Effect of Contact Area and Shape of Anode Current Collectors on Bacterial Community Structure in Microbial Fuel Cells
Abstract
:1. Introduction
2. Results and Discussion
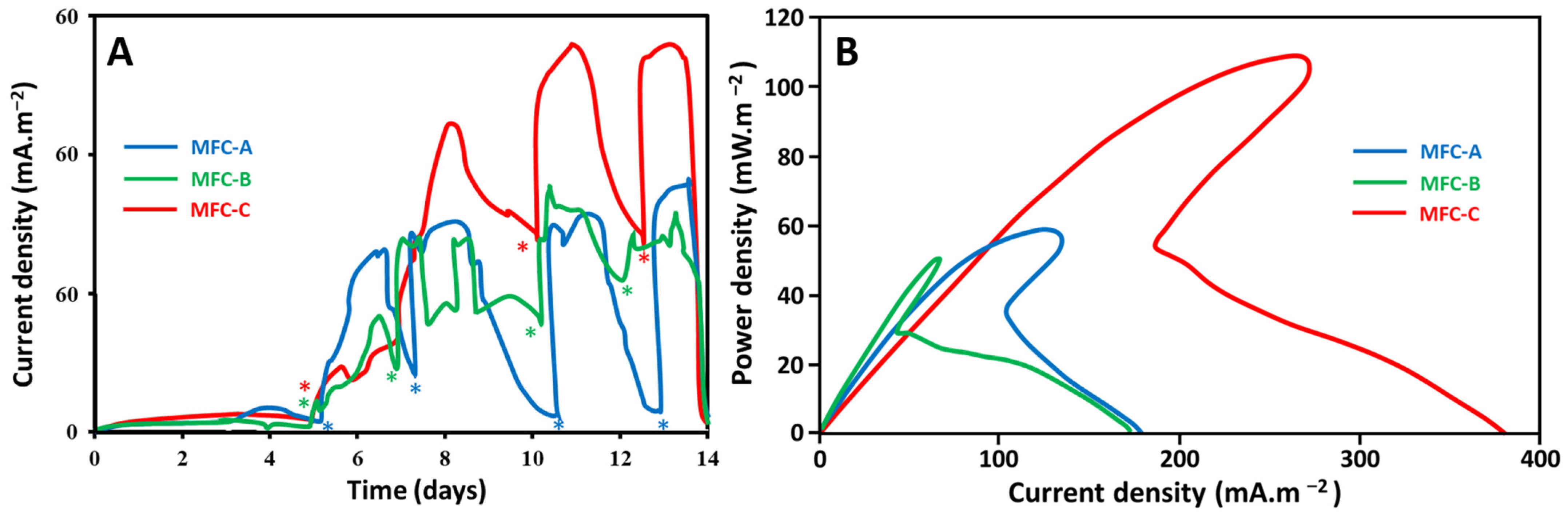
2.1. Effect of Current Collectors on Electricity Production Performance
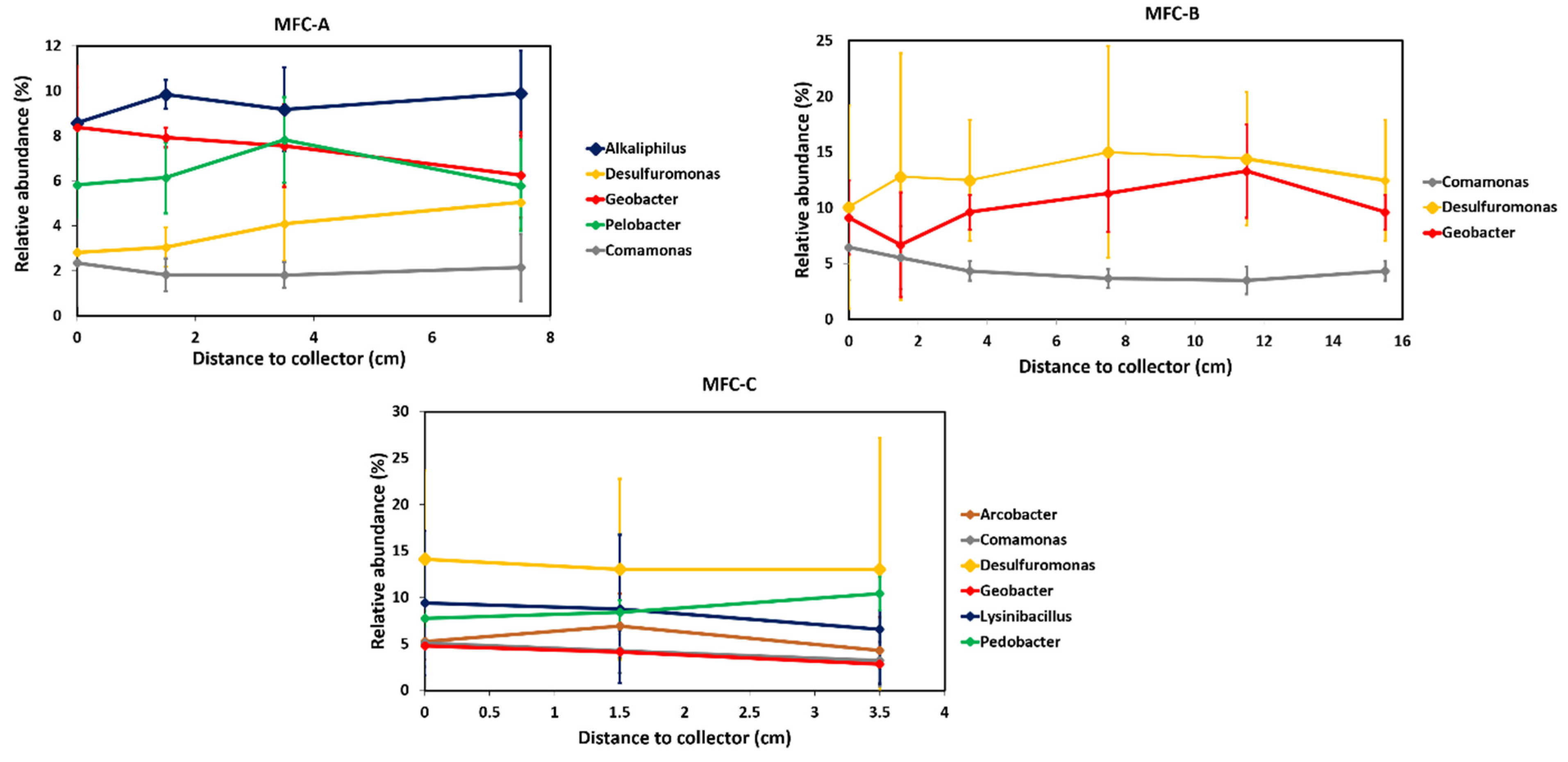
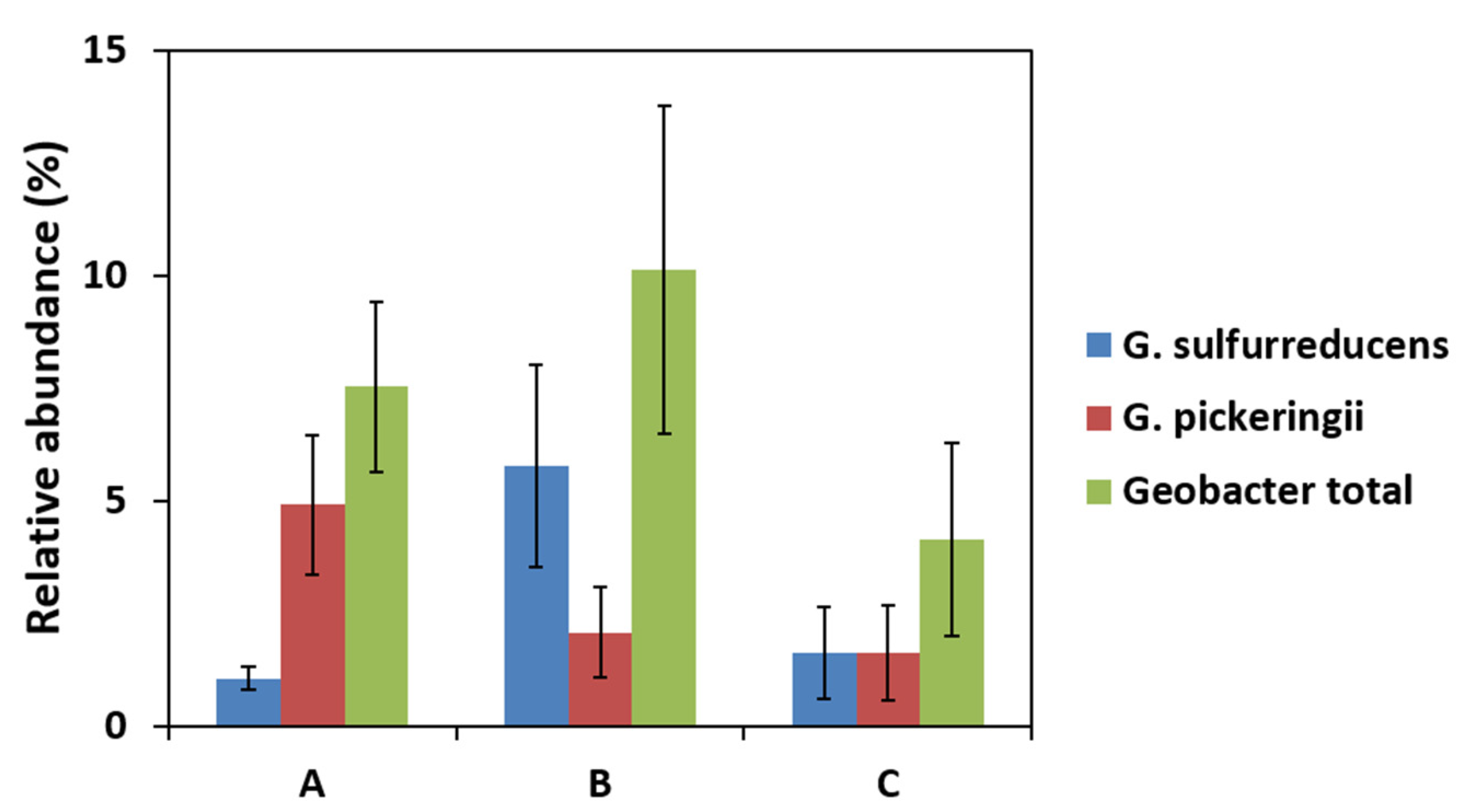
2.2. Effect of Current Collectors on Bacterial Community of Anodic Biofilms
2.3. Influence of Current Collectors on the EIS Response of Mfcs
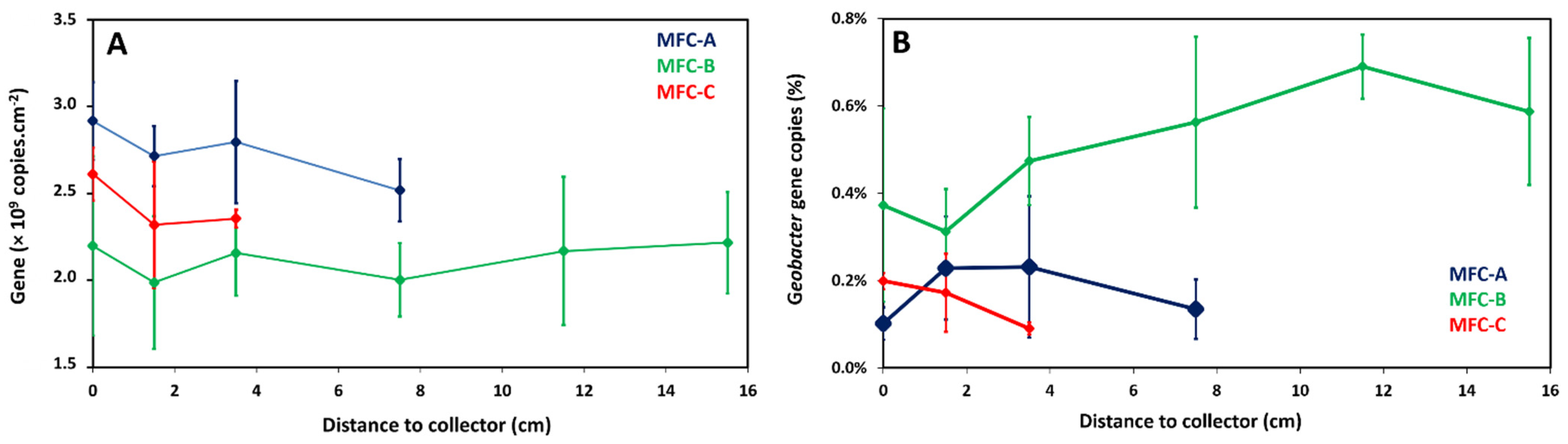
2.4. Local Potential Influences Geobacter Development
2.5. Economic and Technologie Considerations
3. Materials and Methods
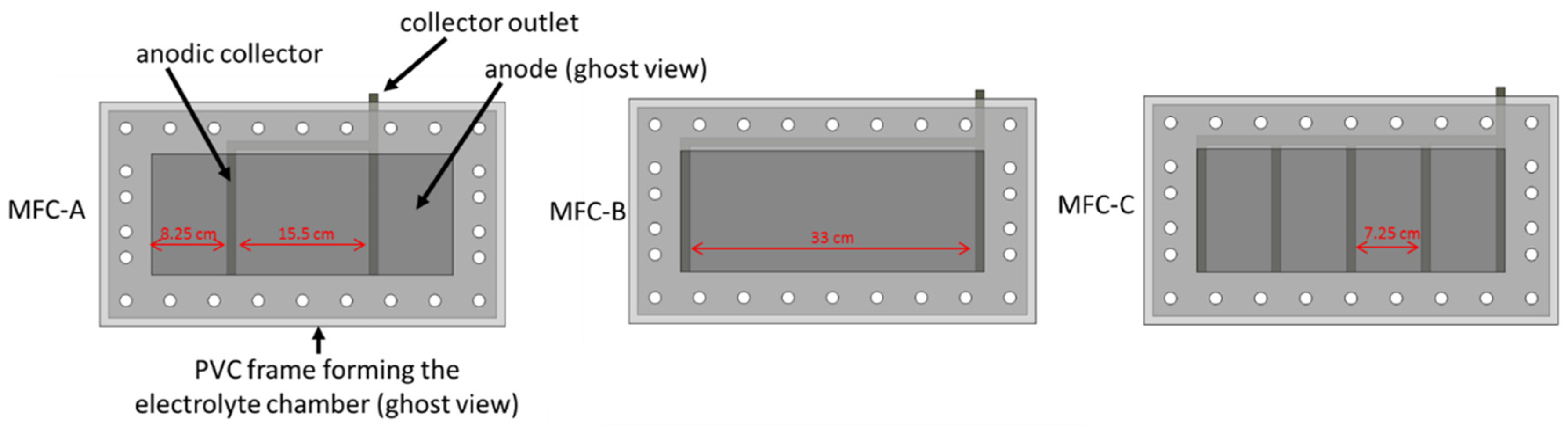
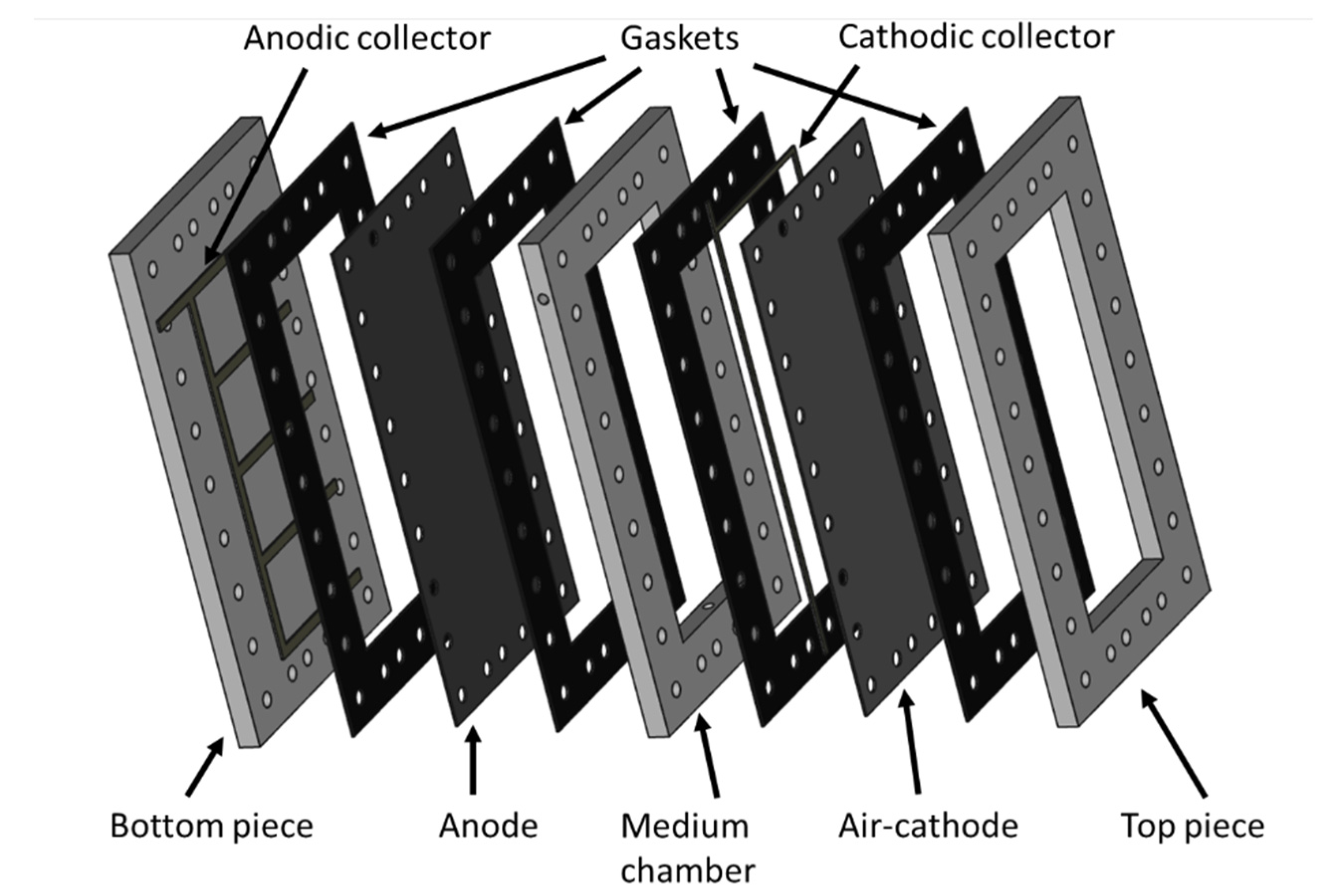
3.1. MFC Constrction and Monitoring
3.2. Electrochemical Impedance Spectroscopy (EIS)
3.3. QPCR Assay
3.4. Gene Suencing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Rahimnejad, M.; Adhami, A. Microbial Fuel Cell as New Technology for Bioelectricity Generation: A Review. Alex. Eng. J. 2015, 54, 745–756. [Google Scholar] [CrossRef] [Green Version]
- Paitier, A.; Godain, A.; Lyon, D.; Haddour, N.; Vogel, T.M.; Monier, J.M. Microbial Fuel Cell Anodic Microbial Population Dynamics during MFC Start-Up. Biosens. Bioelectron. 2017, 92, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Rabaey, K. Conversion of Wastes into Bioelectricity and Chemicals by Using Microbial Electrochemical Technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [Green Version]
- Li, W.W.; Yu, H.Q.; He, Z. Towards Sustainable Wastewater Treatment by Using Microbial Fuel Cells-Centered Technologies. Energy Environ. Sci. 2014, 7, 911–924. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, U.; Jin, W.; Pervez, A.; Bhatti, Z.A.; Tariq, M.; Shaheen, S.; Iqbal, A.; Mahmood, Q. Anaerobic Microbial Fuel Cell Treating Combined Industrial Wastewater: Correlation of Electricity Generation with Pollutants. Bioresour. Technol. 2016, 200, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Z.; Xu, Y.; Tan, X.; Yue, Z.; Ma, K.; Wang, Y. Reduced Graphene Oxide@polydopamine Decorated Carbon Cloth as an Anode for a High-Performance Microbial Fuel Cell in Congo Red/Saline Wastewater Removal. Bioelectrochemistry 2021, 137, 107675. [Google Scholar] [CrossRef] [PubMed]
- Trapero, J.R.; Horcajada, L.; Linares, J.J.; Lobato, J. Is Microbial Fuel Cell Technology Ready? An Economic Answer towards Industrial Commercialization. Appl. Energy 2017, 185, 698–707. [Google Scholar] [CrossRef]
- Feng, Y.; He, W.; Liu, J.; Wang, X.; Qu, Y.; Ren, N. A Horizontal Plug Flow and Stackable Pilot Microbial Fuel Cell for Municipal Wastewater Treatment. Bioresour. Technol. 2014, 156, 132–138. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Dong, Y.; Li, C.; Han, X.; Liu, G.; Liu, J.; Feng, Y. Field Tests of Cubic-Meter Scale Microbial Electrochemical System in a Municipal Wastewater Treatment Plant. Water Res. 2019, 155, 372–380. [Google Scholar] [CrossRef]
- Dong, Y.; He, W.; Liang, D.; Li, C.; Liu, G.; Liu, J.; Ren, N.; Feng, Y. Operation Strategy of Cubic-Meter Scale Microbial Electrochemistry System in a Municipal Wastewater Treatment Plant. J. Power Sources 2019, 441, 227124. [Google Scholar] [CrossRef]
- Liang, P.; Duan, R.; Jiang, Y.; Zhang, X.; Qiu, Y.; Huang, X. One-Year Operation of 1000-L Modularized Microbial Fuel Cell for Municipal Wastewater Treatment. Water Res. 2018, 141, 1–8. [Google Scholar] [CrossRef]
- Lepage, G.; Perrier, G.; Ramousse, J.; Merlin, G. First Steps towards a Constructal Microbial Fuel Cell. Bioresour. Technol. 2014, 162, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Morse, D.E. Miniaturizing Microbial Fuel Cells. Trends Biotechnol. 2011, 29, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Li, L.; Ceylan, C.Ü.; Haynes, A.; Cope, J.; Wilkinson, H.H.; Erbay, C.; de Figueiredo, P.; Han, A. A Microfluidic Microbial Fuel Cell Array That Supports Long-Term Multiplexed Analyses of Electricigens. Lab Chip 2012, 12, 4151–4159. [Google Scholar] [CrossRef] [PubMed]
- Dewan, A.; Beyenal, H.; Lewandowski, Z. Scaling up Microbial Fuel Cells. Environ. Sci. Technol. 2008, 42, 7643–7648. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.; Chadwick, B.; Kagan, J.; Thacher, R.; Wotawa-Bergen, A.; Richter, K. Scale up Considerations for Sediment Microbial Fuel Cells. RSC Adv. 2013, 3, 15947–15954. [Google Scholar] [CrossRef]
- Cheng, S.; Logan, B.E. Increasing Power Generation for Scaling up Single-Chamber Air Cathode Microbial Fuel Cells. Bioresour. Technol. 2011, 102, 4468–4473. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.P.; Kim, E.; Koo, B. Effects of Wire-Type and Mesh-Type Anode Current Collectors on Performance and Electrochemistry of Microbial Fuel Cells. Chemosphere 2018, 209, 542–550. [Google Scholar] [CrossRef]
- Liu, B.; Williams, I.; Li, Y.; Wang, L.; Bagtzoglou, A.; McCutcheon, J.; Li, B. Towards High Power Output of Scaled-up Benthic Microbial Fuel Cells (BMFCs) Using Multiple Electron Collectors. Biosens. Bioelectron. 2016, 79, 435–441. [Google Scholar] [CrossRef] [Green Version]
- Bensalah, F.; Julien, P.; Haddour, N.; Erouel, M.; Buret, F.; Khirouni, K. Carbon Nano-Fiber/PDMS Composite Used as Corrosion-Resistant Coating for Copper Anodes in Microbial Fuel Cells. Nanomaterials 2021, 11, 3144. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.; Mediayati, Y.; van Veelen, H.P.J.; Temmink, H.; Sleutels, T.; Hamelers, B.; Ter Heijne, A. ter The Effect of Intermittent Anode Potential Regimes on the Morphology and Extracellular Matrix Composition of Electro-Active Bacteria. Biofilm 2022, 4, 100064. [Google Scholar] [CrossRef] [PubMed]
- Dennis, P.G.; Virdis, B.; Vanwonterghem, I.; Hassan, A.; Hugenholtz, P.; Tyson, G.W.; Rabaey, K. Anode Potential Influences the Structure and Function of Anodic Electrode and Electrolyte-Associated Microbiomes. Sci. Rep. 2016, 6, 39114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, R.; Luo, C.; Zhou, S.; Wang, Y.; Yuan, Y.; Zhou, S. Anode Potential-Dependent Protection of Electroactive Biofilms against Metal Ion Shock via Regulating Extracellular Polymeric Substances. Water Res. 2020, 178, 115845. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Phung, N.T.; Chang, I.S.; Kim, B.H.; Sung, H.C. Use of Acetate for Enrichment of Electrochemically Active Microorganisms and Their 16S RDNA Analyses. FEMS Microbiol. Lett. 2003, 223, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Holmes, D.E.; Bond, D.R.; O’Neil, R.A.; Reimers, C.E.; Tender, L.R.; Lovley, D.R. Microbial Communities Associated with Electrodes Harvesting Electricity from a Variety of Aquatic Sediments. Microb. Ecol. 2004, 48, 178–190. [Google Scholar] [CrossRef]
- Fogel, G.B.; Collins, C.R.; Li, J.; Brunk, C.F. Prokaryotic Genome Size and SSU RDNA Copy Number: Estimation of Microbial Relative Abundance from a Mixed Population. Microb. Ecol. 1999, 38, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, M.; Kouzuma, A.; Watanabe, K. Effects of NaCl Concentration on Anode Microbes in Microbial Fuel Cells. AMB Express 2015, 5, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyon, D.Y.; Buret, F.; Vogel, T.M.; Monier, J.M. Is Resistance Futile? Changing External Resistance Does Not Improve Microbial Fuel Cell Performance. Bioelectrochemistry 2010, 78, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Call, D.F.; Kiely, P.D.; Wang, A.; Logan, B.E. Syntrophic Interactions Improve Power Production in Formic Acid Fed MFCs Operated with Set Anode Potentials or Fixed Resistances. Biotechnol. Bioeng. 2012, 109, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Steyn, P.L.; Segers, P.; Vancanneyt, M.; Sandra, P.; Kersters, K.; Joubert, J.J. Classification of Heparinolytic Bacteria into a New Genus, Pedobacter, Comprising Four Species: Pedobacter heparinus Comb. Nov., Pedobacter piscium Comb. Nov., Pedobacter africanus sp. Nov. and Pedobacter saltans sp. Nov. Proposal of the Family Sphingobac. Int. J. Syst. Bacteriol. 1998, 48, 165–177. [Google Scholar] [CrossRef]
- Ntougias, S.; Fasseas, C.; Zervakis, G.I. Olivibacter sitiensis Gen. Nov., sp. Nov., Isolated from Alkaline Olive-Oil Mill Wastes in the Region of Sitia, Crete. Int. J. Syst. Evol. Microbiol. 2007, 57, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.J.; Wen, W.; Wu, J.M. Simple Air Calcination Affords Commercial Carbon Cloth with High Areal Specific Capacitance for Symmetrical Supercapacitors. J. Mater. Chem. A 2018, 6, 21078–21086. [Google Scholar] [CrossRef]
- Bernabeu, P.; Caprani, A. Influence of Surface Charge on Adsorption of Fibrinogen and/or Albumin on a Rotating Disc Electrode of Platinum and Carbon. Biomaterials 1990, 11, 258–264. [Google Scholar] [CrossRef]
- Reguera, G.; Nevin, K.P.; Nicoll, J.S.; Covalla, S.F.; Woodard, T.L.; Lovley, D.R. Biofilm and Nanowire Production Leads to Increased Current in Geobacter Sulfurreducens Fuel Cells. Appl. Environ. Microbiol. 2006, 72, 7345–7348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malvankar, N.S.; Lovley, D.R. Microbial Nanowires: A New Paradigm for Biological Electron Transfer and Bioelectronics. ChemSusChem 2012, 5, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Gorby, Y.A.; Yanina, S.; McLean, J.S.; Rosso, K.M.; Moyles, D.; Dohnalkova, A.; Beveridge, T.J.; Chang, I.S.; Kim, B.H.; Kim, K.S.; et al. Electrically Conductive Bacterial Nanowires Produced by Shewanella Oneidensis Strain MR-1 and Other Microorganisms. Proc. Natl. Acad. Sci. USA 2006, 103, 11358–11363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durack, G.; Robinson, J.P. Emerging Tools for Single Cell Analysis; Robinson, J.P., Babcock, G.F., Eds.; Wiley-Liss, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Rabaey, K.; Rozendal, R.A. Microbial Electrosynthesis - Revisiting the Electrical Route for Microbial Production. Nat. Rev. Microbiol. 2010, 8, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Vandieken, V.; Mußmann, M.; Niemann, H.; Jørgensen, B.B. Desulfuromonas svalbardensis sp. Nov. and Desulfuromusa ferrireducens sp. Nov., Psychrophilic, Fe(III)-Reducing Bacteria Isolated from Arctic Sediments, Svalbard. Int. J. Syst. Evol. Microbiol. 2006, 56, 1133–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Commault, A.S.; Lear, G.; Packer, M.A.; Weld, R.J. Influence of Anode Potentials on Selection of Geobacter Strains in Microbial Electrolysis Cells. Bioresour. Technol. 2013, 139, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Ueki, T.; Zhang, T.; Malvankar, N.S.; Shrestha, P.M.; Flanagan, K.A.; Aklujkar, M.; Butler, J.E.; Giloteaux, L.; Rotaru, A.E.; et al. Geobacter. The Microbe Electric’s Physiology, Ecology, and Practical Applications, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2011; Volume 59, ISBN 9780123876614. [Google Scholar]
- Ishii, S.; Suzuki, S.; Norden-Krichmar, T.M.; Phan, T.; Wanger, G.; Nealson, K.H.; Sekiguchi, Y.; Gorby, Y.A.; Bretschger, O. Microbial Population and Functional Dynamics Associated with Surface Potential and Carbon Metabolism. ISME J. 2014, 8, 963–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.; Liu, H.; Logan, B.E. Increased Performance of Single-Chamber Microbial Fuel Cells Using an Improved Cathode Structure. Electrochem. Commun. 2006, 8, 489–494. [Google Scholar] [CrossRef]
- Lefebvre, O.; Uzabiaga, A.; Chang, I.S.; Kim, B.H.; Ng, H.Y. Microbial Fuel Cells for Energy Self-Sufficient Domestic Wastewater Treatment-a Review and Discussion from Energetic Consideration. Appl. Microbiol. Biotechnol. 2011, 89, 259–270. [Google Scholar] [CrossRef]
- Greengenes Database. Available online: http://greengenes.lbl.gov/Download/ (accessed on 22 February 2017).
- Fierer, N.; Jackson, J.A.; Vilgalys, R.; Jackson, R.B. Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl. Environ. Microbiol. 2005, 71, 4117–4120. [Google Scholar] [CrossRef] [Green Version]
- Stults, J.R.; Snoeyenbos-West, O.; Methe, B.; Lovley, D.R.; Chandler, D.P. Application of the 5′ fluorogenic exonuclease assay (TaqMan) for quantitative ribosomal DNA and rRNA analysis in sediments. Appl. Environ. Microbiol. 2001, 67, 2781–2789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- 16s Metagenomic Sequencing Library Preparation; Illumina: San Diego, CA, USA, 2013.


| A | B | C | |||
|---|---|---|---|---|---|
| Genus | Relative Abundance Average (%) | Genus | Relative Abundance Average (%) | Genus | Relative Abundance Average (%) |
| Alkaliphilus * | 9.37 | Desulfuromonas | 13.20 | Desulfuromonas | 13.46 |
| Geobacter | 7.53 | Geobacter | 10.13 | Lysinibacillus | 8.59 |
| Pelobacter *,** | 6.39 | Comamonas | 4.57 | Pedobacter | 8.53 |
| Pedobacter | 5.46 | Sedimentibacter | 4.03 | Arcobacter | 5.74 |
| Parabacteroides * | 5.02 | Alkaliphilus * | 3.21 | Comamonas | 4.38 |
| Sedimentibacter * | 4.90 | Pedobacter | 2.98 | Geobacter | 4.14 |
| Propionigenium * | 4.04 | Xenophilus | 2.68 | Sedimentibacter * | 3.42 |
| Desulfuromonas | 3.75 | Alicycliphilus | 2.58 | Alkaliphilus * | 3.26 |
| Arcobacter | 2.66 | Parabacteroides * | 2.40 | Parabacteroides * | 3.14 |
| Clostridium *,** | 2.37 | Clostridium *,** | 2.37 | Olivibacter | 2.54 |
| MFC-A | MFC-B | MFC-C | |
|---|---|---|---|
| Rohm (Ω) | 1.96 ± 0.06 | 1.62 ± 0.05 | 0.140 ± 0.005 |
| RBio (Ω) | 5.4 ± 0.2 | 0.33 ± 0.01 | 0.66 ± 0.02 |
| CPEBio (F) | (1.42 ± 0.05) × 10−3 | (4.6 ± 0.2) × 10−4 | (7.9 ± 0.3) × 10−6 |
| RCT (Ω) | 3.3 ± 0.1 | 14.3 ± 0.5 | 0.62 ± 0.01 |
| CPEDL (F) | (8.8 ± 0.3) × 10−3 | 0.071 ± 0.003 | 1.37 ± 0.4 |
| Rinternal = Rohm + RBio+ RCT (Ω) | 10.7 ± 0.4 | 16.3 ± 0.6 | 1.42 ± 0.04 |
| Electroactive surface area (m2) | 4.4 × 10−2 | 0.355 | 6.8 |
| Anode Materials | Maximum Power Density (mW·m−2) | Unite Price (€·m−2) | Price per Watt (k€·W−1) |
|---|---|---|---|
| MFC-A | 100 | 700 a | 3 |
| MFC-B | 104 | 700 a | 16.3 |
| MFC-C | 50 | 1000 a | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paitier, A.; Haddour, N.; Gondran, C.; Vogel, T.M. Effect of Contact Area and Shape of Anode Current Collectors on Bacterial Community Structure in Microbial Fuel Cells. Molecules 2022, 27, 2245. https://doi.org/10.3390/molecules27072245
Paitier A, Haddour N, Gondran C, Vogel TM. Effect of Contact Area and Shape of Anode Current Collectors on Bacterial Community Structure in Microbial Fuel Cells. Molecules. 2022; 27(7):2245. https://doi.org/10.3390/molecules27072245
Chicago/Turabian StylePaitier, Agathe, Naoufel Haddour, Chantal Gondran, and Timothy M. Vogel. 2022. "Effect of Contact Area and Shape of Anode Current Collectors on Bacterial Community Structure in Microbial Fuel Cells" Molecules 27, no. 7: 2245. https://doi.org/10.3390/molecules27072245
APA StylePaitier, A., Haddour, N., Gondran, C., & Vogel, T. M. (2022). Effect of Contact Area and Shape of Anode Current Collectors on Bacterial Community Structure in Microbial Fuel Cells. Molecules, 27(7), 2245. https://doi.org/10.3390/molecules27072245







