One-Step Preparation of S, N Co-Doped Carbon Quantum Dots for the Highly Sensitive and Simple Detection of Methotrexate
Abstract
:1. Introduction
2. Results
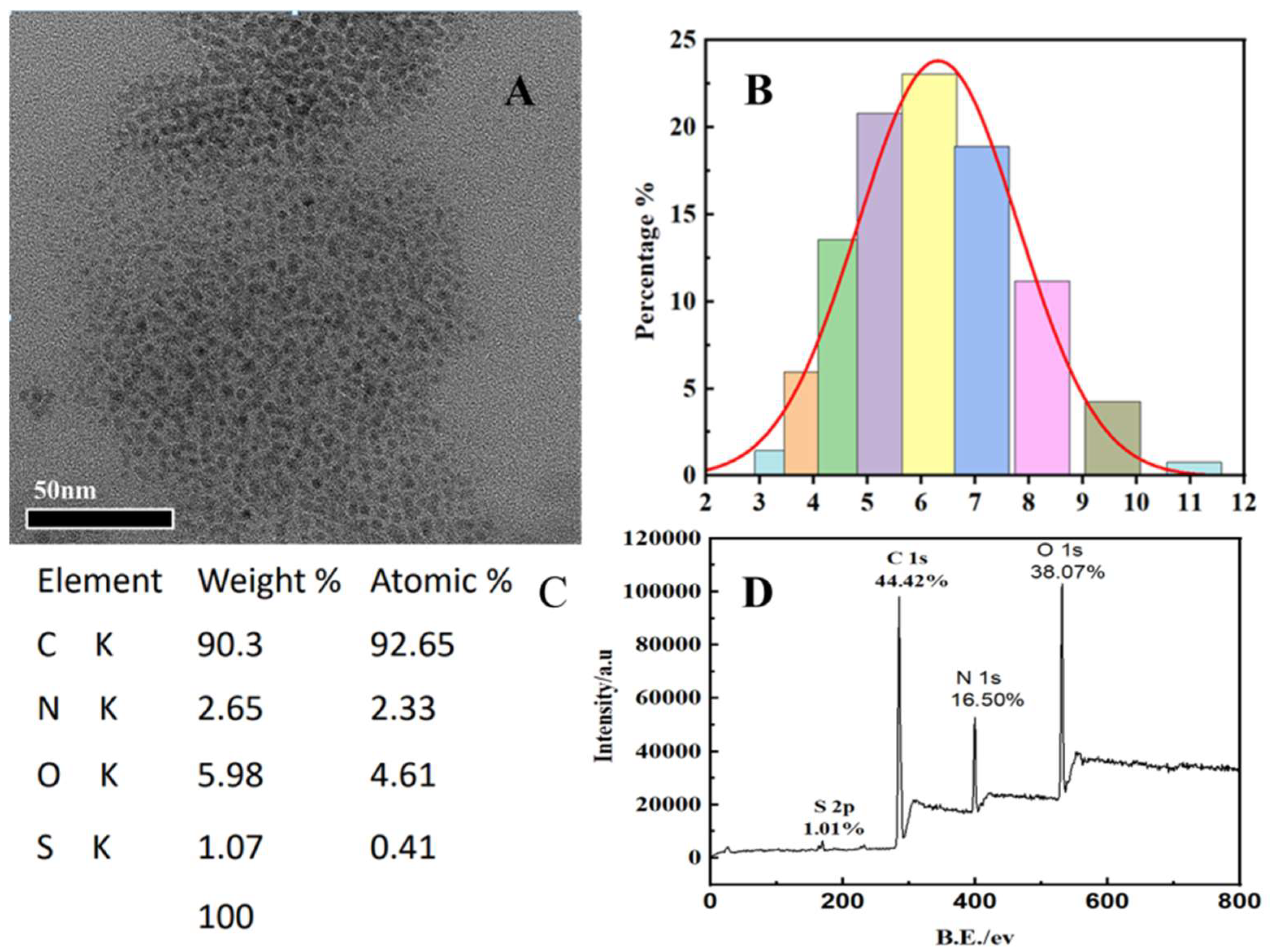
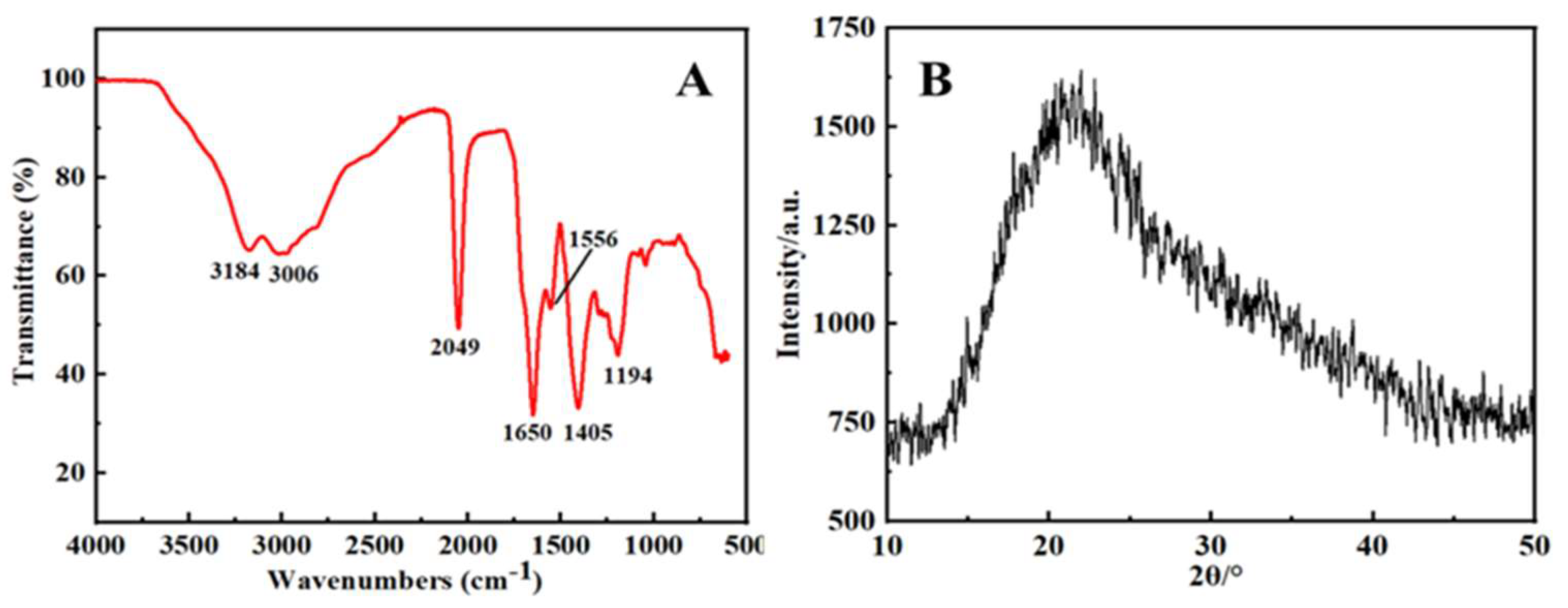
2.1. Morphological and Microstructural Characterization of the Synthesized S, N-CQDs
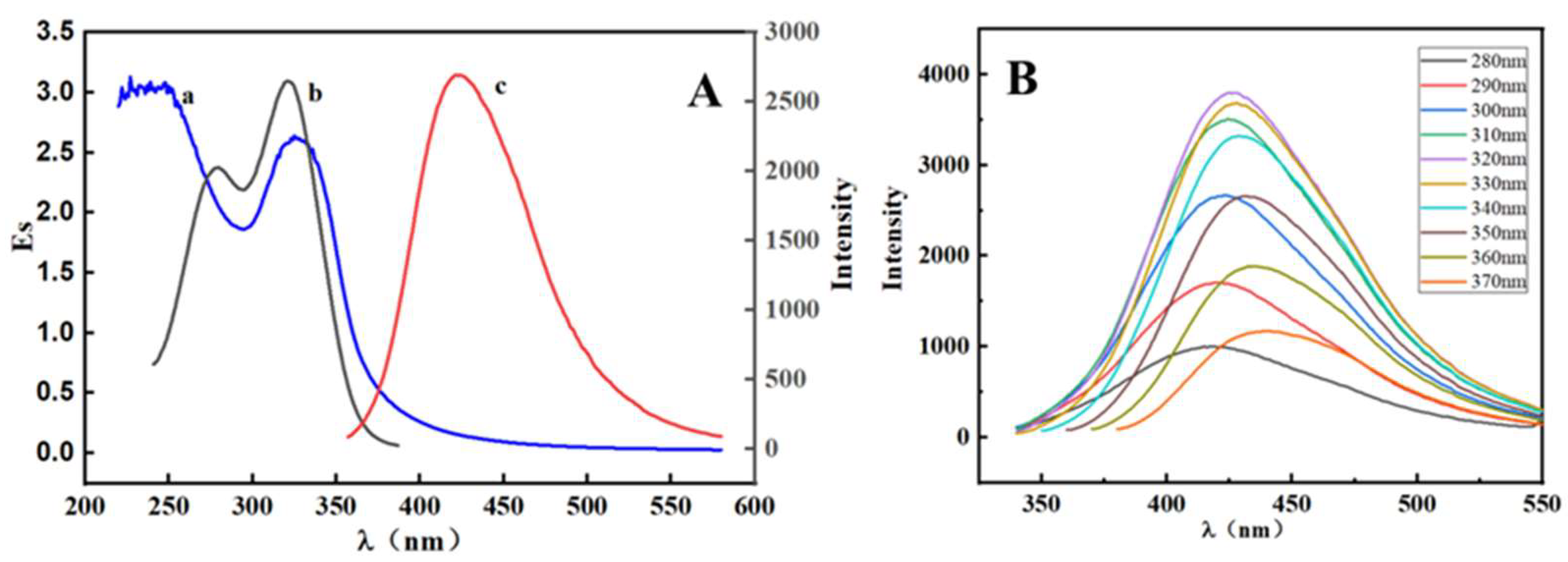
2.2. Optical Properties of the Synthesized S, N-CQDs
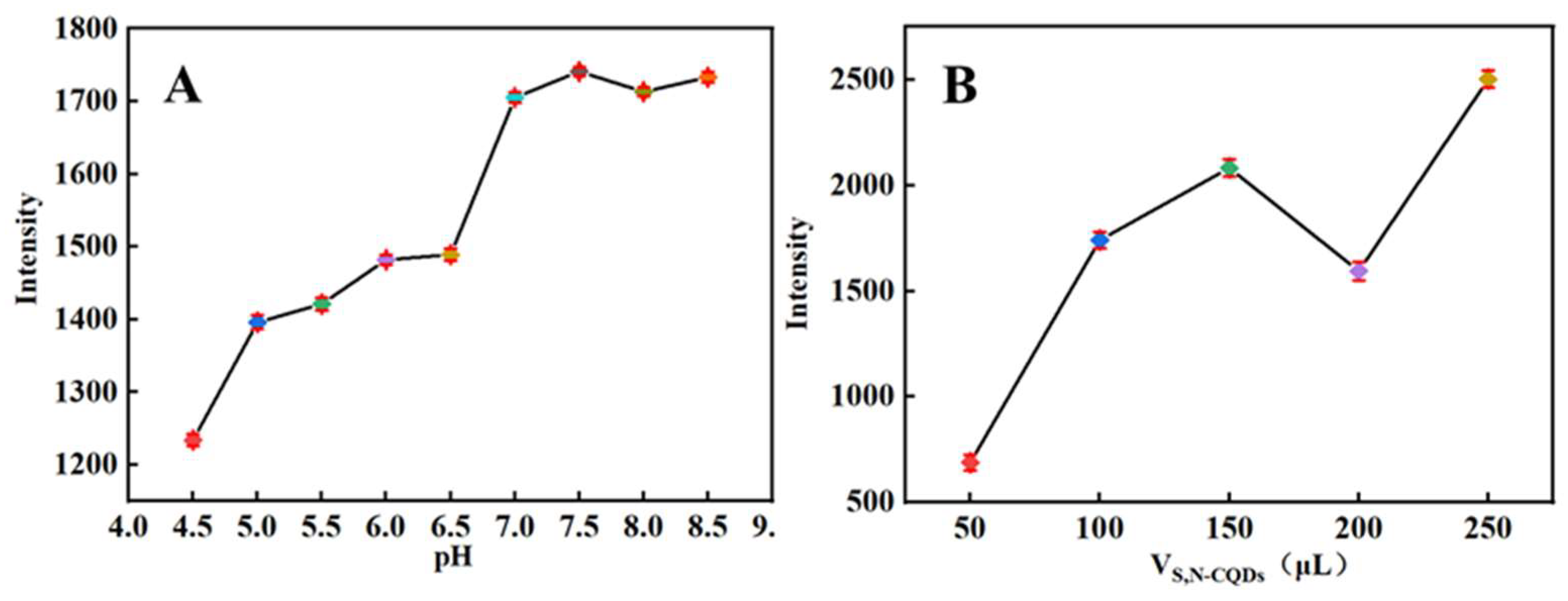
2.3. Conditions Optimization
2.4. Calibration Curve
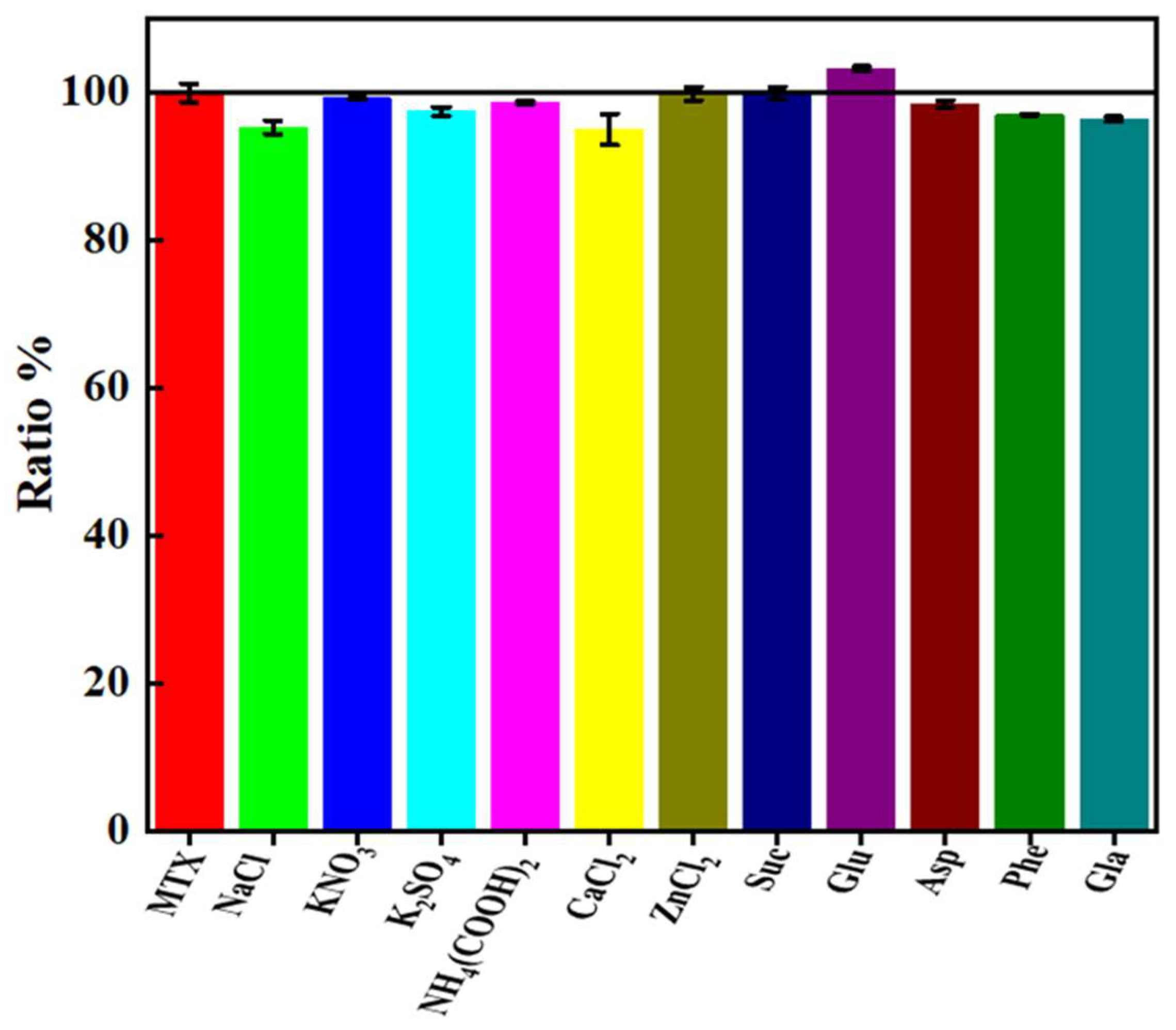
2.5. Interferencing Test
2.6. Reproducibility and Stability
2.7. Analytical Application
3. Materials and Methods
3.1. Materials
3.2. Instrumentation
3.3. Methods
3.3.1. Synthesis of S, N-CQDs
3.3.2. Measurement of Fluorescence Quantum Yield
3.3.3. Pretreatment and Detection of MTX Tablets
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Göksel, Y.; Zor, K.; Rindzevicius, T.; Als-Nielsen, B.E.T.; Schmiegelow, K.; Boisen, A. Quantification of Methotrexate in Human Serum Using Surface-Enhanced Raman Scattering Toward Therapeutic Drug Monitoring. ACS Sens. 2021, 6, 2664–2673. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.S.; Bukar, N.; Toulouse, J.L.; Pelechacz, D.; Robitaille, R.J.; Pelletier, N.; Masson, J.F. Miniature multi-channel SPR instrument for methotrexate monitoring in clinical samples. Biosens. Bioelectron. 2015, 64, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.J.; Wang, L.; Hu, G.F.; Li, C.B.; Peng, M.T. Clinical application of the simultaneous detection of methotrexate and 7-hydroxymethotrexate in the delayed elimination for pediatric acute lymphoblastic leukemia. Chin. Med. J. 2020, 25, 1973–1978. [Google Scholar] [CrossRef]
- Haugeberg, G.; Conaghan, P.G.; Quinn, M.; Emery, P. Bone loss in patients with active early rheumatoid arthritis: Infliximab and methotrexate compared with methotrexate treatment alone. Explorative analysis from a 12-month randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 2009, 68, 1898–1901. [Google Scholar] [CrossRef]
- Widemann, B.C.; Adamson, P.C. Understanding and Managing Methotrexate Nephrotoxicity. Oncologist 2006, 11, 694–703. [Google Scholar] [CrossRef] [Green Version]
- Somensi, C.A.; Simionatto, E.L.; Dalmarco, J.B.; Gaspareto, P.; Radetski, C.M. A comparison between ozonolysis and sonolysis/ozonolysis treatments for the degradation of the cytostatic drugs methotrexate and doxorubicin: Kinetic and efficiency approaches. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2012, 47, 1543–1550. [Google Scholar] [CrossRef]
- Begas, E.; Papandreou, C.; Tsakalof, A.; Daliani, D.; Papatsibas, G.; Asprodini, E. Simple and reliable HPLC method for the monitoring of methotrexate in osteosarcoma patients. J. Chromatogr. Sci. 2014, 52, 590–595. [Google Scholar] [CrossRef]
- Takada, M.; Ohba, Y.; Kabashima, T.; Nakashima, K.; Wada, M. Simple Simultaneous Assay of Methotrexate and Non-Steroidal Anti-Inflammatory Drugs by HPLC. Chromatography 2020, 41, 79–84. [Google Scholar] [CrossRef]
- Zuben, E.S.V.; Oliveira, A.G.; Chorilli, M.; Scarpa, M.V. Development and validation of a rapid reverse-phase HPLC method for the determination of methotrexate from nanostructured liquid crystalline systems. Die Pharm. 2018, 73, 128–132. [Google Scholar] [CrossRef]
- Wei, Y.L.; Luo, L.Q.; Ding, Y.P.; Si, X.J.; Ning, Y.Q. Highly sensitive determination of methotrexate at poly (L-lysine) modified electrode in the presence of sodium dodecyl benzene sulfonate. Bioelectrochemistry 2014, 98, 70–75. [Google Scholar] [CrossRef]
- Ghadimi, H.; Nasiri-Tabrizi, B.; Nia, P.M.; Basirun, W.J.; Tehrani, R.M.A.; Lorestani, F. Nanocomposites of nitrogen-doped graphene decorated with a palladium silver bimetallic alloy for use as a biosensor for methotrexate detection. RSC Adv. 2015, 5, 99555–99565. [Google Scholar] [CrossRef]
- Salandari-Jolge, N.; Ensafi, A.A.; Rezaei, B. A novel three-dimensional network of CuCr2O4/CuO nanofibers for voltammetric determination of anticancer drug methotrexate. Anal. Bioanal. Chem. 2020, 412, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.R.; Wang, Y.F.; Dong, Y.Q.; Xu, K.; Long, H.; Deng, C.; Yin, Y.M.; Eremin, S.A.; Meng, M.; Xi, R.M. A validated chemiluminescence immunoassay for methotrexate (MTX) and its application in a pharmacokinetic study. Anal. Methods 2016, 8, 162–170. [Google Scholar] [CrossRef]
- Ding, C.Q.; Zhu, A.W.; Tian, Y. Functional Surface Engineering of C-Dots for Fluorescent Biosensing and in Vivo Bioimaging. Acc. Chem. Res. 2014, 47, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.A.; Zou, S.Y.; Huo, D.Q.; Hou, C.J.; Yang, M.; Li, J.J.; Bian, M.H. Simple and sensitive fluorescence sensor for methotrexate detection based on the inner filter effect of N, S co-doped carbon quantum dots. Anal. Chim. Acta 2019, 1047, 179–187. [Google Scholar] [CrossRef]
- Janus, L.; Radwan-Pragowska, J.; Piatkowski, M.; Bogdal, D. Smart, Tunable CQDs with Antioxidant Properties for Biomedical Applications—Ecofriendly Synthesis and Characterization. Molecules 2020, 25, 736. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Feng, Y.Q.; Dong, P.P.; Huang, J.F. A Mini Review on Carbon Quantum Dots: Preparation, Properties, and Electrocatalytic Application. Front. Chem. 2019, 7, 671–678. [Google Scholar] [CrossRef]
- Wang, Y.F.; Hu, A.G. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.H.; Lu, W.J.; Zhang, H.L.; Meng, Y.T.; Du, F.F.; Shuang, S.M.; Dong, C. Copper doped carbon dots as the multi-functional fluorescent sensing platform for tetracyclines and pH. Sens. Actuators B 2021, 330, 129360. [Google Scholar] [CrossRef]
- Molae, M.J. A review on nanostructured carbon quantum dots and their applications in biotechnology, sensors, and chemiluminescence. Talanta 2019, 196, 456–478. [Google Scholar] [CrossRef]
- Ji, C.Y.; Zhou, Y.Q.; Leblanc, R.M.; Peng, Z.L. Recent Developments of Carbon Dots in Biosensing: A Review. ACS Sens. 2020, 5, 2724–2741. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.Q.; Pang, H.C.; Yang, H.B.; Guo, C.X.; Shao, J.W.; Chi, Y.W.; Li, C.M.; Yu, T. Carbon-Based Dots Co-doped with Nitrogen and Sulfur for High Quantum Yield and Excitation-Independent Emission. Angew. Chem. Int. Ed. Engl. 2013, 52, 7800–7804. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Zheng, M.; Zhang, L.G.; Zhao, H.F.; Xie, Z.G.; Jing, X.B.; Haddad, R.E.; Fan, H.Y.; Sun, Z.C. Formation mechanism and optimization of highly luminescent N-doped graphene quantum dots. Sci. Rep. 2014, 4, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.T.; Yang, C.L.; Chen, Y.X.; Zhu, Z.B.; Zhou, L.H. Cuttlefish ink-based N and S co-doped carbon quantum dots as a fluorescent sensor for highly sensitive and selective para-nitrophenol detection. Anal. Methods 2021, 44, 5351–5359. [Google Scholar] [CrossRef]
- Li, L.; Dong, T. Correction: Photoluminescence tuning in carbon dots: Surface passivation or/and functionalization, heteroatom doping. J. Mater. Chem. C 2019, 7, 3105. [Google Scholar] [CrossRef] [Green Version]
- Qie, X.W.; Zan, M.H.; Li, L.; Gui, P.; Chang, Z.M.; Ge, M.F.; Wang, R.S.; Guo, Z.Z.; Dong, W.F. High photoluminescence nitrogen, phosphorus co-doped carbon nanodots for assessment of microbial viability. Colloids Surf. B Biointerfaces 2020, 191, 110987. [Google Scholar] [CrossRef]
- Chandra, S.; Bano, D.; Pradhan, P.; Singh, V.K.; Yadav, P.K.; Sinha, D.; Hasan, S.H. Nitrogen/sulfur-co-doped carbon quantum dots: A biocompatible material for the selective detection of picric acid in aqueous solution and living cells. Anal. Bioanal. Chem. 2020, 412, 3753–3763. [Google Scholar] [CrossRef]
- Wang, H.Y.; Lu, Q.J.; Hou, Y.X.; Liu, Y.L.; Zhang, Y.Y. High fluorescence S, N co-doped carbon dots as an ultra-sensitive fluorescent probe for the determination of uric acid. Talanta 2016, 155, 62–69. [Google Scholar] [CrossRef]
- Li, Y.P.; Hu, Y.; Jia, Y.; Jiang, X.H.; Cheng, Z.J. N, S Co-Doped Carbon Quantum Dots for the Selective and Sensitive Fluorescent Determination of N-Acetyl-l-Cysteine in Pharmaceutical Products and Urine. Anal. Lett. 2019, 52, 1711–1731. [Google Scholar] [CrossRef]
- Wang, C.J.; Shi, H.X.; Yang, M.; Yao, Z.R.; Zhang, B.; Liu, E.Z.; Hu, X.Y.; Xue, W.M.; Fan, J. Biocompatible sulfur nitrogen co-doped carbon quantum dots for highly sensitive and selective detection of dopamine. Colloids Surf. B 2021, 205, 111874. [Google Scholar] [CrossRef]
- Lin, M.; Zou, H.Y.; Yang, T.; Liu, Z.X.; Liu, H.; Huang, C.Z. An inner filter effect based sensor of tetracycline hydrochloride as developed by loading photoluminescent carbon nanodots in the electrospun nanofibers. Nanoscale 2016, 8, 2999. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V.K.; Kundu, S.; Ajayan, P.M.; Yadav, R.M.; Shelke, M.T.; Narayanan, N.; Vajtai, R. Synthesis of N, F and S Co-doped Graphene Quantum Dots. Nanoscale 2015, 7, 11515–11519. [Google Scholar] [CrossRef]
- Shahshahanipour, M.; Rezaei, B.; Ensafi, A.A.; Etemadifar, Z. An Ancient Plant for the Synthesis of a Novel Carbon Dot and its Applications as an Antibacterial Agent and Probe for Sensing of An Anti-cancer Drug. Mater. Sci. Eng. C 2019, 98, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, J.P.; Fang, G.Z.; Deng, J.K.; Wang, S. A molecularly imprinted polymer capped nitrogen-doped graphene quantum dots system for sensitive determination of tetracycline in animal-derived food. Chem. Select. 2020, 5, 839–846. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Nasr-Esfahani, P.; Rezaei, B. Simultaneous detection of folic acid and methotrexate by an optical sensor based on molecularly imprinted polymers on dual-color CdTe quantum dots. Anal. Chim. Acta 2017, 996, 64–73. [Google Scholar] [CrossRef]
- Liu, C.B.; Song, Z.L.; Pan, J.M.; Yan, Y.S.; Cao, Z.J.; Wei, X.; Gao, L.; Wang, J.; Dai, J.D.; Meng, M.J.; et al. A simple and sensitive surface molecularly imprinted polymers based fluorescence sensor for detection of λ-Cyhalothrin. Talanta 2014, 125, 14–23. [Google Scholar] [CrossRef]
- Wang, Y.W.; Liu, H.; Wang, F.; Gao, Y.M. Electrochemical oxidation behavior of methotrexate at DNA/SWCNT/Nafion composite film-modified glassy carbon electrode. J. Solid State Electrochem. 2012, 16, 3227–3235. [Google Scholar] [CrossRef]
- Li, H.; Shao, F.Q.; Huang, H.; Feng, J.J.; Wang, A.J. Eco-friendly and rapid microwave synthesis of green fluorescent graphitic carbon nitride quantum dots for vitro bioimaging. Sens. Actuators B 2016, 226, 506–511. [Google Scholar] [CrossRef]
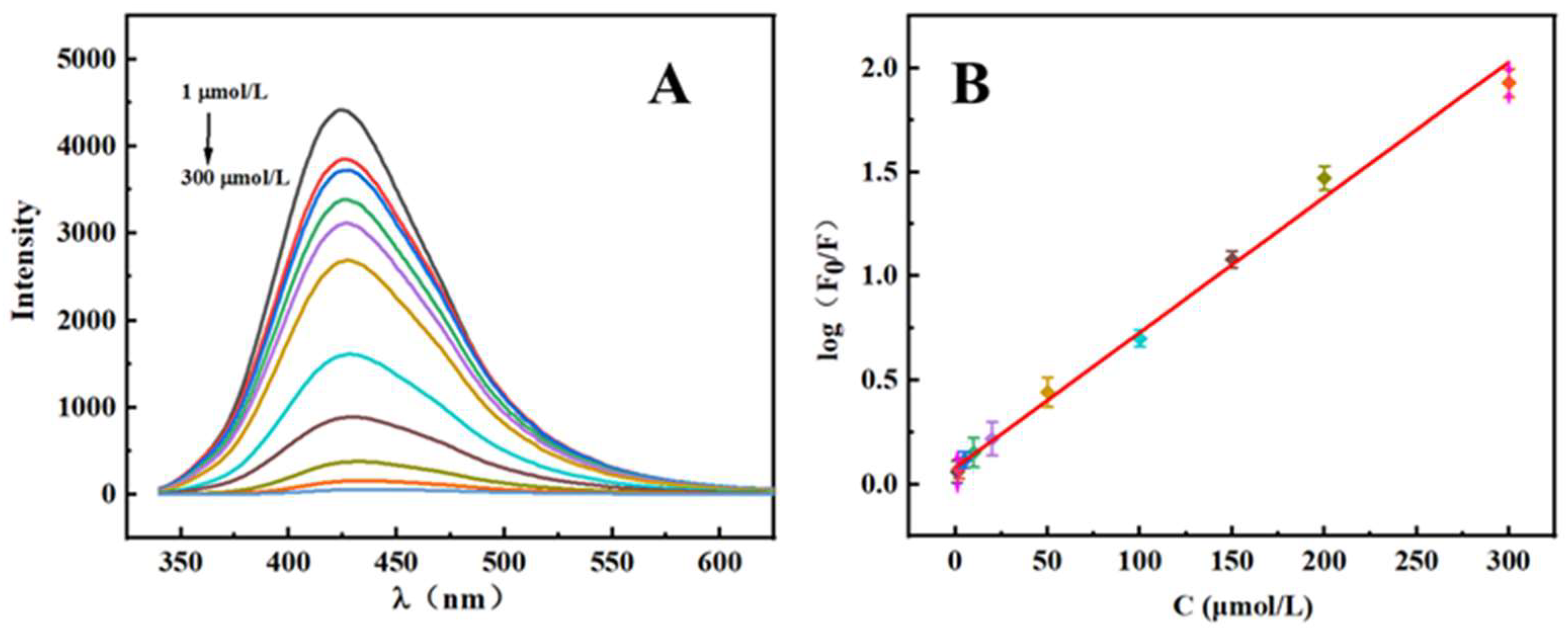
| Method | Linear Range | Detection Limit | References |
|---|---|---|---|
| SPRS | 0–150 μmol/L | 0.6 μmol/L | [1] |
| HPLC | 5.0–150 μg/mL | 3.3 μg/mL | [9] |
| electroanalysis | 0.02–1.5 μmol/L | 8 nmol/L | [37] |
| fluorescence | 0.4–41.3 μg/mL | 12 ng/mL | [28] |
| 1–300 μmol/L | 0.33 μmol/L | This work |
| No. | Detected (μmol/L) | Detected (mg/Piece) | Labeled (mg/Piece) | Labeled Quantity (%) | Average (%) | Relative Standard Deviation (%) |
|---|---|---|---|---|---|---|
| 1 | 6.05 | 2.52 | 2.5 | 100.8 | 99.0 | 1.1 |
| 2 | 5.93 | 2.47 | 98.8 | |||
| 3 | 5.92 | 2.46 | 98.4 | |||
| 4 | 5.90 | 2.45 | 98.0 | |||
| 5 | 5.95 | 2.48 | 99.2 |
| No. | Sample (μmol/L) | Added (μmol/L) | Total (μmol/L) | Recovery (%) | Average (%) | RSD (%) |
|---|---|---|---|---|---|---|
| 1 | 5.95 | 80 | 81.61 | 94.5 | 94.9 | 2.13 |
| 2 | 81.01 | 93.9 | ||||
| 3 | 80.87 | 93.7 | ||||
| 4 | 80.68 | 98.5 | ||||
| 5 | 81.11 | 94.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Si, X.; Han, M.; Bai, C. One-Step Preparation of S, N Co-Doped Carbon Quantum Dots for the Highly Sensitive and Simple Detection of Methotrexate. Molecules 2022, 27, 2118. https://doi.org/10.3390/molecules27072118
Wei X, Si X, Han M, Bai C. One-Step Preparation of S, N Co-Doped Carbon Quantum Dots for the Highly Sensitive and Simple Detection of Methotrexate. Molecules. 2022; 27(7):2118. https://doi.org/10.3390/molecules27072118
Chicago/Turabian StyleWei, Xiaoyi, Xiaojing Si, Mei Han, and Chen Bai. 2022. "One-Step Preparation of S, N Co-Doped Carbon Quantum Dots for the Highly Sensitive and Simple Detection of Methotrexate" Molecules 27, no. 7: 2118. https://doi.org/10.3390/molecules27072118





