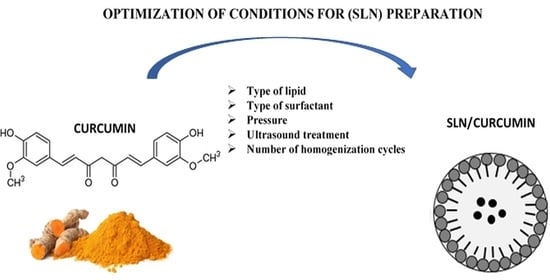
Optimization of the Conditions of Solid Lipid Nanoparticles (SLN) Synthesis
Abstract
:1. Introduction
2. Result and Discussion
2.1. Selection of Lipids
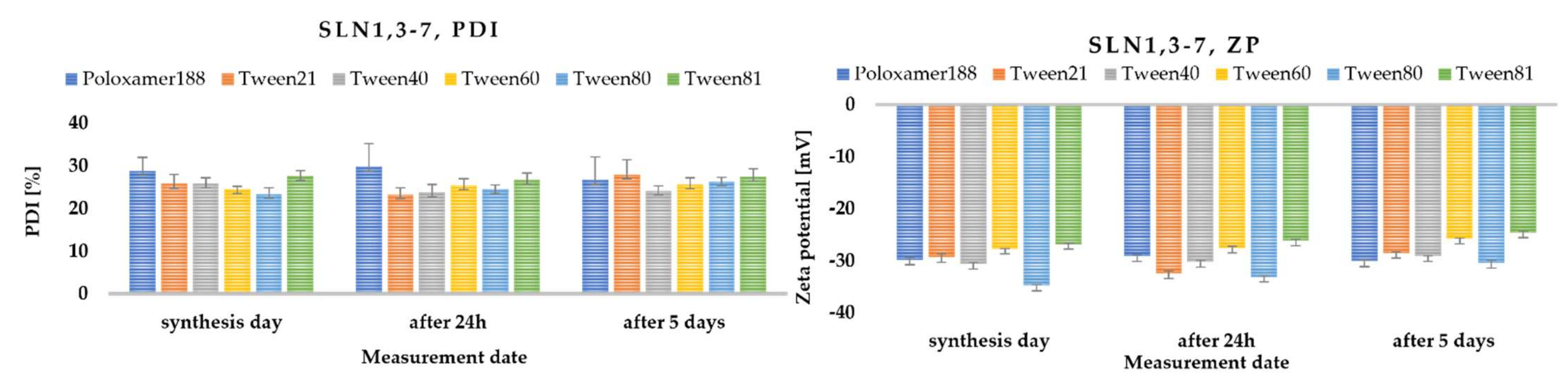
2.2. Surfactant Selection
2.3. Method Selection
2.3.1. Pressure Variation
2.3.2. Sonification
2.3.3. The Effect of Sonification and High-Speed Homogenization (UT)
2.3.4. Influence of the Number of High-Pressure Homogenization Cycles
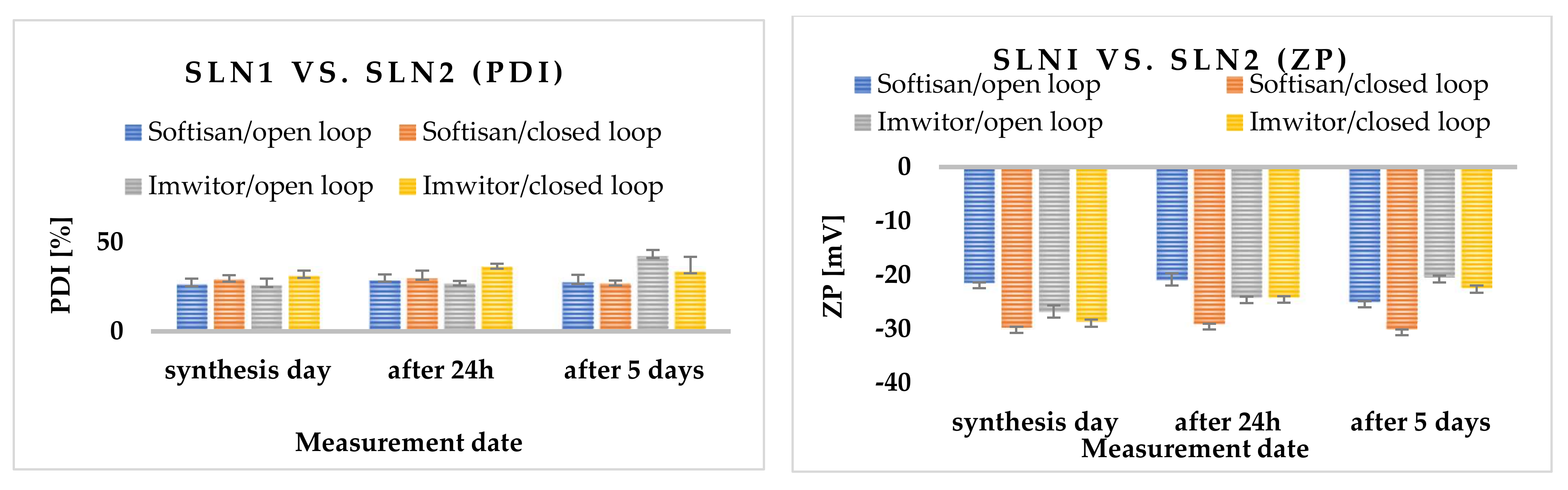
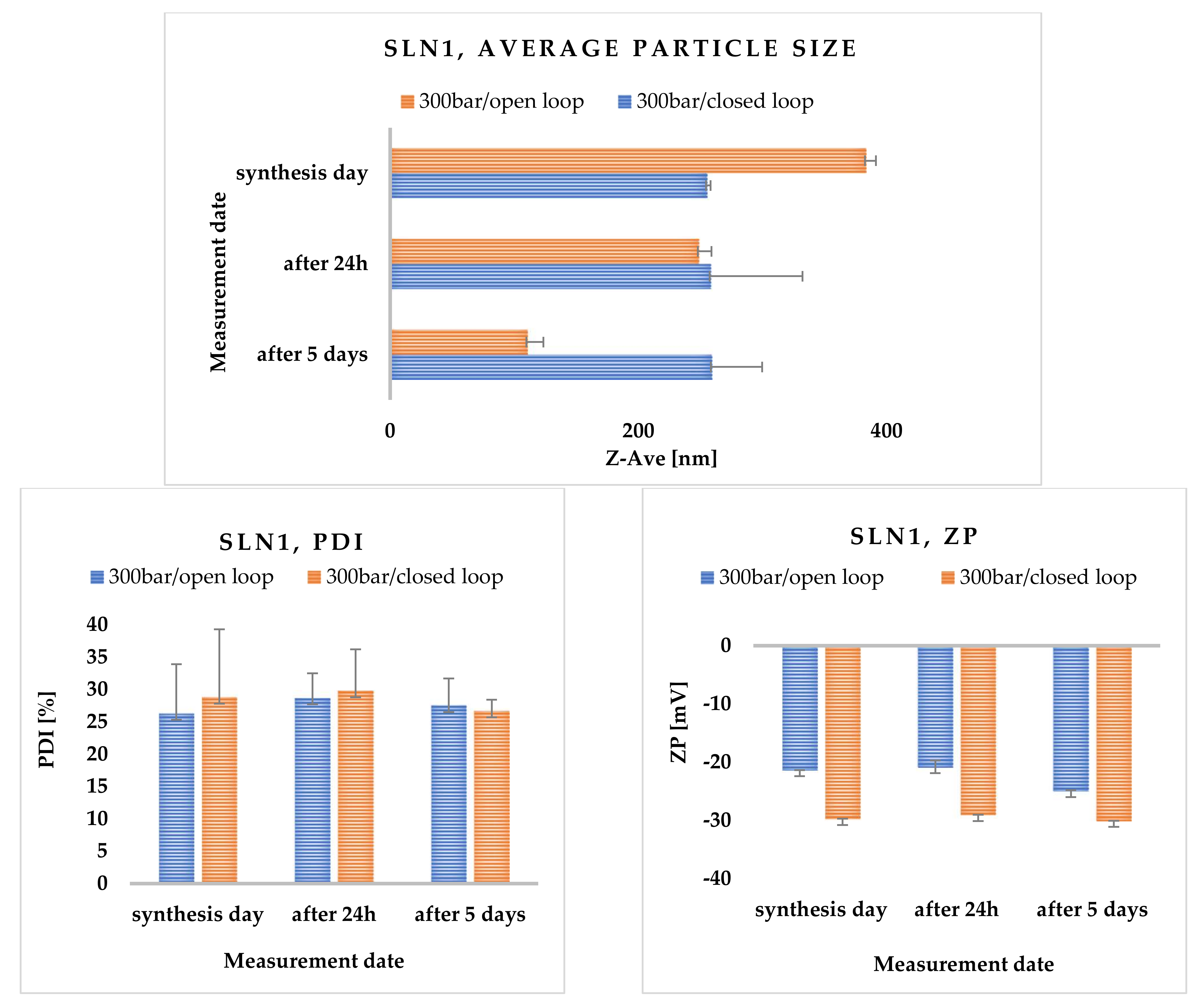
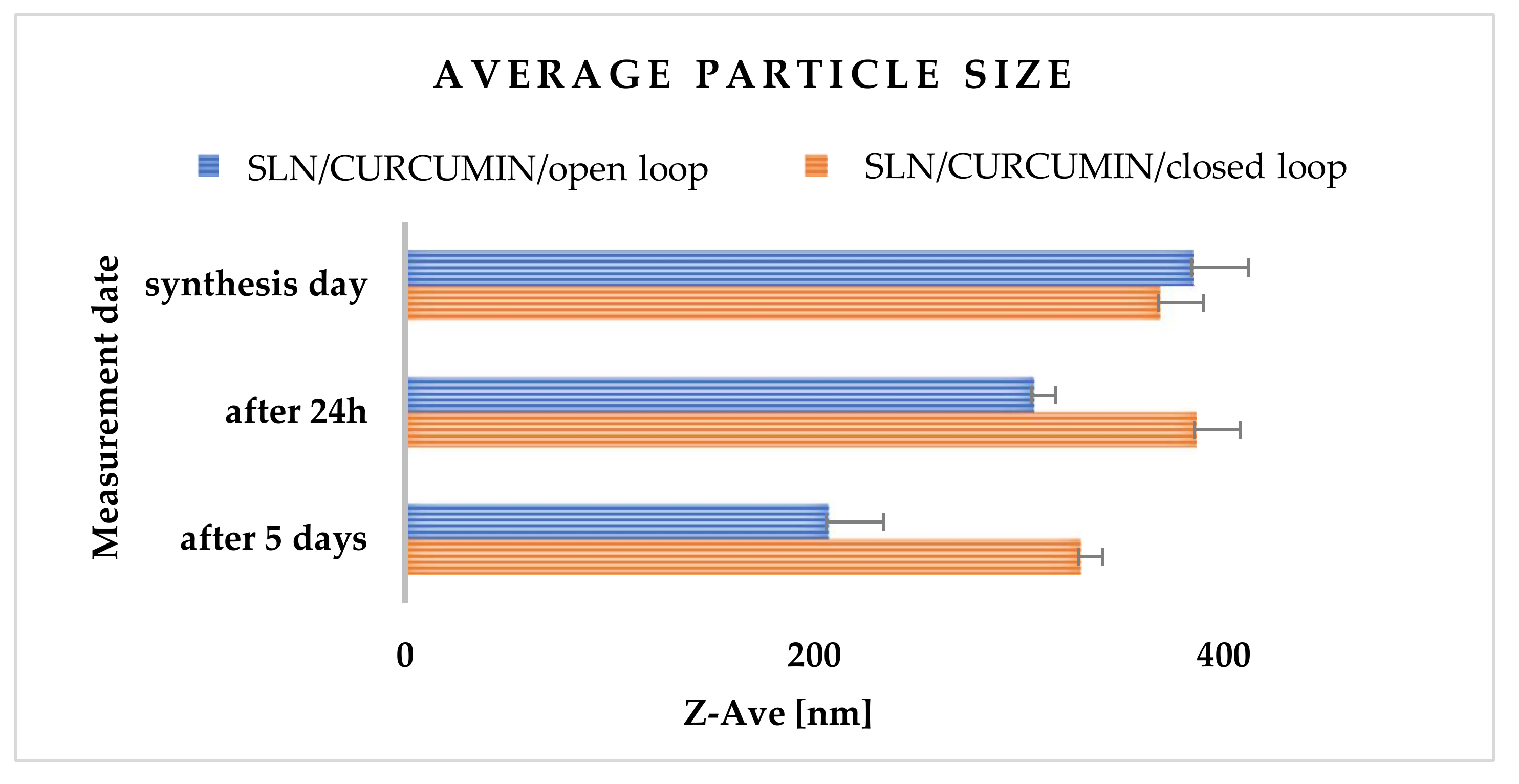
2.3.5. Effects of Open- and Closed-Loop System
2.3.6. Physicochemical Characterization of Solid Lipid Nanoparticles
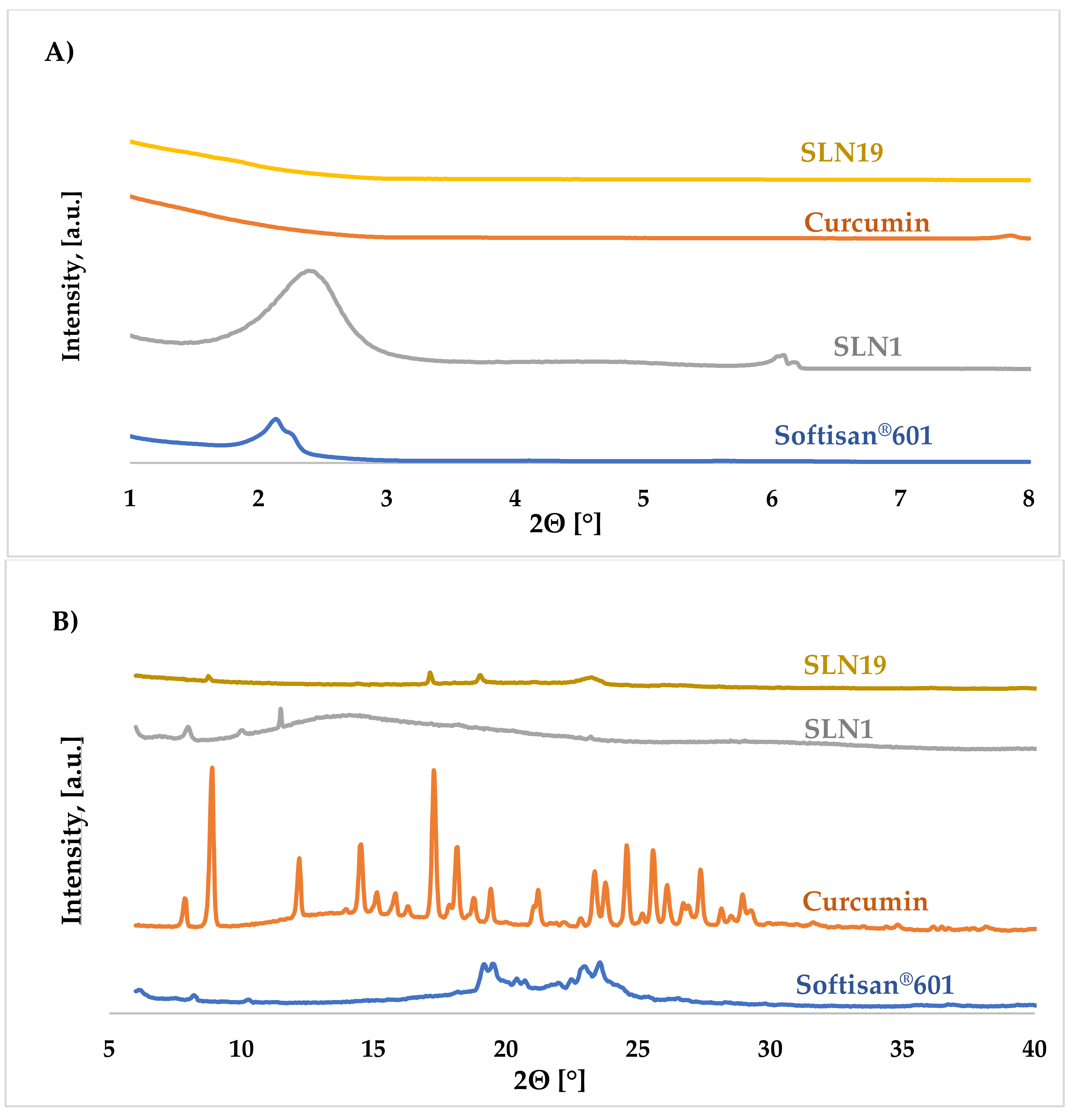
X-ray Diffraction (XRD)
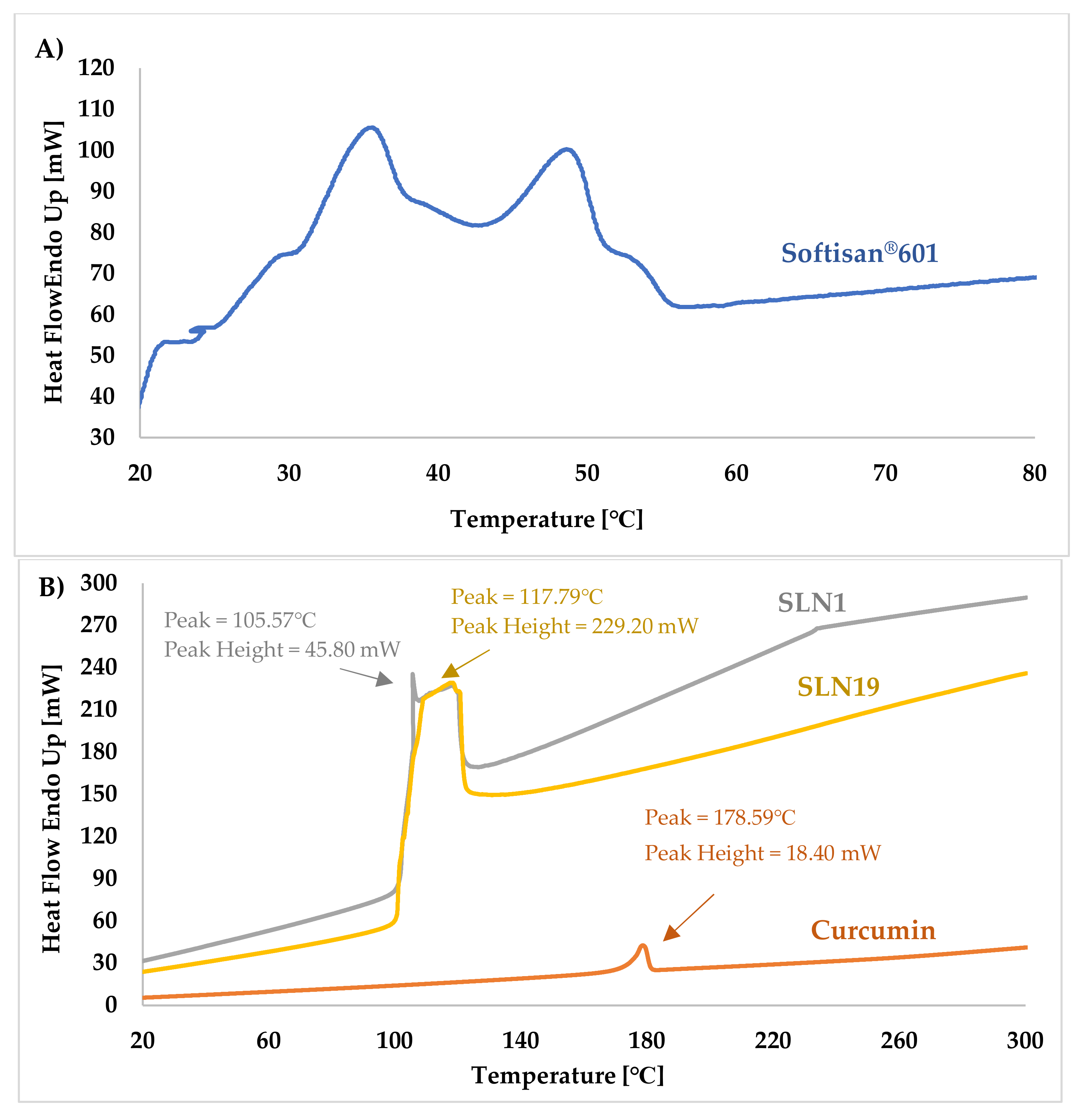
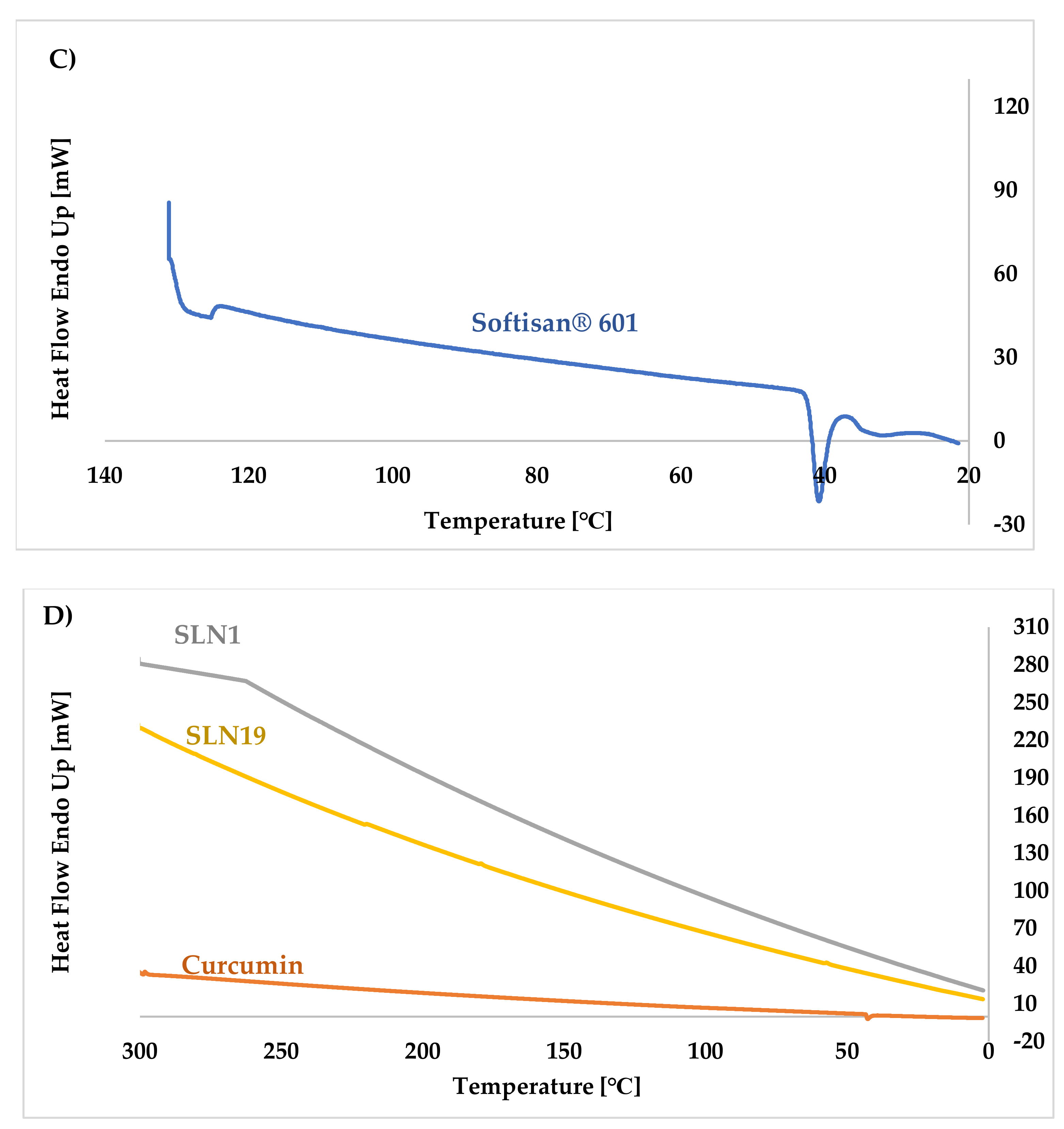
Differential Scanning Calorimetry (DSC)
Scanning Electron Microscopy (SEM)
Confocal Microscopy
2.3.7. Assessment of Encapsulation Efficiency and Loading Capacity of Curcumin
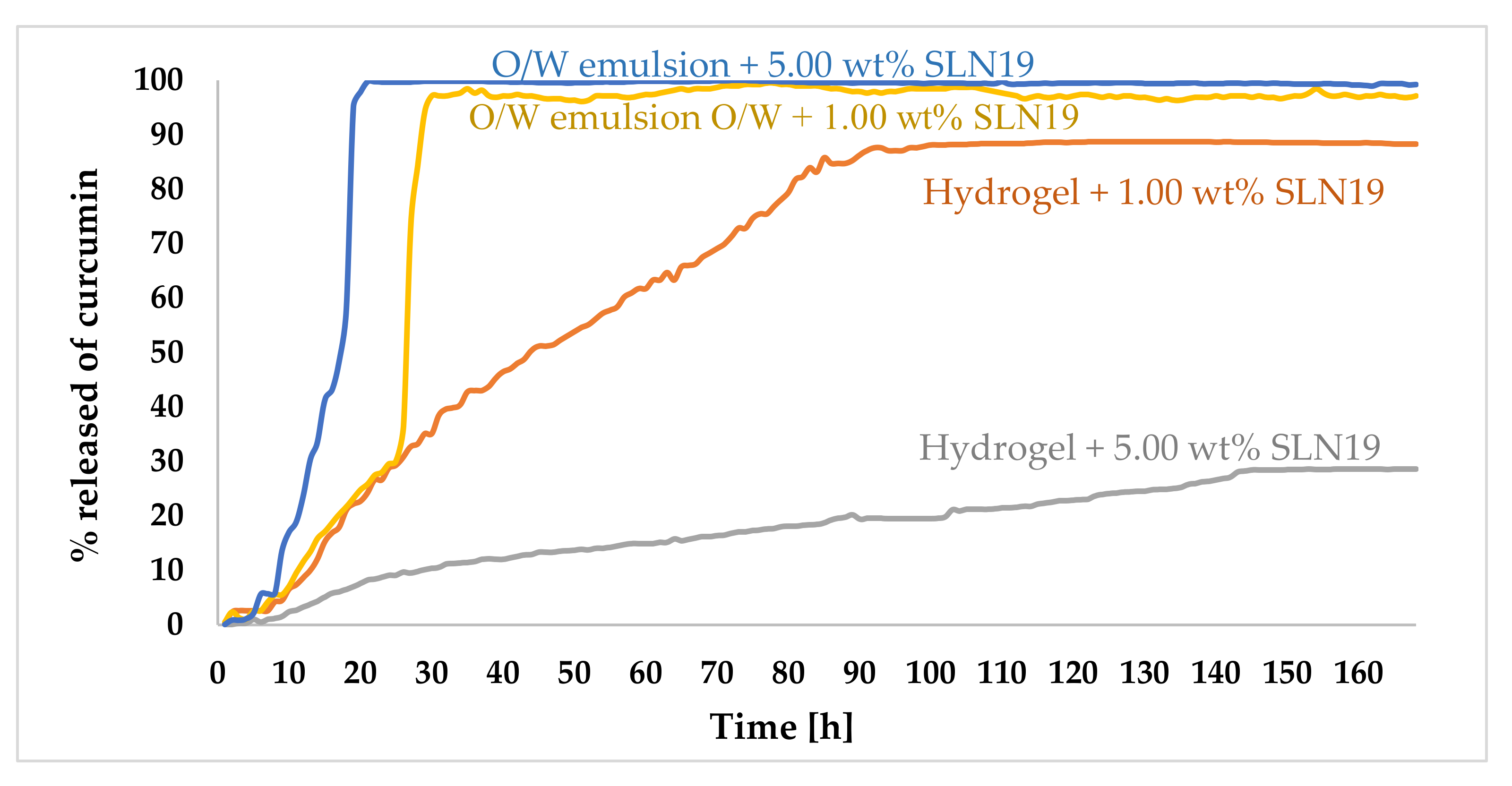
2.3.8. Study of the Release Kinetics of Curcumin
3. Materials and Methods
3.1. Materials
3.2. Methodology
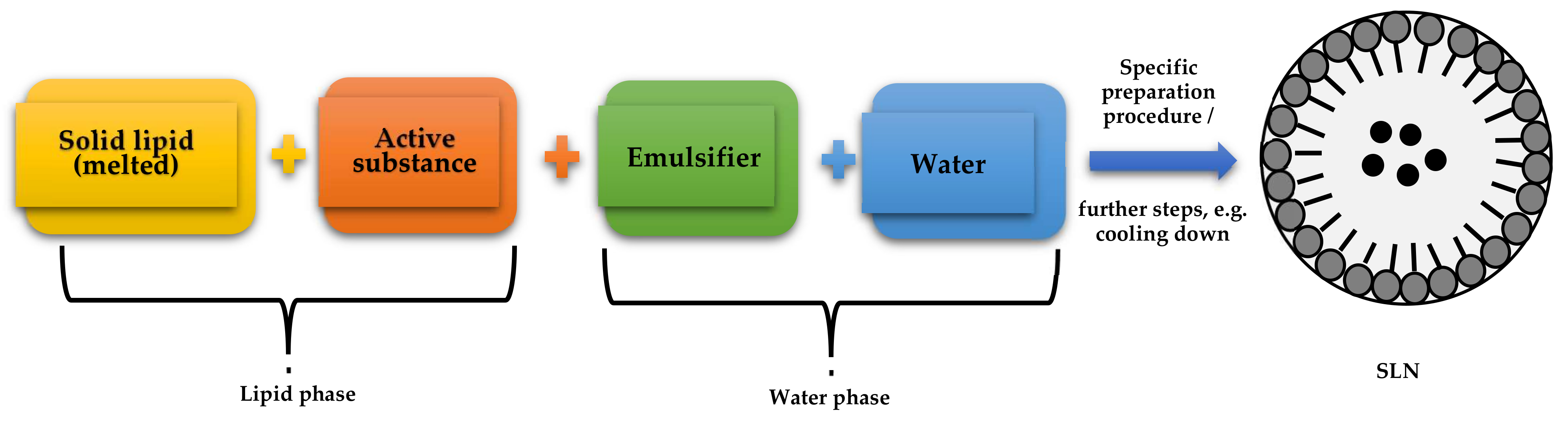
3.2.1. Optimization of SLN-Type Lipid Nanoparticle Synthesis
Lipid Screening
Surfactant Selection
Selection of Synthesis Method
Synthesis of Non-Incorporated SLNs
SLNs Incorporated with Curcumin
3.2.2. Physicochemical Characterization of Basic Parameters Describing Lipid Nanoparticles
Average Particle Size and Polydispersity Index
Zeta Potential (ZP)
X-ray Diffraction (XRD)
Differential Scanning Calorimetry (DSC)
Scanning Electron Microscopy (SEM)
Confocal Microscopy
3.2.3. Encapsulation Efficiency and Loading Capacity
3.2.4. Study of the Kinetics of Curcumin Release from Prepared Cosmetic Formulations
Preparation of Hydrogel Containing 1 wt.% SLN
Preparation of Hydrogel Containing 5 wt.% SLN
Preparation of an o/w Emulsion Containing 1 wt.% SLN
Preparation of an o/w Emulsion Containing 5 wt.% SLN
Release Kinetics of Active Substance from Above-Mentioned Cosmetic Formulations
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Weyhers, H.; Ehlers, S.; Hahn, H.; Souto, E.B.; Müller, R.H. Solid lipid nanoparticles (SLN)--effects of lipid composition on in vitro degradation and in vivo toxicity. Pharmazie 2006, 61, 539–544. [Google Scholar] [PubMed]
- Ekambaram, P.; Sathali, A.A.H.; Priyanka, K. Solid lipid nanoparticles: A review. Sci. Revs. Chem. Commun. 2012, 2, 80–102. [Google Scholar]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN. NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Shegokar, R.; Keck, C.M. 20 Years of Lipid Nanoparticles (SLN & NLC): Present State of Development & Industrial Applications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, 131–155. [Google Scholar] [CrossRef]
- Wissing, S.; Kayser, O.; Müller, R. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef]
- Saupe, A.; Wissing, S.A.; Lenk, A.; Schmidt, C.; Müller, R.H. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC)–structural investigations on two different carrier systems. Biomed. Mater. Eng. 2005, 15, 393–402. [Google Scholar]
- Teeranachaideekul, V.; Müller, R.H.; Junyaprasert, V.B. Encapsulation of ascorbyl palmitate in nanostructured lipid carriers (NLC)–effects of formulation parameters on physicochemical stability. Int. J. Pharm. 2007, 340, 198–206. [Google Scholar] [CrossRef]
- Teixeira, M.; Carbone, C.; Souto, E. Beyond liposomes: Recent advances on lipid based nanostructures for poorly soluble/poorly permeable drug delivery. Prog. Lipid Res. 2017, 68, 1–11. [Google Scholar] [CrossRef]
- Kovacevic, A.; Savic, S.; Vuleta, G.; Müller, R.; Keck, C. Polyhydroxy surfactants for the formulation of lipid nanoparticles (SLN and NLC): Effects on size, physical stability and particle matrix structure. Int. J. Pharm. 2011, 406, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Műller, R.H.; Lucks, J.S. Arzneistofftr/hger aus Festen Lipidteilchen-Feste Lipid Nanosphiiren (SLN). German Patent EP0605497B2; Application, 18 September 1991. P 41 31 562.6. [Google Scholar]
- Műller, R.H.; Mehnert, W.; Lucks, J.S.; Schwarz, C.; Zur Műehlen, A.; Weyhers, H. Solid lipid nanoparticles (SLN)–an alternative colloidal carrier system for controlled drug delivery. Eur. J. Pharm. Biopharm. 1995, 41, 62–69. [Google Scholar]
- Mukherjee, S.; Ray, S.; Thakur, R. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Üner, M. Preparation, characterization and physico-chemical properties of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): Their benefits as colloidal drug carrier systems. Pharm.-Int. J. Pharm. Sci. 2006, 61, 375–386. [Google Scholar]
- Souto, E.; Almeida, A.; Müller, R. Lipid nanoparticles (SLN®, NLC®) for cutaneous drug delivery: Structure, protection and skin effects. J. Biomed. Nanotechnol. 2007, 3, 317–331. [Google Scholar] [CrossRef]
- Lasoń, E.; Ogonowski, J. Stałe Nanocząstki Lipidowe–charakterystyka. zastosowanie i otrzymywanie. Chemk 2011, 65, 960–967. [Google Scholar]
- Wosicka, H.; Lulek, J. Solid lipid nanoparticles and nanostructured lipid carriers in novel cosmetics. Pol. J. Cosmetol. 2009, 12, 23–37. [Google Scholar]
- Doktorovová, S.; Kovačević, A.B.; Garcia, M.L.; Souto, E.B. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: Current evidence from in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2016, 108, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.M.; Zhang, X. The World Medicines Situation 2011. Traditional Medicines: Global Situation. Issues and Challenges; World Health Organization: Geneva, Switzerland, 2011; Available online: http://www.herbalsafety.utep.edu/wp-content/uploads/2016/09/WMS_ch18_wTraditionalMed-2011.pdf (accessed on 18 January 2022).
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical. Trials AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [Green Version]
- Basnet, P.; Skalko-Basnet, N. Curcumin: An Anti-Inflammatory Molecule from a Curry Spice on the Path to Cancer Treatmen. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef] [Green Version]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagahama, K.; Utsumi, T.; Kumano, T.; Maekawa, S.; Oyama, N.; Kawakami, J. Discovery of a new function of curcumin which enhances its anticancer therapeutic potency. Sci. Rep. 2016, 60, 30962. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer, A. Turmeric (curcumin) in biliary diseases. Lancet 1937, 229, 619–621. [Google Scholar] [CrossRef]
- Müller, H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Gasco, M.R. Method for Producing Solid Lipid Microspheres Having a Narrow Size Distribution. US Patent 739,440, 10 May 1993. [Google Scholar]
- Pooja, P.; Tunki, L.; Kulhari, H.; Bharathi, B.; Reddy, B.B.; Sistla, R. Optimization of solid lipid nanoparticles prepared by a single emulsification-solvent evaporation method. Data Brief. 2016, 6, 15–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, R.; Petersen, R.; Hommoss, A.; Pardeike, J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv. Drug Deliv. Rev. 2007, 59, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Santana, M.H.A.; Souto, E.B. Optimizing SLN and NLC by 22 full factorial design: Effect of homogenization technique. Mater. Sci. Eng. C 2012, 32, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Gaumet, M.; Vargas, A.; Gurny, R.; Delie, F. Nanoparticles for drug delivery: The need for precision in reporting particle size parameters. Eur. J. Pharm. Biopharm. 2008, 69, 1–9. [Google Scholar] [CrossRef]
- Jores, K.; Mehnert, W.; Drechsler, M.; Bunjes, H.; Johann, C.; Mäder, K. Investigations on the structure of solid lipid nanoparticles (SLN) and oil-loaded solid lipid nanoparticles by photon correlation spectroscopy, field-flow fractionation and transmission electron microscopy. J. Control. Release 2004, 95, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and zeta potential–What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Doktorovova, S.; Souto, E.B. Nanostructured lipid carrier-based hydrogel formulations for drug delivery: A comprehensive review. Expert Opin. Drug. Deliv. 2009, 6, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, Z.; Nowak, I. Solid lipid Nanoparticles and Nanostructured Nipid Carriers as Novel Carriers for Cosmetic Ingredients. In Nanobiomaterials in Galenic Formulations and Cosmetics; Grumezescu, A.M., Ed.; William Andrew Pub.: Amsterdam, The Netherlands, 2016; pp. 231–255. [Google Scholar]
- Wissing, S.; Müller, R. Solid lipid nanoparticles as carrier for sunscreens: In vitro release and in vivo skin penetration. J. Control. Release 2002, 81, 225–233. [Google Scholar] [CrossRef]
- Silva, A.; González-Mira, E.; García, M.; Egea, M.; Fonseca, J.; Silva, R.; Santos, D.; Souto, E.; Ferreira, D. Preparation, characterization and biocompatibility studies on risperidone-loaded solid lipid nanoparticles (SLN): High pressure homogenization versus ultrasound. Colloids Surf. B 2011, 86, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.C.; Ge, Z.Q. Influence of electrolyte and poloxamer 188 on the aggregation kinetics of solid lipid nanoparticles (SLNs). Drug Dev. Ind. Pharm. 2012, 38, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.; Müller, R.H. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLNTM) dispersions. Int. J. Pharm. 1998, 168, 221–229. [Google Scholar] [CrossRef]
- Kalaycioglu, G.D.; Aydogan, N. Preparation and investigation of solid lipid nanoparticles for drug delivery. Colloids Surf. A 2016, 510, 77–86. [Google Scholar] [CrossRef]
- Lerche, D. Dispersion stability and particle characterization by sedimentation kinetics in a centrifugal field. J. Disper. Sci. Technol. 2002, 23, 699–709. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Daquilano, D.A.; Sgualdino, G. Fundamental Aspects of Equilibrium and Crystallization Kinetics in Crystallization Processes in Fats and Lipid Systems, 1st ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Kumar, S.; Randhawa, J.K. High melting lipid based approach for drug delivery: Solid lipid nanoparticles. Mater. Sci. Eng. 2013, 33, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Pouton, C.W.; Porter, C.J.H. Formulation of lipid-based delivery systems for oral administration: Materials, methods and strategies. Adv. Drug Deliv. Rev. 2008, 60, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Izumikawa, S.; Yoshioka, S.; Aso, Y.; Takeda, Y. Preparation of poly (l-lactide) microspheres of different crystalline morphology and effect of crystalline morphology on drug release rate. J. Control. Release 1991, 15, 133–140. [Google Scholar] [CrossRef]
- Omwoyo, W.N.; Ogutu, B.; Oloo, F.; Swai, H.; Kalombo, L.; Melariri, P.; Mahanga, G.M.; Gathirwa, J.W. Preparation, characterization, and optimization of primaquine-loaded solid lipid nanoparticles. Int. J. Nanomed. 2014, 9, 3865. [Google Scholar]
- Mahato, R.I.; Narang, A.S. Pharmaceutical Dosage Forms and Drug Delivery: Revised and Expanded; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Allais, C.; Keller, G.; Lesieur, P.; Ollivon, M.; Artzner, F. X-ray diffraction/Calorimetry coupling. J. Therm. Anal. Calorim. 2003, 74, 723–728. [Google Scholar] [CrossRef]
- Beck, R.; Guterres, S.; Pohlmann, A. Nanocosmetics and Nanomedicines: New Approaches for Skin Care; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Bunjes, H.; Unruh, T. Characterization of lipid nanoparticles by differential scanning calorimetry, X-ray and neutron scattering. Adv. Drug Deliv. Rev. 2007, 59, 379–402. [Google Scholar] [CrossRef]
- Klančnik, G.; Medved, G.J.; Mrvar, P. Differential thermal analysis (DTA) and differential scanning calorimetry (DSC) as a method of material investigation. RMZ–M&G 2010, 57, 127–142. [Google Scholar]
- Estanqueiro, M.; Conceição, J.; Amaral, M.; Lobo, J.S. Characterization, sensorial evaluation and moisturizing efficacy of nanolipidgel formulations. Int. J. Cosmet. Sci. 2014, 36, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Słowik, G. Fundamentals of Electron Microscopy and Its Selected Applications in Carrier Cathode Characterization in Adsorbents and Catalyst: Selected Technologies in Relation to Technologies; University of Rzeszów: Rzeszów, Poland, 2012. [Google Scholar]
- Ghaffari, S.; Alihosseini, F.; Sorkhabadi, S.M.R.; Bidgoli, S.A.; Mousavi, S.E.; Haghighat, S.; Nasab, A.A.; Kianvash, N. Nanotechnology in Wound Healing; Semisolid Dosage Forms Containing Curcumin-Ampicillin Solid Lipid Nanoparticles, In Vitro, Ex Vivo and In Vivo Characteristics. Adv. Pharm. Bull 2018, 8, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Gumireddy, A.; Christman, R.; Kumari, D.; Tiwari, A.; North, E.J.; Chauhan, H. Preparation, Characterization, and In vitro Evaluation of Curcumin- and Resveratrol-Loaded Solid Lipid Nanoparticles. AAPS Pharm. Sci. Tech. 2019, 20, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Muller, R.H. Lipid nanoparticles (solid lipid nanoparticles and nanostructured lipid carriers) for cosmetic, dermal, and transdermal applications. In Nanoparticulate Drug Delivery Systems; Thassu, D., Ed.; CRC Press: New York, NY, USA, 2007; pp. 213–233. [Google Scholar]
- Kaur, I.P.; Bhandari, R.; Bhandari, S.; Kakkar, V. Potential of solid lipid nanoparticles in brain targeting. J. Control. Release 2008, 127, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, K.L.; Ré, M.I. Lipid Nanoparticles as Carriers for Cosmetic Ingredients: The First (SLN) and the Second Generation (NLC); Springer: Berlin/Heidelberg, Germany, 2011; pp. 101–122. [Google Scholar]
- Joshi, S.A.; Ramteke, K.H. Solid lipid nanoparticle: A review. IOSR J. Pharm. 2012, 2, 34–44. [Google Scholar] [CrossRef]
- Dhillon, S.; Kostrzewski, A. Clinical Pharmacokinetics; Pharmaceutical Press: London, UK, 2006. [Google Scholar]
- Tian, H.; Lu, Z.; Li, D.; Hu, J. Preparation and characterization of citral-loaded solid lipid nanoparticles. Food Chem. 2018, 248, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Ferreira, N.R.; Feliczak-Guzik, A.; Nowak, I.; Souto, E.B. Loading, release profile and accelerated stability assessment of monoterpenes-loaded solid lipid nanoparticles (SLN). Pharm. Dev. Technol. 2018, 25, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, R.; Caputo, O.; Gasco, M.R. Solid lipospheres of doxorubicin and idarubicin. Int. J. Pharm. 1993, 89, 9–12. [Google Scholar] [CrossRef]
- Fangueiro, J.F.; Andreani, T.; Fernandes, L.; Garcia, M.L.; Egea, M.A.; Silva, A.M.; Souto, E.B. Physicochemical characterization of epigallocatechin gallate lipid nanoparticles (EGCG-LNs) for ocular instillation. Colloids Surf. B Biointerfaces 2014, 123, 452–460. [Google Scholar] [CrossRef] [PubMed]


| Lipid + Curcumin (1:100) | Miscibility of Curcumin with Lipid * | ||||
|---|---|---|---|---|---|
| 15 min | 30 min | 1 h | 24 h | 72 h | |
| Compritol® 888 ATO | − | − | − | − | − |
| Imwitor® 900 K | √ | √ | √ | √ | √ |
| Precirol® ATO 5 | − | − | − | − | − |
| Softisan® 601 | √ | √ | √ | √ | √ |
| Sample Name | Pressure [bar] | Measurement Date | Type of Loop System | Z-Ave [nm] ± SD | PDI [%] ± SD | ZP [mV] ± SD |
|---|---|---|---|---|---|---|
| SLN19 | 300 | after synthesis | open | 385.30 ± 26.70 | 25.70 ± 3.50 | |±25.10| ± 0.10 |
| after synthesis | closed | 368.90 ± 21.00 | 21.70 ± 4.30 | |±33.20| ± 0.10 | ||
| after 24 h | open | 307.30 ± 10.30 | 27.70 ± 3.70 | |±27.50| ± 0.30 | ||
| after 24 h | closed | 386.70 ± 21.60 | 24.70 ± 5.40 | |±30.60| ± 0.10 | ||
| after 5 days | open | 206.90 ± 26.70 | 26.10 ± 1.00 | |±23.20| ± 0.20 | ||
| after 5 days | closed | 330.00 ± 10.60 | 25.20 ± 0.70 | |±29.30| ± 0.20 | ||
| SLN20 | 400 | after synthesis | open | 344.40 ± 19.20 | 25.10 ± 2.70 | |±22.50| ± 0.30 |
| after synthesis | closed | 407.50 ± 40.50 | 29.10 ± 2.90 | |±27.20| ± 0.10 | ||
| after 24 h | open | 208.90 ± 6.80 | 25.40 ± 0.90 | |±22.40| ± 0.10 | ||
| after 24 h | closed | 389.60 ± 43.70 | 29.60 ± 2.60 | |±26.70| ± 0.10 | ||
| after 5 days | open | 128.80 ± 10.10 | 23.80 ± 1.10 | |±22.10| ± 0.40 | ||
| after 5 days | closed | 195.40 ± 26.40 | 25.70 ± 1.30 | |±26.30| ± 0.20 | ||
| SLN21 | 500 | after synthesis | open | 389.10 ± 15.00 | 29.70 ± 1.40 | |±23.70| ± 0.20 |
| after synthesis | closed | 515.30 ± 37.70 | 26.60 ± 2.70 | |±25.10| ± 0.10 | ||
| after 24 h | open | 220.40 ± 6.70 | 26.10 ± 1.40 | |±20.40| ± 0.30 | ||
| after 24 h | closed | 450.50 ± 24.00 | 27.90 ± 2.40 | |±24.80| ± 0.20 | ||
| after 5 days | open | 200.20 ± 14.40 | 25.20 ± 3.90 | |±22.90| ± 0.30 | ||
| after 5 days | closed | 390.90 ± 35.40 | 25.40 ± 2.10 | |±24.60| ± 0.20 |
| 20 μm | 10 μm | 2 μm |
|---|---|---|
| Curcumin | ||
 | 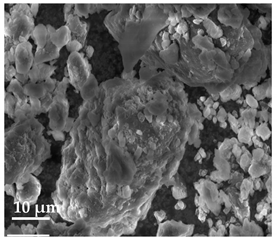 | 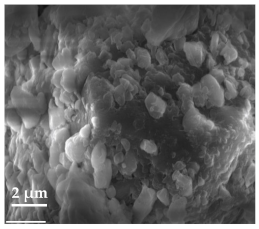 |
| SLN1 | ||
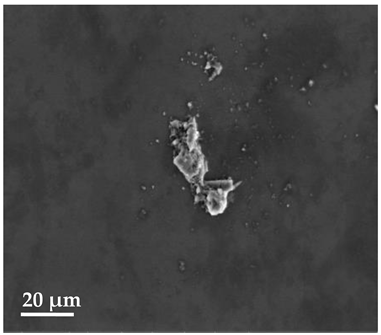 |  | 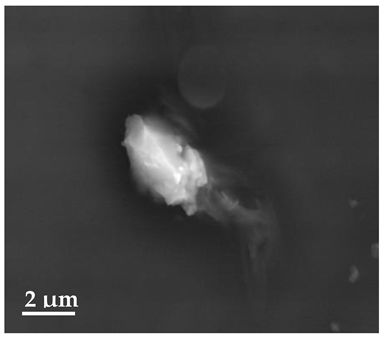 |
| SLN19 | ||
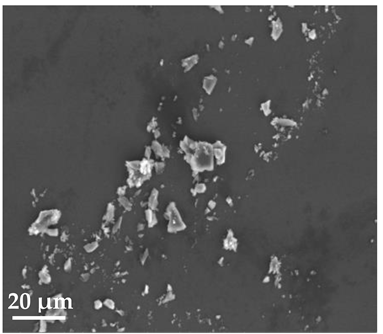 | 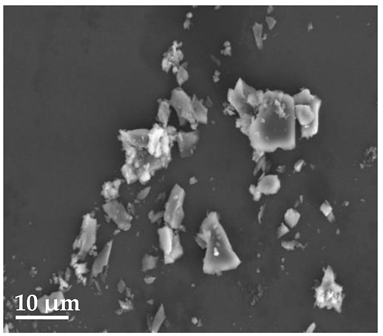 | 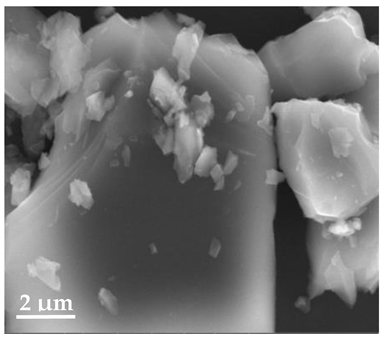 |
| Curcumin 20 μm | SLN1 20 μm |
|---|---|
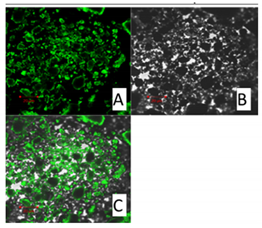 |  |
| SLN19 20 μm | |
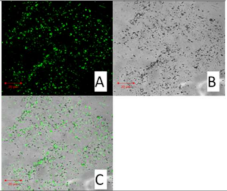 | |
| Sample | Incorporated Active Substance | EE 1 (% ± SD 2) | LC 1 (% ± SD 2) |
|---|---|---|---|
| SLN19 | Curcumin | 84.52 ± 0.62 | 12.89 ± 0.25 |
| Name | Lipid | Surfactant | Technique for Obtaining | Pressure [Bar] | Number of Cycles |
|---|---|---|---|---|---|
| Type of lipid | |||||
| SLN1 SLN2 | Softisan® 601 | Poloxamer 188 | 1 HPH + 2 UT | 300 | 3 cycles |
| Imwitor ® 900 K | |||||
| Type of surfactant | |||||
| SLN1 SLN3 SLN4 SLN5 SLN6 SLN7 | Softisan® 601 | Poloxamer 188 | 1 HPH + 2 UT | 300 | 3 cycles |
| Tween 21 | |||||
| Tween 40 | |||||
| Tween 60 | |||||
| Tween 80 | |||||
| Tween 81 | |||||
| Pressure | |||||
| SLN1 SLN8 SLN9 | Softisan® 601 | Poloxamer 188 | 1 HPH + 2 UT | 300 | 3 cycles |
| 400 | |||||
| 500 | |||||
| Effect of ultrasound treatment | |||||
| SLN10 SLN11 SLN12 | Softisan® 601 | Poloxamer 188 | 1 HPH + sonification 1 min | 300 | 3 cycles |
| 1 HPH + sonification 5 min | |||||
| 1 HPH + sonification 10 min | |||||
| Effect of Ultrasound and Ultra-Turrax | |||||
| SLN13 SLN14 SLN15 | Softisan® 601 | Poloxamer 188 | 1 HPH + sonification 10 min + 2 UT | 300 | 3 cycles |
| 400 | |||||
| 500 | |||||
| Effect of number of homogenization cycles | |||||
| SLN1 | Softisan® 601 | Poloxamer 188 | 1 HPH + 2 UT | 300 | 3 cycles |
| SLN16 | 5 cycles | ||||
| SLN17 | 6 cycles | ||||
| SLN18 | 7 cycles | ||||
| Formulation Component | Mass [g] | % wt. | |
|---|---|---|---|
| Lipid phase | |||
| Active ingredient | Curcumin | 0.50 | 1.00 |
| Lipid | Softisan® 601 | 1.00 | 2.00 |
| Water phase | |||
| Surfactant | Poloxamer 188 | 0.62 | 1.25 |
| Water | Milli-Q® Plus | 47.87 | 95.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musielak, E.; Feliczak-Guzik, A.; Nowak, I. Optimization of the Conditions of Solid Lipid Nanoparticles (SLN) Synthesis. Molecules 2022, 27, 2202. https://doi.org/10.3390/molecules27072202
Musielak E, Feliczak-Guzik A, Nowak I. Optimization of the Conditions of Solid Lipid Nanoparticles (SLN) Synthesis. Molecules. 2022; 27(7):2202. https://doi.org/10.3390/molecules27072202
Chicago/Turabian StyleMusielak, Ewelina, Agnieszka Feliczak-Guzik, and Izabela Nowak. 2022. "Optimization of the Conditions of Solid Lipid Nanoparticles (SLN) Synthesis" Molecules 27, no. 7: 2202. https://doi.org/10.3390/molecules27072202
APA StyleMusielak, E., Feliczak-Guzik, A., & Nowak, I. (2022). Optimization of the Conditions of Solid Lipid Nanoparticles (SLN) Synthesis. Molecules, 27(7), 2202. https://doi.org/10.3390/molecules27072202