Effects of Artemisia macrocephala Jacquem on Memory Deficits and Brain Oxidative Stress in Streptozotocin-Induced Diabetic Mice
Abstract
:1. Introduction
2. Results
2.1. Quantitative Spectrophotometric and Non-Spectrophotometric Phytochemical Analysis
2.2. In Vitro Anticholinesterase Activity
2.3. Acute Toxicity Study
2.4. Y-Maze Spontaneous Alteration
| Treatment/Dose (mg) | Spontaneous Alternation Performance (%) | |
|---|---|---|
| Normal control | 80.11 ± 1.65 | |
| Diabetic control | 39.06 ± 1.78 ### | |
| Crd-Am | 100 | 57.13 ± 1.59 ** |
| 200 | 62.54 ± 1.50 *** | |
| nhex-Am | 75 | 45.22 ± 1.61 ns |
| 150 | 44.78 ± 1.73 ns | |
| Chl-Am | 75 | 68.57 ± 1.65 *** |
| 150 | 73.89 ± 1.77 *** | |
| Et-Am | 75 | 59.37 ± 1.61 ** |
| 150 | 64.69 ± 1.71 *** | |
| But-Am | 75 | 47.25 ± 1.56 ns |
| 150 | 48.19 ± 1.47 * | |
| Aq-Am | 75 | 46.09 ± 1.40 ns |
| 150 | 49.67 ± 1.46 * | |
| Donepezil | 2 | 79.05 ± 1.60 *** |
| Metformin | 50 | 74.29 ± 1.96 *** |
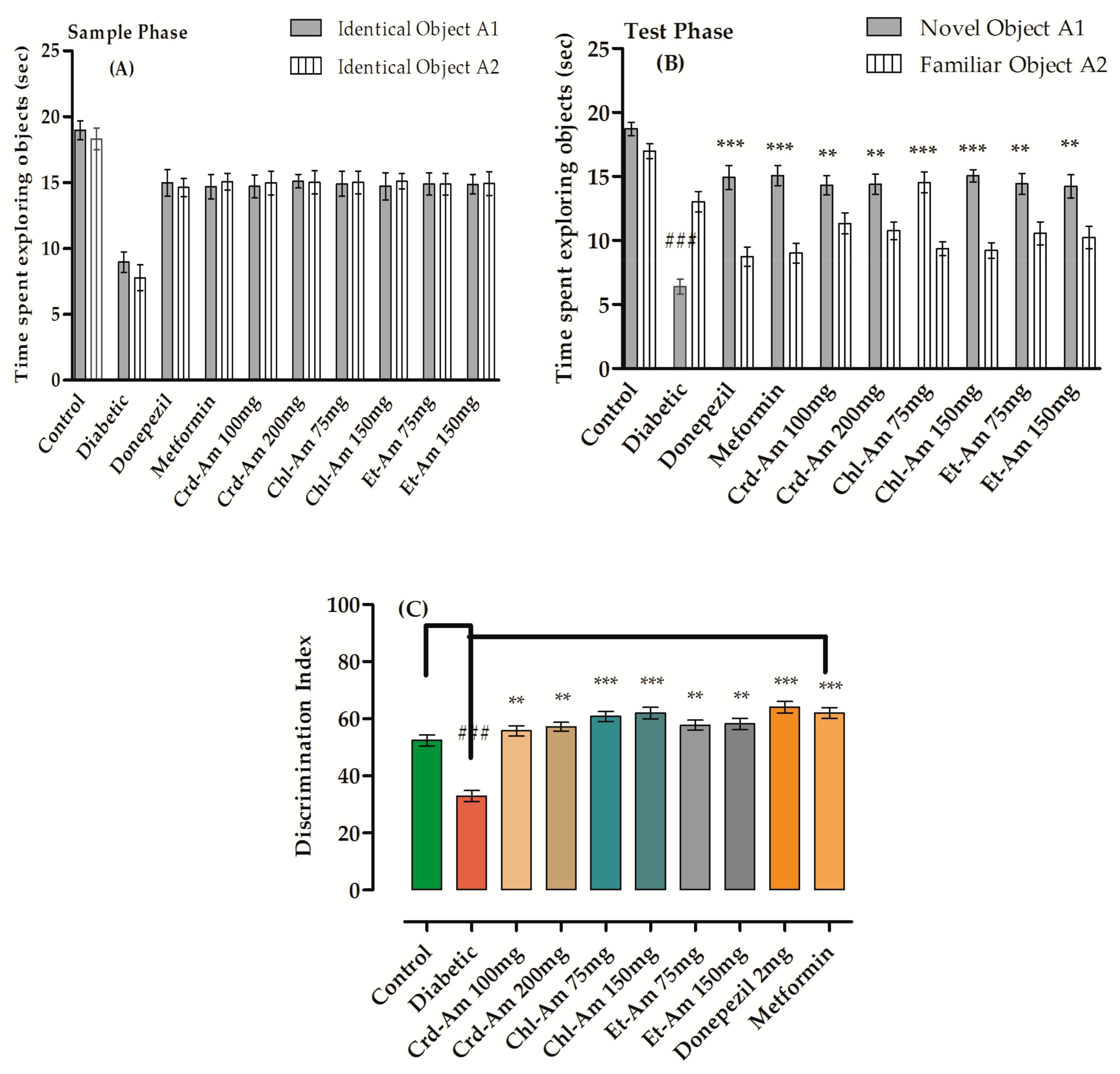
2.5. Novel Object Recognition Test


2.6. Estimation of Blood Glucose Level, Brain and Body Weight
2.7. Biochemical Parameters and Biomarker Level
| Sample Test | SOD (U/mg of Protein) | CAT (U/mg of Protein) | MDA nmol/mg Protein) | GSH (μg/mg of Protein) | |
|---|---|---|---|---|---|
| Control | 9.49 ± 1.37 | 40.18 ± 1.51 | 12.59 ± 1.06 | 56.93 ± 1.71 | |
| Diabetic | 3.18 ± 0.93 ### | 11.16 ± 1.37 ### | 38.27 ± 1.44 ### | 29.38 ± 1.66 ### | |
| Crd-Am | 100 mg | 9.01 ± 1.18 ** | 35.81 ± 1.25 ** | 19.11 ± 1.39 *** | 44.12 ± 1.33 ** |
| 200 mg | 9.17 ± 1.21 ** | 36.30 ± 1.16 ** | 18.93 ± 1.22 ** | 45.61 ± 1.41 ** | |
| Chl-Am | 75 mg | 9.39 ± 1.37 *** | 37.08 ± 1.51 *** | 17.06 ± 1.43 ** | 47.31 ± 1.49 *** |
| 150 mg | 9.66 ± 1.25 *** | 38.22 ± 1.17 *** | 15.81 ± 1.29 *** | 49.17 ± 1.51 *** | |
| Et-Am | 75 mg | 9.05 ± 1.03 ** | 35.73 ± 1.39 ** | 18.0 ± 1.21 ** | 45.98 ± 1.47 ** |
| 150 mg | 9.21 ± 1.27 *** | 36.79 ± 1.44 *** | 17.63 ± 1.11 ** | 47.33 ±1.36 *** | |
| Donepezil | 2 mg | 10.21 ± 1.41 *** | 39.08 ± 1.38 *** | 14.38 ± 1.22 *** | 51.88 ± 1.34 ** |
| Metformin | 50 mg | 10.73 ± 1.37 *** | 40.46 ± 1.33 *** | 13.89 ± 1.35 *** | 54.73 ± 1.61 ** |
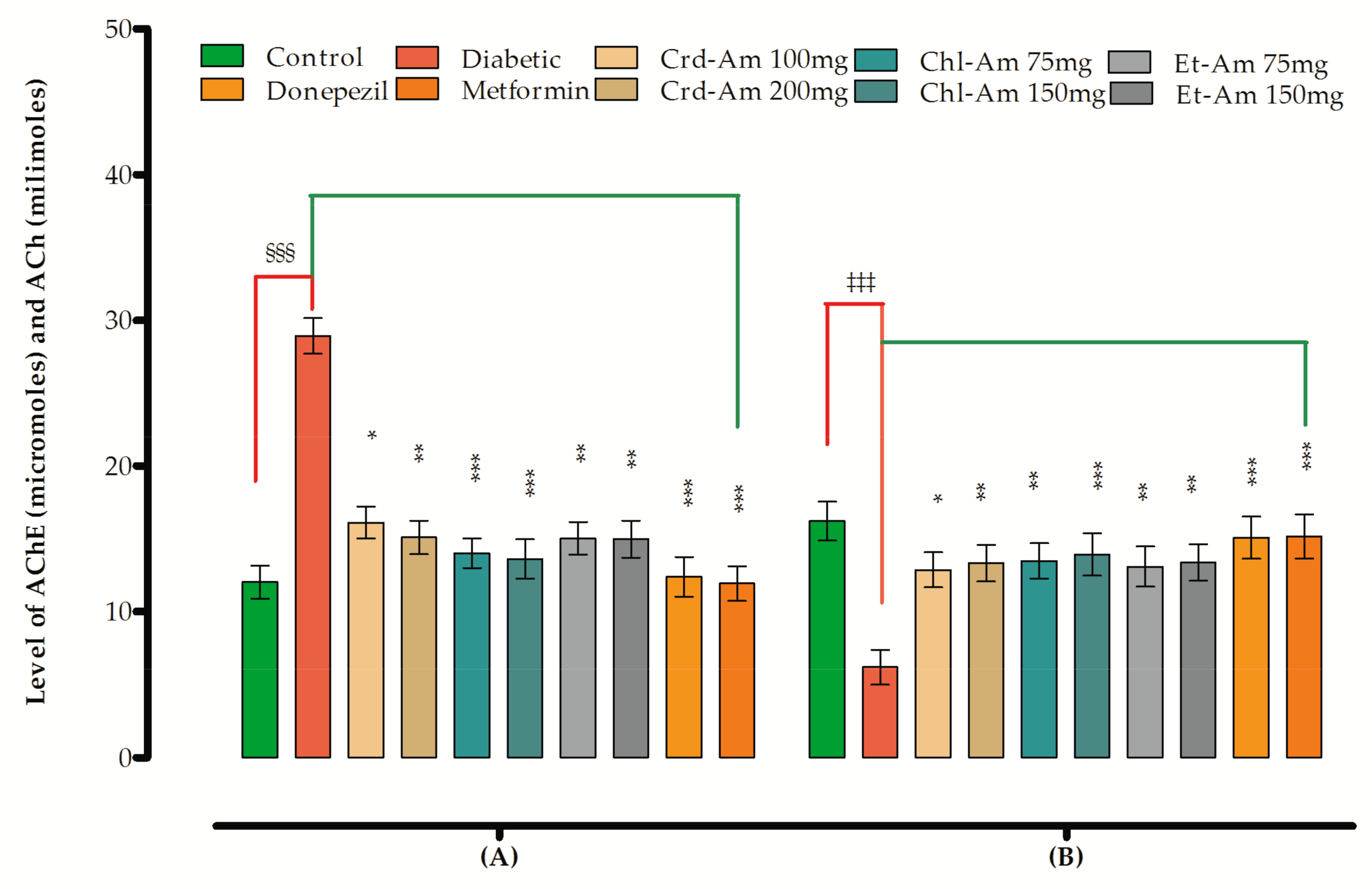
2.8. Effects on Biomarker Levels in Brain
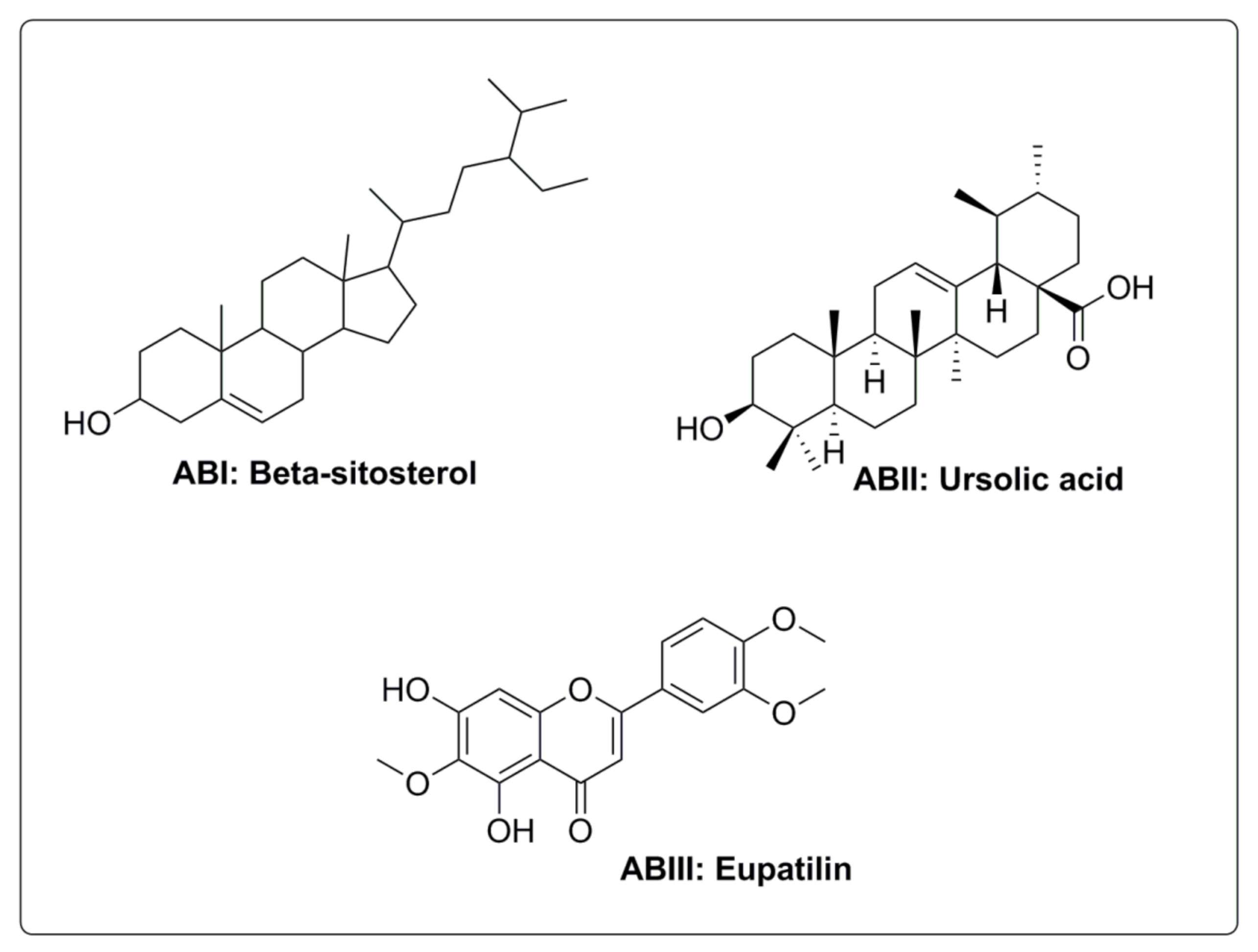
2.9. Isolated Compounds
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Collection of Plant Material and Authentication
4.3. Sample Extraction and Fractionation
4.4. Quantitative Phytochemical Analysis
4.5. Isolation of Bioactive Compounds
4.6. Animals and Ethical Approval
4.7. In Vitro Anticholinesterase Activity
4.8. Acute Toxicity Test
4.9. Induction and Assessment of Diabetes
4.9.1. Drug Treatment and Assessment of Memory
4.9.2. Y-Maze Test
4.9.3. Novel object Recognition Test (NORT)
4.9.4. Estimation of Blood Glucose Level, Brain and Body Weight
4.9.5. Biochemical Estimation
4.9.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Barbosa Filho, J.M.; Medeiros, K.C.P.; Diniz, M.d.F.F.M.; Batista, L.M.; Athayde-Filho, P.F.; Silva, M.S.; da Cunha, E.V.L.; Almeida, J.R.G.S.; Quintans-Júnior, L.J. Natural products inhibitors of the enzyme acetylcholinesterase. Rev. Bras. Farmacogn. 2006, 16, 258–285. [Google Scholar] [CrossRef] [Green Version]
- Bartus, R.T.; Dean, R.L.; Pontecorvo, M.J.; Flicker, C. The cholinergic hypothesis: A historical overview, current perspective, and future directions. Ann. N. Y. Acad. Sci. 1985, 444, 332–358. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T.; Dean, R.L., 3rd; Beer, B.; Lippa, A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, C.M. Type 2 diabetes mellitus, dyslipidemia, and Alzheimer’s disease. J. Alzheimers Dis. 2010, 20, 711–722. [Google Scholar] [CrossRef] [Green Version]
- Velayudhan, L.; Poppe, M.; Archer, N.; Proitsi, P.; Brown, R.G.; Lovestone, S. Risk of developing dementia in people with diabetes and mild cognitive impairment. Br. J. Psychiatry 2010, 196, 36–40. [Google Scholar] [CrossRef]
- Pesce, M.; Tatangelo, R.; La Fratta, I.; Rizzuto, A.; Campagna, G.; Turli, C.; Ferrone, A.; Franceschelli, S.; Speranza, L.; Patruno, A.; et al. Aging-related oxidative stress: Positive effect of memory training. Neuroscience 2018, 370, 246–255. [Google Scholar] [CrossRef]
- Hemmati, A.A.; Alboghobeish, S.; Ahangarpour, A. Effects of cinnamic acid on memory deficits and brain oxidative stress in streptozotocin-induced diabetic mice. Korean J. Physiol. Pharmacol. 2018, 22, 257–267. [Google Scholar] [CrossRef] [Green Version]
- Sultana, R.; Perluigi, M.; Butterfield, D.A. Lipid peroxidation triggers neurodegeneration: A redox proteomics view into the Alzheimer disease brain. Free Radic. Biol. Med. 2013, 62, 157–169. [Google Scholar] [CrossRef] [Green Version]
- Pratico, D. Oxidative stress hypothesis in Alzheimer’s disease: A reappraisal. Trends Pharmacol. Sci. 2008, 29, 609–615. [Google Scholar] [CrossRef]
- Omar, S.H.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. The protective role of plant biophenols in mechanisms of Alzheimer’s disease. J. Nutr. Biochem. 2017, 47, 1–20. [Google Scholar] [CrossRef]
- Zhou, S.-F.; Zhong, W.-Z. Drug Design and Discovery: Principles and Applications. Molecules 2017, 22, 279. [Google Scholar] [CrossRef]
- Muhammad, A.; Nawaz, A. Effects of medicinal plants on Alzheimer’s disease and memory deficits. Neural Regen. Res. 2017, 12, 660–670. [Google Scholar]
- Pandey, A.K.; Singh, P. The Genus Anthemis: A 2012–2017 Literature review on chemical composition, antimicrobial, insecticidal and antioxidant activities of essential oils. Medicines 2017, 4, 68. [Google Scholar] [CrossRef] [Green Version]
- Kshirsagar, S.G.; Rao, R.V. Antiviral and immunomodulation effects of Artemisia. Medicina 2021, 57, 217. [Google Scholar] [CrossRef]
- Obistioiu, D.; Cristina, R.T.; Schmerold, I.; Chizzola, R.; Stolze, K.; Nichita, I.; Chiurciu, V. Chemical characterization by GC-MS and in vitro activity against Candida albicans of volatile fractions prepared from Artemisia dracunculus, Artemisia abrotanum, Artemisia absinthium and Artemisia vulgaris. Chem. Cent. J. 2014, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Semwal, R.B.; Semwal, D.K.; Mishra, S.P.; Semwal, R. Chemical Composition and Antibacterial Potential of Essential Oils from Artemisia capillaris, Artemisia nilagirica, Citrus limon, Cymbopogon flexuosus, Hedychium spicatum and Ocimum tenuiflorum. Nat. Prod. J. 2015, 5, 199–205. [Google Scholar] [CrossRef]
- Bisht, D.; Kumar, D.; Kumar, D.; Dua, K.; Chellappan, D.K. Phytochemistry and pharmacological activity of the genus artemisia. Arch. Pharm. Res. 2021, 24, 1–36. [Google Scholar] [CrossRef]
- Orege, J.I.; Adeyemi, S.B.; Tiamiyu, B.B.; Akinyemi, T.O.; Ibrahim, Y.A.; Orege, O.B. Artemisia and Artemisia-based products for COVID-19 management: Current state and future perspective. Adv. Tradit. Med. 2021, 5, 1–12. [Google Scholar] [CrossRef]
- Nigam, M.; Atanassova, M.; Abhay, P.M.; Pezzani, R.; Devkota, H.P.; Plygun, S.; Salehi, B.; Setzer, W.N.; Sharifi-Rad, J. Bioactive compounds and health benefits of Artemisia species. Nat. Prod. Commun. 2019, 14, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.W.-A.; Ghias, M.; Shoaib, M.; Ali, N.; Shah, I.; Umar, M.N.; Shah, S.M.M.; Shah, S.M.H.; Khan, W.; Khan, S.; et al. Antidiabetic potential of flavonoids from Artemisia macrocephalla Jaquem in streptozotocin-induced diabetic rats: Pharmacological and biochemical approach. Pak. J. Pharm. Sci. 2019, 32, 2865–2871. [Google Scholar]
- Hasanein, P.; Shahidi, S. Effects of Hypericum perforatum extract on diabetes-induced learning and memory impairment in rats. Phytother. Res. 2011, 25, 544–549. [Google Scholar] [CrossRef]
- Hasanein, P.; Shahidi, S. Effects of combined treatment with vitamins C and E on passive avoidance learning and memory in diabetic rats. Neurobiol. Learn. Mem. 2010, 93, 472–478. [Google Scholar] [CrossRef]
- Bachman, D.L.; Wolf, P.A.; Linn, R.; Knoefel, J.E.; Cobb, S.J.; Belanger, A.; D’agostino, R.B.; White, L.R. Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology 1992, 42, 115–119. [Google Scholar] [CrossRef]
- Larrazábal-Fuentes, M.J.; Fernández-Galleguillos, C.; Palma-Ramírez, J.; Romero-Parra, J.; Sepúlveda, K.; Galetovic, A.; González, J.; Paredes, A.; Bórquez, J.; Simirgiotis, M.J.; et al. Chemical profiling, antioxidant, anticholinesterase, and antiprotozoal potentials of Artemisia copa Phil (Asteraceae). Front. Pharmacol. 2020, 4, 1911. [Google Scholar] [CrossRef]
- Kassab, S.; Begley, P.; Church, S.J.; Rotariu, S.M.; Chevalier-Riffard, C.; Dowsey, A.W.; Phillips, A.M.; Zeef, L.A.; Grayson, B.; Neill, J.C.; et al. Cognitive dysfunction in diabetic rats is prevented by pyridoxamine treatment. A multidisciplinary investigation. Mol. Metab. 2019, 28, 107–119. [Google Scholar] [CrossRef]
- Li, J.; Cesari, M.; Liu, F.; Dong, B.; Vellas, B. Effects of diabetes mellitus on cognitive decline in patients with Alzheimer disease: A systematic review. Can. J. Diabetes 2017, 41, 114–119. [Google Scholar] [CrossRef] [Green Version]
- Kamal, A.; Biessels, G.J.; Duis, S.E.; Gispen, W.H. Learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: Interaction of diabetes and ageing. Diabetologia 2000, 43, 500–506. [Google Scholar] [CrossRef] [Green Version]
- Hasanein, P.Z.; Felehgari, A. Emamjomeh, Preventive effects of Salvia officinalis L. against learning and memory deficit induced by diabetes in rats: Possible hypoglycaemic and antioxidant mechanisms. Neurosci. Lett. 2016, 622, 72–77. [Google Scholar] [CrossRef]
- Sharafati-Chaleshtori, R.; Nickdasti, A.; Mortezapour, E.; Pourhanifeh, M.H.; Ghazanfari, M.; Movahedpour, A.; Khatami, A.; Ashrafizadeh, M.; Zarrabi, A.; Mahabady, M.K.; et al. Artemisia Species as a New Candidate for Diabetes Therapy: A Comprehensive Review. Curr. Mol. Med. 2021, 21, 832–849. [Google Scholar] [CrossRef]
- Reddy, V.P.; Zhu, X.; Perry, G.; Smith, M.A. Oxidative stress in diabetes and Alzheimer’s disease. J. Alzheimers Dis. 2009, 16, 763–774. [Google Scholar] [CrossRef] [Green Version]
- Samarghandian, S.; Azimi-Nezhad, M.; Samini, F. Ameliorative effect of saffron aqueous extract on hyperglycemia, hyperlipidemia, and oxidative stress on diabetic encephalopathy in streptozotocin induced experimental diabetes mellitus. BioMed Res. Int. 2014, 2014, 920857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albasher, G.; Aljarba, N.; Al Sultan, N.; Alqahtani, W.S.; Alkahtani, S. Evaluation of the neuro-protective effect of Artemisia judaica extract in a murine diabetic model. J. Food Biochem. 2020, 44, e13337. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, H.B.; Mousa-Al-Reza Hadjzadeh, V.H.; Shiravi, A.; Hosseinian, S.; Vaezi, G. The role of Artemisia turanica extract on renal oxidative and biochemical markers in STZ-induced diabetes in rat. Avicenna J. Phytomed. 2020, 10, 504–512. [Google Scholar]
- Mworia, J.K.; Kibiti, C.M.; Ngugi, M.P.; Ngeranwa, J.N. Antipyretic potential of dichloromethane leaf extract of Eucalyptus globulus (Labill) and Senna didymobotrya (Fresenius) in rats models. Heliyon 2019, 5, e02924. [Google Scholar] [CrossRef] [Green Version]
- Hashmi, W.J.; Hammad, I.; Farrukh, M.; Bushra, M. Neuroprotective, antidiabetic and antioxidant effect of Hedera nepalensis and lupeol against STZ+ AlCl 3 induced rats model. DARU J. Pharm. Sci. 2018, 26, 179–190. [Google Scholar] [CrossRef]
- Bredie, S.J.; Jong, M.C. No significant effect of ginkgo biloba special extract EGb 761 in the treatment of primary Raynaud phenomenon: A randomized controlled trial. J. Cardiovasc. Pharmacol. 2012, 59, 215–221. [Google Scholar] [CrossRef]
- Kharoubi, O.; Slimani, M.; Aoues, A. Neuroprotective effect of wormwood against lead exposure. J. Emerg. Trauma Shock 2011, 4, 82–88. [Google Scholar] [CrossRef]
- Kang, J.Y.; Lee, D.S.; Park, S.K.; Ha, J.S.; Kim, J.M.; Ha, G.J.; Seo, W.T.; Heo, H.J. Cognitive function of Artemisia argyi H. Fermented by Monascus purpureus under TMT-induced learning and memory deficits in ICR mice. Evid.-Based Complement. Altern. 2017, 2017, 5809370. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.-Y.; Li, L.; Hao, Q.-M.; Qin, Y.; Ma, C.-S. β-Sitosterol treatment attenuates cognitive deficits and prevents amyloid plaque deposition in amyloid protein precursor/presenilin 1 mice. Korean J. Physiol. Pharmacol. 2020, 24, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Vezza, T.; Canet, F.; de Marañón, A.M.; Bañuls, C.; Rocha, M.; Víctor, V.M. Phytosterols: Nutritional Health Players in the Management of Obesity and Its Related Disorders. Antioxidants 2020, 9, 1266. [Google Scholar] [CrossRef]
- Bakoyiannis, I.; Daskalopoulou, A.; Pergialiotis, V.; Perrea, D. Phytochemicals and cognitive health: Are flavonoids doing the trick? Biomed. Pharmacother. 2019, 109, 1488–1497. [Google Scholar] [CrossRef]
- Macready, A.L.; Kennedy, O.B.; Ellis, J.A.; Williams, C.M.; Spencer, J.P.E.; Butler, L.T. Flavonoids and cognitive function: A review of human randomized controlled trial studies and recommendations for future studies. Genes Nutr. 2009, 4, 227–242. [Google Scholar] [CrossRef] [Green Version]
- Maciel, R.M.; Carvalho, F.B.; Olabiyi, A.A.; Schmatz, R.; Gutierres, J.M.; Stefanello, N.; Zanini, D.; Rosa, M.M.; Andrade, C.M.; Rubin, M.; et al. Neuroprotective effects of quercetin on memory and anxiogenic-like behavior in diabetic rats: Role of ectonucleotidases and acetylcholinesterase activities. Biomed. Pharmacother. 2016, 84, 559–568. [Google Scholar] [CrossRef]
- Uriarte-Pueyo, I.; Calvo, M.I. Flavonoids as acetylcholinesterase inhibitors. Curr. Med. Chem. 2011, 18, 5289–5302. [Google Scholar] [CrossRef]
- Batool, R.; Khan, M.R.; Sajid, M.; Ali, S.; Zahra, Z. Estimation of phytochemical constituents and in vitro antioxidant potencies of Brachychiton populneus. R. Br. BMC Chem. 2019, 13, 1–15. [Google Scholar]
- Nasim, S.; Shah, M.; Shah, S.W.A.; Ahmed, M.N.; Ahmad, M.; Anwar, N.; Ghias, M. Activity guided isolation and mechanistic approach towards analgesic potential of Chenopodium mediated through opioidergic pathway. Pak. J. Pharm. Sci. 2021, 34, 197–203. [Google Scholar]
- Shoaib, M.; Shah, S.W.A.; Ali, N.; Shah, I.; Umar, M.N.; Ayaz, M.; Tahir, M.N.; Akhtar, S. In vitro enzyme inhibition potentials and antioxidant activity of synthetic flavone derivatives. J. Chem. 2015, 2015, 516878. [Google Scholar] [CrossRef] [Green Version]
- Shoaib, M.; Shah, I.; Ali, N.; Shah, S.W.A. In vitro acetylcholinesterase and butyrylcholinesterase inhibitory potentials of essential oil of Artemisia macrocephala. J. Pharmacol. 2015, 10, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Lorke, D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983, 54, 275–287. [Google Scholar] [CrossRef]
- Ekiert, H.; Świątkowska, J.; Knut, E.; Klin, P.; Rzepiela, A.; Tomczyk, M.; Szopa, A. Artemisia dracunculus (Tarragon): A review of its traditional uses, phytochemistry and pharmacology. Front. Pharmacol. 2021, 12, 653993. [Google Scholar] [CrossRef]
- Szopa, A.; Pajor, J.; Klin, P.; Rzepiela, A.; Elansary, H.O.; Al-Mana, F.A.; Mattar, M.A.; Ekiert, H. Artemisia absinthium L.—Importance in the history of medicine, the latest advances in phytochemistry and therapeutical, cosmetological and culinary uses. Plants 2020, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Babaei-Balderlou, F.S. Zare. Melatonin improves spatial navigation memory in male diabetic rats. In Veterinary Research Forum; Faculty of Veterinary Medicine, Urmia University: Urmia, Iran, 2012. [Google Scholar]
- Callahan, P.M.; Terry, A.V., Jr.; Peitsch, M.C.; Hoeng, J.; Koshibu, K. Differential effects of alkaloids on memory in rodents. Sci. Rep. 2021, 11, 9843. [Google Scholar] [CrossRef] [PubMed]
- Pahaye, D.B.; Bum, E.N.; Taïwé, G.S.; Ngoupaye, G.T.; Sidiki, N.; Moto, F.C.O.; Kouemou, N.; Njapdounke, S.J.K.; Nkantchoua, G.; Kandeda, A.; et al. Neuroprotective and antiamnesic effects of Mitragyna inermis willd (Rubiaceae) on scopolamine-induced memory impairment in mice. Behav. Neurol. 2017, 2017, 5952897. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Sample | TPC (mg GAE/g) | TFC (mg QE/g) | TTC (mg GAE/g) |
|---|---|---|---|
| Crd-Am | 59.11 ± 0.53 | 49.31 ± 0.56 | 66.31 ± 0.91 |
| Nhex-Am | 36.09 ± 0.73 | 28.21 ± 0.71 | 14.09 ± 0.73 |
| Chl-Am | 71.17 ± 0.79 | 59.50 ± 1.01 | 39.70 ± 0.88 |
| Et-Am | 63.89 ± 0.83 | 51.33 ± 0.98 | 38.21 ± 0.67 |
| But-Am | 43.13 ± 0.91 | 33.26 ± 0.67 | 48.67 ± 1.01 |
| Aq-Am | 34.27 ± 0.67 | 28.19 ± 0.89 | 42.15 ± 0.71 |
| Sample | Yield (%) | |||
|---|---|---|---|---|
| Alkaloids | Flavonoids | Saponins | Terpenoids | |
| Crd-Am | 4.11 ± 0.48 | 8.63 ± 0.86 | 4.75 ± 0.73 | 8.03 ± 0.79 |
| nhex-Am | 0.88 ± 0.33 | 2.07 ± 0.61 | 3.72 ± 0.65 | 6.54 ± 0.81 |
| Chl-Am | 3.09 ± 0.28 | 8.58 ± 0.87 | 4.22 ± 0.73 | 7.39 ± 0.79 |
| Et-Am | 2.11 ± 0.39 | 8.01 ± 0.73 | 3.97 ± 0.67 | 7.05 ± 0.71 |
| But-Am | 1.13 ± 0.71 | 4.11 ± 0.67 | 2.90 ± 0.57 | 4.17 ± 0.58 |
| Aq-Am | 1.68 ± 0.67 | 2.39 ± 0.61 | 2.04 ± 0.38 | 5.96 ± 0.71 |
| Sample Test | Dose (mg/kg b.w.) | Body Weight | Brain Weight | Blood Glucose Level |
|---|---|---|---|---|
| Control | 24.21 ± 1.12 | 9.09 ± 2.19 | 104.21 ± 2.19 | |
| Diabetic | 20.39 ± 2.01 | 12.11 ± 1.98 ††† | 244.89 ± 2.95 ††† | |
| Crd-Am | 100 | 23.19 ± 1.76 | 9.79 ± 1.03 *** | 143.94 ± 2.19 * |
| 200 | 24.11 ± 1.14 | 10.51 ± 1.91 *** | 131.18 ± 2.13 ** | |
| Chl-Am | 75 | 23.39 ± 1.06 | 9.96 ± 1.41 * | 135.76 ± 2.22 * |
| 150 | 23.14 ± 1.11 | 10.19 ± 1.21 ** | 130.92 ± 1.84 ** | |
| Et-Am | 75 | 23.29 ± 1.15 | 9.89 ± 1.15 * | 140.86 ± 1.67 ** |
| 150 | 22.59 ± 1.12 | 10.11± 1.97 ** | 135.01 ± 1.91 ** | |
| Donepezil | 2 | 24.18 ± 1.17 | 10.13 ± 1.15 *** | 196.88 ± 2.38 *** |
| Metformin | 50 | 24.89 ± 1.91 | 9.91 ± 1.51 *** | 162.01 ± 2.83 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bari, A.; Shah, S.M.M.; Al-Joufi, F.A.; Shah, S.W.A.; Shoaib, M.; Shah, I.; Zahoor, M.; Ahmed, M.N.; Ghias, M.; Shah, S.M.H.; et al. Effects of Artemisia macrocephala Jacquem on Memory Deficits and Brain Oxidative Stress in Streptozotocin-Induced Diabetic Mice. Molecules 2022, 27, 2399. https://doi.org/10.3390/molecules27082399
Bari A, Shah SMM, Al-Joufi FA, Shah SWA, Shoaib M, Shah I, Zahoor M, Ahmed MN, Ghias M, Shah SMH, et al. Effects of Artemisia macrocephala Jacquem on Memory Deficits and Brain Oxidative Stress in Streptozotocin-Induced Diabetic Mice. Molecules. 2022; 27(8):2399. https://doi.org/10.3390/molecules27082399
Chicago/Turabian StyleBari, Atiqul, Syed Muhammad Mukarram Shah, Fakhria A. Al-Joufi, Syed Wadood Ali Shah, Mohammad Shoaib, Ismail Shah, Muhammad Zahoor, Muhammad Naeem Ahmed, Mehreen Ghias, Syed Muhammad Hassan Shah, and et al. 2022. "Effects of Artemisia macrocephala Jacquem on Memory Deficits and Brain Oxidative Stress in Streptozotocin-Induced Diabetic Mice" Molecules 27, no. 8: 2399. https://doi.org/10.3390/molecules27082399
APA StyleBari, A., Shah, S. M. M., Al-Joufi, F. A., Shah, S. W. A., Shoaib, M., Shah, I., Zahoor, M., Ahmed, M. N., Ghias, M., Shah, S. M. H., & Khalil, A. A. K. (2022). Effects of Artemisia macrocephala Jacquem on Memory Deficits and Brain Oxidative Stress in Streptozotocin-Induced Diabetic Mice. Molecules, 27(8), 2399. https://doi.org/10.3390/molecules27082399






