Impact of Chitosan on the Mechanical Stability of Soils
Abstract
:1. Introduction
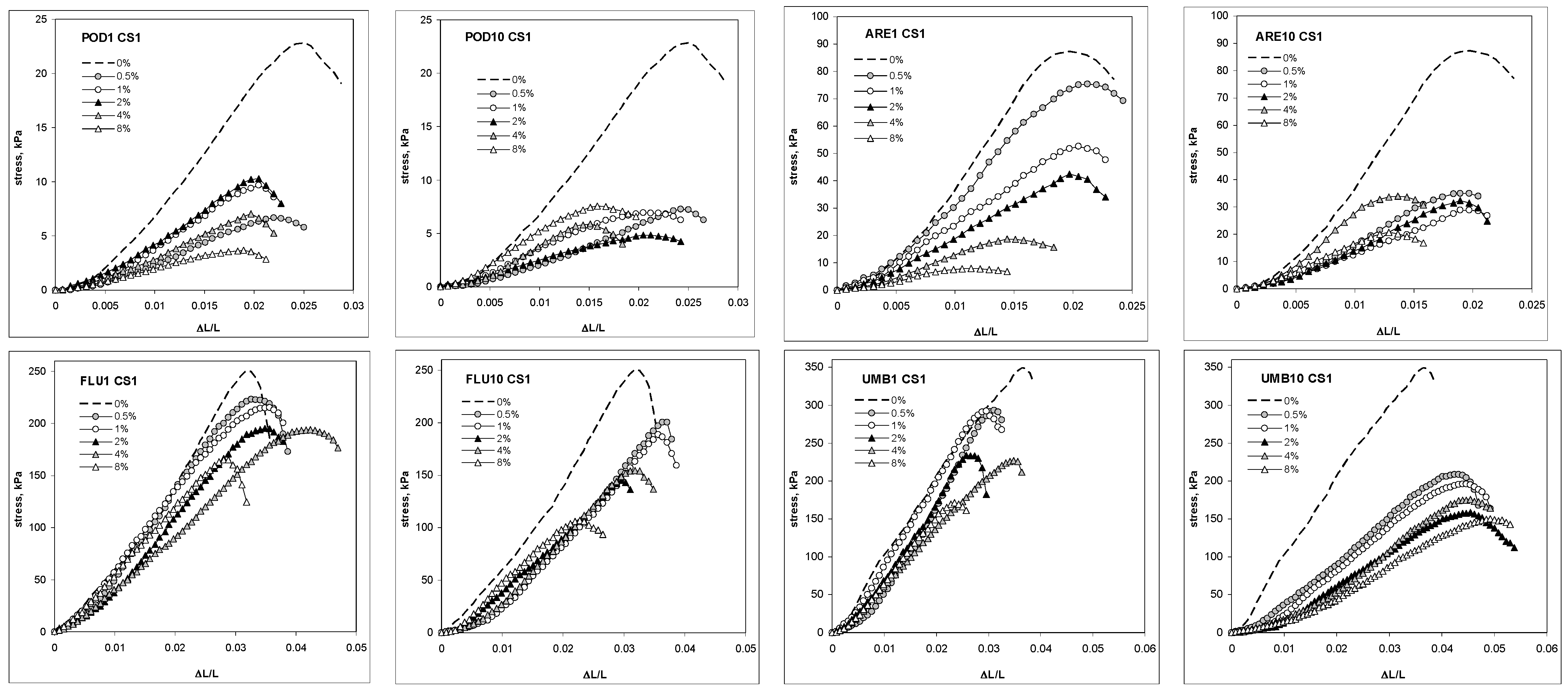
2. Results
3. Discussion
4. Materials and Methods
4.1. Preparation of Soil/Chitosan Aggregates
4.2. Studies of Soil/Chitosan Aggregates
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pal, P.; Pal, A.; Nakashima, K.; Kumar Yadav, B. Applications of chitosan in environmental remediation: A review. Chemosphere 2021, 266, 128934. [Google Scholar] [CrossRef] [PubMed]
- Majeti, N.V.; Kumar, R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Malerba, M.; Cerana, R. Chitosan effects on plant systems. Int. J. Mol. Sci. 2016, 17, 996. [Google Scholar] [CrossRef] [PubMed]
- Jabnoun-Khiareddine, H.; El-Mohamedy, R.S.R.; Abdel-Kareem, F.; Aydi Ben Abdallah, R.; Gueddes-Chahed, M.; Daami-Remadi, M. Variation in chitosan and salicylic acid efficacy towards soil-borne and air-borne fungi and their suppressive effect of tomato wilt severity. J. Plant Pathol. Microbiol. 2015, 6, 1000325. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Li, P. Antimicrobial chitosan conjugates: Current synthetic strategies and potential applications. Int. J. Mol. Sci. 2020, 21, 499. [Google Scholar] [CrossRef] [Green Version]
- Katiyar, D.; Hemantaranjan, A.; Singh, B. Chitosan as a promising natural compound to enhance potential physiological responses in plant: A review. Ind. J. Plant Physiol. 2015, 20, 1–9. [Google Scholar] [CrossRef]
- Rabêlo, V.M.; Magalhães, P.C.; Bressanin, L.A.; Carvalho, D.T.; Oliveira dos Reis, C.; Karam, D.; Doriguetto, A.C.; dos Santos, M.H.; Rodrigues dos Santos Santos Filho, P.; Corrêa de Souza, T. The foliar application of a mixture of semisynthetic chitosan derivatives induces tolerance to water defcit in maize, improving the antioxidant system and increasing photosynthesis and grain yield. Sci. Rep. 2019, 9, 8164. [Google Scholar] [CrossRef]
- Sharif, R.; Mujtaba, M.; Ur Rahman, M.; Shalmani, A.; Ahmad, H.; Anwar, T.; Tianchan, D.; Wang, X. The multifunctional role of chitosan in horticultural crops; a review. Molecules 2018, 23, 872. [Google Scholar] [CrossRef] [Green Version]
- Chouhan, D.; Mandal, P. Applications of chitosan and chitosan based metallic nanoparticles in agrosciences—A review. Int. J. Biol. Macromol. 2021, 166, 1554–1569. [Google Scholar] [CrossRef]
- Basuki, K.T.; Swantomo, D.; Sigit Sanyoto, N.T. Characterization of chitosan-acrylamide hydrogels as soil conditioner. Adv. Mat. Res. 2015, 1112, 414–417. [Google Scholar] [CrossRef]
- Iftime, M.M.; Ailieseia, G.L.; Ungureanub, E.; Marina, L. Designing chitosan based eco-friendly multifunctional soil conditioner systems with urea controlled release and water retention. Carbohydr. Polym. 2019, 223, 115040. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, J.; Yang, J.; Zheng, G.; Chen, T.; Huang, J.; Bian, J.; Meng, X. Water-soluble chitosan enhances phytoremediation efficiency of cadmium by Hylotelephium spectabile in contaminated soils. Carbohydr. Polym. 2020, 246, 116559. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Adnan Ramzani, P.M.; Rasool, B.; Khan, M.A.; Ur-Rahman, M.; Akhtar, I.; Turan, V.; Tauqeer, H.M.; Farhad, M.; Khan, S.A.; et al. Efficacy of chitosan-coated textile waste biochar applied to Cd-polluted soil for reducing Cd mobility in soil and its distribution in moringa (Moringa oleifera L.). J. Environ. Manag. 2021, 284, 112047. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.; Prasidhi, A.K.; Im, J.; Cho, G.C. Soil strengthening using thermo-gelation biopolymers. Constr. Build. Mater. 2015, 77, 430–438. [Google Scholar] [CrossRef]
- Chang, I.; Im, J.; Prasidhi, A.K.; Cho, G.C. Effects of Xanthan gum biopolymer on soil strengthening. Constr. Build. Mater. 2015, 74, 65–72. [Google Scholar] [CrossRef]
- Huang, J.; Kogbara, R.B.; Hariharan, N.; Masad, E.A.; Little, D.N. A state-of-the-art review of polymers used in soil stabilization. Constr. Build. Mater. 2021, 305, 124685. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Kumar, A.; Ranjbar-Mohammadi, M.; Ashrafizadeh, M.; Han, S.S.; Khang, G.; Roveimiab, Z. Recent advances in natural gum-based biomaterials for tissue engineering and regenerative medicine: A review. Polymers 2020, 12, 176. [Google Scholar] [CrossRef] [Green Version]
- Grzadka, E. Influence of surfactants on the adsorption and elektrokinetic properties of the system: Guar gum/manganese dioxide. Cellulose 2013, 20, 1313–1328. [Google Scholar] [CrossRef] [Green Version]
- Gooneh-Farahani, S.; Naghib, S.M.; Naimi-Jamal, M.R.; Seyfoori, A. A pH-sensitive nanocarrier based on BSA-stabilized graphene-chitosan nanocomposite for sustained and prolonged release of anticancer agents. Sci Rep. 2021, 11, 17404. [Google Scholar] [CrossRef]
- Cho, Y.W.; Jang, J.; Park, C.R.; Ko, S.W. Preparation and solubility in acid and water of partially deacetylated chitins. Biomacromolecules 2000, 1, 609–614. [Google Scholar] [CrossRef]
- Kofuji, K.; Qian, C.J.; Nishimura, M.; Sugiyama, I.; Murata, Y.; Kawashima, S. Relationship between physicochemical characteristics and functional properties of chitosan. Eur. Polym. J. 2005, 41, 2784–2791. [Google Scholar] [CrossRef]
- Park, P.J.; Je, J.Y.; Kim, S.K. Free radical scavenging activities of differently deacetylated chitosans using an ESR spectrometer. Carbohydr. Polym. 2004, 55, 17–22. [Google Scholar] [CrossRef]
- Mostafa, H.M.; Sourell, H.; Bockisch, F.J. Mechanical properties of some bioplastics under different soil types used as biodegradable drip tubes. Agric. Eng. Int. CIGR J. 2010, 12, 1–16. [Google Scholar]
- Kassymova, Z.S.; Orazzhanova, L.K.; Klivenko, A.N.; Mussabayeva, B.K.; Aserzhanov, D.K. Preparation and properties of interpolymer complexes capable of soil structuring. Russ. J. Appl. Chem. 2019, 92, 208–217. [Google Scholar] [CrossRef]
- Chang, I.; Lee, M.; Tran, A.T.P.; Lee, S.; Kwon, Y.M.; Im, J.; Cho, G.C. Review on biopolymer-based soil treatment (BPST) technology in geotechnical engineering practices. Transp. Geotech. 2020, 24, 100385. [Google Scholar] [CrossRef]
- Hataf, N.; Ghadir, P.; Ranjbar, N. Investigation of soil stabilization using chitosan biopolymer. J. Clean. Prod. 2018, 170, 1493–1500. [Google Scholar] [CrossRef]
- Orts, W.J.; Sojka, R.E.; Glenn, G.M. Biopolymer additives to reduce erosion-induced soil losses during irrigation. Ind. Crops Prod. 2000, 11, 19–29. [Google Scholar] [CrossRef]
- Renouard, S.; Hano, C.; Ouagne, P.; Blondeau, J.P.; Lainé, E. Protection of flax fiber-based yarns against natural soil degradation by chitosan. Mater. Lett. 2014, 137, 269–273. [Google Scholar] [CrossRef]
- Ramdas, V.M.; Mandree, P.; Mgangira, M.; Mukaratirwa, S.; Lalloo, R.; Ramchuran, S. Review of current and future bio-based stabilisation products (enzymatic and polymeric) for road construction materials. Transp. Geotech. 2021, 27, 100458. [Google Scholar] [CrossRef]
- Plank, J. Applications of biopolymers and other biotechnological products in building materials. Appl. Microbiol. Biotechnol. 2004, 66, 1–9. [Google Scholar] [CrossRef]
- Shariatmadari, N.; Reza, M.; Tasuji, A.; Ghadir, P.; Javadi, A.A. Experimental study on the effect of chitosan biopolymer on sandy soil stabilization. E3S Web Conf. 2020, 195, 06007. [Google Scholar] [CrossRef]
- Aguilar, R.; Nakamatsu, J.; Ramírez, E.; Elgegren, M.; Ayarza, J.; Kim, S.; Pando, M.A.; Ortega-San-Martin, L. The potential use of chitosan as a biopolymer additive for enhanced mechanical properties and water resistance of earthen construction. Constr. Build. Mater. 2016, 114, 625–637. [Google Scholar] [CrossRef]
- Szabó, S.; Szabó, G.; Bihar, Á. Effects of acid loadings on heavy metal mobilization in Cambisols. Ann. Geogr. 2007, 40, 72–79. [Google Scholar]
- Goulding, K.W. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag. 2016, 32, 390–399. [Google Scholar] [CrossRef]
- Getaneh, S.; Kidanemariam, W. Soil Acidity and Its Managements: A Review. Int. J. Adv. Res. Biol. Sci. 2021, 8, 70–79. [Google Scholar]
- Pandey, P.; Kumar Verma, M.; De, N. Chitosan in agricultural context—A review. Bull. Env. Pharmacol. 2018, 7, 87–96. [Google Scholar]
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of chitosan on plant responses with special reference to abiotic stress. Physiol. Mol. Biol. Plants 2019, 25, 313–326. [Google Scholar] [CrossRef]
- Kumararaja, P.; Manjaiah, K.M.; Datta, S.C.; Shabeer, A.T.P.; Sarkar, B. Chitosan-g-poly(acrylic acid)-bentonite composite: A potential immobilizing agent of heavy metals in soil. Cellulose 2018, 25, 3985–3999. [Google Scholar] [CrossRef] [Green Version]
- Kanmani, P.; Aravind, J.; Kamaraj, M.; Sureshbabu, P.; Karthikeyan, S. Environmental applications of chitosan and cellulosic biopolymers: A comprehensive outlook. Bioresour. Technol. 2017, 242, 295–303. [Google Scholar] [CrossRef]
- Babla, M.; Katwal, U.; Yong, M.T.; Jahandari, S.; Rahme, M.; Chen, Z.H.; Tao, Z. Value-added products as soil conditioners for sustainable agriculture. Resour. Conserv. Recycl. 2022, 178, 106079. [Google Scholar] [CrossRef]
- Soldo, A.; Miletić, M.; Auad, M.L. Biopolymers as a sustainable solution for the enhancement of soil mechanical properties. Sci. Rep. 2020, 10, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunthanid, J.; Puttipipatkhachorn, S.; Yamamoto, K.; Peck, G.E. Physical properties and molecular behavior of chitosan films. Drug Dev. Ind. Pharm. 2001, 27, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Kasaai, M.R. Calculation of Mark-Houwink-Sakurada (MHS) equation viscometric constants for chitosan in any solvent-temperature system using experimental reported viscometric constants data. Carbohydr. Polym. 2007, 68, 477–488. [Google Scholar] [CrossRef]
- Gu, B.H.; Doner, H.E. The interaction of polysaccharides with silver hill illite. Clays Clay Miner. 1992, 40, 151–156. [Google Scholar] [CrossRef]
- Qureshi, M.U.; Chang, I.; Al-Sadarani, K. Strength and durability characteristics of biopolymer-treated desert sand. Geomech. Eng. 2017, 12, 785–801. [Google Scholar] [CrossRef]
- Fatehi, H.; Ong, D.E.L.; Yu, J.; Chang, I. Biopolymers as green binders for soil improvement in geotechnical applications: A review. Geosciences 2021, 11, 291. [Google Scholar] [CrossRef]
- Chang, I.; Im, J.; Lee, S.-W.; Cho, G.-C. Strength durability of gellan gum biopolymertreated Korean sand with cyclic wetting and drying. Constr. Build. Mater. 2017, 143, 210–221. [Google Scholar] [CrossRef] [Green Version]
- Kubavat, D.; Trivedi, K.; Vaghela, P.; Prasad, K.; Vijay Anand, G.K.; Trivedi, H.; Patidar, R.; Chaudhari, J.; Andhariya, B.; Ghosh, A. Characterization of a chitosan-based sustained release nanofertilizer formulation used as a soil conditioner while simultaneously improving biomass production of Zea mays L. Land Degrad. Dev. 2020, 31, 2734–2746. [Google Scholar] [CrossRef]
- Józefaciuk, G.; Skic, K.; Adamczuk, A.; Boguta, P.; Lamorski, K. Structure and strength of artificial soils containing monomineral clay fractions. Materials 2021, 14, 4688. [Google Scholar] [CrossRef]
- Horabik, J.; Jozefaciuk, G. Structure and strength of kaolinite-soil silt aggregates: Measurements and modeling. Geoderma 2021, 382, 114687. [Google Scholar] [CrossRef]
- Neto, J.M.; Bellato, C.R.; Milagres, J.L. Preparation and evaluation of chitosan beads immobilized with Iron(III) for the removal of As(III) and As(V) from water. J. Braz. Chem. Soc. 2013, 24, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Yazdani, M.; Virolainen, E.; Conley, K.; Vahala, R. Chitosan-Zinc(II) Complexes as a Bio-Sorbent for the Adsorptive Abatement of Phosphate: Mechanism of Complexation and Assessment of Adsorption Performance. Polymers 2018, 10, 25. [Google Scholar] [CrossRef] [Green Version]
- Zavareh, S.; Behrouzi, Z.; Avanes, A. Cu (II) binded chitosan/Fe3O4 nanocomomposite as a new biosorbent for efficient and selective removal of phosphate. Int. J. Biol. Macromol. 2017, 101, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.W.A. Stabilization of Desert Sand using Water-Born Polymers; United Arab Emirates University: Abu Dhabi, United Arab Emirates, 2004; p. 437. [Google Scholar]
- Moen, D.E.; Richardson, J.L. Ultrasonic dispersion of soil aggregates stabilized by polyvinyl alcohol and T403-glyoxal polymers. Soil Sci. Soc. Am. J. 1984, 48, 628–631. [Google Scholar] [CrossRef]
- Richardson, J.L.; Gunnerson, W.T.; Giles, J.F. Influence of in situ two-phase polymers on aggregate stabilization in various Textured North Dakota Soils. Can. J. Soil Sci. 1987, 67, 209–213. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Ayeldeen, M.; Negm, A.; El Sawwaf, M.; Gadda, T. Laboratory study of using biopolymer to reduce wind erosion. Int. J. Geotech. Eng. 2016, 12, 228–240. [Google Scholar] [CrossRef]
- Adamczuk, A.; Kercheva, M.; Hristova, M.; Jozefaciuk, G. Impact of chitosan on water stability and wettability of soils. Materials 2021, 14, 7724. [Google Scholar] [CrossRef]
- Cassie, A.B.D. Contact angles. Discuss. Faraday Soc. 1948, 3, 11–16. [Google Scholar] [CrossRef]




| Abbreviation | POD | ARE | FLU | UMB | CS1 | CS2 |
|---|---|---|---|---|---|---|
| Material | Podzol | Arenosol | Fluvisol | Umbrisol | Chitosan1 | Chitosan2 |
| Locality E | 22°58′41″ | 22°26′6″ | 22°59′38″ | 21°41′59″ | ||
| Locality N | 51°9′14″ | 51°2′9″ | 51°9′43″ | 50°49′25″ | ||
| pH | 4.1 | 5.5 | 6.5 | 7.7 | ||
| Particle density [g cm−3] | 2.54 | 2.62 | 2.62 | 2.68 | 1.51 | 1.54 |
| Nitrogen [%] | 0.16 | 0.13 | 0.46 | 0.14 | 7.51 | 7.79 |
| Total organic carbon [%] | 0.65 | 1.55 | 3.04 | 0.9 | 41.59 | 41.27 |
| Sand (0.063–2 mm) [%] | 72.4 | 47.1 | 20.2 | 10.4 | ||
| Silt (0.002–0.063 mm) [%] | 25.9 | 46.2 | 52.2 | 72.4 | ||
| Clay (<0.002 mm) [%] | 1.7 | 6.7 | 27.6 | 17.2 | ||
| Grain fraction 0.177–0.105 mm [%] | 50 | 50 | ||||
| Grain fraction 0.105–0.053 mm [%] | 50 | 50 | ||||
| Degree of Deacetylation (DD) | 0.77 | 0.91 | ||||
| Average molecular mass (M), [kDa] | 699 | 280 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczuk, A.; Jozefaciuk, G. Impact of Chitosan on the Mechanical Stability of Soils. Molecules 2022, 27, 2273. https://doi.org/10.3390/molecules27072273
Adamczuk A, Jozefaciuk G. Impact of Chitosan on the Mechanical Stability of Soils. Molecules. 2022; 27(7):2273. https://doi.org/10.3390/molecules27072273
Chicago/Turabian StyleAdamczuk, Agnieszka, and Grzegorz Jozefaciuk. 2022. "Impact of Chitosan on the Mechanical Stability of Soils" Molecules 27, no. 7: 2273. https://doi.org/10.3390/molecules27072273






