Abstract
Nitrobenzenesulfochlorination of β-aminopropioamidoximes leads to a set of products depending on the structure of the initial interacting substances and reaction conditions. Amidoximes, functionalized at the terminal C atom with six-membered N-heterocycles (piperidine, morpholine, thiomorpholine and phenylpiperazine), as a result of the spontaneous intramolecular heterocyclization of the intermediate reaction product of an SN2 substitution of a hydrogen atom in the oxime group of the amidoxime fragment by a nitrobenzenesulfonyl group, produce spiropyrazolinium ortho- or para-nitrobenzenesulfonates. An exception is ortho-nitrobenzenesulfochlorination of β-(thiomorpholin-1-yl)propioamidoxime, which is regioselective at room temperature, producing two spiropyrazolinium salts (ortho-nitrobezenesulfonate and chloride), and regiospecific at the boiling point of the solvent, when only chloride is formed. The para-Nitrobezenesulfochlorination of β-(benzimidazol-1-yl)propioamidoxime, due to the reduced nucleophilicity of the aromatic β-amine nitrogen atom, is regiospecific at both temperatures, and produces the O-para-nitrobenzenesulfochlorination product. The antidiabetic screening of the new nitrobezenesulfochlorination amidoximes found promising samples with in vitro α-glucosidase activity higher than the reference drug acarbose. 1H-NMR spectroscopy and X-ray analysis revealed the slow inversion of six-membered heterocycles, and experimentally confirmed the presence of an unfavorable stereoisomer with an axial N–N bond in the pyrazolinium heterocycle.
1. Introduction
Heterocycles with potential bioactive properties are of great interest, first of all, for medical chemists working in the field of heterocyclic compounds synthesis. Among the new drugs approved by the FDA in 2021, almost 50% are substances with nitrogen-containing heterocycles [1]. Pyrazoline derivatives, as prominent representatives of nitrogen-containing heterocycles, became the subject of a report on the world market of diphenylpyrazolines from Market Strides (global aggregator and publisher of market intelligence research reports). The main segments of the diphenylpyrazoline market are divided into pharmaceutical and industrial, and address textiles, detergents, paper production, cosmetics, plastics, ceramics, and medicines [2].
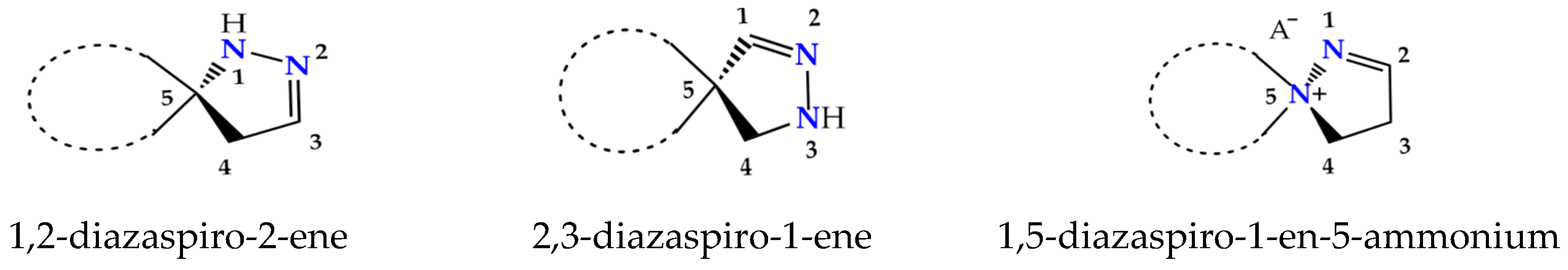
Spiropyrazolines, also termed as spirocyclic hydrazine moiety, are rigid asymmetric heterocyclic structures with a chirality axis and with possible centers of chirality C-5 carbon atoms in the most studied 1,2-diazospiro- and 2,3-diazospiropyrazolines or the ammonium nitrogen atom N(+)-5 in 1,5-diazospiropyrazolinium systems (Figure 1).Thus, these compounds exhibit great synthetic potential due to an enantiomeric advantage, regioisomeric composition, reactivity, and tautomeric transformations. Essentially, 1,2- and 2,3-spiro-1-pyrazolines exist as spiro-2-pyrazolines (∆2 isomers) [3], although, in solutions, they exhibit an equilibrium of the imine and enamine forms [4]. As we know, the spiropyrazolinium salts with a 1,5-diazaspiro-1-en-5-ium fragment that we obtained via the hydrolysis of 3-(β-heteroamino)ethyl-5-aryl-1,2,4-oxadiazoles and the arylsulfochlorination of β-aminopropioamidoximes exist, under standard conditions, as the ∆2 isomer [5,6,7,8]. The thermodynamic advantage of possible tautomers of the key pyrazoline moiety was estimated; it turns out that, in the case of pyrazolines, the ∆2 isomer is much more stable than the others [9].
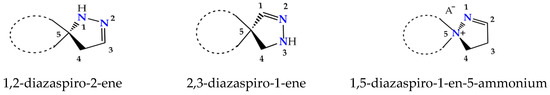
Figure 1.
Structural isomers of spiropyrazolines.
Two comprehensive reviews on functionalized spiropyrazolines were published in 2013 and 2019 [10,11].
The most common methods for the construction of the 1,2- and 2,3-spiro-1-pyrazolines involve the formation of a new ring on an existing carbo- or heterocycle, having exocyclic C=C double bonds. The essential steps in the formation of spiropyrazoline systems in these cases are 1,3-cycloaddition reactions of nitrogen-containing molecules (nitrilimines [12,13,14,15] and diazoalkanes [16,17]) to double bonds, or a condensation reaction of substituted chalcones with hydrazine or its derivatives in an acidic or alkaline medium [18,19].
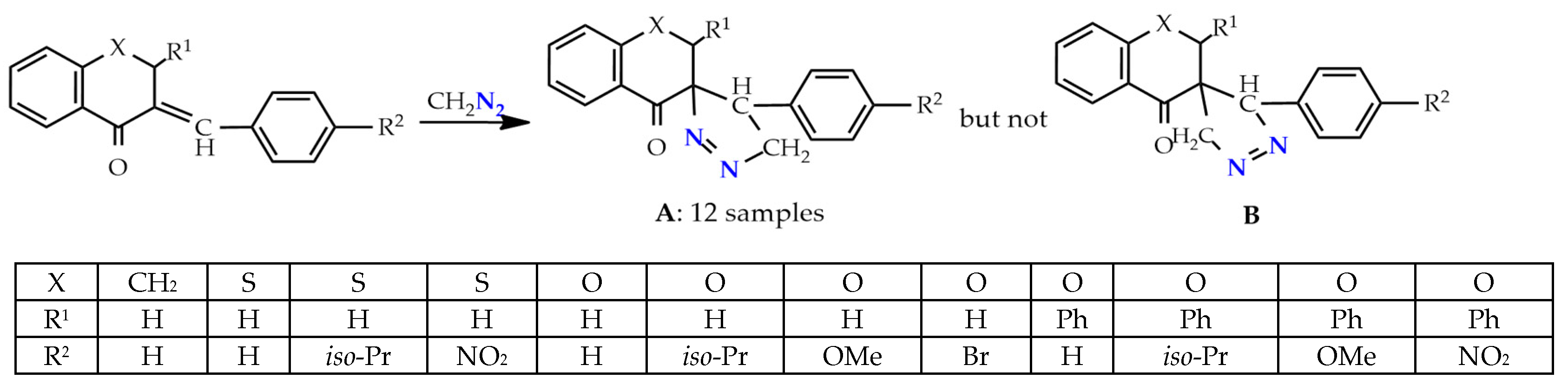
There is information about the regioselective and regiospecific syntheses of spiropyrazolines. The synthesis of only one stereoisomer of spiro-1-pyrazolines A—∆2 1,2-diazospiropyrazolines obtained by the 1,3-dipolar cycloaddition of diazomethane to 3-arylideneflavanones/chromanones proceeds regioselectively in one step (Scheme 1). The alternative structures B were rejected based on the 1H NMR and XRD, data as well as the DFT calculations [20,21].
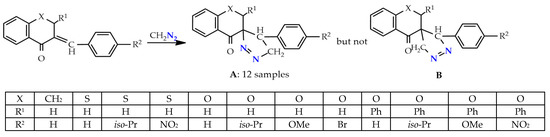
Scheme 1.
1,3-Dipolar cycloaddition of diazomethane to 3-arylideneflavanones.
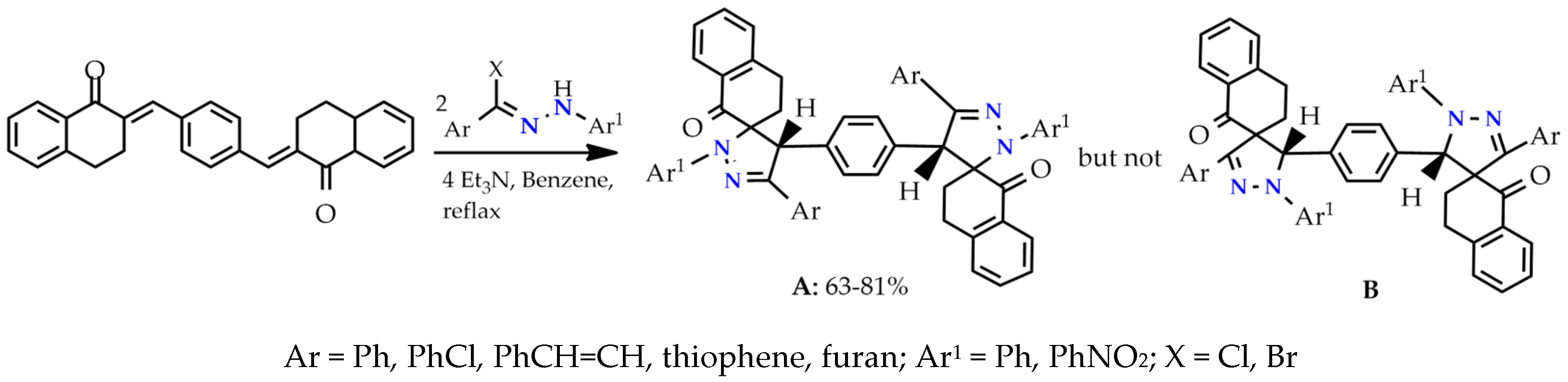
Symmetrical spiropyrazoline systems are formed by the 1,3-dipolar cycloaddition reaction of (2E,2′E)-2,2′-(1,4-phenylene bis(methanylylidene)) bis(3,4-dihydronaphthalen-1(2H)-one) with a hydrazinoyl halides (nitrile imines). The reaction proceeds regioselectively and only one of two possible spiropyrazoline regioisomers (the 1,2-diazaspiropyrazoline, but not 2,3-diazaspiropyrazoline) is formed. The best explanation for the regioselectivity of the reaction and the impossibility of the formation of the 2,3-diazospiropyrazolines adduct is made using the molecular orbital theory in the interaction of HOMO and LUMO reagents (Scheme 2) [22].
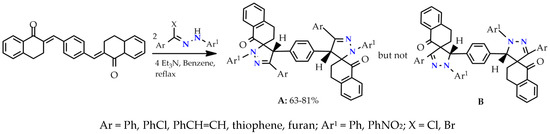
Scheme 2.
Cycloaddition of bis-exocyclic olefins with nitrile imines.
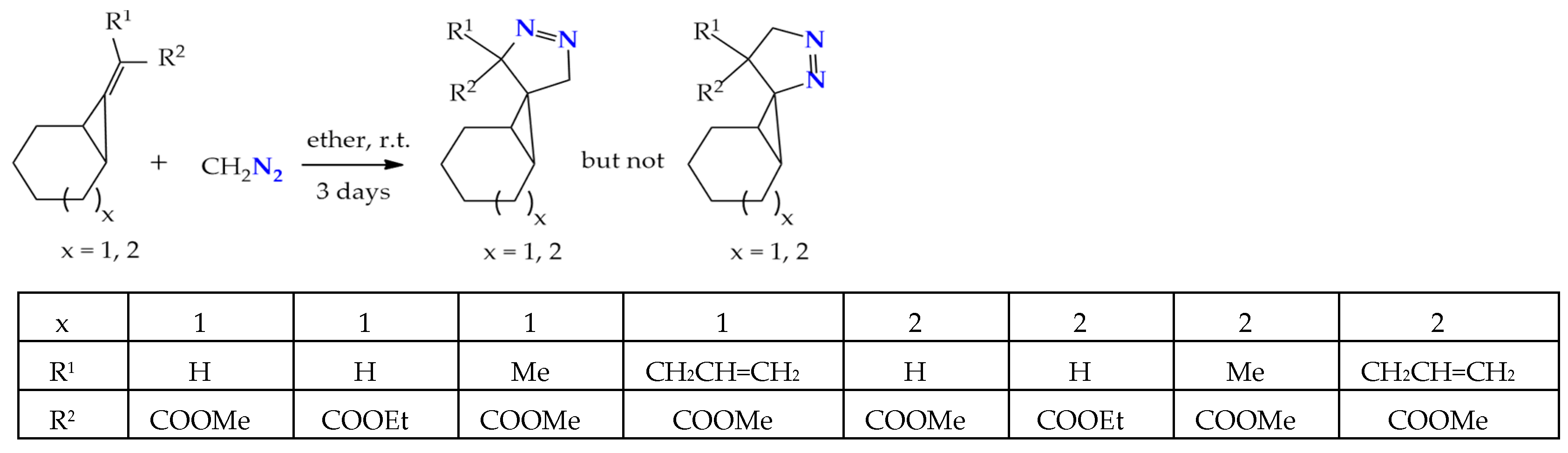
1,3-Dipolar cycloaddition of diazomethane to a double bond activated by an electron-withdrawing group in alkylidenecyclopropanes produces 2,3-diazaspiropyrazolines in excellent amounts (95–99%). The determination of the stereochemistry of spiropyrazolines was carried out by NMR spectroscopy, including NOE experiments, which indicated that the methylene protons of the 2,3-diazaspiropyrazoline ring have NOE effects on cyclopropane protons, and this is possible when the methylene group is in the exo-position (Scheme 3) [23].
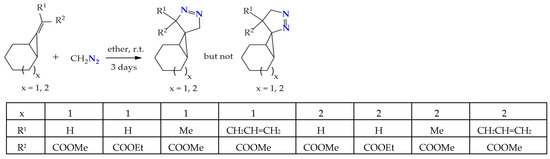
Scheme 3.
Formation of 2,3-diazaspiro-2-ene isomers in the course of the regiospecific addition of diazomethane to alkylidenecyclopropanes.
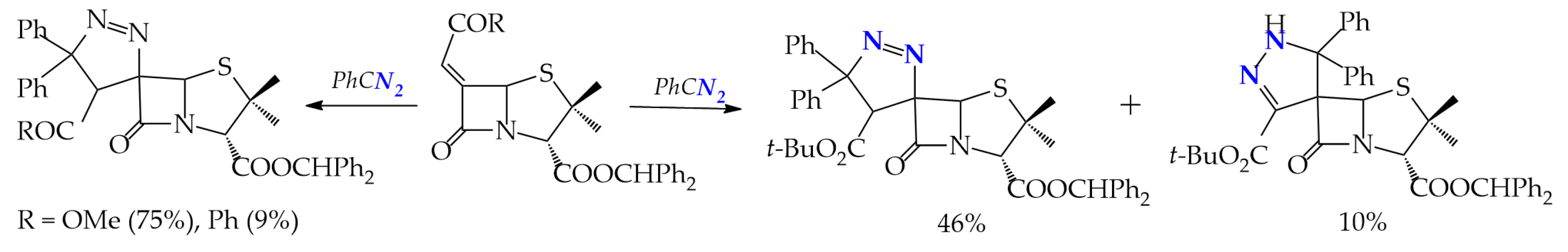
At a 1,3-dipolar addition of diphenyldiazomethane to 6-alkylidenepenicillanates, examples of regioselective and regiospecific syntheses with the formation of 1,2- and 2,3-spiropyrazoline systems are observed. Regio- and stereospecificity is observed in the interaction of diphenyldiazomethane with penicylates having Ph and COMe alkylidene substituents R to form 1,2-spiropyrazolines: (4′S,6S)-3-benzhydryl 4′-methoxycarbonyl- and (4′S,6S)-3-benzhydryl 4′-benzoyl-5′,5′-diphenyl-4′,5′-dihydrospiro[penicillanate-6,3′-(3H-pyrazole)]-3-carboxylates up to 75%. The change of the alkylidene substituent to CO2t-Bu leads to a stereoselective, but regioisomeric, mixture of 1,2- and 2,3-spiropyrazolines: (4′S,6S)-3-benzhydryl 4′-tert-butoxycarbonyl-5′,5′-diphenyl-4′,5′-dihydrospiro[penicillanate-6,3′-(3H-pyrazole)]-3-carboxylate and (6R)-3-benzhydryl 3′-acetyl-1′,5′-dihydrospiro[penicillanate-6,4′-(4H-pyrazole)]-3-carboxylate in a ratio of 46 and 10% (Scheme 4) [24].
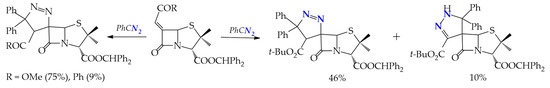
Scheme 4.
1,3-Dipolar addition of diphenyldiazomethane to 6-alkylidenepenicillanates (reaction conditions: DCM, 30 °C, 24 h).
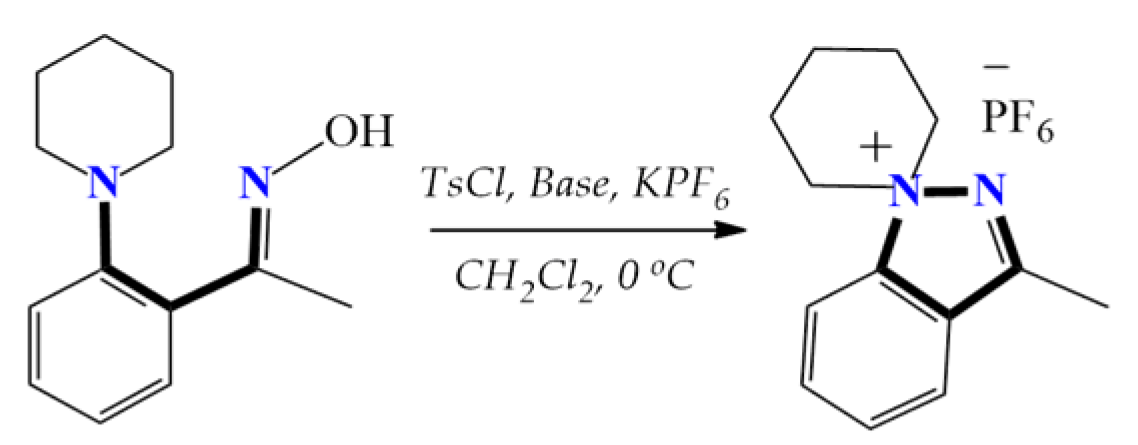
Except for our own studies, no examples of 1,5-diazaspiro-1-en-5-ium spiropyrazolinium salts can be found in the literature. However, stable indazole derivatives, 1,1-disubstituted indazolium hexafluorophosphates with a nitrogen atom at the head of the bridge, were obtained utilizing the tosylation reaction. This transformation is similar to that observed in our previous studies, and includes intramolecular neighboring group participation, finally leading to strong bonding, which follows the SN2 reaction mechanism (Scheme 5) [25].
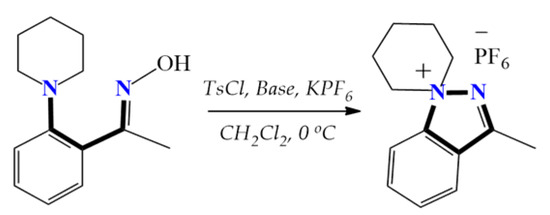
Scheme 5.
The attainment of stable 1,1-disubstituted indazolium hexafluorophosphates.
Previously, we obtained new spiropyrazolium compounds via the hydrolysis of 3-(β-heteroamino)ethyl-5-aryl-1,2,4-oxadiazoles [5,6], by the arylsulfochlorination (Aryl: para-XC6H4SO2Cl; X=CH3O, CH3, H, Br, Cl, NO2) of β-(morpholin-1-yl)aminopropioamidoxime [7] and by the tosylation of β-aminopropioamidoximes (β-amino group: piperidin-1-yl, morpholine-1-yl, thiomorpholin-1-yl, 4-phenylpiperazin-1-yl, benzimidazol-1-yl) at r.t. [8]. In the present study, we are interested in the regioselectivity of β-aminopropioamidoximes ortho-, para-nitrobenzenesulphochlorination at reaction conditions (chloroform (CHCl3), diisopropylamine (DIPEA), room temperature and 70 °C). Depending on the reactant’s structure and reaction temperature, 2-amino-1,5-diazathiospiro[4.5]-dec-1-ene-5-ammonium nitrobenzenesulfonates and chloride and the product of para-nitrobenzenesulfochlorination, on the oxygen atom of β-(benzimidazol-1-yl)propioamidoxime were obtained. Among them, samples with high in vitro antidiabetic activity were found. The significance of the present work is the establishment of the ambiguity of amidoximes sulfochlorination and the possibility of obtaining a wide range of potentially biologically active products.
2. Results and Discussion
2.1. Synthesis and Spectra
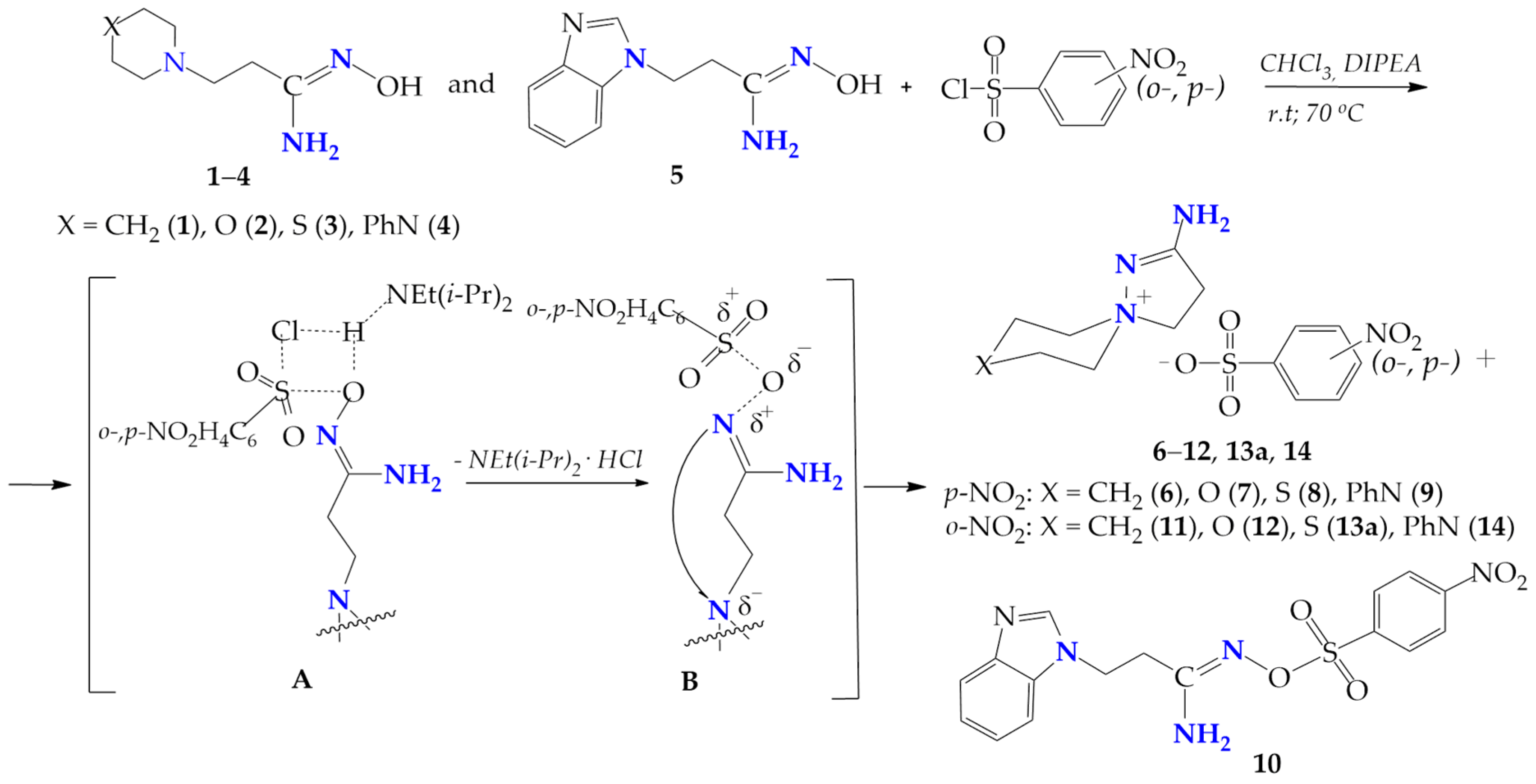
The interaction of β-aminopropioamidoximes (1–5) with ortho-, para-nitrobenzenesulfochlorides in CHCl3 was carried out at room temperature and by heating the reaction mixture to the solvent boiling point. A change in the electronic properties of the sulfochlorinating agent, the transition from tosyl chloride to ortho-, para-nitrobenzenesulfochlores lead to an increase in the reaction time at room temperature from 15–20 h in the case of tosylation [4] to 38–120 h for para-benzenesulfochlorination, and up to 25–104 h for ortho-benzenesulfochlorination.
The heating of the reaction mixture at the CHCl3 boiling point reduces the reaction time to 19–36 h, and to 24 h for para- and ortho-nitrobenzenesulfochlorination, respectively. The completion of β-aminopropioamidoximes arylsulfochlorination was confirmed by the physicochemical data (TLC, elemental analysis, m.p.), and the IR and NMR (1H and 13C) spectra and X-ray data of the isolated products (6–14). On the basis of physicochemical and spectral data, it was concluded that, in the case of the nitrobenzenesulfochlorination of β-aminopropioamidoximes with six-membered heterocycles in the β-position (1–4), the main products were the nitrobenzenesulfonates of spiropyrazoline compounds 6–12, 13a, and 14; however, when the substrate was β-(benzimidazol-1-yl)propioamidoxime (5), only the product of para-nitrobenzensulfochlorination at the oxygen atom of the amidoxime fragment 10 was isolated from the reaction mixture (Scheme 6).
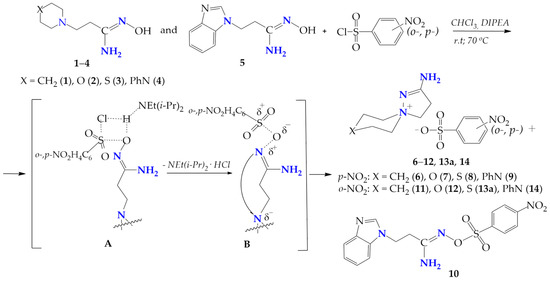
Scheme 6.
Nitrobenzenesulfochlorination of β-aminopropioamidoximes.
It is assumed that, in the case of the nitrobenzenesulfochlorination of β-aminopropioamidoximes 1–4, the O-nitrobenzenesulphonates of β-propioamidoximes formed as intermediate B, due to the thermodynamic advantage, rearranging into spiropyrazoline nitrobenzenesulphonates (6–12, 13a, 14). Only in the case of the benzimidazole derivative, the intermediate B remains stable and produces O-para-nitrobenzenesulphonate 10.
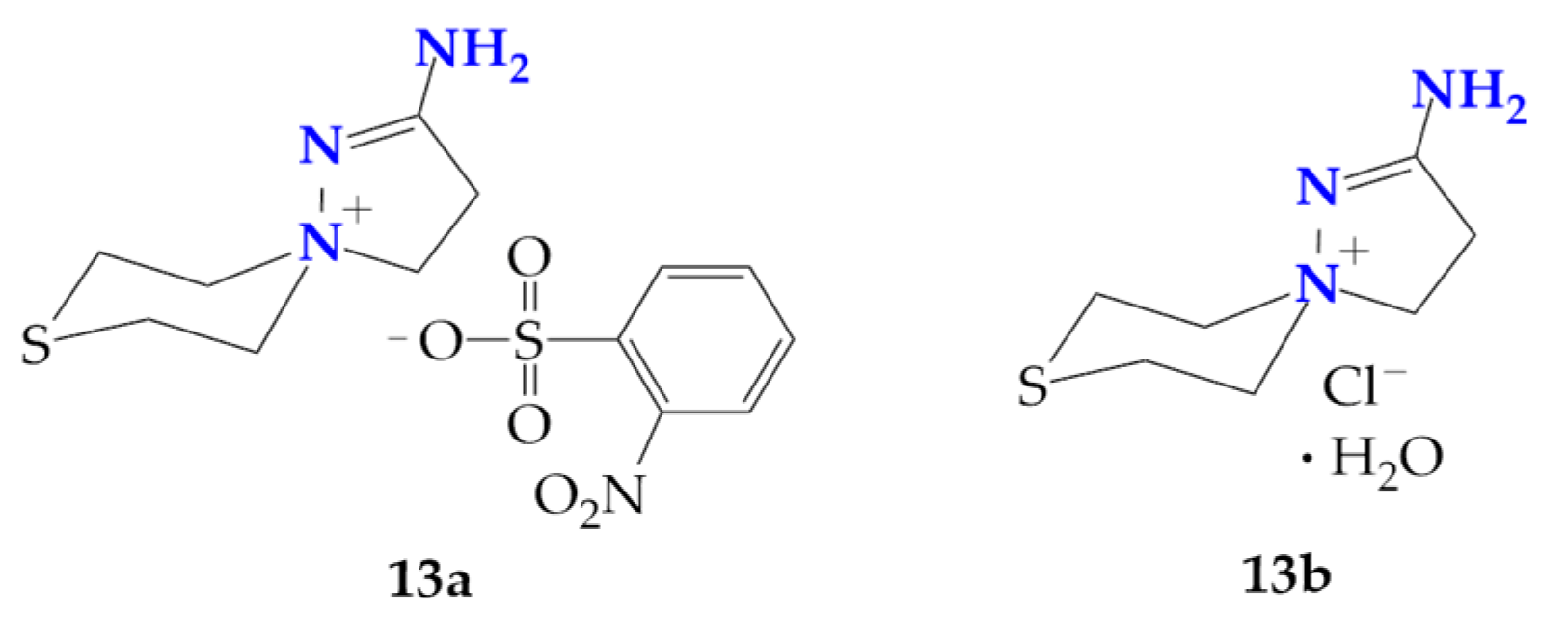
In addition, the ortho-nitrobenzenesulfochlorination of β-(thiomorpholine-1-yl)propioamidoxime (3) has the following features: at room temperature, a mixture of 2-aminospiropyrazolylammonium ortho-nitrobenzenesulfonate and chloride (13a and 13b) were obtained, and carrying out the reaction at 70 °C only produces chloride 13b (Figure 2). Compound 13b was previously characterized by us [26,27].
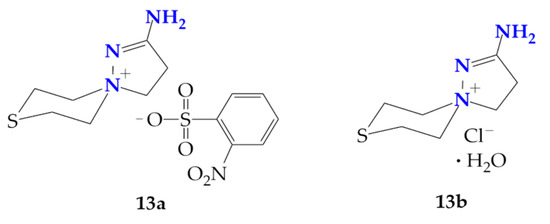
Figure 2.
Salts of spiropyrazolinium compounds 13a and 13b isolated at the ortho-nitrobenzenesufochlorination of β-(thiomorpholine-1-yl)propioamidoxime.
Evidently, in this case, the para-nitrophenylsulfonate anion in the primarily formed 2-amino-1,5-diazaspiro[4.5]dec-1-en-5-ium para-nitrophenylsulfonate (13a) was exchanged for the chloride anion from DIPEA hydrochloride, releasing para-nitrobenzenesulfonic acid and DIPEA.
The stoichiometry of the reaction does not provide for the formation of a hydrate. We believe that the preparation of hydrate 13b can be explained by the prolonged contact of the mother liquor during the preparation of the single crystals of the ortho-nitrophenylsulfochlorination product of amidoxime 3 with atmospheric moisture.
When establishing the structure of nitrobenzenesulfochlorination products, a difference was noted in the values of the mobility index Rf for the products produced by the nitrobenzenesulfochlorination of amidoximes with six-membered nitrogenous heterocycles in the β-position (6–9, 11–14) and for the para-nitrobenzenesulfoderivative of β-(benzimidazol-1-yl)propioamidoxime (10) (Rf 0.01–0.12 and 0.75). Compounds 7 and 13b were previously described in [7] and [26,27], respectively (Table 1).

Table 1.
Physicochemical data on the products as a result of the nitrobenzenesulfochlorination of β-aminopropioamidoximes (6–14).
In the IR spectra of compounds 6–12, 13a, and 14, there are two pairs of bands related to the characteristic stretching vibrations of a strong intensity of the NO2 and SO2 groups at 1515–1549 (as) cm−1 and 1349–1377 (sy) cm−1, and 1207–1240 (as) cm−1 and 1024–1197 (sy) cm−1, respectively, whereas, in the IR spectrum of compound 13b, there are no bands of stretching vibrations for the bonds of the NO2 and SO2 groups.
It is interesting that, in the 1H-NMR spectra of compounds 6–9, 13a, and 13b, it was possible to fix the diastereotopic nature of the geminal protons of the methylene groups located at the ammonium nitrogen atom, which produce pairs of multiplet signals with an intensity of 2 protons at δ: 3.35 m, 3.44 m (6); 3.41 m, 3.65 m (7); 3.60 m, 3.72 m (8); 3.49 m, 3.98 m (9); 3.10 m, 3.68 m (13a); and 3.10 m, 3.68 m (13b).
Similarly, the geminal protons of the methylene groups at the S, O, S, S, and N atoms of the β-heterocycles of compounds 8, 12, 13a, 13b, and 14 also appear as pairs of proton signals with an intensity of 2 protons at δ: 2.87 m, 3.15 m (8); 3.35 m, 3.60 m (12); 2.84 m, 3.55 m (13a); 2.85 m, 3.55 m (13b); and 3.40 m, 3.71 m (14). Evidently, the effect of the slow rotation of β-heterocycles with the possibility of fixing the equatorial and axial protons is observed here. In addition, the diastereotopicity of these protons may be associated with the presence of an asymmetry axis inherent in the spiro compounds.
The signals of Csp3 and Csp2 carbon atoms in the 13C-NMR spectra of compounds 6–14 are present in the characteristic regions.
We obtained a quantum-chemical confirmation of the advantageousness of the formation of spirocyclic tosylation and para-nitrobenzenesulfochlorination products of β-aminopropioamidoximes (1–4), with negative values of the Gibbs energy of the chemical reaction in the range of −119.99–−163.57 kJ/mol and the disadvantage of the formation of a spirostructure for β-(benzimidazole-1-yl)propioamidoxime (5), with positive values of the Gibbs energy for the tosylation and para-nitrobenzenesulfochlorination products as 45.87 and 20.02 kJ/mol, respectively [28].
The thermodynamic advantage of the formation of 2-aminospiropyrazolylammonium sulfonates (tosylates, para- and ortho-nitrobenzenesulfonates) for a number of β-aminopropioamidoximes (1, 2, 4), in comparison to the formation of chloride hydrates, was identified, except for the case when the initial substrate was β-(thiomorpholine-1-yl)propioamidoxime 3; in this case, 2-amino-8-thia-1,5-diazaspiro[4.5]dec-1-en-5-ammonium chloride monohydrate (13b) is preferred by −8.1, −6.18, −3.08 kJ/mol, respectively [29].
It should be noted that we attempted to react β-(benzimidazol-1-yl)propioamidoxime (5) with ortho-nitrobenzenesulfonate several times, under the described conditions (CHCl3, DIPEA, r.t. and 70 °C), but, each time, a resinous reaction mixture was obtained.
2.2. The In Vitro Antidiabetic Screening of 2-Amino-1,5-diazaspiro[4.5]dec-1-en-5-ammonium Nitrobenzenesulphonates and Chloride Hydrate (6–9, 11–14) and 3-(1H-Benzo[d]imidazol-1-yl)-N′-{[(4-nitrophenyl)sulfonyl]oxy}propanimidamide (10)
The α-amylase and α-glucosidase inhibition tests, which hydrolyze starch in postprandial hyperglycemia, are standard tests used in the discovery and development of new antidiabetic drugs. Essentially, the regulation of α-amylase and α-glucosidase biological functions (inhibition) is critical to the treatment regimen. This implies that new drug candidates with a potent inhibition of the α-glucosidase and α-amylase would be valuable to drug discovery and development for diabetes mellitus [30].
The in vitro antidiabetic activity of spiropyrazolilammonium ortho-, para-nitrobenzenesulfonates and chloride 6–9, 11–14, and the product of O-para-nitrobenzenesulfochlorination of β-(benzimidazol-1-yl)propioamidoxime (10) was assessed via the degree of inhibition of the activity of α-amylase and α-glucosidase by the studied substances, compared to the standard drug acarbose (Table 2). The table shows four series of experiments with different experimental values of the in vitro activity of acarbose in relation to α-amylase and α-glucosidase.

Table 2.
In vitro α-amylase and α-glucosidase activity of nitrobenzenesulfochlorination products of β-aminopropioamidoximes (6–14).
All the tested compounds had an average inhibitory activity against α-amylase, the values of which are less than the activity of acarbose. In regard to α-glucosidase, the products of para-nitrobenzenesulfochlorination 8 and 10 had a pronounced inhibitory activity (61.0% and 67.1%). In the same series of experiments, the average inhibitory activity of 36.5% and 48.1% is shown by the compounds 7 and 9. The reference drug acarbose exhibited the standard inhibitory activity of 58.9% and 50.3% in terms of α-glucosidase and α-amylase, respectively. In two other series of experiments, the tested ortho-nitro derivatives (11–14) did not show activity against α-amylase and α-glucosidase, comparable to the activity of acarbose.
The apparent difference in the antidiabetic activity of the subgroups of compounds 6–10 and 11–14 can be explained by the difference in their chemical structure. The first subgroup is derivative of para-nitrophenylsulfonic acid; the second subgroup is an ortho-nitrophenylsulfonic acid derivative. It is likely that binding to the sites of α-amylase and α-glucosidase enzymes responsible for increasing blood sugar is more efficient for para-nitrobenzenesulfonic acid derivatives (6–10), while ortho-nitrophenylsulfonic acid derivatives (10–14) are less efficient for the target reference antidiabetic drug acarbose.
The absence of α-glucosidase activity in representatives of the subgroup of compounds 11–14 may be associated with the previously described trend of a general decrease in antidiabetic activity in the series of ortho-nitrophenylsulfonic acid derivatives (10–14), whereas the absence of α-glucosidase activity for compound 6 may be a random variable.
2.3. X-ray Diffraction
X-ray diffraction studies of all the reaction products were carried out. They confirmed that, regardless of the synthetic conditions, the nitrobenzenesulfochlorination of β-aminopropioamidoximes containing piperidin-1-yl, morpholine-1-yl and 4-phenylpiperazin-1-yl as β-aminogroup affords spiropyrazolium nitrobenzenesulfonates 6, 7, 9 or 11, 12, 14. As a result of the ortho-nitrobenzenesulfochlorination of β-thiomorpholin-1-ylpropioamidoxime, nitrophenylsolfonate 13a and chloride monohydrate 13b of the corresponding spirocation, similar to the one previously reported [29], can be obtained depending on the reaction conditions. No rearrangement was detected for benzimidazol-1-yl-containing product 10 in accord with the B3LYP/6-31++G(d,p) calculations of the standard Gibbs free energies of reaction [28,29] found for N-substituted aminopyrazoles [31,32]. All salts contain one cation and one anion in the asymmetric unit (Figure S1, ESI). The quality of XRD data allowed us to locate all of the hydrogen atoms on difference Fourier maps, and undoubtedly confirmed that none of the sulfonate groups contained any hydrogen atoms.
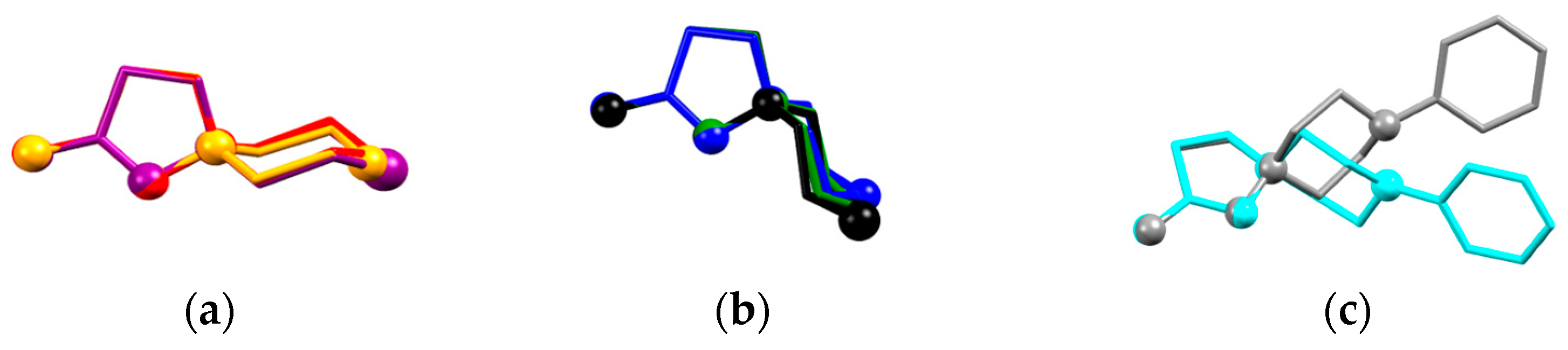
The molecular structures of these compounds are depicted on Figure S1 (ESI), and the main geometry parameters of the cations and anions are listed in Table 3. The X–CH2 bond distances increase and CH2–X–CH2 angles decrease, passing from O to NPh; CH2 and S. Valence angles at the positively charged N1 atom are close to ideal 109.5° values, however only N–CH2 bond distances within the 6-membered ring are typical for a single N–C bond. In 10, a similar N1–CH2 bond is equal to 1.458(2) Å, due to the mesomeric effect of the benzimidazole substituent. The N–N bond in these salts varies from 1.460(2) to 1.470(2) Å, which is longer than 1.42–1.43 Å that is found for N-substituted aminopyrazoles [32,33]. Overall the conformations of the cations in 7, 9, 11,13a, and 13b correspond to one conformation on the six-membered ring towards the five-membered ring, while 6, 8, 12, and 14 are the first examples of the inverted chair conformation (Figure 3) of this ring confirmed by means of X-ray diffraction. The difference in the two conformations manifests itself in the 1H-NMR spectra (see above), and also through the N1–C1 bond length, which is generally shorter in cations with ‘novel’ conformations. In our opinion, the overall conformation of the molecule and elongation of bond distances in the pyrazole ring can be attributed to the anomeric effect [33] of the (hetero)atom in the six-membered ring. The length of the N2=C bond in all the spirocations is nearly the same as the N2=C bond distance of 1.302(2) in 10.

Table 3.
Selected geometrical parameters of spirocations 6–9, 11–14 (Å and °).
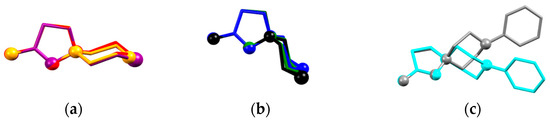
Figure 3.
Molecular conformations of the spirocation in (a) 7 (orange), 11 (red), 13a (violet); (b) 6 (green), 12 (blue), 14 (black); and (c) 9 (grey) and 14 (cyan). The superimposed atoms are N–N=C–C atoms in the 5-membered pyrazole ring.
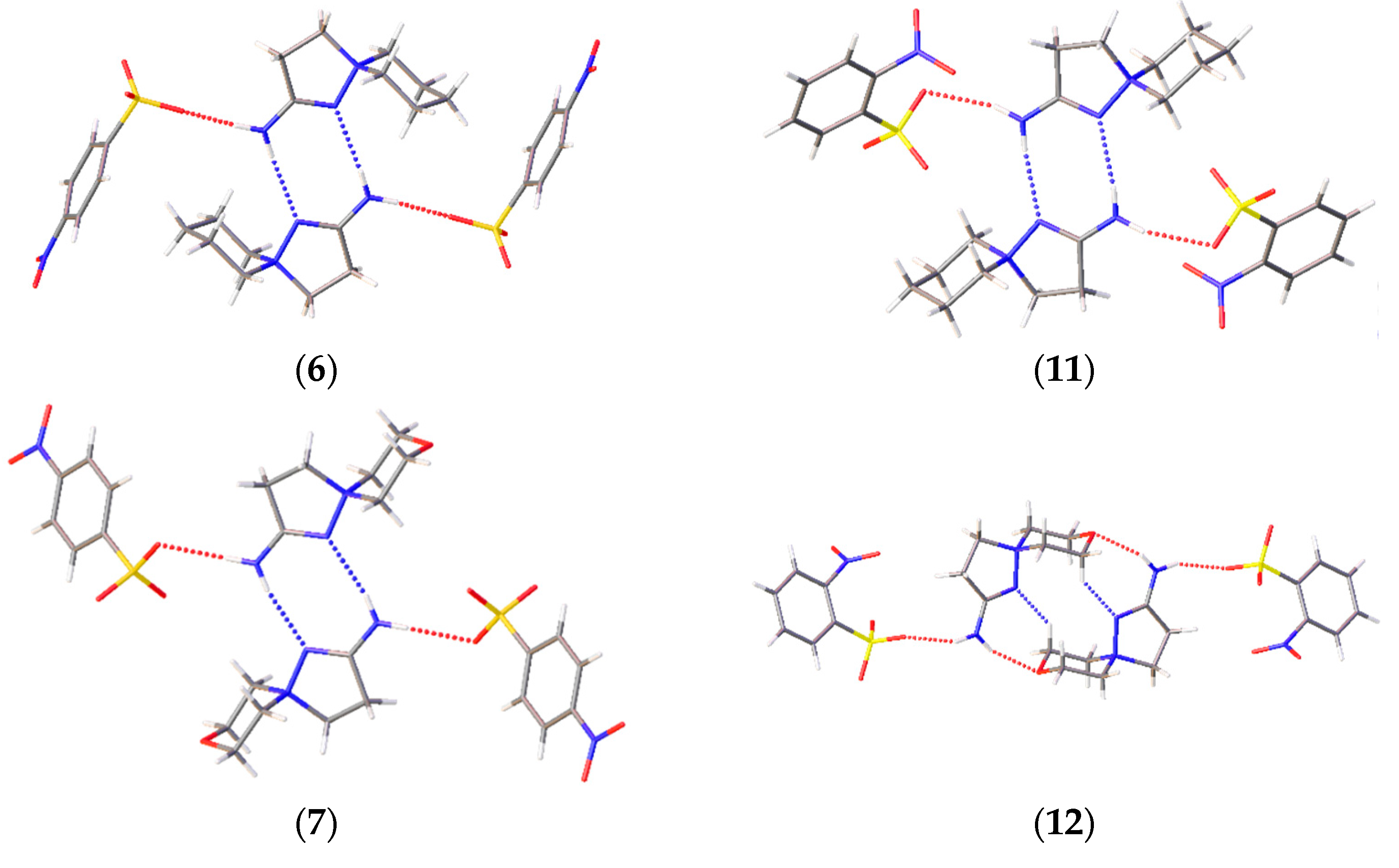
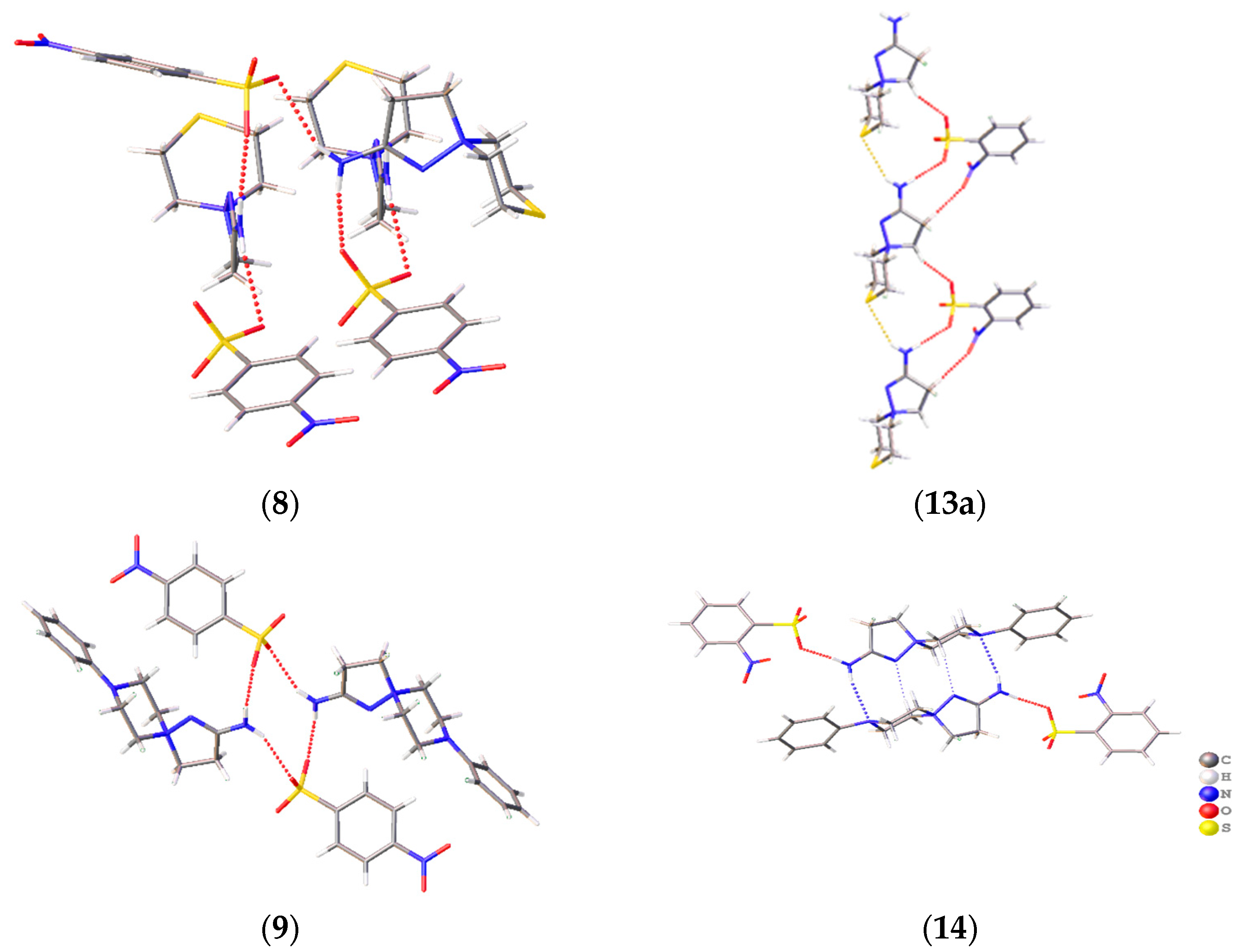
A prominent variation in the biological properties of these compounds can be accounted for the possibility of these cations to realize different conformations and to take part in different types of H bonds [34]. In these solids, the only donor for the H bonds is the amino group, while the sulfonate groups of the anions and heteroatoms of the cations compete to act as acceptors of H bonding. As a result, H-bonded chains are observed in 2-amino-1,5-diazathiospiro[4.5]-dec-1-ene-5-ammonium-containing salts and tetramers in six other salts (Figure 4). The D33(9) tetramers in 6, 7, and 11 are formed through two N–H…N interactions and two N–H…O ones. Similar D33(13) tetramers in 12 and 14 are obtained through a similar N–H…O cation…anion interactions, but the cations are shifted along each other to allow for the bonding with a heteroatom of the six-membered ring. The R44(12) cycles in 9 and the H-bonded chains in 8 and 13 are formed by the interactions of the amino group with sulfonate groups of two neighboring molecules. The Gad(n) notation of H-bonded architectures is presented in terms of [33], where G represents the type of pattern (C for chain, S for intramolecular hydrogen bonds, R for ring, and D for finite), a is the number of acceptors, d is the number of donors, and n the number of atoms in the pattern. Note that, despite the similar crystal parameters and space group of 11–13 (Table S1, ESI), these compounds cannot be regarded as isostructural, as they realize different packing and intermolecular (including H-bonding) interactions. Crystal packing demonstrates that, for these spirocations, heteroatoms of the six-membered cycle can take part in H bonding, and different conformations can be stabilized by means of intermolecular bonding.
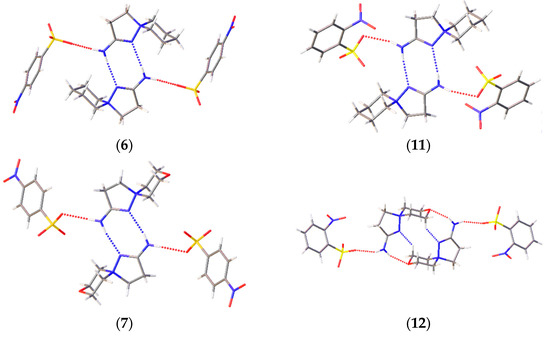
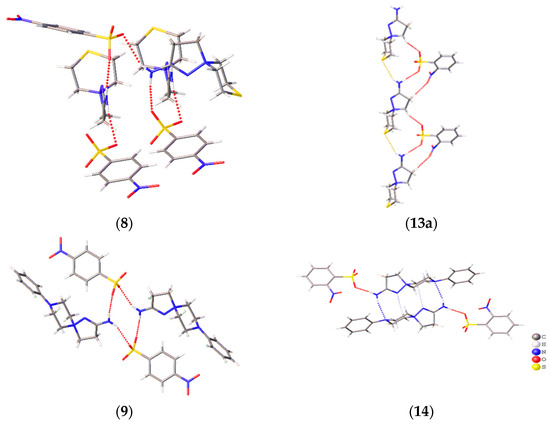
Figure 4.
H-bonding patterns in the crystal structures of the studied compounds. H bonds are depicted with dotted lines.
3. Materials and Methods
3.1. Synthesis
The reagents were purchased from different chemical suppliers and were purified
Before use. FT-IR spectra were obtained on a Thermo Scientific Nicolet 5700 FTIR instrument (Thermo Fisher Scientific, Inc., Waltham, MA, USA) in KBr pellets. The 1H- and 13C-NMR spectra of compounds 6–10 were acquired on a Bruker Avance III 500 MHz NMR spectrometer (Bruker, BioSpin GMBH, Rheinstetten, Germany) and 1H- and 13C-NMR spectra of compounds 11–14 on a Jeol JNM-ECA 500 (Jeol, Tokyo 196-8558, JAPAN), (500 and 126 MHz, respectively).
The signals of the residual undeuterated solvents were used as a reference for the 1H-NMR (2.50 ppm) and 13C-NMR (39.5 ppm) spectra. Elemental analysis was carried out on a CE440 elemental analyzer (Exeter Analytical, Inc., Shanghai, China). The melting points were determined in glass capillaries on a PTP(M) apparatus (Khimlabpribor, Klin, Russia). The reaction progress and purity of the obtained products were controlled using Sorbfil (Sorbpolymer, Krasnodar, Russia) TLC plates coated with CTX-1A silica gel, grain size 5–17 μm, containing UV-254 indicator. The eluent for TLC analysis was a mixture of benzene–EtOH, 1:3. The solvents for the synthesis, recrystallization, and TLC analysis (ethanol, 2-PrOH, benzene, DMF, and acetone) were purified according to the standard techniques.
3.1.1. A General Procedure for the Synthesis of 2-Amino-1,5-diazaspiro[4.5]-dec-1-ene-5-ammonium para-Nitrobezenesulfonates (6–9) and 3-(1H-Benzo[d]imidazol-1-yl)-N′-{[(4-nitrobenzene)sulfonyl]oxy}propanimidamide (10)
The synthesis of β-aminopropioamidoximes4-nitrobenzenesulfochlorinationproducts 6–10(general method). To a solution of 0.0029 mol of β-aminopropioamidoximes 1–5 in 20 mL of CHCl3, 0.0029 mol of DIPEA was added. The reaction mixture was cooled to −1 °C, and a solution of 0.0029 mol of 4-nitrobenzenesulfochloride in 2 mL of CHCl3 was added dropwise with stirring. The reaction mixture was then allowed to warm to r.t. (or heated to the boiling point (b.p.) of CHCl3) and stirred until the completion of the reaction. The progress of the reaction was monitored by TLC. The formed white precipitates of the products 6–10 were filtered off and recrystallized from 2-PrOH.
2-Amino-1,5-diazaspiro[4.5]dec-1-en-5-ammonium 4-nitrobenzenesulfonate (6). The reaction mixture consisting of 0.5 g (0.0029 mol) of compound 1 in 20 mL of CHCl3 and 0.38 g (0.0029 mol) of DIPEA was cooled to -1℃. Then, 0.64 g (0.0029 mol) of 4-nitrobenzenesulfochloride was added dropwise. When the reaction mixture was kept at r.t. for 38 h, 0.80 g (77%) of white solid 6 was obtained (when the reaction mixture was kept at CHCl3 b.p. for 27 h, 0.48 g (46%) of white solid 6 was obtained); m.p. 151 °C, Rf 0.12. IR (KBr, cm−1): 1665 (C=N), 1607 (C=C), 1232 (SO2 as) and 1190 (SO2 sy), 1529 (NO2 as) and 1352 (NO2 sy), 3302 [N(-H)2], 2938, 2864, 2815 (Csp3−H), 3032, 3257 (Csp2−H). 1H-NMR (500 MHz, DMSO-d6): 3.10 (t, J = 7.0 Hz, 2H, α-CH2), 3.81 (t, J = 7.0 Hz, 2H, β-CH2), 1.55 m, 1.75 m, 1.87 m, [6H, (CH2)3], 3.35 [m, 2H, N(+)(CHax)2] and 3.44 [m, 2H, N(+)(CHeq)2], 7.23 (s, 2H, NH2), 7.83−8.21 (m, 4H, C(sp2)H). 13C-NMR (126 MHz, DMSO-d6): 21.0, 21.9, 31.5, 60.7, 64.3 (2C), 126.0 (2C), 128.5 (2C), 138.0 (1C), 145.3 (1C), 168.5. Anal. Calcd for C14H20N4O5S (356.40): C, 47.18; H, 5.66. Found: C, 47.36; H, 5.37.
2-Amino-8-oxa-1,5-diazaspiro[4.5]dec-1-ene-5-ammonium 4-nitrobenzenesulfonate (7).The reaction mixture consisting of 0.5 g (0.0029 mol) of compound 2 in 20 mL of CHCl3 and 0.38 g (0.0029 mol) of DIPEA was cooled to −1 °C. Then, 0.64 g (0.0029 mol) of 4-nitrobenzenesulfochloride was added dropwise. When the reaction mixture was kept at r.t. for 120 h, 0.73 g (70%) of white solid 7 was obtained (when the reaction mixture was kept at CHCl3 bp for 24 h, 0.77 g (74%) of white solid 7 was obtained); m.p. 187–188 °C, Rf 0.10. IR (KBr, cm−1): 1650 (C=N), 1600 (C=C), 1231 (SO2 as) and 1194 (SO2 sy), 1520 (NO2 as) and 1362 (NO2 sy), 3466 [N(-H)2], 2967, 2854 (Csp3−H), 3236, 3328, 3388 (Csp2−H). 1H-NMR (500 MHz, DMSO-d6): 3.13 (t, J = 7.0 Hz, 2H, α-CH2), 3.92 (t, J = 7.0 Hz, 2H, β-CH2), 3.32 [m, 4H, O(CH2)2], 3.41 [m, 2H, N(+)(CHax)2] and 3.65 [m, 2H, N(+)(CHeq)2], 7.28 (s, 2H, NH2), 7.80−8.19 (m, 4H, C(sp2)H). 13C-NMR (126 MHz, DMSO-d6): 31.4, 62.1, 62.4, 63.2, 123.8 (2C), 127.4 (2C), 147.7 (1C), 154.8 (1C), 170.1. Anal. Calcd for C13H18N4O6S (358.37): C, 43.57; H, 5.06. Found: C, 43.48; H, 5.13.
2-Amino-8-thia-1,5-diazaspiro[4.5]dec-1-en-5-ammonium 4-nitrobenzenesulfonate (8). The reaction mixture consisting of 0.55 g (0.0029 mol) of compound 3 in 20 mL of CHCl3 and 0.38 g (0.0029 mol) of DIPEA was cooled to −1 °C. Then, 0.64 g (0.0029 mol) of 4-nitrobenzenesulfochloride was added dropwise. When the reaction mixture was kept at r.t. for 84 h, 0.74 g (68%) of white solid 8 was obtained (when the reaction mixture was kept at CHCl3 b.p. for 21 h, 0.51 g (47%) of white solid 8 was obtained); m.p. 230 °C, Rf 0.10. IR (KBr, cm−1): 1642 (C=N), 1588 (C=C), 1229 (SO2 as) and 1197 (SO2 sy), 1519 (NO2 as) and 1349 (NO2 sy), 3308, 3396 [N(-H)2], 2944 (Csp3−H), 3105, 3199, 3245 (Csp2−H). 1H-NMR (500 MHz, DMSO-d6): 3.11 (t, J = 7.0 Hz, 2H, α-CH2), 3.86 (t, J = 7.0 Hz, 2H, β-CH2), 2.87 [m, 2H, S(CHax)2] and 3.15 [m, 2H, S(CHeq)2], 3.60 [m, 2H, N(+)(CHax)2] and 3.72 [m, 2H, N(+)(CHeq)2], 7.37 (s, 2H, NH2), 7.83−8.20 (m, 4H, C(sp2)H). 13C-NMR (126 MHz, DMSO-d6): 23.2, 31.4, 62.5, 64.7, 123.8 (2C), 127.3 (2C), 147.7 (1C), 154.8 (1C), 169.0. Anal. Calcd for C13H18N4O5S2 (374.44): C, 41.70; H, 4.85. Found: C, 41.59; H, 4.33.
2-Amino-8-phenyl-1,5,8-triazaspiro[4.5]dec-1-en-5-ammonium 4-nitrobenzenesulfonate (9). The reaction mixture consisting of 0.72 g (0.0029 mol) of compound 4 in 20 mL of CHCl3 and 0.38 g (0.0029 mol) of DIPEA was cooled to −1 °C. Then, 0.64 g (0.0029 mol) of 4-nitrobenzenesulfochloride was added dropwise. When the reaction mixture was kept at r.t. for 60 h, 0.88 g (70%) of white solid 9 was obtained (when the reaction mixture was kept at CHCl3 b.p. for 19 h, 0.87 g (69%) of white solid 9 was obtained); m.p. 203 °C, Rf 0.15. IR (KBr, cm−1): 1649 (C=N), 1599 (C=C), 1240 (SO2 as) and 1189 (SO2 sy), 1515 (NO2 as) and 1350 (NO2 sy), 3421 [N(-H)2], 2790, 2880, 2915 (Csp3−H), 3118, 3290 (Csp2−H). 1H-NMR (500 MHz, DMSO-d6): 3.17 (t, J = 7.0 Hz, 2H, α-CH2), 3.95 (t, J = 7.0 Hz, 2H, β-CH2), 3.56 [m, 4H, N(CH2)2], 3.49 [m, 2H, N(+)(CHax)2] and 3.98 [m, 2H, N(+)(CHeq)2], 7.25 (s, 2H, NH2), 7.81−8.53 (m, 9H, C(sp2)H). 13C-NMR (126 MHz, DMSO-d6): 31.5, 44.5, 61.5, 62.9, 115.3 (2C), 120.4 (1C), 123.8 (2C), 127.4 (2C), 129.5 (2C), 147.7 (1C), 149.9 (1C), 156.7 (1C), 169.0. Anal. Calcd for C19H23N5O5S (433.48): C, 52.64; H, 5.35. Found: C, 52.35; H, 5.21.
3-(1H-benzo[d]imidazol-1-yl)-N′-{[(4-nitrophenyl)sulfonyl]oxy}propanimidamide (10). The reaction mixture consisting of 0.59 g (0.0029 mol) of compound 5 in 20 mL of CHCl3 and 0.38 g (0.0029 mol) of DIPEA was cooled to −1 °C. Then, 0.64 g (0.0029 mol) of 4-nitrobenzenesulfochloride was added dropwise. When the reaction mixture was kept at r.t. for 80 h, 0.93 g (82%) of white solid 10 was obtained (when kept at CHCl3 b.p. for 36 h, 0.41 g (36%) of white solid 10 was obtained); m.p. 158 °C, Rf 0.75. IR (KBr, cm−1): 1648 (C=N), 1617 (C=C), 1240 (SO2 as) and 1187 (SO2 sy), 1520 (NO2 as) and 1365 (NO2 sy), 3417 [N(-H)2], 2791, 2920 (Csp3−H), 3110, 3237 (Csp2−H). 1H-NMR (500 MHz, DMSO-d6): 2.50 (t, J = 7.0 Hz, 2H, α-CH2), 4.33 (t, J = 7.0 Hz, 2H, β-CH2), 6.50 (s, 2H, NH2), 7.78−8.44 (m, 8H, C(sp2)H), 8.05 (s, 1H, C(sp2)H). 13C-NMR (126 MHz, DMSO-d6): 31.2, 42.9, 110.8 (2C), 119.8 (2C), 121.9 (1C), 122.7 (1C), 128.5 (2C), 130.0 (2C), 133.5 (2C), 134.0 (1C), 143.7 (1C), 144.3 (1C), 144.7 (1C), 160.0. Anal. Calcd for C16H15N5O5S (389.39): C, 49.35; H, 3.88. Found: C, 49.28; H, 3.45.
3.1.2. A General Procedure for the Synthesis of 2-Amino-1,5-diazaspiro[4.5]-dec-1-ene-5-ammonium 2-Nitrobezenesulfonate (11–13a, 14) and 2-Amino-8-thia-1,5-diazaspiro[4.5]dec-1-en-5-ium Chloride Hydrate (13b)
The synthesis of β-aminopropioamidoximes2-nitrobenzenesulfochlorinationproducts6–10(general method). To a solution of 0.0029 mol of β-aminopropioamidoximes 1–5 in 20 mL of CHCl3, 0.0029 mol of DIPEA was added. The reaction mixture was cooled to −1 °C, and a solution of 0.0029 mol of 2-nitrobenzenesulfochloride in 2 mL of CHCl3 was added dropwise with stirring. The reaction mixture was then allowed to warm to r.t. (or up to the b.p. of CHCl3) and stirred until the completion of the reaction. The progress of the reaction was monitored by TLC. The formed white precipitates of the products were filtered off and recrystallized from 2-PrOH.
2-Amino-1,5-diazaspiro[4.5]dec-1-en-5-ammonium 2-nitrobenzenesulfonate (11). The reaction mixture consisting of 0.5 g (0.0029 mol) of compound 1 in 20 mL of CHCl3 and 0.38 g (0.0029 mol) of DIPEA was cooled to −1 °C. Then, 0.64 g (0.0029 mol) of 2-nitrobenzenesulfochloride was added dropwise. When the reaction mixture was kept at r.t. for 35 h, 0.80 g (77%) of white solid 11 was obtained (when the reaction mixture was kept at CHCl3 b.p. for 29 h, 0.78 g (75%) of white solid 11 was obtained); m.p. 153 °C, Rf 0.05. IR (KBr, cm−1): 1657 (C=N), 1593 (C=C), 1221, 1232 (SO2 as) and 1024 (SO2 sy), 1541 (NO2 as) and 1377 (NO2 sy), 3399 [N(-H)2], 2949 (Csp3−H), 3204, 3398 (Csp2−H). 1H-NMR (500 MHz, DMSO-d6): 3.05 (t, J = 7.95 Hz, 2H, α-CH2), 3.77 (t, J = 7.95 Hz, 2H, β-CH2), 1.54m, 1.71m, 1.84m, [6H, (CH2)3], 3.35 [m, 4H, N(+)(CH2)2], 7.20d, 7.46−7.81 (m, 4H, C(sp2)H). 13C-NMR (126 MHz, DMSO-d6): 21.0, 21.9(2C), 31.6, 60.7, 64.3, 122.0, 129.5, 130.5, 131.3, 140.0, 148.3, 168.6. Anal. Calcd for C14H20N4O5S (356.40): C, 47.18; H, 5.66. Found: C, 47.57; H, 5.33.
2-Amino-8-oxa-1,5-diazaspiro[4.5]dec-1-ene-5-ammonium 2-nitrobenzenesulfonate (12). The reaction mixture consisting of 0.5 g (0.0029 mol) of compound 2 in 20 mL of CHCl3 and 0.38 g (0.0029 mol) of DIPEA was cooled to −1 °C. Then, 0.64 g (0.0029 mol) of 2-nitrobenzenesulfochloride was added dropwise. When the reaction mixture was kept at r.t. for 25 h, 0.97 g (93%) of white solid 12 was obtained (when the reaction mixture was kept at CHCl3 b.p. for 24 h, 0.67 g (64%) of white solid 12 was obtained); m.p. 148 °C, Rf 0.01. IR (KBr, cm−1): 1637 (C=N), 1593 (C=C), 1207, 1228 (SO2 as) and 1022 (SO2 sy), 1549 (NO2 as) and 1377 (NO2 sy), 3387 [N(-H)2], 2907 (Csp3−H), 3250, 3306, 3328 (Csp2−H). 1H-NMR (500 MHz, DMSO-d6): 3.09 (t, J = 7.95 Hz, 2H, α-CH2), 3.88 (m, 2H, β-CH2), 3.35 [m, 2H, O(CHax)2] and 3.60 [m, 2H, O(CHeq)2], 3.88 [m, 4H, N(+)(CH2)2], 7.20 (s, 2H, NH2), 7.45−7.81 (m, 4H, C(sp2)H). 13C-NMR (126 MHz, DMSO-d6): 31.5, 62.2 (2C), 63.2, 122.0, 129.5, 130.6, 131.3, 140.0, 148.3, 169.2. Anal. Calcd for C13H18N4O6S (358.37): C, 43.57; H, 5.06. Found: C, 43.89; H, 5.47.
2-Amino-8-thia-1,5-diazaspiro[4.5]dec-1-en-5-ammonium 2-nitrobenzenesulfonate (13a) and 2-Amino-8-thia-1,5-diazaspiro[4.5]dec-1-en-5-ium chloride hydrate (13b). The reaction mixture consisting of 0.55 g (0.0029 mol) of compound 3 in 20 mL of CHCl3 and 0.38 g (0.0029 mol) of DIPEA was cooled to −1 °C. Then, 0.64 g (0.0029 mol) of 2-nitrobenzenesulfochloride was added dropwise. When the reaction mixture was kept at r.t. for 104 h, the white precipitate of the mixture of products 13a and 13b was obtained. After recrystallization, 0.16 g (25%) of 13b was obtained. After the evaporation of the filtrate from the recrystallization to ½ volume. 0.27 g (25%) of 2-nitrobenzenesulfonate 13a was formed. When the reaction mixture was kept at CHCl3 b.p. for 24 h, only 0.37 g (56%) of white solid 13b was obtained.
13a: m.p. 138−140 °C, Rf 0.08. IR (KBr, cm−1): 1647 (C=N), 1604 (C=C), 1207, 1215 (SO2 as) and 1024 (SO2 sy), 1545 (NO2 as) and 1373 (NO2 sy), 3497 [N(-H)2], 2936, 2964 (Csp3−H), 3152, 3258, 3302 (Csp2−H). 1H-NMR (500 MHz, DMSO-d6): 3.10 (t, J = 7.0 Hz, 2H, α-CH2), 3.83 (t, J = 7.95 Hz, 2H, β-CH2), 3.10 [m, 2H, S(CHax)2] and 3.55 [m, 2H, S(CHeq)2], 3.10 [m, 2H, N(+)(CHax)2] and 3.68 [m, 2H, N(+)(CHeq)2], 7.26 (s, 2H, NH2), 7.47−7.81 (m, 4H, C(sp2)H). 13C-NMR (126 MHz, DMSO-d6): 23.2, 31.5, 62.6, 64.7, 122.9, 129.5, 130.6, 131.3, 139.7, 148.3, 169.1. Anal. Calcd for C13H18N4O5S2 (374.44): C, 41.70; H, 4.85. Found: C, 41.67; H, 4.46.
13b: m.p. > 280 °C, Rf 0.08. IR (KBr, cm−1): 1659 (C=N); 1612 [H−N; (H)2−O]; 670 [S−C]; 3135, 3230, 3380, 3384 (H−O, H−N). 1H-NMR (500 MHz, DMSO-d6): 3.10 (t, J = 7.0 Hz, 2H, α-CH2), 3.85 (t, J = 7.95 Hz, 2H, β-CH2), 2.85 [m, 2H, S(CHax)2] and 3.55 [m, 2H, S(CHeq)2], 3.10 [m, 2H, N(+)(CHax)2] and 3.68 [m, 2H, N(+)(CHeq)2], 7.26 (s, 2H, NH2). 13C-NMR (126 MHz, DMSO-d6): 23.2, 31.5, 62.6, 64.7, 169.1. Anal. Calcd for C7H16ClN3OS (225.74): C, 37.24; H, 7.14. Found: C, 37.52; H, 7.48.
2-Amino-8-phenyl-1,5,8-triazaspiro[4.5]dec-1-en-5-ammonium 2-nitrobenzenesulfonate (14). The reaction mixture consisting of 0.72 g (0.0029 mol) of compound 4 in 20 mL of CHCl3 and 0.38 g (0.0029 mol) of DIPEA was cooled to −1 °C. Then, 0.64 g (0.0029 mol) of 2-nitrobenzenesulfochloride was added dropwise. When the reaction mixture was kept at r.t. for 34 h, 0.99 g (79%) of white solid 14 was obtained (when reaction mixture was kept at CHCl3 b.p. for 24 h 1.02 g (81%) white solid 14 was obtained); m.p. 185−187 °C, Rf 0.08. IR (KBr, cm−1): 1647 (C=N), 1593 (C=C), 1217, 1224 (SO2 as) and 1024 (SO2 sy), 1535 (NO2 as) and 1366 (NO2 sy), 3373 [N(-H)2], 2837 (Csp3−H), 3161, 3300 (Csp2−H). 1H-NMR (500 MHz, DMSO-d6): 3.12 (t, J = 7.91 Hz, 2H, α-CH2), 3.91 (t, J = 7.91 Hz, 2H, β-CH2), 3.40 [m, 2H, N(CHax)2], 3.71 [m, 2H, N(CHeq)2], 3.54 [m, 4H, N(+)(CH2)2], 6.82−7.81 (m, 11H, NH2, C(sp2)H). 13C-NMR (126 MHz, DMSO-d6): 31.6, 44.6, 61.5, 62.9, 116.4, 120.5, 122.9, 129.5, 129.7, 130.6, 131.3, 139.8, 148.3, 150.0, 169.2. Anal. Calcd for C19H23N5O5S (433.48): C, 52.64; H, 5.35. Found: C, 52.92; H, 5.63.
When reaction mixture was kept at r.t. for 34 h, 0.99 g (79%) white solid 14 was obtained [when reaction mixture was kept at CHCl3 b.p. for 24 h 1.02 g (81%) white solid 14 was obtained].
3.2. Screening
The in vitro antidiabetic activity of samples 6–14 was assessed by the degree of inhibition of α-amylase and α-glucosidase activity. Pure DMSO was used as a solvent. The final concentration of the sample substances was 10 mg/mL. A total of 100 µL of α-amylase or α-glucosidase (1 U/mL) and 200 µL of the test sample solution (10 mg/mL) were added to 500 µL of phosphate buffer (0.1 M; pH 6.8). The resulting mixture was incubated for 15 min at +37 °C, and 200 µL of P-NPG solution (5 mM) was added.
Then, the resulting mixture was incubated again at +37 °C for 20 min. The reaction was stopped by the addition of 500 μL of sodium carbonate (0.1 M). Since the samples showed too much absorbance at 405 nm, they were diluted 5 times with 5 mL of water and 1 mL of sodium carbonate solution (0.1 M). A solution of α-amylase or α-glucosidase (1 U/mL) was used as a blank. As a negative control, 200 μL of pure DMSO was used in triplicate. As a reference drug, acarbose was obtained at a concentration of 10.0 mg/mL (positive control).
Simultaneously, a negative control was placed without the addition of the test compounds. All the samples were examined in triplets. The inhibitory activity was expressed as a percentage (%) of the degree of inhibition of α-glucosidase, in comparison to the negative control.
3.3. Single-Crystal X-ray Diffraction
The X-ray diffraction data of 8, 9, and 11–14 were collected on a Bruker Apex II diffractometer (Bruker AXS, Inc., Madison, WI, USA) equipped with an Oxford Cryostream cooling unit and a graphite monochromated Mo anode (λ = 0.71073 Å). The intensities of the reflections for 10 were collected at 100 K at the “Belok” beamline of the Kurchatov Synchrotron Radiation Source (NRC “Kurchatov Institute”, Moscow, Russia), at the wavelength of 0.745 Å using a MAR CCD 165 detector. The image integration was performed using the iMosflm software [35]. The integrated intensities were empirically corrected for the absorption using the Scala program [36]. The crystal structures were solved using SHELXT [37] program and refined with SHELXL [38] using OLEX2 software [39]. The structures were refined by the full-matrix least-squares procedure against F2. Non-hydrogen atoms were refined anisotropically. The H(C) positions were calculated, the H(N) and H(O) atoms were located on difference Fourier maps and refined using the riding model. The details of the experiment and the crystal parameters are presented in Tables S1 and S2 (Electronic Supporting Information).
4. Conclusions
The set of the reaction products of β-aminopropioamidoximes nitrobenzenesulfochlorination depends on the structure of the initial substrates and temperature. β-Aminopropioamidoximes with six-membered heterocycles in the β-aminogroup produce good yields of 2-amino-1,5-diazaspiro[4.5]-dec-1-ene-5-ammonium nitrobenzenesulfonates at r.t. and CHCl3 b.p. An exception is the ortho-nitrobenzensulfochlorination of β-(thiomorpholin-1-yl)propioamidoxime, when the reaction is regioselective at r.t., as two products are formed: 2-amino-1,5-diazathiospiro[4.5]-dec-1-ene-5-ammonium ortho-nitrobezenesulfonate and chloride hydrate. Heating leads to a regiospecific course of the reaction with the formation of only chloride hydrate.
The para-Nitrobenzenesulfochlorination of β-(benzimidazol-1-yl)propioamidoxime produces the O-para-nitrobenzenesulfochlorination product. The reaction time when the reaction mixture is heated is reduced by 2–3 times. The in vitro screening of the library of nitrobenzenelsulfochlorination products for antidiabetic activity reveals two samples with high α-glucosidase activity exceeding the activity of the acarbose standard: products of para-nitrobenzeneulfochlorination of β-(thiomorpholin-1-yl)- and β-(benzimidazol-1-yl)propioamidoximes. The arsenal of the physicochemical and spectral methods made it possible to establish the structural features of the studied spiropyrazolinium organic salts. Thus, in DMSO-d6 solutions in the 1H NMR spectra, the slow inversion of six-membered nitrogen-containing β-heterocycles can be observed. These spiropyrazoline derivatives can be interesting objects in dynamic NMR spectroscopy, which would allow for the rotational barriers of six-membered heterocycles to be measured. It is assumed that the bulky substituted arylsulfonate anion anchors the spiroheterocycles in the most thermodynamically favorable chair-like position. According to X-ray diffraction data, the axial location of the N–N bond in the spiropyrazoline heterocycles is unambiguously determined. The NMR and XRD data demonstrate that two various conformations of spirocation are present both in solution and in solids. The cation can take part in different types of intermolecular interactions, depending on the conformation and the nature of the six-membered cycle.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27072181/s1, Figure S1: Asymmetric units of the X-rayed compounds in a representation of atoms with thermal ellipsoids (p = 50%); Table S1: Crystallographic data and the experimental details for compounds 6 and 8–10; Table S2: Crystallographic data and the experimental details for compounds 11–14; Compounds 6 and 8–14 are registered in CCDC with the numbers 2154973-2154980. Crystallographic information files are available from the Cambridge Crystallographic Data Center upon request (http://www.ccdc.cam.ac.uk/structures).
Author Contributions
Conceptualization, L.K.; methodology, G.B., E.Y. and A.K.; software, A.V., Z.S. (Zhanar Shaimerdenova); investigation, A.V., P.D., G.B., E.Y., A.K. and Z.S. (Zarina Shulgau), S.A., Z.S. (Zhanar Shaimerdenova) and K.A.; data curation, L.K.; writing—original draft preparation, L.K. and A.V.; writing—review and editing, L.K.; visualization, L.K., A.V. and E.Y.; project administration, E.Y.; funding acquisition, L.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Committee of Science of the Ministry of Education and Science of the Republic of Kazakhstan (grant AP08856440). A. Vologzhanina acknowledges the support of the Ministry of Education and Science of Russia.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
Single-crystal X-ray diffraction data were measured using the equipment of the Center for Molecular Studies of INEOS RAS (Moscow, Russia). SCXRD data for compound 10 were measured at the “Belok” beamline of the Kurchatov Synchrotron Radiation Source (NRC “Kurchatov Institute”, Moscow, Russia).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 6–14 are available from the authors.
References
- de la Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2021. An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules 2022, 27, 1075. [Google Scholar] [CrossRef] [PubMed]
- Global Diphenyl Pyrazoline Market Research Report 2021—Impact of COVID-19 on the Market. Available online: https://www.businessgrowthreports.com/TOC/19091429 (accessed on 26 March 2022).
- Lévai, A.; Simon, A.; Jenei, A.; Kálmán, G.; Jekő, J.; Tóth, G. Synthesis of spiro-1-pyrazolines by the reaction of exocyclic α,β,γ,δ-unsaturated ketones with diazomethane. Arkivoc 2009, 12, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Dadiboyena, S.; Valente, E.J.; Hamme, A.T., II. Synthesis and Tautomerism of Spiro-Pyrazolines. Tetrahedron Lett. 2014, 5514, 2208–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayukova, L.A.; Uzakova, A.B.; Vologzhanina, A.V.; Akatan, K.; Shaymardan, E.; Kabdrakhmanova, S.K. Rapid Boulton–Katritzky rearrangement of 5-aryl-3-[2-(piperidin-1-yl)ethyl]-1,2,4-oxadiazoles upon exposure to water and HCl. Chem. Heterocycl. Compd. 2018, 54, 643–649. [Google Scholar] [CrossRef]
- Kayukova, L.; Vologzhanina, A.; Praliyev, K.; Dyusembaeva, G.; Baitursynova, G.; Uzakova, A.; Bismilda, V.; Chingissova, L.; Akatan, K. Boulton-Katritzky Rearrangement of 5-Substituted Phenyl-3-[2-(morpholin-1-yl)ethyl]-1,2,4-oxadiazoles as a Synthetic Path to Spiropyrazoline Benzoates and Chloride with Antitubercular Properties. Molecules 2021, 26, 967. [Google Scholar] [CrossRef]
- Kayukova, L.A.; Praliyev, K.D.; Myrzabek, A.B.; Kainarbayeva, Z.N. Arylsulfochlorination of β-aminopropioamidoximes giving 2-aminospiropyrazolylammonium arylsulfonates. Rus. Chem. Bul. (Int. Ed.) 2020, 69, 496–503. [Google Scholar] [CrossRef]
- Kayukova, L.A.; Baitursynova, G.P.; Yergaliyeva, E.M.; Zhaksylyk, B.A.; Yelibayeva, N.S.; Kurmangaliyeva, A.B. Arylsulphonates of spiropyrazolines and O-tosilate-β-(benzimidazol-1-yl)propioamidoxime as the products of β-aminopropioamidoximestosylation. Chem. J. Kaz. 2021, 2, 22–32. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J. The tautomerism of pyrazolines (Dihydropyrazoles). J. Chil. Chem. Soc. 2015, 60, 2966–2970. [Google Scholar] [CrossRef] [Green Version]
- Dadiboyena, S. Cycloadditions and condensations as essential tools in spiropyrazoline synthesis. Eur. J. Med. Chem. 2013, 63, 347–377. [Google Scholar] [CrossRef]
- Sadiq Zubi; Naz Sadia; Hussain Akbar Erum; Aslam Umbreen. Spiropyrazolines: A Worthy Insight into the Recent Strategiesand Synthetic Applications. Lett. Org. Chem. 2019, 16, 357–391. [Google Scholar] [CrossRef]
- Liu, H.; Jia, H.; Wang, B.; Xiao, Y.; Guo, H. Synthesis of Spirobidihydropyrazole through Double 1,3-Dipolar Cycloaddition of Nitrilimines with Allenoates. Org. Lett. 2017, 19, 4714–4717. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, Â.; Gonçalves, L.M.; Santos, M.M.M. Synthesis of novel spiropyrazoline oxindoles and evaluation of cytotoxicity in cancer cell lines. Eur. J. Med. Chem. 2014, 79, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Rouatbi, F.; Mhiri, C.; Askri, M.; Knorr, M.; Rousselin, Y.; Kubicki, M.M. Regioselective Synthesis of Mono- and Dispiropyrazoline Derivatives via 1,3-dipolar Cycloaddition with Nitrilimines. J. Heterocycl. Chem. 2016, 54, 1152–1160. [Google Scholar] [CrossRef]
- Singh, V.; Singh, V.; Batra, S. Straightforward Strategy for the Stereoselective Synthesis of Spiro-Fused (C-5)Isoxazolino- or (C-3)Pyrazolino-(C-3)quinolin-2-ones from Baylis–Hillman Adducts by 1,3-Dipolar Cycloaddition and Reductive Cyclization. Eur. J. Org. Chem. 2008, 2008, 5446–5460. [Google Scholar] [CrossRef]
- Verma, D.; Mobin, S.; Namboothiri, I.N.N. Highly Selective Synthesis of Pyrazole and Spiropyrazoline Phosphonates via Base-Assisted Reaction of the BestmannOhira Reagent with Enones. J. Org. Chem. 2011, 76, 4764–4770. [Google Scholar] [CrossRef]
- Adamus-Grabicka, A.A.; Markowicz-Piasecka, M.; Cieślak, M.; Królewska-Golińska, K.; Hikisz, P.; Kusz, J.; Małecka, M.; Budzisz, E. Biological Evaluation of 3-Benzylidenechromanones and Their Spiropyrazolines-Based Analogues. Molecules 2020, 25, 1613. [Google Scholar] [CrossRef] [Green Version]
- Dandia, A.; Joshi, R.; Sehgal, V.; Sharma, C.; Saha, M. Synthesis of novel 3-spiro indulines containing benz(g) indazole, benz(h)pyrazolo(3,4-b)quinoline and naphthisoxazol moieties. Heterocycl. Commun. 1996, 2, 281–286. [Google Scholar] [CrossRef]
- Ibrahim, M.N.; El-Messmary, M.; Elarfi, M.G.A. Synthesis of Spiro Heterocyclic Compounds. E-J. Chem. 2010, 7, 55–58. [Google Scholar] [CrossRef]
- Toth, G.; Szollosy, A. Synthesis and Stereochemistry of Spiropyrazolines. J. Chem. Soc. Perkin Trans. II 1986, 12, 1895–1898. [Google Scholar] [CrossRef]
- Budzisz, E.; Paneth, P.; Geromino, I.; Muzioł, T.; Rozalski, M.; Krajewska, U.; Pipiak, P.; Ponczek, M.B.; Małecka, M.; Kupcewicz, B. The cytotoxic effect of spiroflavanone derivatives, their binding ability to human serum albumin (HSA) and a DFT study on the mechanism of their synthesis. J. Mol. Struct. 2017, 1137, 267–276. [Google Scholar] [CrossRef]
- Farghaly, T.; Abbas, I.; Hassan, W.; Lotfy, M. Study on regioselective synthesis of bioactive bis-spiropyrazolines using molecular orbital calculations. Eur. J. Chem. 2014, 5, 577–583. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; Senboku, H.; Tokuda, M. A New Synthesis of Ring-Fused Alkylidenecyclobutanes by Ring-Enlargement Reaction of Bicyclo[n.1.0]alkylidene Derivatives. Tetrahedron Lett. 2003, 44, 3329–3332. [Google Scholar] [CrossRef]
- Santos, B.S.; Gomes, C.S.B.; Pinho e Melo, T.M.V.D. Synthesis of chiral spiropyrazoline-b-lactams and spirocyclopropyl b-lactams from 6-alkylidenepenicillanates. Tetrahedron 2014, 70, 3812–3821. [Google Scholar] [CrossRef]
- Ning, Y.; Kawahata, M.; Yamaguchi, K.; Otani, Y.; Ohwada, T. Synthesis, Structure and N-N Bonding Character of 1,1-Disubstituted Indazolium Hexafluorophosphate. Chem. Commun. 2018, 54, 1881–1884. [Google Scholar] [CrossRef]
- Kayukova, L.A.; Orazbaeva, M.A.; Gapparova, G.I.; Beketov, K.M.; Espenbetov, A.A.; Faskhutdinov, M.F.; Tashkhodjaev, B.T. Rapid acid hydrolysis of 5-aryl-3-(β-thiomorpholinoethyl)-1,2,4-oxadiazoles. Chem. Heterocycl. Compd. 2010, 46, 879–886. [Google Scholar] [CrossRef]
- Kayukova, L.A.; Yergaliyeva, E.M.; Vologzhanina, A.V. Redetermination of the structure of 2-amino-8-thia-1,5-di aza spiro [4.5]dec-1-en-5-ium chloride monohydrate. Acta Cryst. 2022, E78, 164–168. [Google Scholar] [CrossRef]
- Yergaliyeva, E.M.; Kayukova, L.A.; Bazhykova, K.B.; Gubenko, M.A.; Langer, P. Computational studies of the products of tosylation and para-nitrobenzenesulfochlorination. J. Struct. Chem. 2021, 62, 1969–1975. [Google Scholar] [CrossRef]
- Yergaliyeva, E.M.; Kayukova, L.A.; Gubenko, M.A.; Baitursynova, G.P.; Uzakova, A.B. Free energies of 2-amino-1,5-diazaspiro[4.5]dec-1-en-5-ium chlorides monohydrates and arylsulfonates formation at β-aminopropioamidoximes arylsulfochlorination. Chem. J. Kaz. 2022, 75. submitted. [Google Scholar]
- Akinyede, K.A.; Oyewusi, H.A.; Hughes, G.D.; Ekpo, O.E.; Oguntibeju, O.O. In Vitro Evaluation of the Anti-Diabetic Potential of Aqueous Acetone Helichrysum petiolare Extract (AAHPE) with Molecular Docking Relevance in Diabetes Mellitus. Molecules 2022, 27, 155. [Google Scholar] [CrossRef]
- Sbit, M.; Dupont, L.; Dideberg, O.; Goblet, M.; Dejardin, J.V. Structures de l′amino-3 phényl-1 pyrazoline-2 et de l′amino-3 (m-trifluorométhylphényl)-1 pyrazoline-2. Acta Cryst. 1988, C44, 909–912. [Google Scholar] [CrossRef]
- Claramunt, R.M.; Cozzini, P.; Domiano, P.; Elguero, J.; Forfar, I.; Fruchier, A. Structure of 3-amino-4,5-dihydropyrazoles in acid media: X-ray structure of 3-amino-1-phenyl-4,5-dihydropyrazol-2-ium picrate and the origin of broad signals in 1H NMR spectroscopy. J. Chem. Soc. Perkin Trans. 2 1995, 1875–1881. [Google Scholar] [CrossRef]
- Alabugin, I.V.; Kuhn, L.; Krivoshchapov, N.V.; Mehaffy, P.; Medvedev, M.G. Anomeric effect, hyperconjugation and electrostatics: Lessons from complexity in a classic stereoelectronic phenomenon. Chem. Soc. Rev. 2021, 50, 10212–10252. [Google Scholar] [CrossRef] [PubMed]
- Motherwell, W.D.S.; Shields, G.P.; Allen, F.H. Automated assignment of graph-set descriptors for crystallographically symmetric molecules. Acta. Cryst. 2000, B56, 466–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battye, T.G.G.; Kontogiannis, L.; Johnson, O.; Powell, H.R.; Leslie, A.G.W. iMOSFLM: A new graphical interface for diffraction-image processing with MOSFLM. Acta Cryst. 2011, D67, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Evans, P. Scaling and assessment of data quality. Acta Cryst. 2006, 62, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).