Abstract
Diabetes mellitus is a major health issue that has posed a significant challenge over the years. Gymnanthemum amygdalinum is a well-known plant that can be potentially used to treat this disease. Therefore, this study aimed to evaluate the inhibitory effect of its root, stem bark, leaves, and flower extracts on alpha-glucosidase using an in vitro inhibition assay to isolate the bioactive compounds and determine their levels in the samples. The air-dried plant parts were extracted by maceration using methanol. The results showed that the flower extract had the greatest inhibitory effect (IC50 47.29 ± 1.12 µg/mL), followed by the leaves, roots, and stem bark. The methanolic flower extract was further fractionated with different solvents, and the ethyl acetate fraction showed the strongest activity (IC50 19.24 ± 0.12 µg/mL). Meanwhile, acarbose was used as a positive control (IC50 73.36 ± 3.05 µg/mL). Characterization based on UV, 1H-, and 13C-NMR established that the ethyl acetate fraction yielded two flavonoid compounds, namely, luteolin and 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-methoxy-4H-chromen-4-on, which had IC50 values of 6.53 ± 0.16 µg/mL and 39.95 ± 1.59 µg/mL, respectively. The luteolin levels in the crude drug, methanolic extract, and ethyl acetate fraction were 3.4 ± 0.2 mg (0.3%), 32.4 ± 0.8 mg (3.2%), and 68.9 ± 3.4 mg (6.9%) per 1 g samples, respectively. These results indicated that the G. amygdalinum flower extract exerted potent inhibitory alpha-glucosidase activity.
1. Introduction
As a metabolic disease, type 2 diabetes mellitus is reported in more than 90% of diabetes cases worldwide [1] and is characterized by elevated postprandial blood glucose levels [2]. Therefore, one effective strategy to control postprandial hyperglycemia is delaying glucose absorption using alpha-glucosidase inhibitors (AGIs). Examples of these drugs include acarbose, miglitol, and voglibose [3]. Since AGIs cause side effects such as gastrointestinal disorders [4,5], an effective alternative treatment with fewer side effects and proven safety is needed [6]. Consequently, searching for new AGI sources is very important for therapeutic applications. Traditionally, several plants are used as potential alpha-glucosidase inhibitors [7]. One of these is Gymnanthemum amygdalinum, also known as Vernonia amygdalina, which belongs to the Asteraceae family. It possesses various pharmacological activities. It is for constipation, diarrhea, skin wounds, scabies, ascariasis, tonsillitis, fever, and malaria [8,9,10]. Several studies reported that G. amygdalinum was traditionally used for managing diabetes mellitus in Nigeria [11,12]. Some secondary metabolites can be isolated from G. amygdalinum leaves, such as vernonioside A1, vernonioside A2, vernonioside B1, vernonioside B2, vernodalin, vernolepin, vernomygdin, vernodalol, vernodalinol, vernoamyoside, luteolin, luteolin 7-O-β-glucoside, and luteolin 7-O-glucuronide [10]. Furthermore, the flavonoid-rich fraction of G. amygdalinum leaf extracts show a significant antidiabetic effect [9]. Luteolin is a flavonoid compound that is well known to be an alpha-glucosidase inhibitor. Previous studies show that the number of phenolic hydroxyl groups on the B ring is correlated with inhibitory activity [13]. This property is possibly due to the alpha-glucosidase inhibitory activity found in the leaf extract, but this is yet to be confirmed for the plant’s other parts. Therefore, this study aimed to evaluate the in vitro AGI activity of G. amygdalinum roots, stem barks, leaves, and flowers. The active compounds were isolated from the selected parts, and their levels were determined through thin-layer chromatography (TLC) densitometry.
2. Results
2.1. Phytochemical Screening
The phytochemical screening of methanolic flower extracts detected alkaloid, flavonoid, tannin, quinone, and triterpenoid.
2.2. In Vitro Alpha-Glucosidase Inhibitory Activity
The alpha-glucosidase inhibitory activities of the methanolic crude extracts of G. amygdalinum roots, stem bark, leaves, and flowers were determined using pNPG for the substrate (Table 1). Each extract’s test results are shown by percentage inhibition values, while the data for the extract with the most significant inhibitory effect are also presented with IC50 values. Based on the results, the flower extract had better activity than acarbose. The flower fractions’ alpha-glucosidase inhibitory activity showed that ethyl acetate had the greatest inhibitory effect, followed by n-hexane and the water fraction (Table 2). The flower extract with an IC50 value of 47.29 ± 1.12 µg/mL (Table 3) had the greatest inhibitory effect, followed by the leaf, stem bark, and root extracts (Table 1). This value was lower compared to the positive control, acarbose, which recorded 72.99 ± 2.77 µg/mL (Table 3).

Table 1.
The extracts’ alpha-glucosidase inhibitory activity assay.

Table 2.
The flower fractions’ alpha-glucosidase inhibitory activity assay.

Table 3.
The IC50 values of extract, fraction, and isolated compound.
Based on its activity, the flower’s crude methanolic extract was further fractionated successively in different polar and non-polar solvents. The ethyl acetate fraction with an IC50 value of 19.24 ± 0.12 µg/mL (Table 3) exerted a significant inhibitory effect on alpha-glucosidase. It yielded two flavonoids, with this being followed by n-hexane and water (Table 2). Further, the bioactive compounds’ structures were analyzed using UV and NMR spectral analysis, as shown in Figure 1.
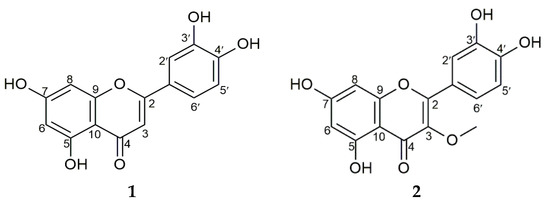
Figure 1.
Structures of compounds 1 and 2.
Compound 1 was obtained as a yellow crystal, eluted using n-hexane/ethyl acetate/formic acid (4:1:0.05), with this being followed by its visualization in a yellow spot form by spraying it with 10% H2SO4 and citroborate on TLC silica gel F254. This compound showed that UV maxima at 269 and 355 nm supported the presence of the flavonoid skeleton. Compound 1’s 1H-NMR spectrum (DMSO-d6) showed a signal at δ 12.98 (1H, s), which served as evidence for the presence of 5-OH, with the chelated hydroxy group causing this. The singlet peak at δ 6.67 (1H, s, H-3) was identified as hydrogen at C-3 in the flavones. Furthermore, three aromatic proton signals were detected at δ 7.42 (1H, d, J = 2.35 Hz, H-2′), 6.89 (1H, d, J = 8.25 Hz, H-5′), and 7.40 (1H, dd, J = 2.35, 7.50 Hz, H-6′), respectively. The other aromatic proton signals were detected at δ 6.19 (1H, d, J = 2.15 Hz, H-6) and 6.44 (1H, d, J = 2.10 Hz, H-8). The presence of α, β-unsaturated ketone was evident from the appearance of carbonyl carbon signals at δ 181.7 (C-8). Additionally, the 13C-NMR spectrum (DMSO-d6) was observed at δ: 93.8 (C-8), 98.8 (C-6), 102.9 (C-3), 103.7 (C-10), 113.4 (C-2′), 116.0 (C-5′), 119.0 (C-6′), 121.5 (C-1′), 145.7 (C-3′), 149.7 (C-4′), 157.3 (C-9), 161.5 (C-5), 163.9 (C-2), and 164.1 (C-7). The presence of 15 carbon resonances was shown by this expression, which strongly agrees with a previous report on 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4-one [(luteolin) (C15H10O6)] [14].
Compound 2 was obtained as a yellow crystal, eluted thrice using n-hexane/ethyl acetate/formic acid (35:15:0.5) as an eluent, and visualized as a dark spot under UV at λ 366 nm in TLC silica gel F254. The dark spot changed to yellow after it was sprayed with citroborate. These results show that both compounds have a flavonoid structure but do not have a free OH group on C-3. Further, the absorption maxima at 269 and 355 nm confirmed the compound’s flavonol nature. The proton NMR spectrum analysis detected three aromatic proton signals at δ 7.63 (1H, d, J = 2.20 Hz, H-2′), 6.91 (1H, d, J = 8.45 Hz, H-5′), and 7.54 (1H, dd, J = 2.20; 8.50 Hz, H-6′), with these signals being typical of flavonoids with 3′,4′-disubstituted B rings. The other aromatic proton signals at δ 6.20 (1H, d, J = 2.10 Hz, H-6) and 6.40 (1H, d, J = 2.05 Hz, H-8) were apparently due to meta-coupled protons on the flavonoid’s A ring, while one methoxy group was evident at δ 3.78 (3H, s). Other signals in the 13C-NMR (CD3OD) were observed at δ 94.7 (C-8), 99.7 (C-6), 105.8 (C-10), 116.4 (C-2′), 116.4 (C-5′), 122.3 (C-6′), 122.9 (C-1′), 139.5 (C-3), 146.5 (C-3′), 150.0 (C-4′), 158.0 (C-9), 158.4 (C-2), 163.1 (C-5), 166.0 (C-7), and 180.0 (C-4). The carbon resonance at δ 60.5 was due to the presence of the methoxy group. These data agree with literature reports on 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-methoxy-4H-chromen-4-one [(3-O-methyl quercetin) (C16H12O7)] [15,16]. They were also confirmed by 1H-13C correlations in heteronuclear single quantum correlation spectroscopy (HSQC) measurement, which showed that three hydrogen atoms were directly attached to the carbon.
The alpha-glucosidase inhibition test of compounds 1 and 2 showed that compound 1 had a significant impact compared to compound 2, with the compounds recording IC50 values of 6.53 ± 0.16 µg/mL and 39.95 ± 1.59 µg/mL, respectively (Table 3).
2.3. Luteolin Determination
TLC-densitometry was one of the methods used to measure active substance amounts. It was also used to determine the luteolin levels, with silica F254 being used as the stationary phase. Each sample was eluted thrice at the same TLC plate using n-hexane/ethyl acetate/formic acid (35:15:0.5). The area under curve (AUC) values were determined by scanning the dried spot with a TLC scanner at a 360 nm wavelength. A linear regression equation (y = 48.534x − 27,148, R² = 0.99) was derived by plotting the luteolin concentrations against the AUC values. The luteolin concentrations of 600, 700, 800, 900, and 1000 µg/mL produced AUC values of 2466.4, 6030.5, 12,330, 15,643, and 21,927 AU, respectively. These were used to calculate the luteolin levels in the crude drug, methanolic extract, and ethyl acetate fraction. In the triplicate test, the results showed that 20,000 µg/mL of the crude drug flower solution yielded values of 7481.1, 5810.5, and 4015.5, the methanolic flower extract yielded values of 3827.9, 5127.5, and 3793, and the acetate flower fraction yielded values of 4429.1, 7016.2, and 7456.4. Additionally, one gram of the crude drug, methanolic extract, and ethyl acetate fraction was found to contain 3.4 ± 0.2 mg (0.3%), 32.4 ± 0.8 mg (3.2%), and 68.9 ± 3.4 mg (6.9%) of luteolin, respectively.
3. Discussion
The alpha-glucosidase inhibitory activity of various parts of G. amygdalinum and its isolated compounds was investigated. A previous in vitro study indicated the leaves’ hypoglycemic effect. The MeOH extract of the G. amygdalinum leaf was reported to have significantly inhibited alpha-glucosidase [17]. Despite numerous reports on the secondary metabolite profile of the leaf, no alpha-glucosidase inhibition activity is yet observed in G. amygdalinum flower extracts and fractions. The activity of these plant extracts can be attributed to the phytoconstituents present in them. In this study, the methanolic flower extract of G. amygdalinum showed outstanding inhibitory alpha-glucosidase activity. It was further fractionated in different polarity solvents [18]. Among its fractions, the ethyl acetate fraction showed the maximum inhibitory activity. The ethyl acetate fraction is known to be rich in flavonoids. Flavonoids are phenolic substances with many health properties that frequently exhibit inhibitory effects against alpha-glucosidase enzymes [19]. In general, although previous studies reported on luteolin and its pharmacological activity [20,21,22], more analyses are performed on the leaves than the flower. Furthermore, four compounds that are reported to be isolated from flower extracts are tricosane, vernolide, isorhamnetin, and luteolin [20]. Most of the content in the ethyl acetate fraction is known as luteolin. This flavone is commonly found in Asteraceae [23]. It was found to contribute to the effect of alpha-glucosidase inhibitory activity [13], but to date, there are still no reports on luteolin in G. amygdalinum’s inhibitory effect against alpha-glucosidase. A previous report showed that luteolin has a non-competitive inhibition mechanism type [24]. Luteolin can also be found in Vernonia cinera, but its bioactivity is shown to be anti-inflammatory [12]. The OCH3 group presence in 3-O-methyl quercetin seems to be closely associated with these effects as well. Other plants that also contain luteolin are Brassica oleracea, Capsicum annum, Capscium frustescens, Allium fistulosum, Averhoa bilimbi, Phaseolus vulgaris, Daucus carota, Raphanus sativus, Apium graveolens, and Garcinia atroviridus. The luteolin content in those plants ranged between 9–1035.0 mg/kg; this fact explains why those edible plants have a lot of benefits for health maintenance [25].
4. Materials and Methods
4.1. Chemicals
Bovine serum albumin (EC Number 232-936-2, A8806), α-glucosidase (EC Number 3.2.1.20 Saccharomyces cerevisiae, G5003), and p-nitrophenyl-α-D-glucopyranoside (pNPG, N1377) were purchased from Sigma-Aldrich (St. Louis, MO, USA), while acarbose was purchased from TCI Chemicals (Hydrabad, India). A specific spray reagent, citroborate (5 g citric acid, 5 g boric acid, and 100 mL ethanol), and 10% (v/v) H2SO4 in a MeOH solution were used to identify flavonoids and other compounds. Additionally, during extraction and separation, the entire solvents were distilled before use, making them of technical grade.
4.2. Plant Materials
G. amygdalinum parts, namely roots, stem barks, leaves, and flowers, were collected on 30 October 2019, from Bandung, located in West Java, Indonesia. The plant specimens were identified and authenticated at the Herbarium Bogoriense of the Indonesian Institute of Sciences Research Center for Biology. The specimen number is B-139/2021.
4.3. Phytochemical Screening
The phytochemical screening was performed in methanolic flower extracts using the standard procedure [26].
4.4. Plant Extraction
The powder of G. amygdalinum dried roots (600 g), stem barks (600 g), leaves (1.6 kg), and flowers (400 g) were separated and extracted by maceration at room temperature in absolute MeOH (5 L × 3 times). Each crude extract was evaporated with a rotary evaporator, then filtered and concentrated to yield 52 g, 100 g, 273 g, and 80 g, respectively.
4.5. Inhibitory Assay of Alpha-Glucosidase
Alpha-glucosidase inhibition was assessed using the adopted method with slight modifications [27]. Phosphate buffer solution (36 μL), a sample solution with different concentrations (30 mL), and pNPG substrate with 6 mM concentration (17 μL) were successively added to 96 microplate wells. The incubation of this mixture was performed at 37 °C for 5 min. In each well, 17 μL of alpha-glucosidase solution at 0.2 U/mL was added to obtain a total volume of 100 mL, which was followed by incubation at 37 °C for 15 min. Furthermore, the initial reaction was brought to completion by the addition of Na2CO3 solution (100 μL) at 200 mM. At 400 nm, the entire absorbances were measured in triplicate with a Microplate Reader (Tecan®), while the positive control was utilized as acarbose. Percentage inhibition was used to express the inhibitory activity of alpha-glucosidase, while the calculation followed the formula below:
Inhibition percentage (%) = (B1–B2)/B1 × 100%.
B1 = Blank absorbance * − control of blank absorbance **
B2 = Sample absorbance − control of sample absorbance ***
* Blank contains PBS + pNPG + enzyme + Na2CO3
** Control of blank contains PBS + Na2CO3, without enzyme
*** Control of sample contains extract + PBS + Na2CO3, without enzyme
The IC50 was calculated using a linear regression equation (y = a + bx) in which the sample concentration was represented on the x-axis and the percentage inhibition was represented on the y-axis.
4.6. Isolation and Structure Determination of Active Compounds
The dried flowers of G. amygdalinum were extracted through maceration with MeOH (5 L × 3 times). The total extraction (80 g) of MeOH was suspended in 20% MeOH in 200 mL of water and partitioned sequentially with n-hexane and ethyl acetate, yielding n-Hexane (A 10g), ethyl acetate (B 25 g), and water (C 35 g). The ethyl acetate fraction (24 g) was subjected to silica gel vacuum column chromatography with a gradient of n-hexane (100%), n-hexane–ethyl acetate (9:1; 8:2; 7:3; 6:4; 5:5; 4:6; 3:7; 2:8; 1:9 for each step), and MeOH (100%) to produce 20 fractions. The compound profiles were analyzed using TLC under UV at λ 254 and 366 nm by spraying the compounds with citroborate. Fractions 5, 6, and 7 were combined and isolated with a gradient of n-hexane (100%), n-hexane–chloroform (9:1; 8:2; 7:3; 6:4; 5:5; 4:6; 3:7; 2:8; 1:9 for each step), and MeOH (100%) to produce 50 fractions (Fr. B1-50). Subfraction B40 was separated using silica gel column chromatography with an isocratic of n-hexane–ethyl acetate (4:1) to produce compound 1 and compound 2.
4.7. Determination of Luteolin by TLC-Densitometric Method
About 3 µL of each calibration sample was spotted on TLC silica gel F254. Further, various luteolin concentrations were prepared for a calibration curve. Then, each stock solution of the crude drug (50 g in 50 mL MeOH), methanolic extract (100 mg in 5 mL MeOH), and ethyl acetate fraction (100 mg in 5 mL MeOH) was prepared, and the experiments were performed in triplicate. Furthermore, the TLC-densitometry by Camag TLC Scanner 3® was used.
4.8. Statistical Analysis
The alpha-glucosidase inhibitory activity data of plant extracts and isolated compounds were presented as mean ± standard deviation (S.D.). The data were further analyzed using the independent-samples t-test or the Mann–Whitney U test and the one-way analysis of variances (ANOVA) using SPSS version 25.0 software. p-values less than 0.05 were indicated as significant.
5. Conclusions
The activity assays showed that the G. amygdalinum extracts, fractions, and isolated compounds exerted alpha-glucosidase inhibitory activities compared to the positive control, acarbose. The flower extract had a significant inhibitory effect, while the bioactive compounds included luteolin and 3-O-methyl quercetin. This is the first report on the AGI activity and 3-O-methyl quercetin isolation of the G. amygdalinum flower.
Author Contributions
Data investigation, writing—original draft preparation, S.V.; project administration and funding acquisition, R.H.; conceptualization and supervision, M.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by PPMI-ITB.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the School of Pharmacy-ITB for its support.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Liu, S.; Yu, Z.; Zhu, H.; Zhang, W.; Chen, Y. In vitro α-glucosidase inhibitory activity of isolated fractions from water extract of Qingzhuan dark tea. BMC Complement. Altern. Med. 2016, 16, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shori, A.B. Screening of antidiabetic and antioxidant activities of medicinal plants. J. Integr. Med. 2015, 13, 297–305. [Google Scholar] [CrossRef]
- Meena, S.N.; Kumar, U.; Naik, M.M.; Ghadi, S.C.; Tilve, S.G. α-Glucosidase inhibition activity and in silico study of 2-(benzo[d][1,3]dioxol-5-yl)-4H-chromen-4-one, a synthetic derivative of flavone. Bioorg. Med. Chem. 2019, 27, 2340–2344. [Google Scholar] [CrossRef] [PubMed]
- Kadouh, H.C.; Sun, S.; Zhu, W.; Zhou, K. α-Glucosidase inhibiting activity and bioactive compounds of six red wine grape pomace extracts. J. Funct. Foods 2016, 26, 577–584. [Google Scholar] [CrossRef]
- Hu, X.J.; Wang, X.B.; Kong, L.Y. α-Glucosidase inhibitors via green pathway: Biotransformation for bicoumarins catalyzed by Momordica charantia peroxidase. J. Agric. Food Chem. 2013, 61, 1501–1508. [Google Scholar] [CrossRef]
- Alongi, M.; Anese, M. Effect of coffee roasting on in vitro α-glucosidase activity: Inhibition and mechanism of action. Food Res. Int. 2018, 111, 480–487. [Google Scholar]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharm. Rev. 2011, 5, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Swamy, J.; Prabhakar, G.; Rasingam, L.; Kamalakar, P. Gymnanthemum amygdalinum (Asteraceae)—A New Addition to the Flora of Peninsular India. Int. J. Adv. Res. Sci. Technol. 2015, 4, 449–451. [Google Scholar]
- Kaur, D.; Kaur, N.; Chopra, A. A comprehensive review on phytochemistry and pharmacological activities of Vernonia amygdalina. J. Pharm. Phytochem. 2019, 8, 2629–2636. [Google Scholar]
- Alara, O.R.; Abdurahman, N.H.; Abdul, M.S.K.; Olalere, O.A. Phytochemical and pharmacological properties of Vernonia amygdalina: A review. J. Chem. Eng. Ind. Biotechnol. 2017, 2, 80–96. [Google Scholar] [CrossRef]
- Ijeh, I.I.; Ejike, C.E. Current perspectives on the medicinal potentials of Vernonia amygdalina Del. J. Med. Plants Res. 2011, 5, 1051–1061. [Google Scholar]
- Toyang, N.J.; Verpoorte, R. A review of the medicinal potentials of plants of the genus Vernonia (Asteraceae). J. Ethnopharmacol. 2013, 146, 681–723. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, G.; Pan, J.; Wang, Y. α-Glucosidase by Luteolin: Kinetics, interaction and molecular docking. Int. J. Biol. Macromol. 2014, 64, 213–223. [Google Scholar] [CrossRef]
- Cuong, D.T.D.; Dat, H.T.; Duan, N.T.; Thuong, P.D. Isolation and characterization of six flavonoids from the leaves of Sterculia foetida Linn. Vietnam J. Chem. 2019, 57, 438–442. [Google Scholar] [CrossRef]
- Schwingel, L.C.; Schwingel, G.O.; Storch, N.; Barreto, F.; Bassani, V.L. 3-O-Methylquercetin from organic Nicotiana tabacum L. trichomes: Influence of the variety, cultivation and extraction parameters. Ind. Crops Prod. 2014, 55, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.D.N.; Bevara, G.B.; Kaja, L.K.; Badana, A.K.; Malla, R.R. Protective effect of 3-O-methyl quercetin and kaempferol from Semecarpus anacardium against H2O2 induced cytotoxicity in lung and liver cells. BMC Complement. Altern. Med. 2016, 16, 376. [Google Scholar] [CrossRef] [Green Version]
- Anh, H.L.T.; Vinh, L.B.; Lien, L.T.; Cuong, P.V.; Arai, M.; Ha, T.P. In vitro study on α-amylase inhibitory and α-glucosidase of a new stigmastane-type steroid saponin from the leaves of Vernonia amygdalina. Nat. Prod. Res. 2021, 35, 873–879. [Google Scholar] [CrossRef]
- Khan, S.; Khan, T.; Shah, A.J. Total phenolic and flavonoid contents and antihypertensive effect of the crude extract and fractions of Calamintha vulgaris. Phytomedicine 2018, 47, 174–183. [Google Scholar] [CrossRef]
- Proença, C.; Freitas, M.; Ribeiro, D.; Oliveira, E.F.T.; Sousa, J.L.C.; Tomé, S.M. α-Glucosidase inhibition by flavonoids: An in vitro and in silico structure-activity relationship study. J. Enzym. Inhib. Med. Chem. 2017, 32, 1216–1228. [Google Scholar] [CrossRef] [Green Version]
- Hlila, M.B.; Majouli, K.; Jannet, H.B.; Mastouri, M.; Aouni, M.B.S. Antioxidant and anti α-glucosidase of luteolin and luteolin 7-O-glucoside isolated from Scabiosa arenaria Forssk. J. Coast Life Med. 2017, 5, 317–320. [Google Scholar] [CrossRef]
- Habtamu, A.; Melaku, Y. Antibacterial and antioxidant compounds from the flower extracts of Vernonia amygdalina. Adv. Pharmacol. Sci. 2018, 2018, 4083736. [Google Scholar] [CrossRef] [Green Version]
- Erukainure, O.L.; Chukwuma, C.I.; Sanni, O.; Matsabisa, M.G.; Islam, M.S. Histochemistry, phenolic content, antioxidant, and anti-diabetic activities of Vernonia amygdalina leaf extract. J. Food Biochem. 2019, 43, e12737. [Google Scholar] [CrossRef]
- Panda, S.K.; Luyten, W. Antiparasitic activity in asteraceae with special attention to ethnobotanical use by the tribes of Odisha India. Parasite 2018, 25, 10. [Google Scholar] [CrossRef] [Green Version]
- Djeujo, F.M.; Raggazi, E.; Urettini, M.; Sauro, B.; Chicero, E.; Tonelli, M.; Froldi, G. Magnolol and luteolin inhibition of α-glucosidase activity: Kinetics and type of interaction detected by in vitro and in silico studies. Pharmaceuticals 2022, 15, 205. [Google Scholar]
- Miean, K.H.; Mohamed, S. Flavonoid (myrisetin, quersetin, kaempferol, luteolin and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef]
- Harborne, J.B. Phytochemical methods. In Applied Phytochemistry Method; Kosasih, P., Soediro, I., Eds.; ITB Press: Bandung, Indonesia, 1987. [Google Scholar]
- Triadisti, N.; Sauriasari, R.; Elya, B. Fractionation and α-glucosidase Inhibitory Activity of Fractions from Garcinia hombroniana Pierre Leaves Extracts. Pharm. J. 2017, 9, 488–492. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).