Kinetic Modeling of Hydrogen Production by Dehydrogenation of Polycyclic Naphthenes with Varying Degrees of Condensation
Abstract
:1. Introduction
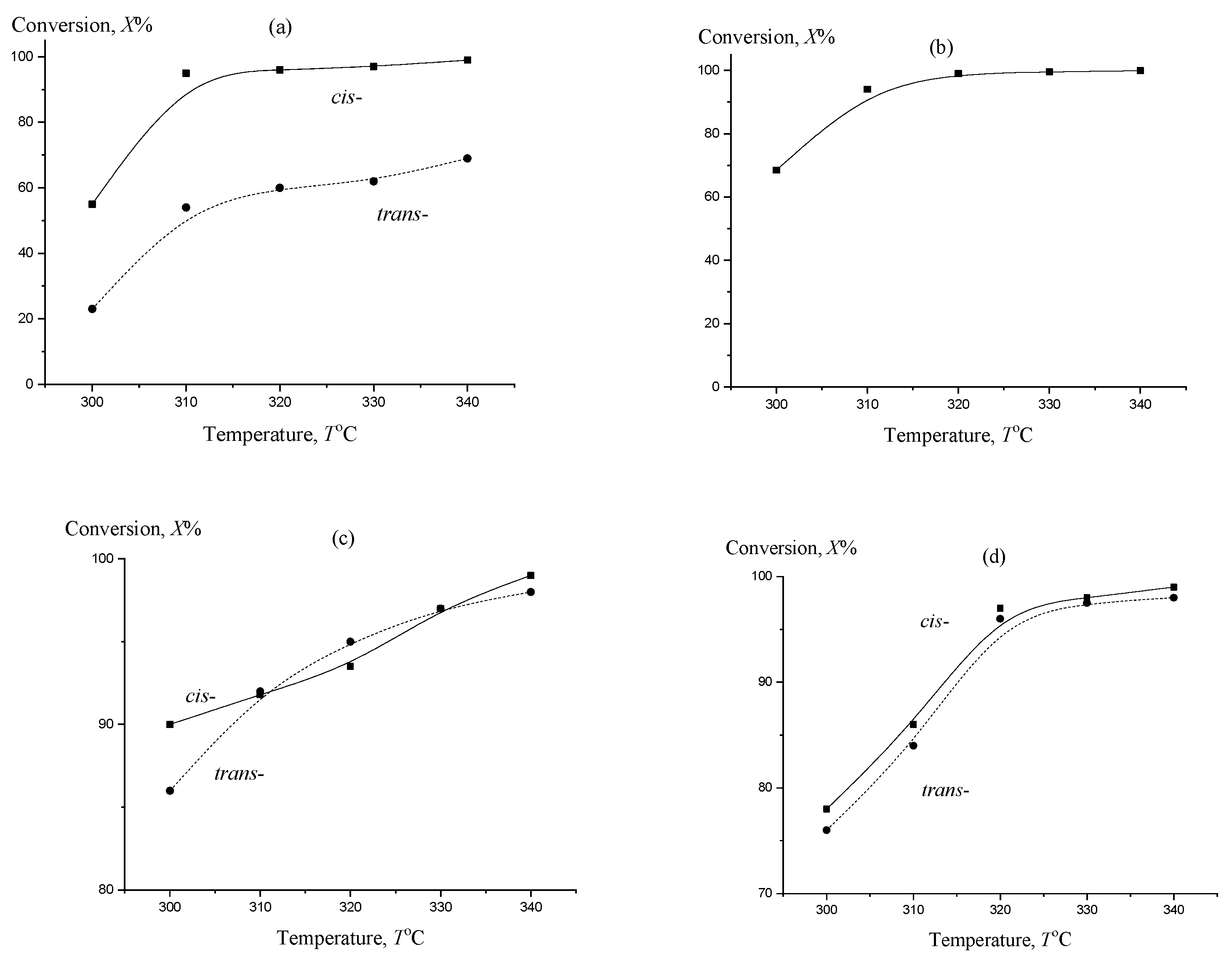
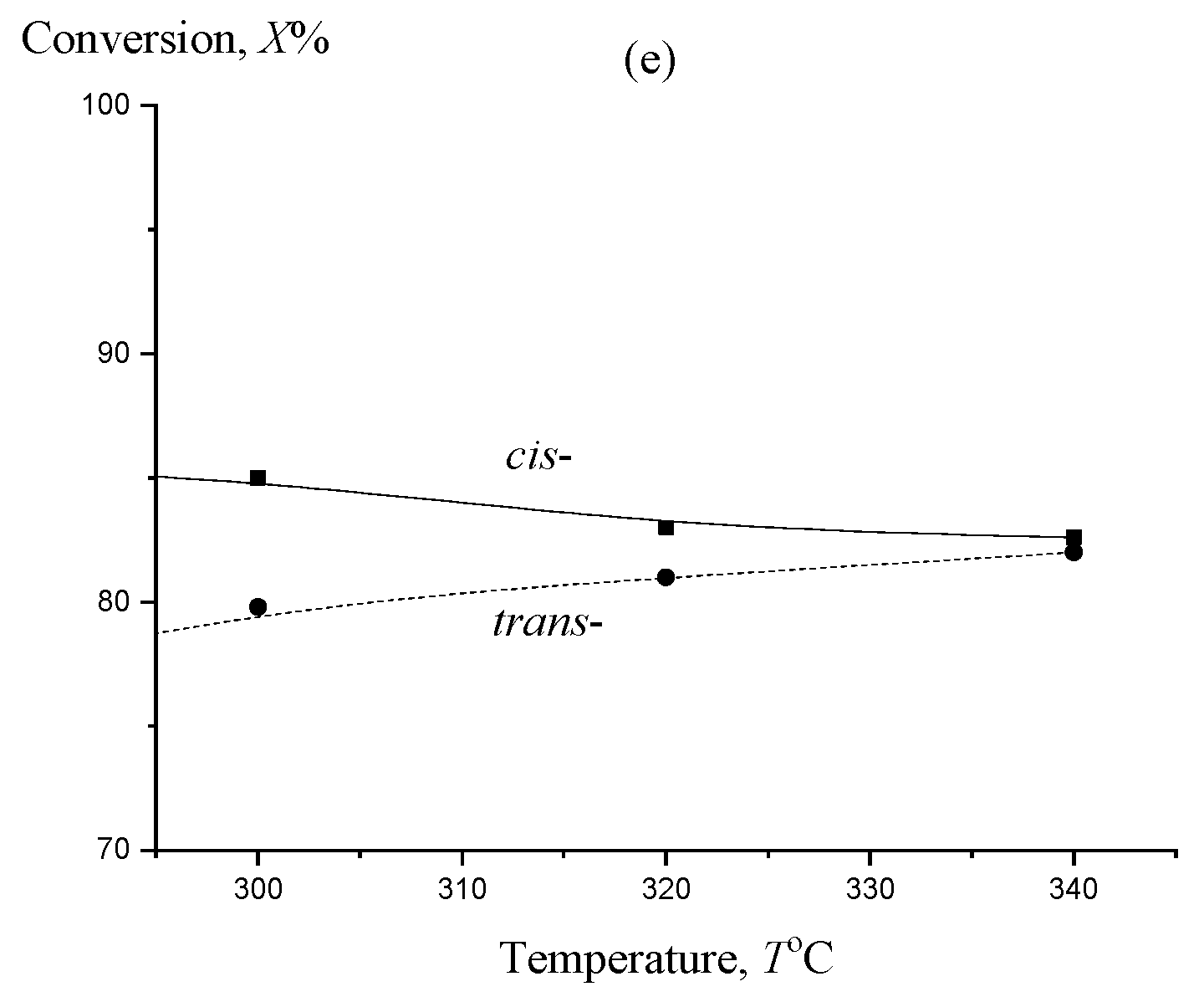
2. Results and Discussion
3. Materials and Methods
3.1. Methods of Conducting Catalytic Dehydrogenation Reactions
3.2. Chromatographic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bourane, A.; Elanany, M.; Pham, T.V.; Katikaneni, S.P. An overview of organic liquid phase hydrogen carriers. Int. J. Hydrogen Energy 2016, 41, 23075. [Google Scholar] [CrossRef]
- Biniwale, R.B.; Rayalu, S.; Devotta, S.; Ichikawa, M. Chemical hydrides: A solution to high capacity hydrogen storage and supply. Int. J. Hydrogen Energy 2008, 33, 360. [Google Scholar] [CrossRef]
- Cooper, B.H.; Donnis, B.B.L. Aromatic saturation of distillates: An overview. Appl. Catal. A Gen. 1996, 137, 203. [Google Scholar] [CrossRef]
- Stanislaus, A.; Cooper, B.H. Aromatic hydrogenation catalysis: A review. Catal. Rev. Sci. Eng. 1994, 36, 75. [Google Scholar] [CrossRef]
- Geburtig, D.; Preuster, P.; Bosmann, A.; Muller, K.; Wasserscheid, P. Chemical utilization of hydrogen from fluctuating energy sources-Catalytic transfer hydrogenation from charged liquid organic hydrogen carrier systems. Int. J. Hydrogen Energy 2016, 41, 1010. [Google Scholar] [CrossRef] [Green Version]
- Gleichweit, C.; Amende, M.; Höfert, O.; Xu, T.; Späth, F.; Brückner, N.; Wasserscheid, P.; Libuda, J.; Steinrück, H.P.; Papp, C. Surface reactions of dicyclohexylmethane on Pt (111). J. Phys. Chem. C 2015, 119, 20299. [Google Scholar] [CrossRef]
- Hodoshima, S.; Hiroshi, A.; Shigeki, T.; Yasukazu, S. Catalytic decalin dehydrogenation/naphthalene hydrogenation pair as a hydrogen source for fuel-cell vehicle. Int. J. Hydrogen Energy 2003, 28, 1255. [Google Scholar] [CrossRef]
- Kustov, L.M.; Kalenchuk, A.N.; Bogdan, V.I. Systems for accumulation, storage and release of hydrogen. Rus. Chem. Rev. 2020, 89, 897. [Google Scholar] [CrossRef]
- Kalenchuk, A.N.; Bogdan, V.I.; Dunaev, S.F.; Kustov, L.M. Influence of steric factors on reversible reactions of hydrogenation-dehydrogenation of polycyclic aromatic hydrocarbons on a Pt/C catalyst in hydrogen storage systems. Fuel 2020, 280, 118625. [Google Scholar] [CrossRef]
- Kalenchuk, A.N.; Bogdan, V.I.; Dunaev, S.F.; Kustov, L.M. Dehydrogenation of Polycyclic Naphthenes on a Pt/C Catalyst for hydrogen storage in liquid organic hydrogen carriers. Fuel Proc. Tech. 2018, 169, 94. [Google Scholar] [CrossRef]
- Parker, D.H.; Pettiette-Hall, C.L.; Li, Y.; McIver, R.T.; Hemminger, J.C. Kinetic study of the initial stages of dehydrogenation of cyclohexane on the Pt(111) surface. J. Phys. Chem. 1992, 96, 1888. [Google Scholar] [CrossRef]
- Pettiette-Hall, C.; Land, D.P.; McIver, R.T.; Hemminger, J.C. Identification of multiple steps in the dehydrogenation of cyclic C6 hydrocarbons to benzene on Pt(111). J. Am. Chem. Soc. 1991, 113, 2755. [Google Scholar] [CrossRef]
- Herz, R.K.; Gillispie, W.D.; Petersen, E.E.; Somorjai, G.A. The structure sensitivity of cyclohexane dehydrogenation and hydrogenolysis catalyzed by platinum single crystals at atmospheric pressure. J. Catal. 1981, 67, 371. [Google Scholar] [CrossRef] [Green Version]
- Kalenchuk, A.N.; Bogdan, V.I.; Dunaev, S.F.; Kustov, L.M. Effect of surface hydrophilization on Pt/Sibunite catalytic activity in bicyclohexyl dehydrogenation in hydrogen storage application. Int. J. Hydrogen Energy 2018, 43, 6191. [Google Scholar] [CrossRef]
- Kalenchuk, A.N.; Smetneva, D.N.; Bogdan, V.I.; Kustov, L.M. Kinetics of decalin dehydrogenation on Pt/C catalyst. Rus. Chem. Bull. Int. Ed. 2015, 64, 2642. [Google Scholar] [CrossRef]
- Eremin, V.V.; Kargov, S.I.; Uspenskaya, I.A.; Kuzmenko, N.E.; Lunin, V.V. The Fundamentals of Physical Chemistry. Theory and Tasks. M: Examen. 2005. Available online: https://moodle.kstu.ru>mod/book/tool/print/index.php (accessed on 20 February 2022).
- Rodgers, J.L.; Nicewander, W.A. Thirteen ways to look at the correlation coefficient. Am. Stat. 1988, 42, 59. [Google Scholar] [CrossRef]
- Weitkamp, A.W. Stereochemistry and mechanism of Hydrogenation of naphthalenes on transition metal catalysts and conformational analysis of the products. Adv. Catal. 1968, 18, 1. [Google Scholar]
- Wang, B.; Goodman, D.W.; Froment, G.F. Kinetic modeling of pure hydrogen production from decalin. J. Catal. 2008, 253, 229. [Google Scholar] [CrossRef]
- Kustov, L.M.; Kalenchuk, A.N.; Dunaev, S.F.; Bogdan, V.I. Effect of Isomerization on the performance of aromatic hydrogen storage systems with different condensation extents. Mend. Commun. 2019, 29, 25. [Google Scholar] [CrossRef]
| Hydrocarbon | Cis- | Trans- | ||
|---|---|---|---|---|
| C, % | m. p., °C | C, % | m. p., °C | |
| Decalin | 39 | 195 | 61 | 186 |
| Perhydro-o-terphenyl | 25 | 16–19 | 75 | 47 |
| Perhydro-m-terphenyl | 20 | 20–25 | 80 | 62 |
| Perhydro-p-terphenyl | 55 | 48 | 45 | 164 |
| Reaction Order | Formula for the Rate Constant |
|---|---|
| 1 | kI = 1/t × ln(Co/C) |
| 2 | kII = 1/t × (Co − C)/(Co × C) |
| T, °C | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 250 | 270 | 280 | 290 | 300 | 310 | 320 | 330 | 340 | |
| X, % | 29 | 40 | 55.8 | 62 | 81 | 82 | 97 | 98.4 | 99 |
| kI, h−1 | 0.223 | 0.511 | 0.816 | 0.968 | 1.661 | 1.714 | 3.506 | 4.135 | 4.605 |
| kII, h−1 | 0.025 | 0.007 | 0.013 | 0.016 | 0.043 | 0.046 | 0.323 | 0.615 | 0.990 |
| Substrate | T, °C | |||||
|---|---|---|---|---|---|---|
| 300 | 310 | 320 | 330 | 340 | ||
| Bicyclohexyl | kI, h−1 | 1.152 | 2.813 | 4.605 | 5.298 | 6.908 |
| kII, h−1 | 0.022 | 0.157 | 0.990 | 1.990 | 9.990 | |
| cis-Decalin | kI, h−1 | 0.800 | 3.000 | 3.219 | 3.507 | 4.605 |
| kII, h−1 | 0.012 | 0.190 | 0.240 | 0.323 | 0.990 | |
| trans-Decalin | kI, h−1 | 0.261 | 0.777 | 0.916 | 0.968 | 1.171 |
| kII, h−1 | 0.003 | 0.012 | 0.015 | 0.016 | 0.022 | |
| cis-Perhydro-para-terphenyl | kI, h−1 | 2.501 | 2.303 | 2.733 | 3.507 | 4.605 |
| kII, h−1 | 0.090 | 0.112 | 0.144 | 0.323 | 0.990 | |
| trans-Perhydro-para-terphenyl | kI, h−1 | 1.966 | 2.526 | 3.000 | 3.507 | 3.912 |
| kII, h−1 | 0.061 | 0.115 | 0.190 | 0.323 | 0.490 | |
| cis-Perhydro-meta-terphenyl | kI, h−1 | 1.514 | 1.966 | 3.507 | 3.912 | 4.605 |
| kII, h−1 | 0.035 | 0.061 | 0.323 | 0.490 | 0.990 | |
| trans-Perhydro-meta-terphenyl | kI, h−1 | 1.427 | 1.833 | 3.219 | 3.689 | 4.200 |
| kII, h−1 | 0.032 | 0.053 | 0.240 | 0.390 | 0.657 | |
| cis-Perhydro-ortho-terphenyl | kI, h−1 | 1.897 | 1.833 | 1.772 | 1.743 | 1.715 |
| kII, h−1 | 0.057 | 0.053 | 0.049 | 0.047 | 0.046 | |
| trans-Perhydro-ortho-terphenyl | kI, h−1 | 1.599 | 1.619 | 1.660 | 1.687 | 1.714 |
| kII, h−1 | 0.040 | 0.041 | 0.043 | 0.044 | 0.046 | |
| Substrate | Correlation Coefficients | ||
|---|---|---|---|
| RI | RII | RI/RII | |
| Cyclohexane | 0.956 | 0.772 | 1.24 |
| cis-Decalin | 0.924 | 0.881 | 1.05 |
| trans-Decalin | 0.929 | 0.963 | 0.97 |
| Bicyclohexyl | 0.992 | 0.822 | 1.21 |
| cis-Perhydro-para-terphenyl | 0.941 | 0.839 | 1.12 |
| trans-Perhydro-para-terphenyl | 0.979 | 0.975 | 1.02 |
| cis-Perhydro-meta-terphenyl | 0.979 | 0.948 | 1.03 |
| trans-Perhydro-meta-terphenyl | 0.978 | 0.968 | 1.01 |
| cis-Perhydro-ortho-terphenyl | −0.981 | −0.976 | 1.004 |
| trans-Perhydro-ortho-terphenyl | 0.996 | 0.996 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalenchuk, A.N.; Kustov, L.M. Kinetic Modeling of Hydrogen Production by Dehydrogenation of Polycyclic Naphthenes with Varying Degrees of Condensation. Molecules 2022, 27, 2236. https://doi.org/10.3390/molecules27072236
Kalenchuk AN, Kustov LM. Kinetic Modeling of Hydrogen Production by Dehydrogenation of Polycyclic Naphthenes with Varying Degrees of Condensation. Molecules. 2022; 27(7):2236. https://doi.org/10.3390/molecules27072236
Chicago/Turabian StyleKalenchuk, Alexander N., and Leonid M. Kustov. 2022. "Kinetic Modeling of Hydrogen Production by Dehydrogenation of Polycyclic Naphthenes with Varying Degrees of Condensation" Molecules 27, no. 7: 2236. https://doi.org/10.3390/molecules27072236





