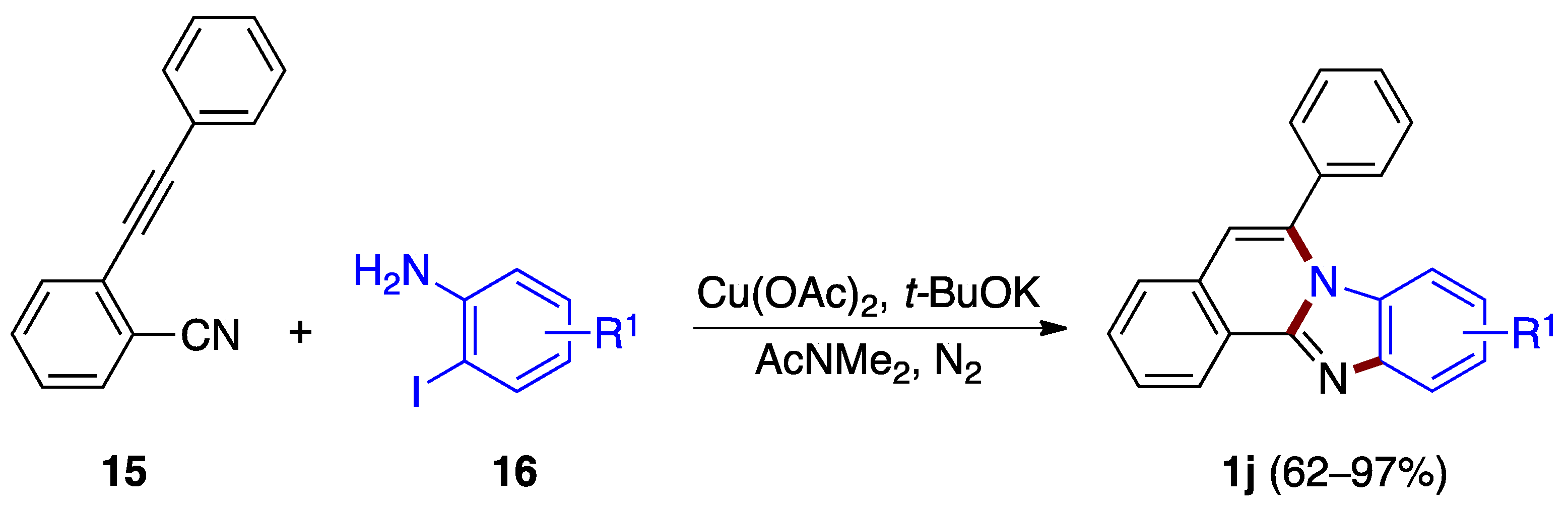
A copper(II) acetate-catalyzed cascade cyclization reaction between o-alkynylbenzonitrile 15 and o-iodoanilines 16 was reported by Deng, Liang et al. [27]. The reaction allows the synthesis of benzimidazo[2,1-a]isoquinolines 1j in good to excellent yields (62–97%), featuring the sequential formation of three different C–N bonds in the presence of a base (Figure 13).
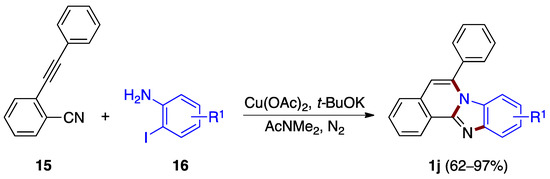
Figure 13.
Copper-catalyzed annulation between o-alkynylbenzonitrile 15 and o-iodoanilines 16.
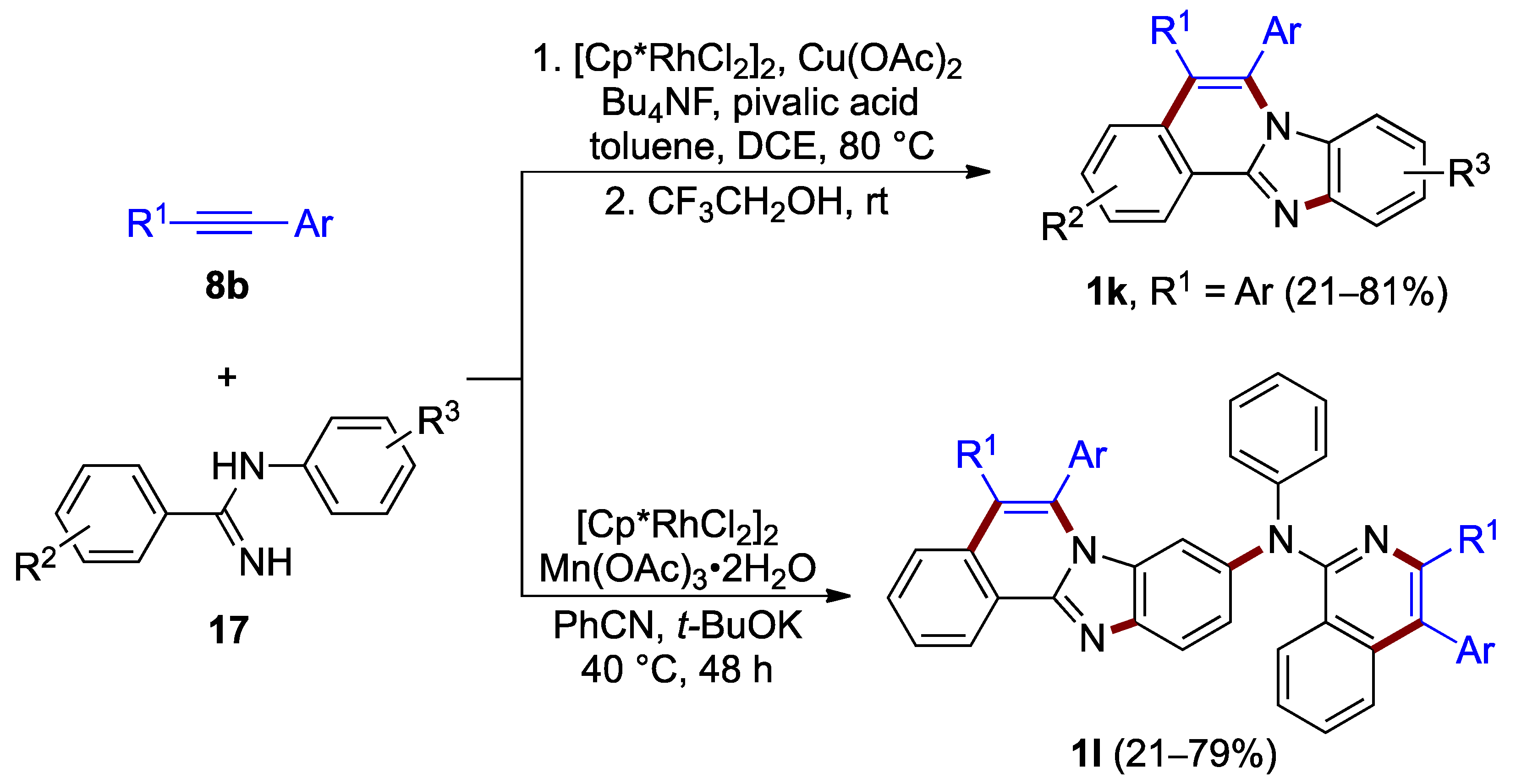
The rhodium(III)-catalyzed oxidative annulation of N-(2-phenyl)benzimidamide 17, which proceeds with use of aryl acetylene 8b at 80 °C, was elaborated by Xu et al. (21–81% yield; Figure 14, top) [28]. This double C–H activation process proceeds by a one-pot reaction, presumably with C–H bond cleavage occurring preferentially at the N-phenyl ring. The resulting benzimidazo[2,1-a]isoquinoline 1k features identical aryl substituents at C–5 and C–6 (R1 = Ar). Xu, Liu, Du et al. achieved a more architecturally complex structure using alternative reagents and solvents, including manganese(III) acetate as an oxidant instead of copper(II) acetate. The synthetic path for this process comprises multiple C–H activation, an intermolecular meta-selective C–H amination, an intramolecular C–H amination between amidines 17 and alkynes 8b, and two different Rh(I)–Rh(III) catalytic cycles to yield the 9-arylamino-substituted product 1l (21–79% yield; Figure 14, bottom) [29].
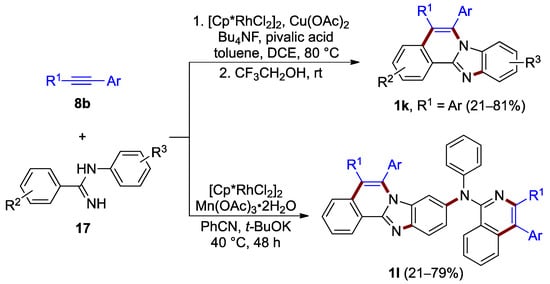
Figure 14.
Rhodium-catalyzed annulation (and amination, bottom) of amidines 17 with alkynes 8b.
5. Ring B and C Formation Using o-Phenylenediamines
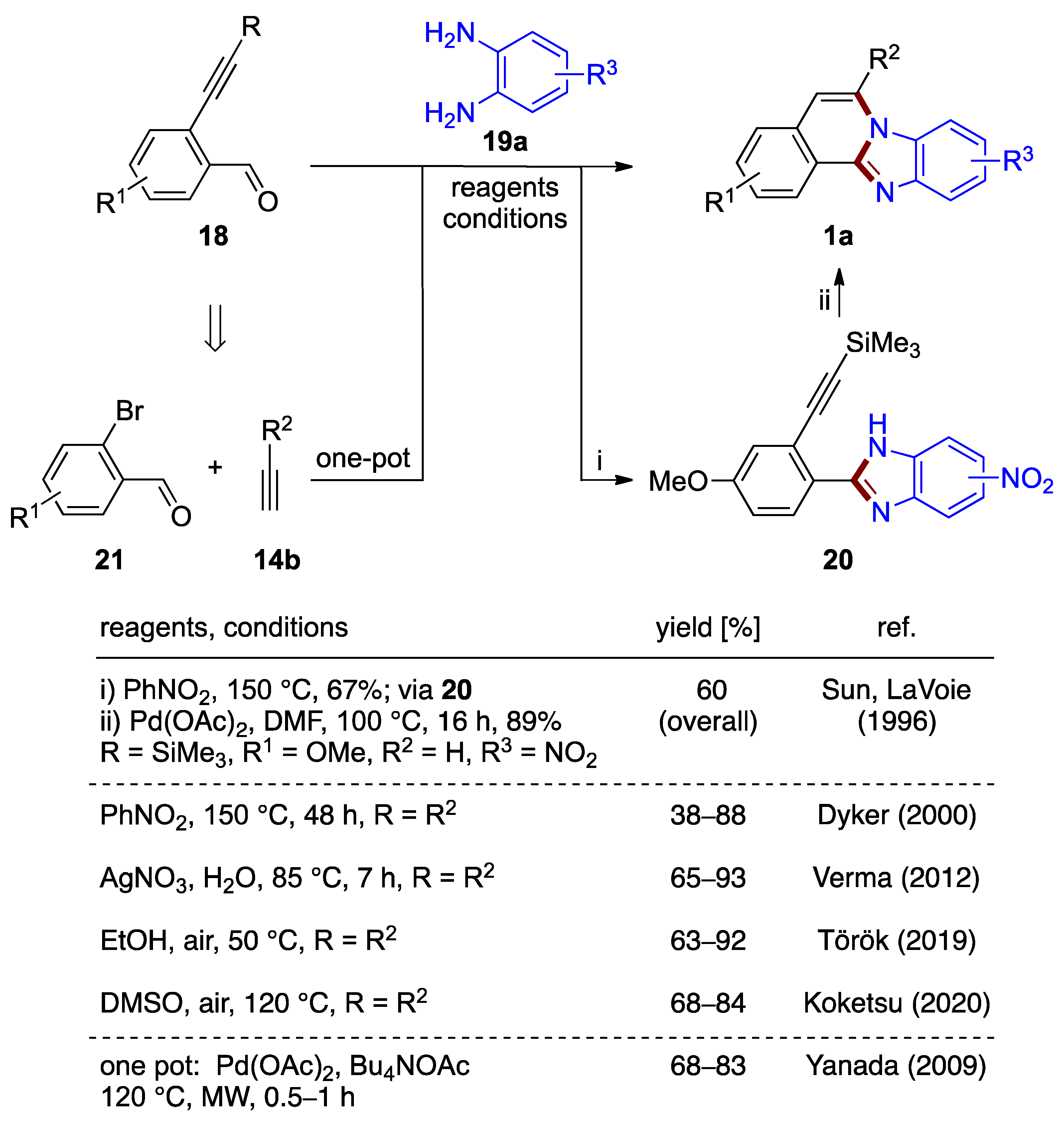
Dyker et al. investigated the tandem cyclization of substituted 2-ethynylbenzaldehydes 18 with o-phenylenediamines 19a (Figure 15, top pathway) [30]. The preparation of several benzimidazo[2,1-a]isoquinolines 1a in 38–88% yields was reported using nitrobenzene as the solvent and oxidizing agent at elevated temperature (150 °C, 2 d). This significant development offers a metal-catalyst-free preparation for this class of compounds [31].
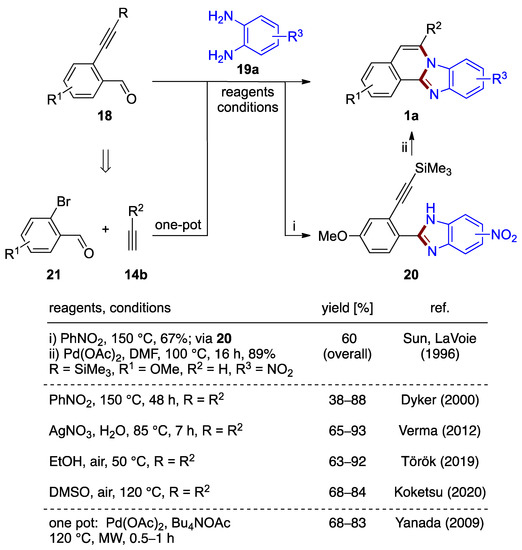
Figure 15.
Synthesis of benzimidazo[2,1-a]isoquinoline 1a by reaction of 2-ethynylbenzaldehyde 18 and o-phenylenediamine 19a (top) and by a one-pot reaction of bromobenzaldehydes 21, terminal acetylenes 14b, and o-phenylenediamines 19a (bottom) [1,30,32,33,34,35].
Dyker’s synthetic design closely relates to the palladium(II) acetate-catalyzed reaction described earlier by Sun and LaVoie [1]. In this prior report, trimethysilyl substituted 2-ethynylbenzaldehyde 18 serves as the starting material in a two-step cyclization/oxidation process via isolated phenylbenzimidazole derivative 20 to form methoxy-nitro-substituted benzimidazo[2,1-a]isoquinolines 1a (R1 = OMe, R2 = H, R3 = NO2; 60% overall yield; Figure 15). Though an important contribution, this approach was not studied beyond a reaction that yielded a mixture of two regiomeric 9- and 10-nitro derivatives [1].
Yanada et al. [32] achieved an improved yield (68%) over Dyker et al. (41%) [30] for the synthesis of an unsubstituted benzimidazo[2,1-a]isoquinoline via a microwave-assisted protocol using palladium(II) acetate in DMF. This result motivated the further development of a protocol based on o-phenylenediamines that combines a one-pot synthesis tandem cyclization and Sonogashira coupling of o-bromobenzaldehydes 21. In this work, a variety of benzimidazo[2,1-a]isoquinolines 1a were produced with 68–83% yields (Figure 15). 2-Ethynylbenzaldehyde 18 forms in situ in the presence of tetrabutylammonium acetate (Bu4NOAc), thus eliminating the need to prepare the precursor separately. The palladium(II) acetate catalyst needed for the alkyne coupling process remains in the reaction medium during the cyclization steps. However, it was not reported if the final cyclization would proceed in the absence of catalyst in the experimental conditions used [32]. This protocol is associated with a stoichiometric amount of base, removal of the high-boiling solvent (DMF), and elevated temperature (120 °C). More recently, Verma et al. described an analogous silver-catalyzed protocol in water (65–93% yield; Figure 15) [33].
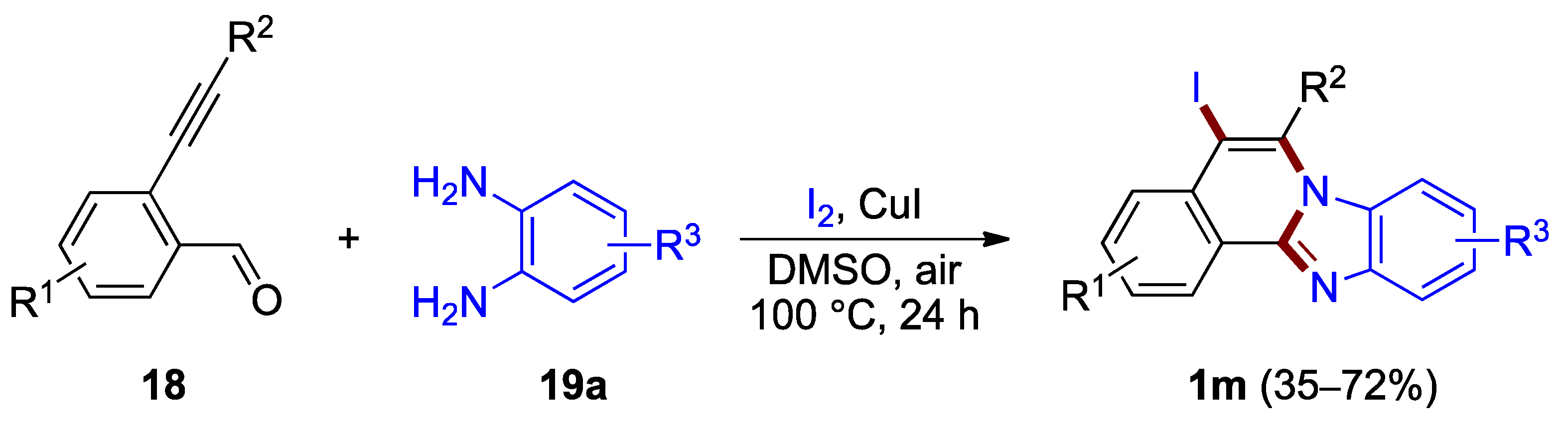
Zhong, Li et al. developed a one-pot method for the synthesis of iodine-functionalized benzimidazo[2,1-a]isoquinolines (Figure 16) [36]. A protocol involving a copper(I) iodide catalyst in the presence of iodine produces 5-iodo-substituted benzimidazo[2,1-a]isoquinolines 1m from 2-ethynylbenzaldehydes 18 and o-phenylenediamines 19a in moderate to good yield (35–72%). The analogous bromination reaction using N-bromosuccinimide as a halogen source has been reported in one example (45% yield) [36].
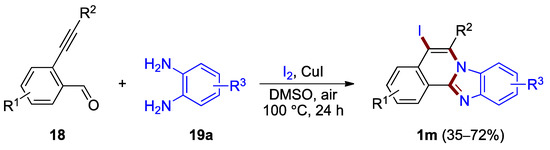
Figure 16.
Synthesis of 5-iodobenzimidazo[2,1-a]isoquinolines 1m.
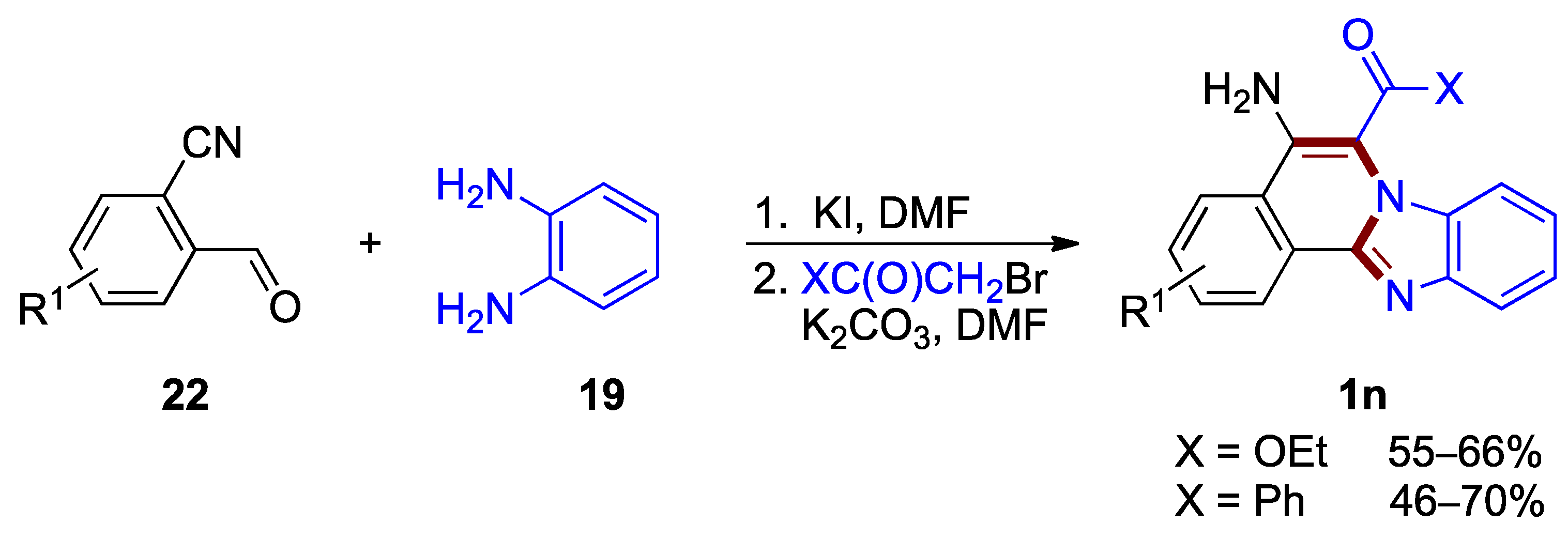
Other functionalization of the benzimidazo[2,1-a]isoquinoline core can also be achieved by a three-component, one-pot protocol developed for the synthesis of 5-amino- and 6-carbonyl-functionalized benzimidazo[2,1-a]isoquinolines 1n [37]. This transformation, elaborated by Kurth et al., proceeds via a transition-metal free protocol using commercially available o-cyanobenzaldehydes 22, o-phenylenediamines 19, and α-bromoacetate or α-bromoacetophenone (46–70% yields; Figure 17).
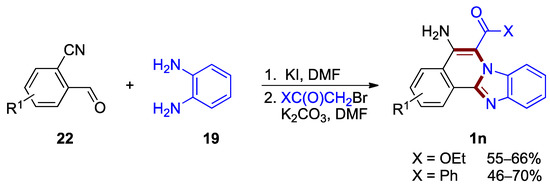
Figure 17.
One-pot tandem cyclization leading to 5-amino- and 6-carbonyl functionalized benzimi-dazo[2,1-a]isoquinolines 1n.
5-Cyano-functionalized benzimidazo[2,1-a]isoquinoline 1o has been synthesized from acetyliminoisocoumarin derivative 23 and o-phenylenediamine 19b in the protocol elaborated by Deady et al. (60%; Figure 18) [38]. A related approach utilizes isocoumarin derivatives (30–33%; not illustrated) [3].
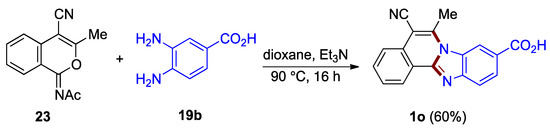
Figure 18.
Reaction of acetyliminoisocoumarin 23 and o-phenylenediamine 19b.
The development of catalyst-free protocols that utilize cost-effective conditions and less hazardous solvents has been pursued by Dembinski and Török. Extended studies of the reactivity of alkynes with amines (or related nitrogen nucleophiles [39]) have established that benzodiazepines can be synthesized by the reaction of alkynones and o-phenylenediamines in ethanol without a catalyst [40,41]. These experiments confirmed that traditional acid-catalyzed or high-temperature syntheses of heterocycles could readily proceed at mild temperatures without a catalyst to give the intended benzimidazo[2,1-a]isoquinolines using substituted o-phenylenediamines.
As shown by Török et al., the catalyst-free reaction between a variety of substituted 2-ethynylbenzaldehydes 18 and o-phenylenediamines 19a, at 50 °C, produced the desired products 1a in 63–92% yields (Figure 15) [34]. The protocol was shown to tolerate strong electron-withdrawing substituents, such as fluorine. The successful installation of a cyclopropyl group at C–6 suggests that the presence of an aryl substituent at the alkyne is not essential to the process. The reaction of 2-ethynylpyridine-3-carbaldehyde demonstrates the utility of the protocol to incorporate additional heteroatoms into the benzimidazo[2,1-a]isoquinoline skeleton. Soon after, Koketsu et al. reported also metal-free protocols using the same reagents 18 and 19a (DMSO, 120 °C; Figure 15), in which produced benzimidazo[2,1-a]isoquinolines 1a (68–84% yields) were subjected to photophysical studies [35].
The reactivity of bifunctional reagents often requires a specific molecular scaffold with pendant functional groups. Regarding the competitive reactivity of nucleophilic aromatic amines versus a carbonyl group or alkyne group, a prior effort suggests that the first step may include the creation of a C–N bond adjacent to the alkyne [40,41]. Alternatively, the first step of the reaction sequence may involve the reaction of the carbonyl group leading to imine formation.
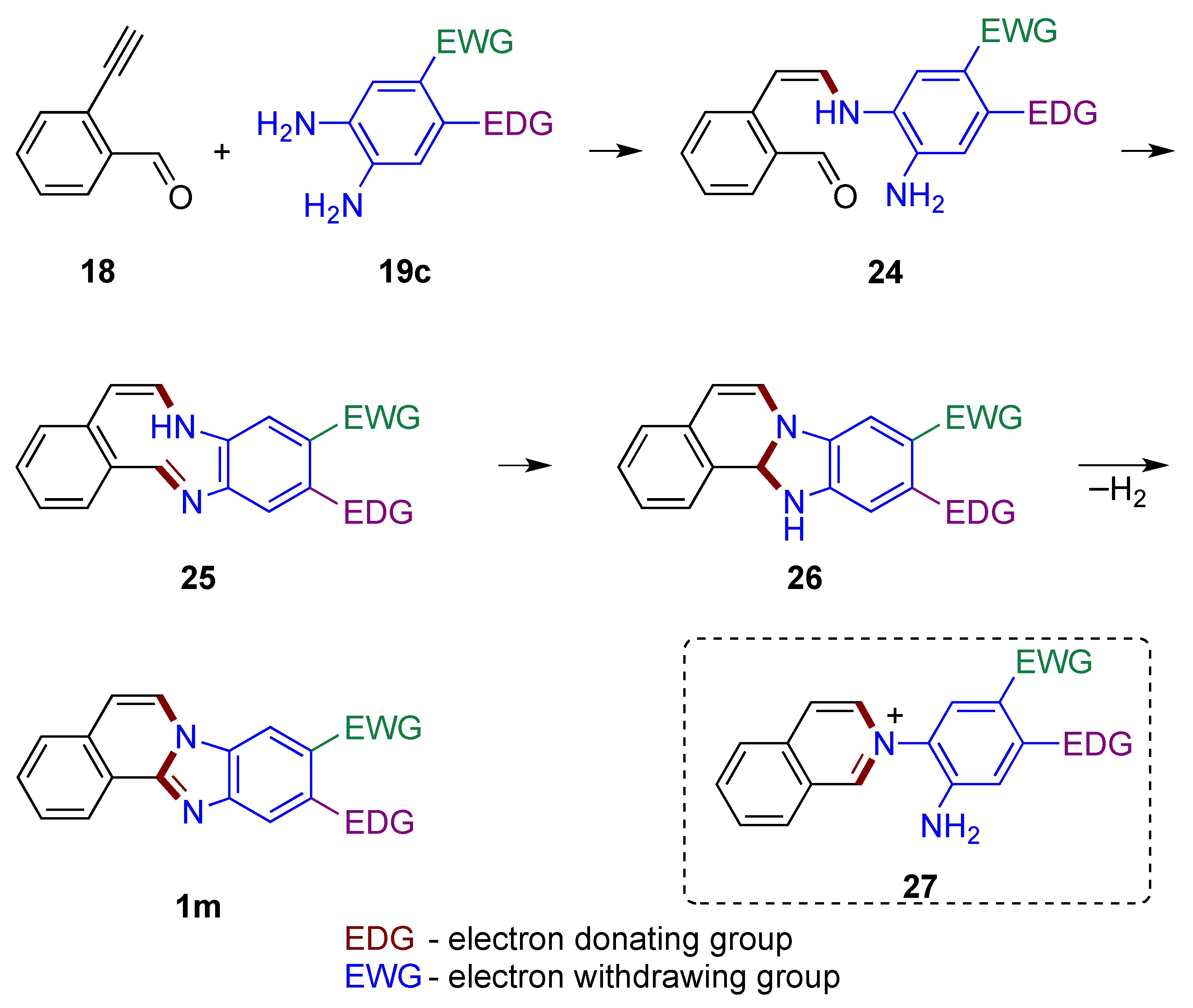
If alkyne 18 were to first react with o-phenylenediamine 19c, enamine 24 would form adjacent to the aldehyde group (Figure 19). The presence of electron donating group (EDG) and electron withdrawing group (EWG) in non-symmetrical o-phenylenediamines affects the nucleophilicity of the amino groups, and in turn determines the regioselectivity of the products. The unreacted amine function of the o-phenylenediamine is then available to attack the carbonyl group, forming an imine 25 embedded within the conjugated 9-membered ring system. Nucleophilic attack of the amine on the imine group yields tetracyclic structure 26, which is further oxidized to the final product 1m by atmospheric oxygen. The route (not illustrated) leading to the formation of a six-membered ring from attack of the carbonyl group by enamine 24 to give potentially reactive isoquinolinium intermediate 27 should be considered as less likely [33].
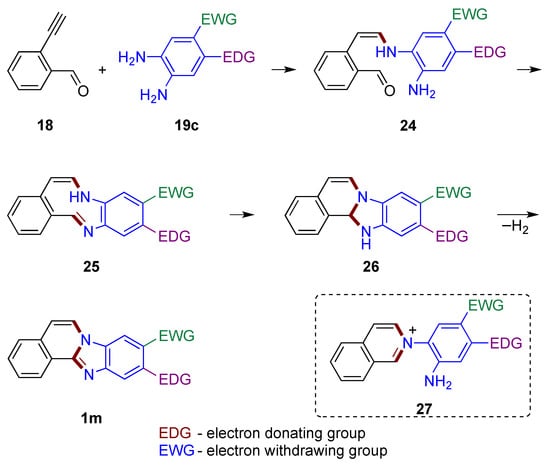
Figure 19.
Mechanistic outline illustrating one of the amino groups of o-phenylenediamine reacting with the alkyne first.
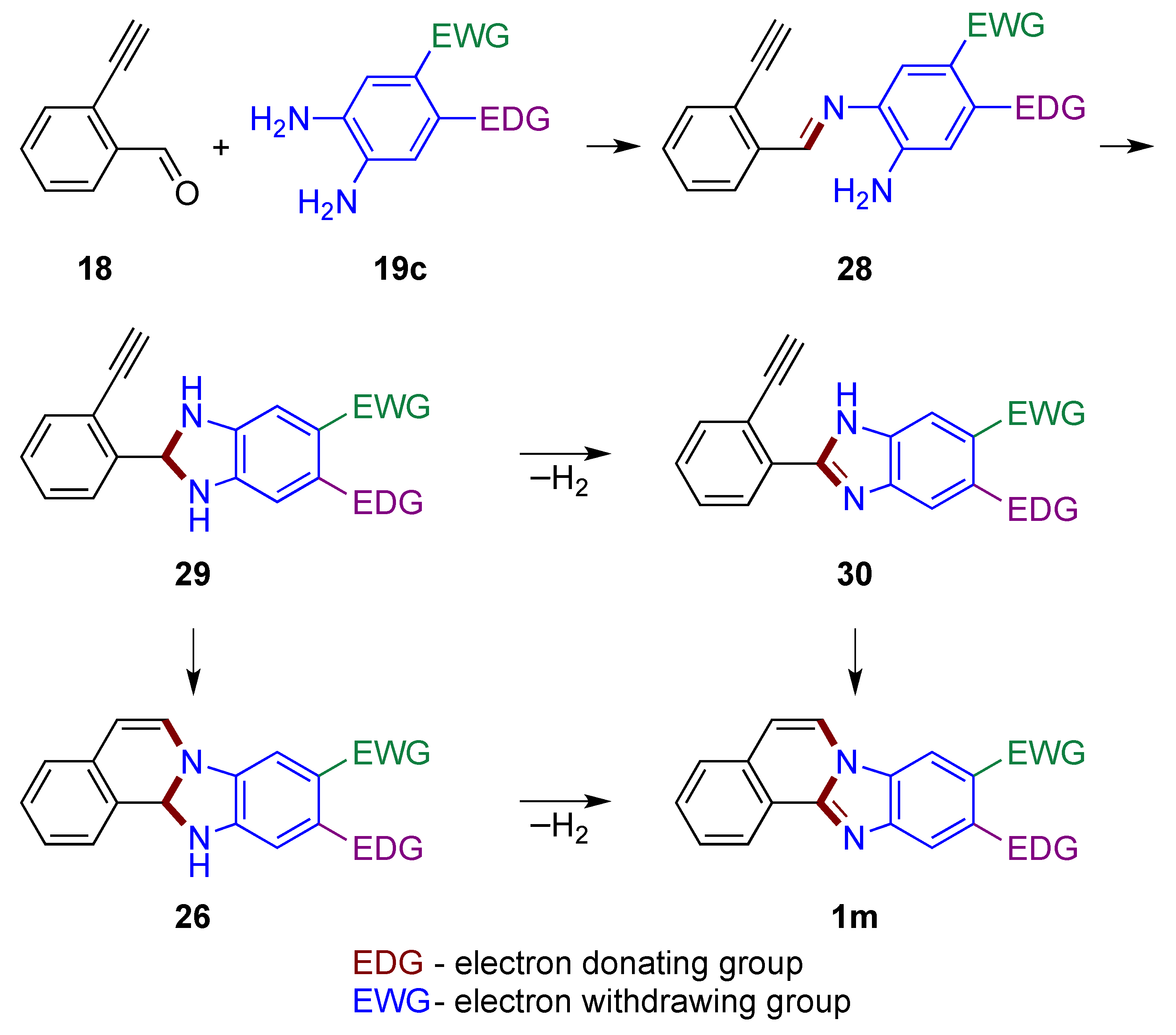
A plausible pathway in which the carbonyl group reacts first is shown in Figure 20. In the first step, the reaction of aldehyde 18 with o-phenylenediamine 19c yields imine 28. The imine’s carbonyl carbon then connects to the second amino group of the phenylenediamine to form a dihydrobenzimidazole 29. Oxidation to the benzimidazole 30 (similar to structure 20, Figure 15) may take place [1,33], which is followed by the attack of the triple bond by the enamine nitrogen atom leading to the final product 1m. If attack of the triple bond by nitrogen takes place first, leading to the dihydro derivative 26, subsequent oxidation by atmospheric oxygen yields the final product 1m. It should be noted that regioselective induction may occur by attack on the alkyne by the more nucleophilic nitrogen in both structures 29 and 30 (possible rotation around a single bond). The EDG and EWG locations illustrated in 19c impact o-phenylenediamine nucleophilic nitrogen atoms in a synergistic manner for both discussed mechanisms (Figure 19 and Figure 20).
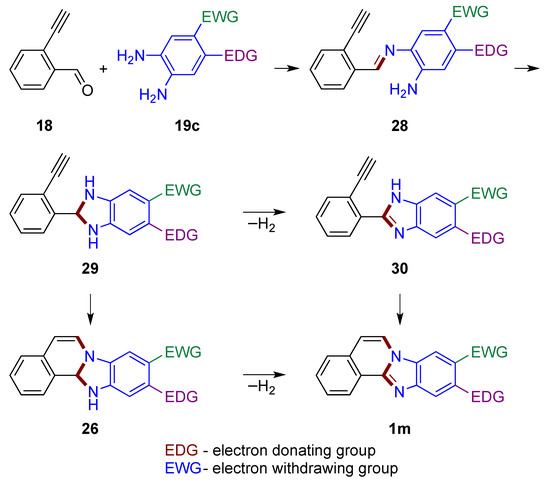
Figure 20.
Mechanistic outline for the formation of benzimidazo[2,1-a]isoquinolines 1m with carbonyl group reacting first, illustrating regioselectivity of a reaction using EDG- and EWG-substituted phenylenediamine.
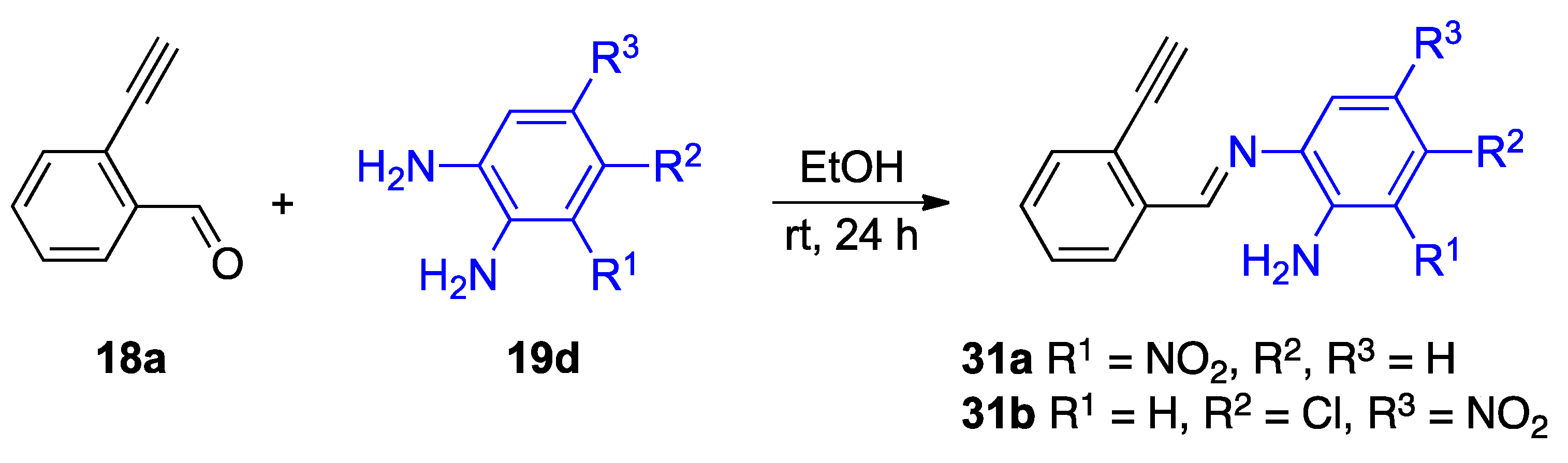
The isolation of several intermediates supports the mechanism provided in Figure 20. The reaction of unsubstituted 2-ethnylbenzaldehyde 18a with selected o-phenylenediamines 19d results in an imine intermediate before a reaction involving the alkyne moiety (Figure 21) [34]. The detection of intermediate 31a,b suggests the reaction at the carbonyl group occurs before the alkyne. Given the electron-withdrawing character of the nitro substituent, the isolation of imines 31a,b also supports the hypothesis that the more electron-rich amino group of the o-phenylenediamine reacts soonest [34].
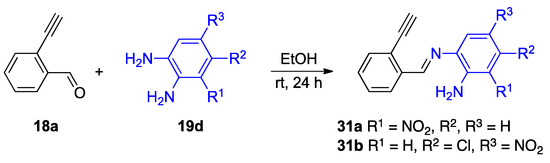
Figure 21.
Isolation of mechanistically relevant imine intermediates 31a,b.
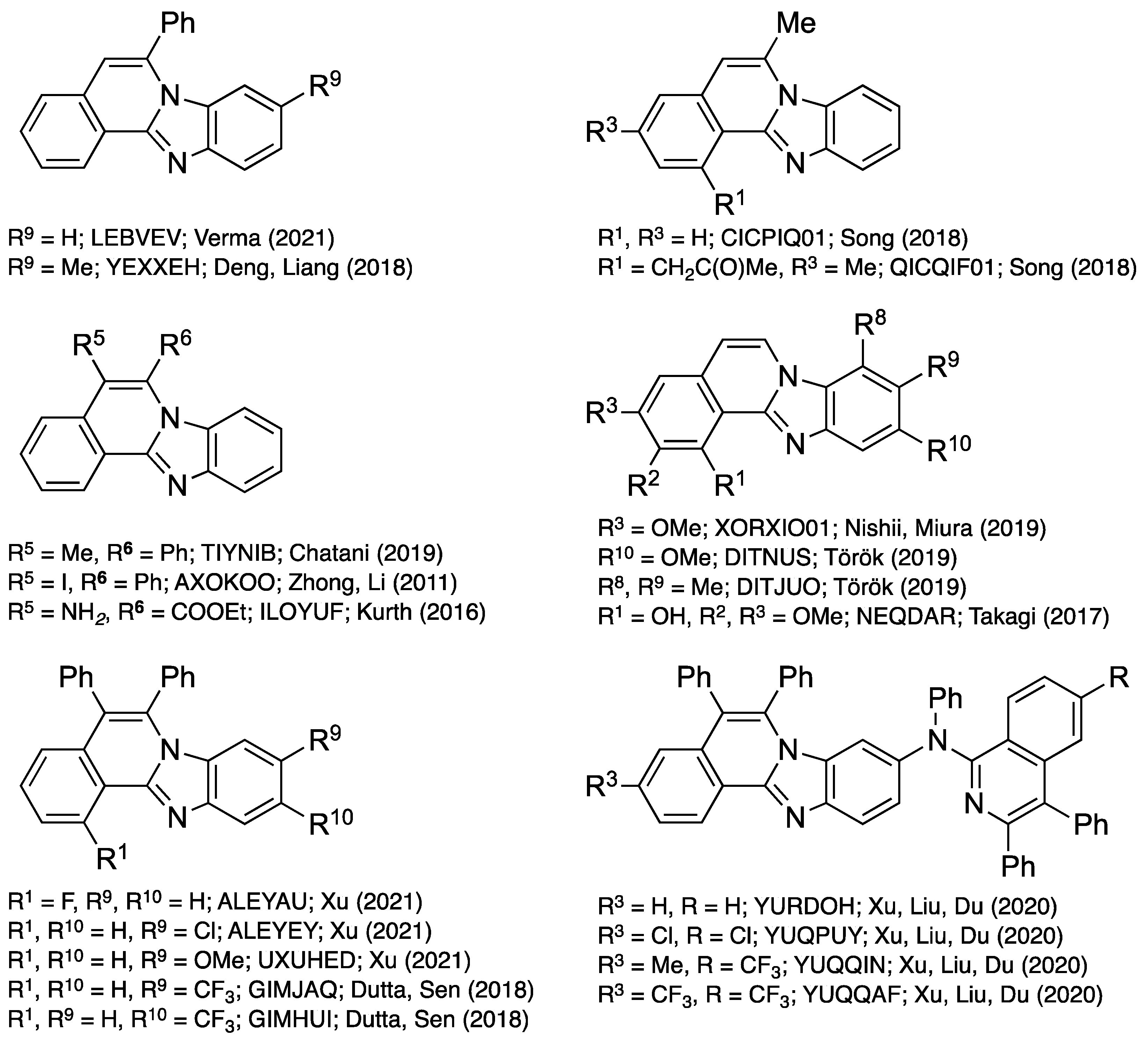
The regioselectivity of the reaction of 2-ethnylbenzaldehyde 18 with the 4-methoxy-o-phenylenediamine 19d (EDG = OMe, EWG = H), documented by X-ray crystallography, provides some support for the mechanisms illustrated in Figure 19 and Figure 20. Unambiguous determination of the structure of the major products (CSD ref. code: DITNUS; Figure 22) suggests that the electron donating ability of the methoxy substituent para to the amino group may contribute to the regioselectivity observed. 3,4-Dimethyl-o-phenylenediamine was also investigated as a reactant to determine the effect of nucleophilicity on regiochemistry. The regioselectivity observed in the isolated major benzimidazo[2,1-a]isoquinoline was confirmed by X-ray crystallography (CSD ref. code: DITJUO; Figure 22). Regioselective induction was observed in other works cited in this review when non-symmetric o-phenylenediamines were used as starting materials, demonstrating the formation of regioisomeric mixtures with different degrees of control.
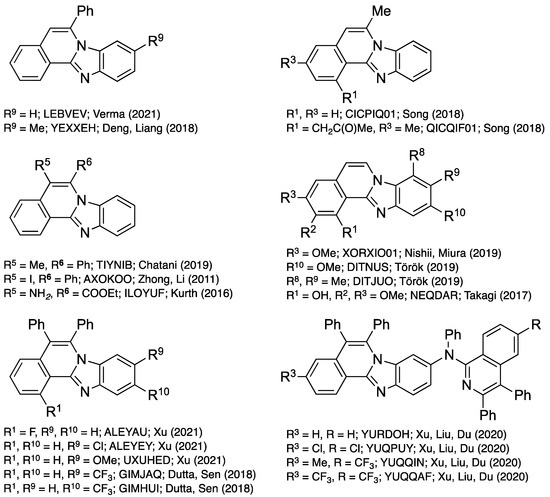
Figure 22.
Structures and CSD reference codes of crystallographically characterized derivatives of benzimidazo[2,1-a]isoquinolines 1 [10,11,12,13,20,21,27,28,29,33,34,36,37].
6. Crystallographically Characterized Benzimidazo[2,1-a]isoquinolines
A search of the CSD shows that several substituted benzimidazo[2,1-a]isoquinolines have been crystallographically characterized (Figure 22) [42]. The retrieved 20 structures were sorted by substituent types attached to C–5 and C–6 to review their influence on bond character (length). The substituents’ R numbering in Figure 22 reflects molecules 1 illustrated in Figure 1. The review of bond distance averages is compiled in Table 1, and provides evidence of aromaticity, with pronounced fluctuation of the bond lengths along the derivatives of 1. Specifically, the C5–C6 bond distance ranges from 1.327(8) to 1.385(2) Å (average 1.35 Å), indicating double bond character, while C4a–C5 and C6–N7 bond distance profiles indicate greater single bond character (1.407(4)–1.464(2) and 1.385(2)–1.418(2)Å, average 1.44 and 1.40 Å, respectively). Presumably, the C5–C6 bond will be the most reactive in the molecule of 1. The C12a–C12b bond length is notably long with 1.426(2)–1.450(2) Å (average 1.44 Å) bond distances that closely follow C–C bond patterns. The crystal structures also reflect the N12–C12a double bond character (1.307(3)–1.344(3), average 1.32 Å); the distance of single bonds N7–C6, N7–C7a, and N7–C12a was consistent, with averages of 1.40, 1.40, and 1.40 Å, respectively (Table 1).

Table 1.
Bond length range and averages [Å] from crystallographic data (for numbering see Figure 1). Bridging internal bonds are highlighted.
7. Summary and Conclusions
Recently, significant emphasis was placed on the environmental compatibility of the synthesis of heterocycles [43,44], including benzimidazo[2,1-a]isoquinolines. Generally, phenyl-substituted benzimidazoles produce benzimidazo[2,1-a]isoquinolines in reactions using either ketone or alkyne reagents. Moreover, benzimidamides or ethynylbenzaldehydes can be used to obtain the target compounds through similar reactions with alkynes and diamines, respectively. Widespread interest in the synthesis of benzimidazo[2,1-a]isoquinolines has generated a range of effective synthetic protocols, often utilizing alkynes, that differ by the degree of compliance to current environmental and safety standards. This development of synthetic protocols has helped to expand current approaches towards benign and catalyst-free methods [34,35,41,42]. In general, the collected synthetic procedures show broad substituent tolerance and are potentially more amenable to sustainable synthesis than earlier protocols. The green nature of the highlighted method developed in the author’s laboratory is reflected by: (i) catalyst-free protocols; (ii) low temperatures; (iii) renewable solvents; (iv) near quantitative yields; (v) high atom economy; and (vi) readily accessible and cost-effective reagents. Most of the reviewed methods offer vast opportunities for structure enrichment by incorporating, for example, an additional nitrogen heteroatom into ring A and/or D by implementing pyridine-derived starting materials, or for extensions of structures to larger aromatic systems.
Author Contributions
All authors wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
Acknowledgment is made to the Donors of the American Chemical Society Petroleum Research Fund (PRF #52858) for the support of this research. The NSF awards (CHE1827313, CHE-0821487, and CHE-1048719), as well as OU Research Excellence Fund and CBMiM PAN statutory funds are also acknowledged.
Acknowledgments
We are grateful to Mitchell Aikens for assembly and review of crystallographic data.
Conflicts of Interest
The author declares no conflict of interest.
References
- Sun, Q.; LaVoie, E.J. Synthesis of Benzimidazo[2,1-a]isoquinolines and 5,6-Dihydrobenzimidazo[2,1-a]isoquinolines. Heterocycles 1996, 43, 737–743. [Google Scholar] [CrossRef]
- Lu, Z.X.; Quazi, N.H.; Deady, L.W.; Polya, G.M. Selective Inhibition of Cyclic AMP-Dependent Protein Kinase by Isoquinoline Derivatives. Biol. Chem. Hoppe-Seyler 1996, 377, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Deady, L.W.; Rodemann, T.; Finlay, G.J.; Baguley, B.C.; Denny, W.A. Synthesis and cytotoxic activity of carboxamide derivatives of benzimidazo[2,1-a]isoquinoline and pyrido[3’,2’:4,5]imidazo[2,1-a]isoquinoline. Anti-Cancer Drug Des. 2000, 15, 339–346. Available online: https://www.ingentaconnect.com/contentone/cog/antcan/2000/00000015/00000005/art00005 (accessed on 16 February 2022).
- Garrett, C.E.; Prasad, K. The Art of Meeting Palladium Specifications in Active Pharmaceutical Ingredients Produced by Pd-Catalyzed Reactions. Adv. Synth. Catal. 2004, 346, 889–900. [Google Scholar] [CrossRef]
- Miao, W.-Q.; Liu, J.-Q.; Wang, X.-S. An efficient synthesis of 6-arylbenzo[4,5]imidazo[2,1-a]isoquinolines via sequential α-arylation of carbonyl and deacylation catalyzed by CuI. Org. Biomol. Chem. 2017, 15, 5325–5331. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.W.; Dao, P.D.Q.; Yoon, N.S.; Cho, C.S. Copper-catalyzed C-C coupling and cyclization: Synthesis of benzo[4,5]imidazo[1,2-a]pyridines and benzo[4,5]imidazo[2,1-a]isoquinolines. J. Organomet. Chem. 2017, 851, 136–142. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z. Copper-Catalyzed Construction of Benzo[4,5]imidazo[2,1-a]isoquinolines Using Calcium Carbide as a Solid Alkyne Source. Org. Lett. 2021, 23, 8407–8412. [Google Scholar] [CrossRef]
- Peng, J.; Shang, G.; Chen, C.; Miao, Z.; Li, B. Nucleophilic Addition of Benzimidazoles to Alkynyl Bromides/Palladium-Catalyzed Intramolecular C–H Vinylation: Synthesis of Benzo[4,5]imidazo[2,1-a]isoquinolines. J. Org. Chem. 2013, 78, 1242–1248. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, R.; Wu, X.; Sun, S.; Yu, J.-T. Rhodium-Catalyzed Annulation of 2-Arylimidazoles and α-Aroyl Sulfoxonium Ylides toward 5-Arylimidazo[2,1-a]isoquinolines. Synthesis 2018, 50, 3487–3492. [Google Scholar] [CrossRef]
- Mai, S.; Luo, Y.; Huang, X.; Shu, Z.; Li, B.; Lan, Y.; Song, Q. Diversity-oriented synthesis of imidazo[2,1-a]isoquinolines. Chem. Commun. 2018, 54, 10240–10243. [Google Scholar] [CrossRef]
- Tang, Z.; Mai, S.; Zhou, Y.; Song, Q. Divergent synthesis of α-aryl ketones/esters via rhodium-catalyzed selective deesterification and decarbonylation of diazo compounds. Org. Chem. Front. 2018, 5, 2583–2587. [Google Scholar] [CrossRef]
- Dutta, P.K.; Sen, S. (Benz)Imidazole-Directed Cobalt(III)-Catalyzed C-H Activation of Arenes: A Facile Strategy to Access Polyheteroarenes by Oxidative Annulation. Eur. J. Org. Chem. 2018, 2018, 5512–5519. [Google Scholar] [CrossRef]
- Obata, A.; Sasagawa, A.; Yamazaki, K.; Ano, Y.; Chatani, N. Nickel-catalyzed oxidative C–H/N–H annulation of N-heteroaromatic compounds with alkynes. Chem. Sci. 2019, 10, 3242–3248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavitha, N.; Sukumar, G.; Kumar, V.P.; Mainkar, P.S.; Chandrasekhar, S. Ruthenium-catalyzed benzimidazoisoquinoline synthesis via oxidative coupling of 2-arylbenzimidazoles with alkynes. Tetrahedron Lett. 2013, 54, 4198–4201. [Google Scholar] [CrossRef]
- Yang, L.; Steinbock, R.; Scheremetjew, A.; Kuniyil, R.; Finger, L.H.; Messinis, A.M.; Ackermann, L. Azaruthena(II)-bicyclo[3.2.0]heptadiene: Key Intermediate for Ruthenaelectro(II/III/I)-catalyzed Alkyne Annulations. Angew. Chem. Int. Ed. 2020, 59, 11130–11135. [Google Scholar] [CrossRef]
- Karthik, S.; Ajantha, J.; Nagaraja, C.M.; Easwaramoorthi, S.; Gandhi, T. Synthesis and photophysics of extended π-conjugated systems of substituted 10-aryl-pyrenoimidazoles. Org. Biomol. Chem. 2016, 14, 10255–10266. [Google Scholar] [CrossRef]
- For related phenanthroimidazoloisoquinolines derivatives, see: Zheng, L.; Hua, R. Modular Assembly of Ring-Fused and π-Extended Phenanthroimidazoles via C–H Activation and Alkyne Annulation. J. Org. Chem. 2014, 79, 3930–3936. [Google Scholar] [CrossRef]
- For related phenanthroimidazoloisoquinolines derivatives, see: Yu, T.; Li, X.; Zhao, Y.; Yang, Q.; Li, Y.; Zhang, H.; Li, Z. Phenanthro[9′,10′:4,5]imidazo[2,1-a]isoquinoline derivatives containing phenoxazine moiety: Synthesis and photophysical properties. J. Photochem. Photobiol. A Chem. 2018, 360, 58–63. [Google Scholar] [CrossRef]
- For related arylimidazo[2,1-a]isoquinolines derivatives, see: Dias, G.G.; Paz, E.R.S.; Kadooca, J.Y.; Sabino, A.A.; Cury, L.A.; Torikai, K.; de Simone, C.A.; Fantuzzi, F.; da Silva Júnior, E.N. Rhodium(III)-Catalyzed C–H/N–H Alkyne Annulation of Nonsymmetric 2-Aryl (Benz)imidazole Derivatives: Photophysical and Mechanistic Insights. J. Org. Chem. 2021, 86, 264–278. [Google Scholar] [CrossRef]
- Ghosh, K.; Nishii, Y.; Miura, M. Rhodium-Catalyzed Annulative Coupling Using Vinylene Carbonate as an Oxidizing Acetylene Surrogate. ACS Catal. 2019, 9, 11455–11460. [Google Scholar] [CrossRef]
- Takagi, K.; Ito, K.; Yamada, Y.; Nakashima, T.; Fukuda, R.; Ehara, M.; Masu, H. Synthesis and Optical Properties of Excited-State Intramolecular Proton Transfer Active π-Conjugated Benzimidazole Compounds: Influence of Structural Rigidification by Ring Fusion. J. Org. Chem. 2017, 82, 12173–12180. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wu, H.; Zheng, J.; Bao, W. Palladium-Catalyzed Oxidative Intramolecular C-C Bond Formation via Double sp2 C-H Activation between the 2-Position of Imidazoles and a Benzene Ring. Adv. Synth. Catal. 2012, 354, 835–838. [Google Scholar] [CrossRef]
- Reddy, V.P.; Iwasaki, T.; Kambe, N. Synthesis of imidazo and benzimidazo[2,1-a]isoquinolines by rhodium-catalyzed intramolecular double C–H bond activation. Org. Biomol. Chem. 2013, 11, 2249–2253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Huang, J.; Liang, D.; Liu, L.; Zhu, Q. C–H cycloamination of N-aryl-2-aminopyridines and N-arylamidines catalyzed by an in situ generated hypervalent iodine(iii) reagent. Chem. Commun. 2013, 49, 7352–7354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Wang, Y.; Peng, C.; Zhang, J.; Zhu, Q. A Direct Intramolecular C−H Amination Reaction Cocatalyzed by Copper(II) and Iron(III) as Part of an Efficient Route for the Synthesis of Pyrido[1,2-a]benzimidazoles fromN-Aryl-2-aminopyridines. J. Am. Chem. Soc. 2010, 132, 13217–13219. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Xing, Q.; Shan, Z.; Xiao, F.; Deng, G.-J. Nickel-Catalyzed Annulation of o-Haloarylamidines with Aryl Acetylenes: Synthesis of Isoquinolone and 1-Aminoisoquinoline Derivatives. Adv. Synth. Catal. 2019, 361, 1896–1901. [Google Scholar] [CrossRef]
- Liu, X.; Deng, G.; Liang, Y. Selective synthesis of benzo[4,5]imidazo[2,1-a]isoquinolines via copper-catalyzed tandem annulation of alkynylbenzonitriles with 2-Iodoanilines. Tetrahedron Lett. 2018, 59, 2844–2847. [Google Scholar] [CrossRef]
- Meng, Y.-Y.; Zhu, W.-J.; Song, Y.-Y.; Bu, G.-G.; Zhang, L.-J.; Xu, F. Rhodium(III)-Catalyzed Oxidative Annulation of Amidines with Alkynes via Sequential C−H Bond Activation. Eur. J. Org. Chem. 2021, 2021, 1290–1294. [Google Scholar] [CrossRef]
- Xu, F.; Song, Y.-Y.; Zhu, W.-J.; Liu, C.-S.; Lu, Y.-Z.; Du, M. Rhodium-catalyzed multiple C–H activation/highlymeta-selective C–H amination between amidines and alkynes. Chem. Commun. 2020, 56, 11227–11230. [Google Scholar] [CrossRef]
- Dyker, G.; Stirner, W.; Henkel, G. Oxidative Heterocyclization of 2-Alkynylbenzaldehydes with 1,2-Phenylenediamine. Eur. J. Org. Chem. 2000, 2000, 1433–1441. [Google Scholar] [CrossRef]
- Analogous, metal-free synthesis of benzimidazo[2,1-a]ellipticines (DMF, 100 °C, 2 h) was reported: Chaitanya, T.K.; Prakash, K.S.; Nagarajan, R. Metal-free synthesis of benzimidazo[2,1-a]ellipticines via tandem inter and intramolecular cyclization. Tetrahedron 2011, 67, 6934–6938. [Google Scholar] [CrossRef]
- Okamoto, N.; Sakurai, K.; Ishikura, M.; Takeda, K.; Yanada, R. One-pot concise syntheses of benzimidazo[2,1-a]isoquinolines by a microwave-accelerated tandem process. Tetrahedron Lett. 2009, 50, 4167–4169. [Google Scholar] [CrossRef]
- Rustagi, V.; Tiwari, R.; Verma, A.K. AgI-Catalyzed Cascade Strategy: Regioselective Access to Diversely Substituted Fused Benzimidazo[2,1-a]isoquinolines, Naphthyridines, Thienopyridines, and Quinoxalines in Water. Eur. J. Org. Chem. 2012, 2012, 4590–4602. [Google Scholar] [CrossRef]
- Mishra, M.; Twardy, D.; Ellstrom, C.; Wheeler, K.A.; Dembinski, R.; Török, B. Catalyst-free ambient temperature synthesis of isoquinoline-fused benzimidazoles from 2-alkynylbenzaldehydes via alkyne hydroamination. Green Chem. 2019, 21, 99–108. [Google Scholar] [CrossRef]
- Sonawane, A.D.; Sonawane, R.A.; Win, K.M.N.; Ninomiya, M.; Koketsu, M. In situ air oxidation and photophysical studies of isoquinoline-fused N-heteroacenes. Org. Biomol. Chem. 2020, 18, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.-C.; Tang, R.-Y.; Zhong, P.; Zhang, X.-G.; Li, J.-H. CuI/I2-Promoted Electrophilic Tandem Cyclization of 2-Ethynylbenzaldehydes with ortho-Benzenediamines: Synthesis of Iodoisoquinoline-Fused Benzimidazoles. J. Org. Chem. 2011, 76, 223–228. [Google Scholar] [CrossRef]
- Bagdasarian, A.L.; Nguyen, H.H.; Palazzo, T.A.; Fettinger, J.C.; Haddadin, M.J.; Kurth, M.J. One-Pot Synthesis of Benzo[4,5]imidazo[2,1-a]isoquinolines and Isoquinolino[3,4-b]quinoxalines via Tandem Cyclization Strategies. J. Org. Chem. 2016, 81, 3924–3928. [Google Scholar] [CrossRef]
- Deady, L.W.; Loria, P.M.; Rodemann, T. Studies on the Synthesis of Benzimidazo[2,1-a]isoquinolines. Aust. J. Chem. 1998, 51, 941–946. [Google Scholar] [CrossRef]
- Borkin, D.A.; Puscau, M.; Carlson, A.; Solan, A.; Wheeler, K.A.; Török, B.; Dembinski, R. Synthesis of diversely 1,3,5-trisubstituted pyrazoles via 5-exo-dig cyclization. Org. Biomol. Chem. 2012, 10, 4505–4508. [Google Scholar] [CrossRef]
- Solan, A.; Nişanci, B.; Belcher, M.; Young, J.; Schäfer, C.; Wheeler, K.A.; Török, B.; Dembinski, R. Catalyst-free chemo-/regio-/stereo-selective amination of alk-3-ynones. Synthesis of 1,5-benzodiazepines and 3-amino-2-alkenones. Green Chem. 2014, 16, 1120–1124. [Google Scholar] [CrossRef]
- Young, J.; Schäfer, C.; Solan, A.; Baldrica, A.; Belcher, M.; Nişanci, B.; Wheeler, K.A.; Trivedi, E.A.; Török, B.; Dembinski, R. Regioselective “hydroamination” of alk-3-ynones with non-symmetrical o-phenylenediamines. Synthesis of diversely substituted 3H-1,5-benzodiazepines via (Z)-3-amino-2-alkenones. RSC Adv. 2016, 6, 107081–107093. [Google Scholar] [CrossRef]
- CCDC. Available online: www.ccdc.cam.ac.uk (accessed on 10 January 2022).
- Schäfer, C.; Cho, H.; Török, B. Green Synthesis of Common Heterocycles. In Green Chemistry in Drug Discovery: From Academia to Industry; Le, P.T., Richardson, P.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 3–33. [Google Scholar] [CrossRef]
- Schäfer, C.; Cho, H.; Vlocskó, R.B.; Xie, G.; Török, B. Recent Advances in the Green Synthesis of Heterocycles: From Building Blocks to Biologically Active Compounds. Curr. Org. Synth. 2022, 17. (Online ahead of print). [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).